CHAPTER 94 Immunization and Prophylaxis
CHAPTER 94 Immunization and Prophylaxis
IMMUNIZATION
Childhood immunization has markedly reduced the impact of major infectious diseases. Active immunization induces immunity by vaccination with a vaccine or toxoid (inactivated toxin). Passive immunization includes transplacental transfer of maternal antibodies and the administration of antibody, either as immunoglobulin or monoclonal antibody.
Vaccinations may be with live attenuated viruses (measles, mumps, rubella, varicella, nasal influenza), inactivated or killed viruses (polio, hepatitis A, intramuscular influenza), recombinant products (hepatitis B], human papillomavirus), reassortants (rotavirus), or immunogenic components of bacteria (pertussis, Haemophilus influenzae type b, and Streptococcus pneumoniae), including toxoids (diphtheria, tetanus). Many purified polysaccharides are T-independent antigens that initiate B-cell proliferation without involvement of CD4 T lymphocytes and are poor immunogens in children younger than 2 years of age. Conjugation of a polysaccharide to a protein carrier induces a T-dependent response in infants and creates immunogenic vaccines for H. influenzae type b, S. pneumoniae, and Neisseria meningitidis.
Childhood immunization standards and recommendations in the United States (Figs. 94-1 and 94-2) are formulated by the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP). In the United States, due to state laws requiring immunization for school entry, approximately 95% of children entering kindergarten are vaccinated for the common infectious diseases. The ACIP recommends that children in the United States routinely receive vaccines against 16 diseases (see Fig. 94-1). This schedule includes up to 21 injections in four to five visits by 18 months of age. Children and adolescents who are at increased risk for pneumococcal infections should receive the pneumococcal polysaccharide vaccine, as well. Children who are behind in immunization should receive catch-up immunizations as rapidly as feasible. Infants born prematurely, regardless of birth weight, should be vaccinated at the same chronologic age and according to the same schedule as full-term infants and children. The single exception to this practice is providing hepatitis B vaccine for infants weighing less than 2000 g if the mother is hepatitis B virus surface antigen (HBsAg)–negative at 1 month instead of at birth. Vaccines for adolescents should be given at 11 to 12 years of age. Human papillomavirus vaccine is recommended for all girls at 11 to 12 years of age for the prevention of cervical cancer, precancerous or dysplastic lesions, and genital warts caused by human papillomavirus types 6, 11, 16, and 18. This vaccine may be administered to girls as young as 9 years of age and to women up to 26 years of age. Many colleges and universities require or recommend meningococcal vaccine before matriculation because of the increased risk of meningococcal disease, especially for freshmen living in dormitories.

Figure 94-1 Recommended immunization schedule for persons 0 to 6 years of age, United States (2010). Recommended immunization schedules are approved by the Advisory Committee on Immunization Practices (http://www.cdc.gov/nip/acip), the American Academy of Pediatrics (http://www.aap.org), and the American Academy of Family Physicians (http://www.aafp.org).
(From http://aapredbook.aappublications.org/resources/2010_0-6yrs_Schedule_FINAL.pdf)
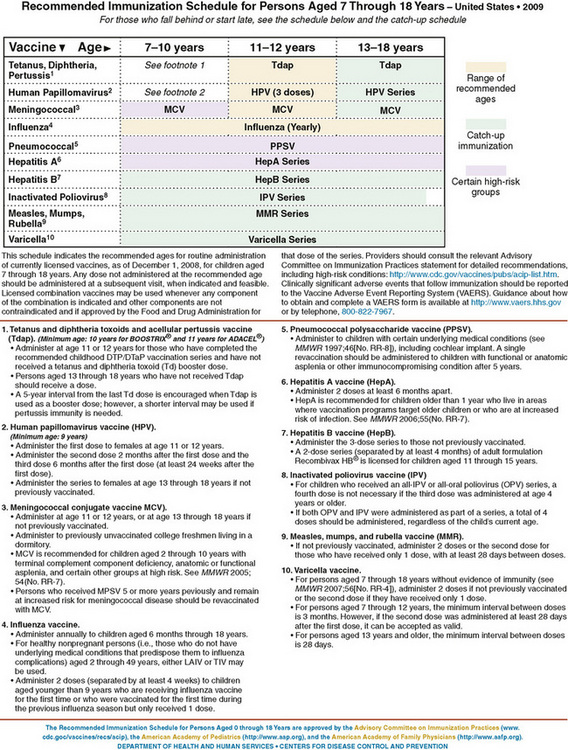
Figure 94-2 Recommended immunization schedule for persons 7 to 18 years of age, United States (2010). Recommended immunization schedules are approved by the Advisory Committee on Immunization Practices (http://www.cdc.gov/nip/acip), the American Academy of Pediatrics (http://www.aap.org), and the American Academy of Family Physicians (http://www.aafp.org).
(From http://aapredbook.aappublications.org/resources/2010_7-18yrs_Schedule_FINAL.pdf)
Vaccines should be administered after obtaining informed consent. The National Childhood Vaccine Injury Act requires that all health care providers provide parents or patients with copies of Vaccine Information Statements prepared by the Centers for Disease Control and Prevention (http://www.cdc.gov/vaccines/pubs/vis/default.htm) before administering each vaccine dose.
Most vaccines are administered by intramuscular or subcutaneous injection. The preferred sites for administration are the anterolateral aspect of the thigh in infants and the deltoid region in children and adults. Multiple vaccines can be administered simultaneously at anatomically separate sites (different limbs, or separated by >1 in) without diminishing the immune response. Measles, mumps, and rubella (MMR) and varicella vaccines should be administered simultaneously or more than 30 days apart. Administration of blood products and immunoglobulin can diminish response to live virus vaccines if administered before the recommended interval.
General contraindications to vaccination include serious allergic reaction (anaphylaxis) after a previous vaccine dose or to a vaccine component, immunocompromised states or pregnancy (live virus vaccines), and moderate or severe acute illness with or without fever. History of anaphylactic-like reactions to eggs is a contraindication to influenza and yellow fever vaccines, which are produced in embryonated chicken eggs. Current preparations of measles and mumps vaccines, which are produced in chick embryo fibroblast tissue culture, do not contain significant amounts of egg proteins and may be administered without testing children with history of egg allergy. Mild acute illness, with or without fever, convalescent phase of illness, recent exposure to infectious diseases, current antimicrobial therapy, breastfeeding, mild to moderate local reaction or low-grade to moderate fever after previous vaccination, and history of penicillin or other nonvaccine allergy or receiving allergen extract immunotherapy are not contraindications to immunization.
Severe immunosuppression resulting from congenital immunodeficiency, human immunodeficiency virus (HIV) infection, leukemia, lymphoma, cancer therapy, or a prolonged course of high-dose corticosteroids (>2 mg/kg/day for ≥2 weeks) predisposes to complications and is a contraindication for live virus vaccines. For HIV-infected children who do not have evidence of severe immunosuppression, MMR vaccination is recommended at 12 months of age with a second dose 1 month later rather than waiting until 4 to 6 years of age. Varicella vaccine is contraindicated for persons with cellular immunodeficiency but is recommended for persons with impaired humoral immunity (hypogammaglobulinemia or dysgammaglobulinemia) and at 12 months of age for HIV-infected children without evidence of severe immunosuppression, given as two doses 3 months apart.
The National Childhood Vaccine Injury Act requires that clinically significant adverse events after vaccination be reported to the Vaccine Adverse Event Reporting System (VAERS) (http://vaers.hhs.gov or 800-822-7967). Suspected cases of vaccine-preventable diseases should be reported to state or local health departments. The Act also established the National Vaccine Injury Compensation Program, a no-fault system in which persons thought to have suffered an injury or death as a result of administration of a covered vaccine can seek compensation.
PROPHYLAXIS
Prophylaxis may include antibiotics, immunoglobulin or monoclonal antibody, vaccine, or in combination and may be used postexposure, for perinatal exposure, and pre-exposure for persons at increased risk for infection. Primary prophylaxis is used to prevent infection before a first occurrence. Secondary prophylaxis is used to prevent recurrence after a first episode.
Meningococcus
Primary prophylaxis to all contacts of index cases of N. meningitidis infection should be administered as soon as possible (see Chapter 100). Prophylaxis is recommended for all household contacts, especially young children; child care or nursery school contacts in the previous 7 days; for direct exposure to the index patient’s secretions through kissing or sharing of toothbrushes or eating utensils; and for mouth-to-mouth resuscitation or unprotected contact during endotracheal intubation within 7 days before onset of illness. Prophylaxis is also recommended for contacts who frequently sleep or eat in the same dwelling as the index patient or passengers seated directly next to the index case during airline flights lasting longer than 8 hours. Chemoprophylaxis is not recommended for casual contacts with no history of direct exposure to the patient’s oral secretions (school or work mate), indirect contact with the index patient, or medical personnel without direct exposure to the patient’s oral secretions. Rifampin twice daily for 2 days, ceftriaxone once, and ciprofloxacin once (>18 years of age) are the recommended regimens.
Tetanus
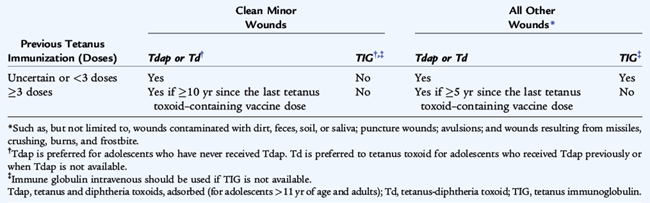
All postexposure wound treatment begins with immediate, thorough cleansing using soap and water, removal of foreign bodies, and débridement of devitalized tissue. Tetanus prophylaxis after wounds and injuries includes vaccination of persons with incomplete immunization and tetanus immunoglobulin for contaminated wounds (soil, feces, saliva), puncture wounds, avulsions, and wounds resulting from missiles, crushing, burns, and frostbite (Table 94-1).
Rabies
Rabies immune globulin (RIG) and rabies vaccine are extremely effective for prophylaxis after exposure to rabies but are of no known benefit after symptoms appear. Because rabies is one of the deadliest infections, recognition of potential exposure and prophylaxis are crucial. Any healthy-appearing domestic animal responsible for an apparently unprovoked bite should be observed for 10 days for signs of rabies, without immediate treatment of the victim. Prophylaxis should be administered if the animal is rabid or suspected to be rabid, or if the animal develops signs of rabies while under observation. A captured wild animal should be euthanized (by animal control officials) without a period of observation and its brain examined for evidence of rabies. If the biting animal is not captured, particularly if it is a wild animal of a species known to harbor the virus in the region, rabies should be presumed and prophylaxis administered to the victim. Skunks, raccoons, foxes, woodchucks, most other carnivores, and bats are regarded as rabid unless proved negative by testing. Prophylaxis also should be provided following exposure to a bat for persons who might be unaware or unable to relate that a bite or direct contact has occurred, such as a mentally disabled person, a sleeping child, or an unattended infant.
All rabies postexposure management begins with immediate thorough cleansing of the bite using soap and water and, if available, irrigation with a virucidal agent such as povidone-iodine. RIG at a dose of 20 U/kg should be administered, with the full dose of RIG infiltrated subcutaneously into the area around the wound, if possible. Any remaining RIG that cannot be infiltrated into the wound should be administered as an intramuscular injection. Inactivated rabies vaccine should be administered simultaneously as soon as possible, with additional vaccine doses at 3, 7, and 14 days.