CHAPTER 143 Acyanotic Congenital Heart Disease
CHAPTER 143 Acyanotic Congenital Heart Disease
ETIOLOGY AND EPIDEMIOLOGY
Congenital heart disease occurs in 8 per 1000 births. The spectrum of lesions ranges from asymptomatic to fatal. Although most cases of congenital heart disease are multifactorial, some lesions are associated with chromosomal disorders, single gene defects, teratogens, or maternal metabolic disease (see Table 139-2).
Congenital heart defects can be divided into three pathophysiologic groups (Table 143-1):
Acyanotic congenital heart disease includes left-to-right shunts resulting in an increase in pulmonary blood flow (patent ductus arteriosus [PDA], ventricular septal defect [VSD], atrial septal defect [ASD]) and obstructive lesions (aortic stenosis, pulmonary stenosis, coarctation of the aorta), which usually have normal pulmonary blood flow.
VENTRICULAR SEPTAL DEFECT
Etiology and Epidemiology
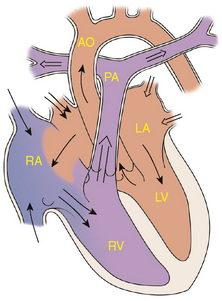
The ventricular septum is a complex structure that can be divided into four components. The largest component is the muscular septum. The inlet or posterior septum comprises endocardial cushion tissue. The subarterial or supracristal septum comprises conotruncal tissue. The membranous septum is below the aortic valve and is relatively small. VSDs occur when any of these components fails to develop normally (Fig. 143-1). VSD, the most common congenital heart defect, accounts for 25% of all congenital heart disease. Perimembranous VSDs are the most common of all VSDs (67%).
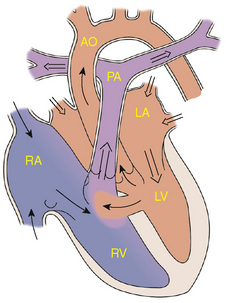
Figure 143-1 Ventricular septal defect. AO, aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle.
Although the location of the VSD is important prognostically and in approach to repair, the amount of flow crossing a VSD depends on the size of the defect and the pulmonary vascular resistance. Large VSDs are not symptomatic at birth because the pulmonary vascular resistance is normally elevated at this time. As the pulmonary vascular resistance decreases over the first 6 to 8 weeks of life the amount of shunt increases, and symptoms may develop.
Clinical Manifestations
The size of the VSD affects the clinical presentation. Small VSDs with little shunt are often asymptomatic but have a loud murmur. Moderate to large VSDs result in pulmonary overcirculation and heart failure, presenting as fatigue, diaphoresis with feedings, and poor growth. The typical physical finding with a VSD is a pansystolic murmur, usually heard best at the lower left sternal border. There may be a thrill. Large shunts increase flow across the mitral valve causing a mid-diastolic murmur at the apex. The splitting of S2 and intensity of P2 depend on the pulmonary artery pressure.
Imaging Studies
Electrocardiogram (ECG) and chest x-ray findings depend on the size of the VSD. Small VSDs usually have normal studies. Larger VSDs cause volume overload to the left side of the heart, resulting in ECG findings of left atrial and ventricular enlargement and hypertrophy. A chest x-ray may reveal cardiomegaly, enlargement of the left ventricle, an increase in the pulmonary artery silhouette, and increased pulmonary blood flow. Pulmonary hypertension due to either increased flow or increased pulmonary vascular resistance may lead to right ventricular enlargement and hypertrophy.
Treatment
Approximately one third of all VSDs close spontaneously. Small VSDs usually close spontaneously and, if they do not close, surgical closure may not be required. Initial treatment for moderate to large VSDs includes diuretics, digoxin, and afterload reduction. Continued poor growth or pulmonary hypertension despite therapy requires closure of the defect. Most VSDs are closed surgically, but some VSDs, especially muscular defects, can be closed with devices placed at cardiac catheterization.
ATRIAL SEPTAL DEFECT
Etiology and Epidemiology
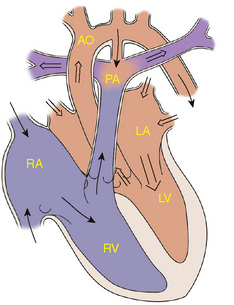
During the embryologic development of the heart, a septum grows toward the endocardial cushions to divide the atria. Failure of septal growth or excessive reabsorption of tissue leads to ASDs (Fig. 143-2). ASDs represent approximately 10% of all congenital heart defects. A secundum defect, with the hole in the region of the foramen ovale, is the most common ASD. A primum ASD, located near the endocardial cushions, may be part of a complete atrioventricular canal defect or may be present with an intact ventricular septum. The least common ASD is the sinus venosus defect, which may be associated with anomalous pulmonary venous return.
Clinical Manifestations
Regardless of the site of the ASD, the pathophysiology and amount of shunting depend on the size of the defect and the relative compliance of the both ventricles. Even with large ASDs and significant shunts, infants and children are rarely symptomatic. A prominent right ventricular impulse at the left lower sternal border (LLSB) often can be palpated. A soft (grade I or II) systolic ejection murmur in the region of the right ventricular outflow tract and a fixed split S2 (due to overload of the right ventricle with prolonged ejection into the pulmonary circuit) are often audible. A larger shunt may result in a mid-diastolic murmur at the left lower sternal border as a result of the increased volume passing across the tricuspid valve.
Imaging Studies
ECG and chest x-ray findings reflect the increased blood flow through the right atrium, right ventricle, pulmonary arteries, and lungs. The ECG may show right axis deviation and right ventricular enlargement. A chest radiograph may show cardiomegaly, right atrial enlargement, and a prominent pulmonary artery.
Treatment
Medical management is rarely indicated. If a significant shunt is still present at around 3 years of age, closure is usually recommended. Many secundum ASDs can be closed with an ASD closure device in the catheterization laboratory. Primum and sinus venosus defects require surgical closure.
PATENT DUCTUS ARTERIOSUS
Etiology and Epidemiology
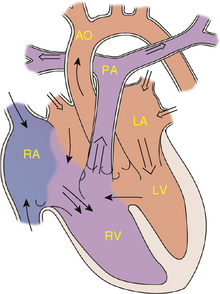
The ductus arteriosus allows blood to flow from the pulmonary artery to the aorta during fetal life. Failure of the normal closure of this vessel results in a PDA (Fig. 143-3). With a falling pulmonary vascular resistance after birth, left-to-right shunting of blood and increased pulmonary blood flow occur. Excluding premature infants, PDAs represent approximately 5% to 10% of congenital heart disease.
Clinical Manifestations
Symptoms depend on the amount of pulmonary blood flow. The magnitude of the shunt depends on the size of the PDA (diameter, length, and tortuosity) and the pulmonary vascular resistance. Small PDAs are asymptomatic; moderate to large shunts can produce the symptoms of heart failure as the pulmonary vascular resistance decreases.
The physical examination findings depend on the size of the shunt. A widened pulse pressure is often present as a result of the runoff of blood into the pulmonary circulation during diastole. A continuous, machine-like murmur can be heard at the left infraclavicular area. The murmur radiates along the pulmonary arteries and is often well heard over the left side of the back. Larger shunts with increased flow across the mitral valve may result in a mid-diastolic murmur at the apex and a hyperdynamic precordium. Splitting of S2 and intensity of P2 depend on the pulmonary artery pressure. A thrill may be palpable.
Imaging Studies
ECG and chest x-ray findings are normal with small PDAs. Moderate to large shunts may result in a full pulmonary artery silhouette and increased pulmonary vascularity. ECG findings vary from normal to evidence of left ventricular hypertrophy. If pulmonary hypertension is present, there is also right ventricular hypertrophy.
Treatment
Spontaneous closure of a PDA after a few weeks of age is uncommon in full-term infants. Moderate and large PDAs may be managed initially with diuretics, but eventually require closure. Elective closure of small, hemodynamically insignificant PDAs is controversial. Most PDAs can be closed in the catheterization laboratory by either coil embolization or a PDA closure device.
ENDOCARDIAL CUSHION DEFECT
Etiology and Epidemiology
Endocardial cushion defects, also referred to as atrioventricular canal defects, may be complete or partial (Fig. 143-4). Abnormal development of the endocardial cushion tissue results in failure of the septum to fuse with the endocardial cushion; this results in abnormal atrioventricular valves as well. The complete defect results in a primum ASD, a posterior or inlet VSD, and clefts in the anterior leaflet of the mitral and septal leaflet of the tricuspid valves. In addition to left-to-right shunting at both levels, there may be atrioventricular valve insufficiency.
Clinical Manifestations
The symptoms of heart failure usually develop as the pulmonary vascular resistance decreases over the first 6 to 8 weeks of life. Symptoms may be earlier and more severe with significant atrioventricular valve insufficiency. Pulmonary hypertension resulting from increased pulmonary circulation often develops early. The presence of murmurs varies depending on the amount of shunting at both atrial and ventricular levels. If there is a large VSD component, S2 will be single. Growth is usually poor. Some children with Down syndrome have complete endocardial cushion defects.
PULMONARY STENOSIS
Etiology
Pulmonary stenosis accounts for approximately 10% of all congenital heart disease and can be valvular, subvalvular, or supravalvular in nature. Pulmonary stenosis results from the failure of the development, in early gestation, of the three leaflets of the valve, insufficient resorption of infundibular tissue, or insufficient canalization of the peripheral pulmonary arteries.
Clinical Manifestations
Symptoms depend on the degree of obstruction present. Infants and children with mild pulmonary stenosis are asymptomatic. Moderate to severe stenosis results in exertional dyspnea and easy fatigability. Newborns with severe stenosis may be more symptomatic and even cyanotic because of right-to-left shunting at the atrial level.
Pulmonary stenosis causes a systolic ejection murmur at the second left intercostal space which radiates to the back. A thrill may be present. S2 may be widely split with a quiet pulmonary component. With more severe pulmonary stenosis, an impulse at the lower left sternal border results from right ventricular hypertrophy. Valvular stenosis may result in a click that varies with respiration. Worsening stenosis causes an increase in the duration of the murmur and a higher frequency of the sound. Murmurs of peripheral pulmonary stenosis vary with the location of the lesions. The systolic ejection murmur is heard distal to the obstruction along the course of blood flow in the pulmonary circulation including radiation to the back.
Imaging Tests
ECG and chest x-ray findings are normal in mild stenosis. Moderate to severe stenosis results in right axis deviation and right ventricular hypertrophy. The heart size is usually normal on chest x-ray, although the main pulmonary artery segment may be prominent because of poststenotic dilation of the main pulmonary artery. Echocardiography provides assessment of the site of stenosis, degree of hypertrophy, and valve morphology and an estimate of the pressure gradient.
Treatment
Valvular pulmonary stenosis usually does not progress, especially if it is mild. Balloon valvuloplasty is usually successful in reducing the gradient to acceptable levels for more significant or symptomatic stenosis. Surgical repair is required if balloon valvuloplasty is unsuccessful or when subvalvular (muscular) stenosis is present.
AORTIC STENOSIS
Etiology and Epidemiology
Valvular, subvalvular, or supravalvular aortic stenosis represents approximately 5% of all congenital heart disease. Lesions result from failure of development of the three leaflets or failure of resorption of tissue around the valve.
Clinical Manifestations
Symptoms depend on the degree of stenosis. Mild to moderate obstructions cause no symptoms. More severe stenosis results in easy fatigability, exertional chest pain, and syncope. Infants with critical aortic stenosis may present with symptoms of heart failure.
A systolic ejection murmur is heard at the right second intercostal space along the sternum and radiating into the neck. The murmur increases in length and becomes higher in frequency as the degree of stenosis increases. With valvular stenosis, a systolic ejection click often is heard, and a thrill may be present at the right upper sternal border or in the suprasternal notch. The aortic component of S2 may be decreased in intensity.
Imaging Studies
ECG and chest x-ray findings are normal with mild degrees of stenosis. Left ventricular hypertrophy develops with moderate to severe stenosis and is detected on the ECG and chest x-ray. Poststenotic dilation of the ascending aorta or aortic knob may be seen on chest radiographs. Echocardiography shows the site of stenosis, valve morphology, and presence of left ventricular hypertrophy and allows an estimate of the pressure gradient.
Treatment
The degree of aortic stenosis frequently progresses with growth and age. Aortic insufficiency often develops or progresses. Serial follow-up with echocardiography is indicated. Balloon valvuloplasty is usually the first interventional procedure for significant stenosis. It is not as successful as pulmonary balloon valvuloplasty and has a higher risk of significant valvular insufficiency. Surgical management is necessary when balloon valvuloplasty is unsuccessful, or significant valve insufficiency develops.
COARCTATION OF THE AORTA
Etiology and Epidemiology
Coarctation of the aorta occurs in approximately 10% of all congenital heart defects. It is almost always juxtaductal in position. During development of the aortic arch, the area near the insertion of the ductus arteriosus fails to develop correctly, resulting in a narrowing of the aortic lumen.
Clinical Manifestations
Timing of presentation depends on the severity of obstruction and associated cardiac defects. Infants presenting with coarctation of the aorta frequently have hypoplastic aortic arches, abnormal aortic valves, and VSDs. They may be dependent on a patent ductus arteriosus to provide descending aortic flow. Symptoms develop when the aortic ampulla of the ductus closes. Less severe obstruction causes no symptoms because blood flow into the descending aorta is not dependent on the ductus.
Symptoms, including poor feeding, respiratory distress, and shock, may develop before 2 weeks of age. Classically the femoral pulses are weaker and delayed compared with the right radial pulse. The blood pressure in the lower extremities is lower than that in the upper extremities. If cardiac function is poor, however, these differences may not be as apparent until appropriate resuscitation is accomplished. In this situation, there may be no murmur, but an S3 is often present.
Older children presenting with coarctation of the aorta are usually asymptomatic. There may be a history of leg discomfort with exercise, headache, or epistaxis. Decreased or absent lower extremity pulses, hypertension (upper extremity), or a murmur may be present. The murmur is typically best heard in the left interscapular area of the back. If significant collaterals have developed, continuous murmurs may be heard throughout the chest. An abnormal aortic valve is present approximately 50% of the time, causing a systolic ejection click and systolic ejection murmur of aortic stenosis.
Imaging Studies
The ECG and chest x-ray show evidence of right ventricular hypertrophy in infantile coarctation with marked cardiomegaly and pulmonary edema. Echocardiography shows the site of coarctation and associated lesions. In older children, the ECG and chest x-ray usually show left ventricular hypertrophy and a mildly enlarged heart. Rib notching may also be seen in older children (>8 years of age) with large collaterals. Echocardiography shows the site and degree of coarctation, presence of left ventricular hypertrophy, and aortic valve morphology and function.
Treatment
Management of an infant presenting with cardiac decompensation includes intravenous infusion of prostaglandin E1 (chemically opens the ductus arteriosus), inotropic agents, diuretics, and other supportive care. Balloon angioplasty has been done, especially in critically ill infants, but surgical repair of the coarctation is most commonly performed. Ballooning and stenting of older patients with coarctation has also been performed, but surgical repair remains the most common form of management.