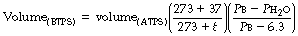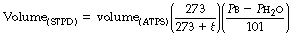Appendix C Conversion factors for gas volumes
Gas volumes are usually measured at ambient (or environmental) temperature and pressure, either dry (e.g. from a cylinder passing through a rota-meter) or saturated with water vapour at ambient temperature (e.g. an expired gas sample). Customary abbreviations are ATPD (ambient temperature and pressure, dry) and ATPS (ambient temperature and pressure, saturated).
Conversion of Gas Volume – ATPS to BTPS
Gas volumes measured by spirometry and other methods usually indicate the volume at ambient temperature and pressure, saturated (ATPS). Tidal volume, minute volume, dead space, lung volumes, and ventilatory gas flow rates, etc. should be converted to the volumes they would occupy in the lungs of the patient at body temperature and pressure, saturated (BTPS).
Conversion from ATPS to BTPS is based on Charles’ and Boyle’s laws (Appendix B), and conversion factors are listed in Table C.1.
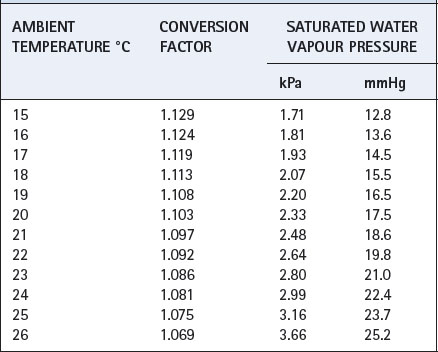
Table C.1 Factors for conversion of gas volumes measured under conditions of ambient temperature and pressure, saturated (ATPS) to volumes that would be occupied under conditions of body temperature and pressure, saturated (BTPS)
Derivation of Conversion Factors
Pb is barometric pressure (kPa) and Table C.1 has been prepared for a barometric pressure of 100 kPa (750 mmHg): variations in the range 99–101 kPa (740–760 mmHg) have a negligible effect on the factors.
t is ambient temperature (°C). Table C.1 has been prepared for a body temperature of 37°C: variations in the range 35–39°C are of little importance.
Ph2o is the water vapour pressure of the sample (kPa) at ambient temperature (see Table C.1).
Conversion of Gas Volume – ATPS to STPD
In measurement of absolute amounts of gases such as oxygen uptake, carbon dioxide output and the exchange of ‘inert’ gases, we need to know the actual quantity (i.e. number of molecules) of gas exchanged and this is most conveniently expressed by stating the gas volume as it would be under standard conditions; i.e. 0°C, 101.3 kPa (760 mmHg) pressure and dry (STPD). Under these conditions, one mole of an ideal gas occupies 22.4 litres.
Conversion from ATPS to STPD is again by application of Charles’ and Boyle’s laws, as follows:
Pb is barometric pressure (kPa).
t is ambient temperature (°C).
Ph2o is the saturated water vapour pressure of the sample (kPa) at ambient temperature (see Table C.1).