Diseases of the Kidney
Renal Aplasia, Hypoplasia, and Dysplasia: Renal aplasia (agenesis) is the failure of development of one or both kidneys so that there is no recognizable renal tissue present. In these cases, the ureter may be present or absent. If present, the cranial extremity of the ureter begins as a blind pouch. A familial tendency for renal aplasia has been observed in Doberman pinscher and beagle dogs. Because life can be sustained when more than one-fourth of renal function is maintained, unilateral aplasia is compatible with life, provided that the other kidney is normal. Unilateral aplasia can go unnoticed during life and be recognized at necropsy. Bilateral aplasia is obviously incompatible with life and occurs sporadically.
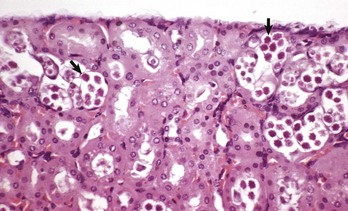
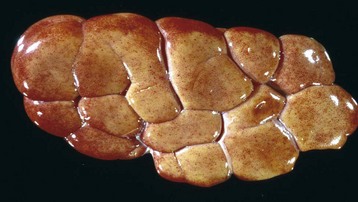
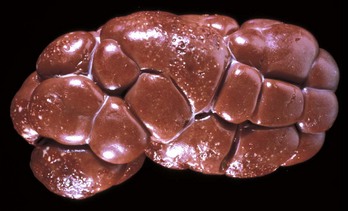
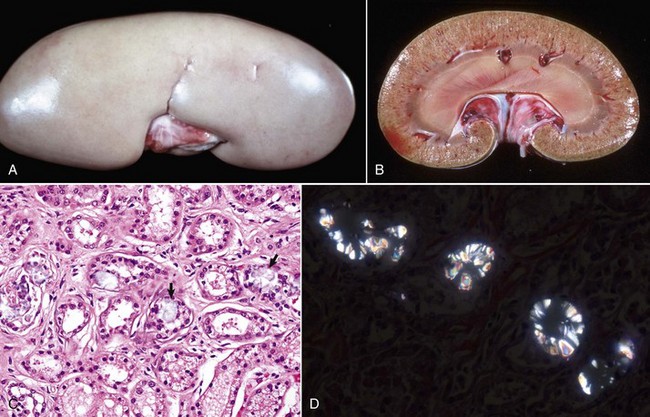
Renal hypoplasia designates incomplete development of the kidneys in a variety of species so that fewer than normal nephrons are present at birth. Renal hypoplasia has been documented as an inherited disease of purebred or crossbred Large White pigs in New Zealand and described in foals of various breeds, as well as in dogs (Fig. 11-32, A) and cats (Fig. 11-32, B). Hypoplasia can be unilateral (Fig. 11-32, B) or bilateral; it is rare and difficult to diagnose subtle cases at necropsy or microscopically. In cattle and pigs, the number of renal papillae in the hypoplastic kidney can be compared with those in a normal kidney. Hypoplastic kidneys from pigs and foals have a notable reduction in the number of glomeruli. In foals, for example, 5 to 12 glomeruli are present per low-power field in affected kidneys compared with 30 to 35 glomeruli per low-power field in normal adult kidneys. Unless significant renal mass is compromised by this condition, hypoplasia is clinically silent.
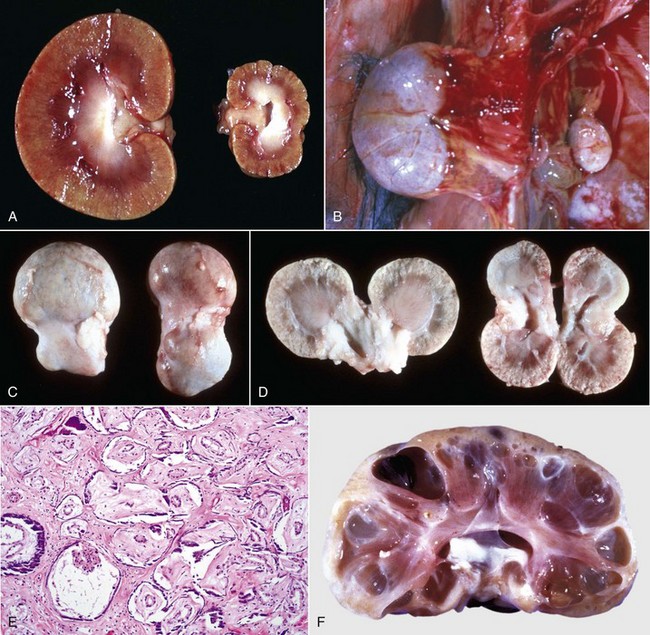
Fig. 11-32 Types of congenital developmental anomalies, kidney.
A and B, Unilateral hypoplastic kidneys, young dogs. A, Dorsal sections. B, The grossly affected right kidney is nearly identical in structure to the left kidney but smaller (hypoplasia). C, Juvenile progressive nephropathy, young dog. Bilateral abnormally shaped firm kidneys. D, Juvenile progressive nephropathy, dorsal sections, dog. Section of the kidneys from C. E, Juvenile progressive nephropathy, chronic, dog. Note the interstitial fibrosis, tubular atrophy, dilated urinary space, and mineralization. H&E stain. F, Polycystic disease, dorsal section, cat. Numerous variably sized tubular cysts are present in the cortex and medulla. The cysts contain clear colorless fluid. This condition is hereditary, and Persian cats are predisposed. (A courtesy Dr. B. Weeks, College of Veterinary Medicine, Texas A&M University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy Dr. M. Miller, College of Veterinary Medicine, University of Missouri; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. C and D courtesy College of Veterinary Medicine, University of Illinois. E courtesy Dr. S.J. Newman, College of Veterinary Medicine, University of Tennessee. F courtesy Dr. A. Confer, College of Veterinary Medicine, Oklahoma State University.)
Occasionally, some bovine kidneys are found to have reduced numbers of external lobes, but these kidneys are not hypoplastic and are microscopically and functionally normal; the reduction in external lobes merely represents fusion of the lobes. The shrunken, pitted kidneys in young animals, particularly dogs, are often diagnosed as hypoplastic. However, in most of these cases, these small kidneys are due to the following:
Renal dysplasia is an abnormality of altered structural organization resulting from abnormal differentiation and the presence of structures not normally present in nephrogenesis. Cystic renal dysplasia has been described in sheep and is inherited as an autosomal dominant trait. Renal dysplasia occurs infrequently and like renal hypoplasia, must be differentiated from renal fibrosis and progressive juvenile nephropathy. Dysplastic changes can be unilateral or bilateral and can involve much of an affected kidney or occur only as focal lesions. Dysplastic kidneys can be small, misshapen, or both. Microscopically, five primary features of dysplasia are described as follows:
• Asynchronous differentiation of nephrons inappropriate for the age of the animal—aggregates of small hypercellular glomeruli in the cortex
• Persistence of primitive mesenchyme so that the interstitial connective tissue has a myxomatous appearance
• Persistence of metanephric ducts
Interstitial fibrosis, renal cysts, and a few enlarged hypercellular glomeruli (compensatory hypertrophy) are changes seen secondarily to the primary dysplastic changes. The number of nephrons, lobules, and calyces are normal. Bilateral renal dysplasia characterized by persistent mesenchyme and atypical tubular development has been described in foals.
Progressive juvenile nephropathy (familial renal disease) of Lhasa Apso, Shih Tzu, golden retriever dogs, and perhaps other canine breeds could be examples of renal dysplasia (Fig. 11-32, C to E). Asynchronous differentiation is often seen and to a lesser extent several other features of dysplasia. However, until these hereditary lesions of dogs are better characterized, it is probably best to retain the diagnostic term of progressive juvenile nephropathy (see the section on Disorders of Dogs).
Ectopic and Fused Kidneys: Ectopic kidneys are misplaced from their normal sublumbar location because of abnormal migration during fetal development. Ectopic kidneys occur most frequently in pigs and dogs and usually involve only one kidney. Ectopic locations often include the pelvic cavity or inguinal position. Although ectopic kidneys are usually structurally and functionally normal, malposition of the ureters predisposes them to obstruction, which results in secondary hydronephrosis. Fused (horseshoe) kidneys result from the fusion of the left and right cranial or left and right caudal poles of the kidneys during nephrogenesis. This fusion results in the appearance of one large kidney with two ureters. The histologic structure and function of the fused kidneys are usually normal.
Renal Cysts: Renal cysts are spherical, thin-walled, variably sized distentions principally of the cortical or medullary renal tubules and are filled with clear, watery fluid. Congenital renal cysts can occur as a primary entity or in cases of renal dysplasia. The pathogenesis of primary renal cysts is not entirely understood. Cysts are likely derived from normal or noncystic segments of the nephron, most commonly the renal tubules, the collecting ducts, and Bowman’s (uriniferous) space. Although genetic mechanisms can be involved in the pathogenesis of renal cysts, experiments with toxic chemicals indicate that genetic predisposition is not a requirement. The following four mechanisms of renal cystic dilation are considered plausible:
• Obstruction of nephrons can cause increased luminal pressure and secondary dilation (called cystic dilation when it is well-developed).
• Modifications in ECM and cell-matrix interactions result in weakened tubular basement membranes allowing saccular dilation of tubules.
• Focal tubular epithelial hyperplasia with production of new basement membranes, increased tubular secretion, and increased intratubular pressure causes development of enlarged, dilated tubules.
• Dedifferentiation of tubular epithelial cells results in loss of polarity of cells with abnormal cell arrangements in tubules, reduced tubular fluid absorption, increased intratubular pressure, and dilation of tubules.
These mechanisms are not mutually exclusive, and several mechanisms often work in concert to create renal cysts.
Cysts range in size from barely visible to several centimeters in diameter. Cysts are usually spherical, delineated by a thin fibrous connective tissue wall lined by flattened epithelium, and are filled with clear, watery fluid. The sources of fluid are glomerular filtrate, transepithelial secretions, or both. When viewed from the renal surface, the cyst wall is pale gray, smooth, and translucent. Cysts can arise anywhere along the nephron and be located in either cortex or medulla. Kidneys can have single or multiple cysts. Some cysts cause no alteration in renal function and therefore are considered incidental findings. Such incidental renal cysts are common in pigs and calves and must be differentiated from hydronephrosis. Acquired renal cysts can occur as a result of renal interstitial fibrosis or other renal diseases that cause intratubular obstruction. These cysts are usually small (1 to 2 mm in diameter) and occur primarily in the cortex.
Polycystic Kidneys: Polycystic kidneys have many cysts that involve numerous nephrons. Congenital polycystic kidneys occur sporadically in many species but can be inherited as an autosomal dominant lesion in pigs and lambs and inherited along with cystic biliary disease in Cairn and West Highland white terriers. The lesion, termed polycystic kidney disease (PKD), is inherited as an autosomal dominant trait in families of Persian cats and bull terriers. Although less well characterized in animals than in humans, this autosomal dominant, high penetrance, heritable condition is thought to be related to mutations in one or more genes (PKD-1 and/or PKD-2) and altered function of the related proteins, principally polycystin-1 and polycystin-2. Manifestation of tubular cysts occurs after mutation of both alleles of these genes, the first of which is a germ-line mutation, and the second is somatic. Polycystin-1 is a cell membrane–associated protein with a large extracellular domain. Polycystin-1, the product of PKD-1, is involved in normal cell proliferation and apoptosis pathways. Although the exact mechanisms for cyst formation are not known, polycystin-1 mutations allow cells either to enter a differentiation pathway that results in tubule formation or to become susceptible to apoptosis. PKD-1 regulates tubular morphology throughout life, but the pathologic consequences are defined by developmental status of the organ with those incurred before the end of terminal renal maturation process, resulting in more severe lesions. Additionally, polycystin-1 is known to be important in both cell adhesion and cell signaling because it is an essential component of desmosomes. Loss of polycystin-1 from its basolateral location may alter critical pathways controlling normal tubulogenesis, thus contributing to cyst formation. Similarly, polycystin-2 functions principally as a localized plasma membrane calcium channel. Additionally, reduced levels of renal cyclic adenosine monophosphate (cAMP) are known to inhibit growth of renal cysts in animal models of PKD so there is an energy-dependent process involved in cyst formation. Further study of these mutated proteins will allow us to narrow the mechanistic pathway of the inherited form and potentially extrapolate to the acquired and sporadic congenital cystic lesions documented. A polycystic renal disease with cysts arising from glomeruli has been described in collie puppies. The gross appearance of the cut surface of a polycystic kidney has been described as “Swiss cheese” (Fig. 11-32, F). As cysts enlarge, they compress the adjacent parenchyma. When extensive regions of renal parenchyma are polycystic, renal function can be impaired.
Diseases of the Glomerulus
Immune-Mediated Glomerulonephritis: GN most often results from immune-mediated mechanisms, most notably after the deposition of soluble immune complexes within the glomeruli and less commonly after the formation of antibodies directed against antigens within the GBM. Antibodies to the basement membrane (anti-GBM disease) bind and damage the glomerulus through fixation of complement and resulting leukocyte infiltration. This mechanism of GN has been well documented in humans and nonhuman primates but only rarely in other domestic animals. To confirm the diagnosis of anti-GBM disease, Ig and complement (C3) must be demonstrated within glomeruli. Antibodies must be eluted from the kidneys and found to bind to normal GBMs of the appropriate species.
Immune-complex GN occurs in association with persistent infections or other diseases that characteristically have a prolonged antigenemia that enhances the formation of soluble immune complexes. In domestic animals, immune-complex GN occurs most commonly in dogs and cats. Immune-complex GN is associated with specific viral infections, such as feline leukemia virus (FeLV) or feline infectious peritonitis (FIP) virus; chronic bacterial infections, such as pyometra or pyoderma; chronic parasitism, such as dirofilariasis; autoimmune diseases, such as canine systemic lupus erythematosus; and neoplasia (Box 11-8). In addition to the role of persistent infections, a familial tendency for development of immune-complex GN has been described in a group of related Bernese mountain dogs.
Immune-complex GN is initiated by the formation of soluble immune complexes (antigen-antibody complexes) in the presence of antigen-antibody equivalency or slight antigen excess, which then do the following:
• Selectively deposit in the glomerular capillaries.
• Stimulate complement fixation with formation of C3a, C5a, and C567, which are chemotactic for neutrophils.
• Damage the basement membrane through neutrophil release of proteinases, arachidonic acid metabolites (such as thromboxane), and oxidants, particularly oxygen-derived free radicals and hydrogen peroxide.
• Continue to damage the glomeruli by the release of biologically active molecules from monocyte infiltrations in the later stages of inflammation (Fig. 11-33, A).
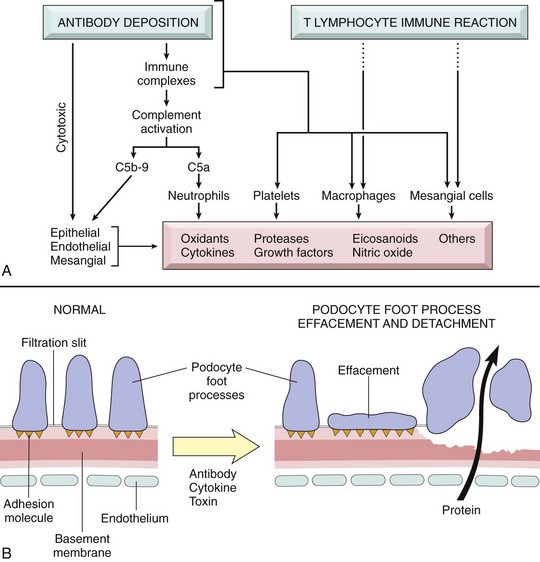
Fig. 11-33 Schematic diagram of the mediators of immune glomerular injury and epithelial cell injury.
A, Mediators of immune glomerular injury. B, Epithelial cell injury. The postulated sequence is a consequence of antibodies to epithelial cell antigens, with subsequent toxins, cytokines, or other factors causing injury and detachment of epithelial cells, resulting in protein leakage through the defective glomerular basement membrane and filtration slits. (From Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
Although circulating immune complexes may contribute to this process, antibody binding to endogenous glomerular antigens or entrapped nonspecific antigens is more common. Direct action of C5b to C9 on the glomerular components results in activation of both glomerular epithelial cells and mesangial cells to produce damaging mediators, such as oxidants and proteases.
Many specific factors determine the extent of deposition of soluble immune complexes in the glomerular capillary walls. These include persistence of appropriate quantities of immune complexes in the circulation, glomerular permeability, the size and molecular charge of the soluble complexes, and the strength of the bond between the antigen and antibody (avidity). Small or intermediate complexes are the most damaging because large complexes are removed from circulation through phagocytosis by cells of the monocyte-macrophage system in the liver and spleen. An increase in local glomerular vascular permeability is necessary for immune complexes to leave the microcirculation and deposit in the glomerulus. This process is usually facilitated via vasoactive amine release from mast cells, basophils, or platelets (see Fig. 11-33, A). Mast cells or basophils release vasoactive amines as a result of the interaction of the immune complexes with antigen-specific IgE on the surface of these cells, by stimulation of the mast cells or basophils by cationic proteins released from neutrophils, or by the anaphylatoxin activity of C3a and C5a. Platelet-activating factor (PAF) is released from immune complex–stimulated mast cells, basophils, or macrophages and causes platelets to release vasoactive amines.
Localization of the complexes within the various levels of the basement membrane or in subepithelial locations depends on their molecular charge and avidity. Once small, soluble immune complexes are deposited within the capillary wall, they can become greatly enlarged as a result of interactions of immune complexes with free antibodies, free antigens, complement components, or other immune complexes.
After immune-complex deposition, glomerular injury can also occur from the aggregation of platelets and activation of Hageman factor, which results in the formation of fibrin thrombi that produce glomerular ischemia. Furthermore, glomerular epithelial cell and ECM damage can result directly from the terminal membrane attack complex of the activated complement cascade (C5 to C9). This can result in epithelial detachment (causing proteinuria) and GBM thickening subsequent to upregulation of epithelial cell receptors for transforming growth factor (Fig. 11-33, B). Cell-mediated cytotoxic responses (from sensitized T lymphocytes) to glomerular antigens or complexes may exacerbate renal lesions. Complexes themselves may modulate the immune response through interaction with receptors on various cells.
Finally, if exposure of the glomerulus to immune complexes is short lived, as in a transient infection, such as infectious canine hepatitis, glomerular immune complexes will be phagocytosed by macrophages or mesangial cells and removed, and the glomerular lesions and clinical signs may resolve. Conversely, continual exposure of glomeruli to soluble immune complexes, such as in persistent viral infections (e.g., FeLV infection) and chronic heartworm disease, can produce progressive glomerular injury, with severe lesions and clinical manifestation of glomerular disease (Box 11-9).
Ultrastructurally, immune complexes either in the GBM or in a subepithelial location appear as electron-dense bodies. Complexes that are poorly soluble, fairly large, or of high avidity often enter the mesangium, where they can be phagocytosed by macrophages and appear ultrastructurally as dense granular deposits within the mesangial stroma or within macrophages. Other ultrastructural changes commonly seen are loss or effacement of visceral epithelial cell foot processes, cytoplasmic vacuolation, retraction and detachment of visceral epithelium, and infiltrates of neutrophils and monocytes within the mesangium.
A diagnosis of immune-mediated GN can be made by immunofluorescent or immunohistochemical demonstration of immunoglobulin and complement components, usually C3, in glomerular tufts. In dogs, IgG or IgM are the most common immunoglobulin isotypes demonstrated in GN; however, combinations of IgG, IgM, and IgA also occur in the glomeruli of some dogs. In one study, IgA was the only immunoglobulin found in three dogs with immune-complex GN. Both Ig and C3 are usually demonstrated in a granular (“lumpy-bumpy”) pattern using immunofluorescent or immunohistochemical techniques (Fig. 11-34); however, in anti-GBM disease, as reported in humans and horses, the antibody deposits have a linear distribution conforming to the basement membranes. It is important to remember that fluorescing deposits indicate the presence of immunoglobulin or complement but do not specifically indicate the presence of disease. Additionally, immunofluorescence may be negative when all reactive binding sites are occupied, thus complicating diagnosis of this condition.
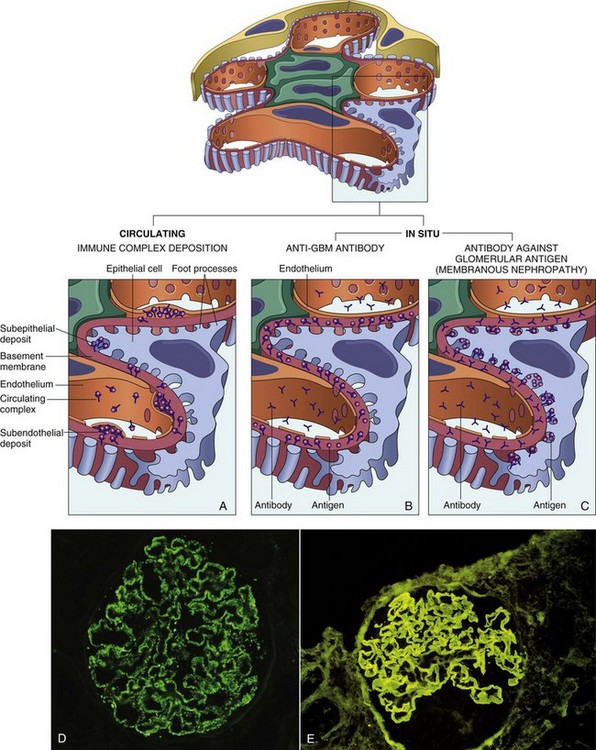
Fig. 11-34 Schematic diagrams of the antibody-mediated glomerular injury.
Antibody-mediated glomerular injury can result either from the deposition of circulating immune complexes (A) or from formation of complexes in situ (B and C). Antiglomerular basement membrane (anti-GBM) disease (B) or antitubular deposits (C) are characterized by linear immunofluorescence patterns, whereas lesions caused by immune complexes reveal granular patterns. D and E, Two patterns of deposition of immune complexes as seen by immunofluorescence microscopy: granular, characteristic of circulating and in situ immune complex nephritis (D); and linear, characteristic of classic anti-GBM disease (E). (From Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
The diagnosis of preformed immune-complex GN can be confirmed only by demonstrating that the antibodies from the immune complexes, eluted from glomeruli, are not capable of binding to normal glomerular elements and hence represent deposition of preformed circulating complexes. Once this has been done, the ideal situation would be to identify the causative antigen present in the immune complexes. This process is accomplished by eluting antibodies from diseased glomeruli and attempting to identify their specificity for suspected antigens. For example, antibodies eluted from the glomeruli of dogs with GN associated with severe heartworm disease bind to several Dirofilaria immitis antigens, including the body wall of adult worms, parasitic uterine fluid, and microfilaria. In most cases of immune-complex GN, the specific causative antigen usually escapes determination. Demonstration of electron-dense deposits in mesangial, subepithelial, or subendothelial locations by electron microscopy is also supportive of the diagnosis of immune-mediated GN.
Gross lesions of acute immune-complex GN are usually subtle. The kidneys are often slightly swollen, have a smooth capsular surface, are of normal color or pale, and have glomeruli that are visible as pinpoint red dots on the cut surface of the cortex (Fig. 11-35). The normal glomeruli of horses are usually visible, so this feature of pinpoint red dots for glomeruli cannot be used for diagnosis in that species. If lesions do not resolve but become subacute to chronic, the renal cortex becomes somewhat shrunken and the capsular surface has a generalized fine granularity. On cut surface, the cortex can be thinned and its surface granular, and glomeruli can appear as pinpoint pale gray dots. With time, more severe scarring can develop throughout the cortex (see the section on Renal Fibrosis).
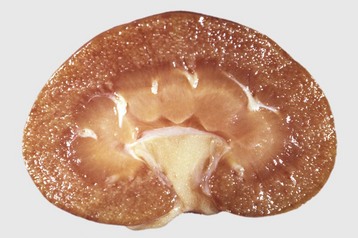
Fig. 11-35 Proliferative glomerulonephritis (GN), kidney, dorsal section, dog.
The small, white, round foci in the cortex are enlarged glomeruli. (Courtesy Dr. S.J. Newman, College of Veterinary Medicine, University of Tennessee.)
Microscopically, immune-complex GN has several histopathologic forms. Although various classifications of GN have been published, the following simple classification is well understood among veterinary pathologists. Lesions in glomeruli may be described as proliferative, membranous, or membranoproliferative (Fig. 11-36). Glomerular lesions can be distributed diffusely, when most of the glomeruli are involved; focally, when only a certain proportion of glomeruli are involved; globally, when an entire glomerular tuft is involved; and segmentally, when only a portion of the glomerular tuft is affected. Most of the lesions in immune-complex GN are diffuse, but within an individual affected glomerulus, the lesions can be either global or segmental.
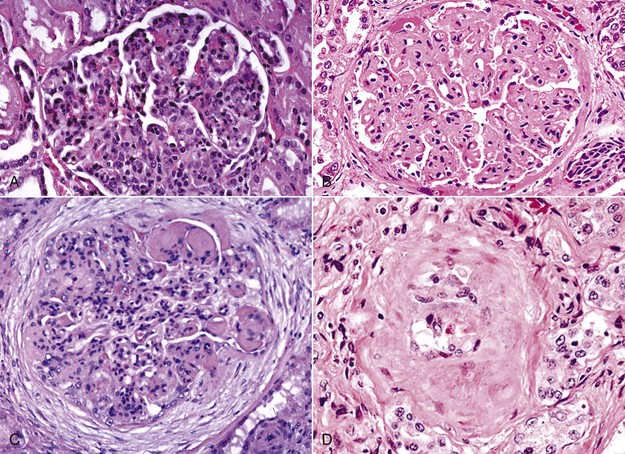
Fig. 11-36 Types of glomerulonephritis (GN).
A, Proliferative GN, pig. The lesion is characterized principally by hypercellularity of the glomerulus due to increased numbers of mesangial cells. H&E stain. B, Membranous GN, dog. The lesion is characterized by generalized hyaline thickening of glomerular capillary basement membranes. It can occur in dogs with dirofilariasis. H&E stain. C, Membranoproliferative GN, horse. Membranoproliferative GN has histologic features of both proliferative GN and membranous GN. Abundant periglomerular fibrosis surrounds this hypercellular glomerulus (mesangial cells). Mesangial matrix is prominent in the top-right area of the glomerulus. H&E stain. D, Glomerulosclerosis, dog. Note the hypocellularity, shrinkage, and hyalinization due to an increase in fibrous connective tissue and mesangial matrix and almost complete loss of glomerular capillaries. In glomerulosclerosis (the end-stage of chronic GN), glomeruli are essentially nonfunctional. H&E stain. (A and C courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B and D courtesy Dr. S.J. Newman, College of Veterinary Medicine, University of Tennessee.)
In the more chronic form, a variety of glomerular tuft changes will be noted, depending on whether the damage is related to mesangial proliferation, membranous proliferation, or both. There is typically enlargement of the tuft by the presence of abundant mesangial matrix, a reduction in cellularity, enhancement of the capillary outlines within the tuft, proliferation of the parietal epithelial cells, expansion of Bowman’s space by high protein ultrafiltrate, and variable thickening of Bowman’s capsule. Glomerulosclerosis is the stage where there is a reduction in the number of functional glomeruli with replacement by large amounts of fibrous connective tissue and subsequent obliteration of Bowman’s space due to capsular fibrosis.
Additionally, in protein-losing diseases, the proximal tubular cells often have microscopic eosinophilic intracytoplasmic bodies referred to as hyaline droplets, which represent accumulations of intracytoplasmic protein absorbed from the filtrate.
Microscopic details of each type of glomerular disease are outlined in the next sections.
Proliferative Glomerulonephritis: Proliferative GN is a form of immune-complex glomerular disease characterized by increased cellularity of the glomerular tufts caused by proliferation of glomerular endothelial, epithelial, and mesangial cells and an influx of neutrophils and other leukocytes and involves both the capillary loops and the mesangium (see Fig. 11-36, A). This form is the most common variant in horses but only rarely does it result in chronic renal failure. Equine infectious anemia and streptococcal antigen are the only antigens in horses proved to be associated with proliferative GN.
Membranous Glomerulonephritis: Membranous GN is characterized by diffuse glomerular capillary basement membrane thickening because of the presence of subepithelial immunoglobulin deposits, as the predominant change (Fig. 11-37; also see Fig. 11-36, B). These deposits are separated by protrusions of GBM matrix that eventually encompass these deposits. After removal of the deposited material, cavities are left in the GBM and later these fill with GBM-like material, which results in sclerotic change within the glomerular tuft. This is characterized by increased deposition of positive material (periodic acid–Schiff [PAS]) and a lesser amount of fibrosis. This variation is the most common form of immune-complex GN in cats.
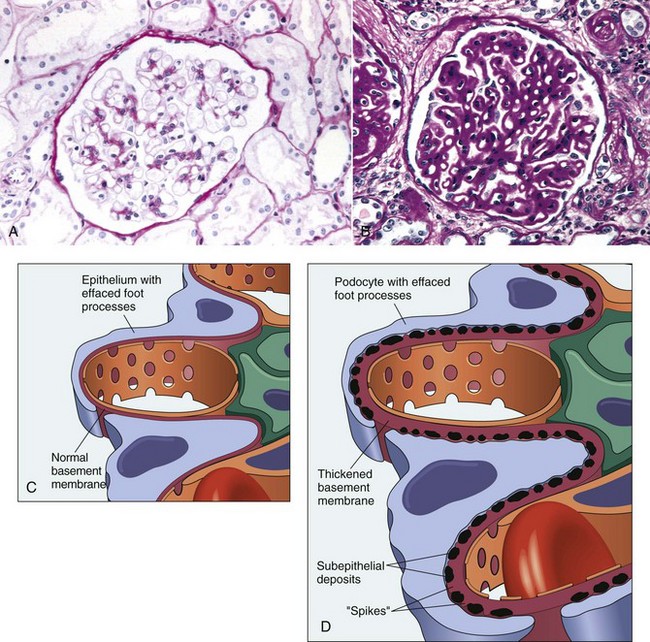
Fig. 11-37 Lupoid nephrosis (A and C) and membranous glomerulonephritis (GN) (B and D).
A, Lupoid nephrosis. The glomerulus appears normal, with a thin basement membrane. PAS reaction. B, Membranous GN. The glomerular basement membrane is diffusely thickened. PAS reaction. C, Schematic diagram of lupoid nephrosis. Diffuse loss of foot processes of visceral epithelial cells. D, Schematic diagram of membranous GN. Membranous GN is characterized by subepithelial deposits, which by transmission electron microscopy are electron dense, and by loss of foot processes. (From Cotran RS, Rennke H, Kumar V: The kidney and its collecting system, ed 7, Philadelphia, 2002, Saunders.)
Membranoproliferative Glomerulonephritis: Membranoproliferative GN (mesangioproliferative, mesangiocapillary) is characterized by hypercellularity following proliferation of glomerular cells and thickening of the capillary basement membrane and mesangium (Fig. 11-38; also see Fig. 11-36, C). This variant appears to be the most common morphologic form of immune-complex GN in the dog. Light microscopy fails to detect differences evident by immunofluorescent and electron microscopy. The latter allows subcategorization of membranoproliferative GN into type I and type II (see Fig. 11-38). Type I is characterized by the presence of subendothelial deposits and a granular pattern after deposition of C3 and lesser quantities of IgG, C1q, and C4. Type I disease appears to be secondary to deposition of circulating immune complexes. Type II is also referred to as dense deposit disease because electron-dense material of unknown composition and smaller quantities of C3 form an irregular deposit within the subendothelial space and the lamina densa. Type II disease appears to be a form of autoimmune disease, but its pathogenesis is not clear.
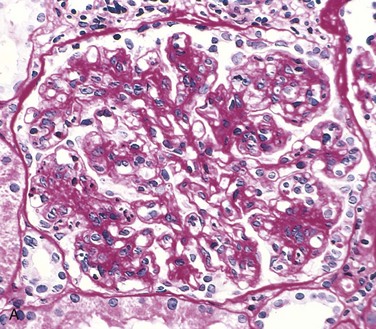
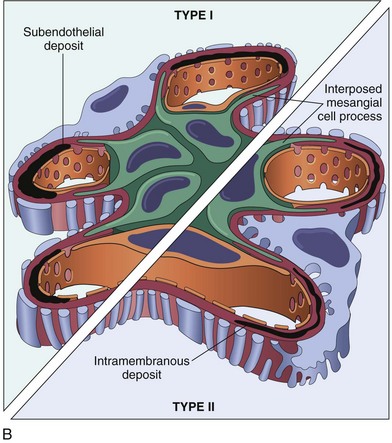
Fig. 11-38 Membranoproliferative glomerulonephritis (GN), glomerulus, kidney.
A, Note the increased mesangial matrix and thickened and focally split basement membranes (stained dark red). The glomeruli are also infiltrated by leukocytes (not visible here). Periodic acid Schiff reaction. B, Schematic representation of patterns in the two types of membranoproliferative GN. In type I there are subendothelial deposits; type II is characterized by intramembranous dense deposits (dense deposit disease). In both, mesangial interposition gives the appearance of split basement membranes when viewed in the light microscope. (From Cotran RS, Rennke H, Kumar V: The kidney and its collecting system, ed 7, Philadelphia, 2002, Saunders.)
Several other changes in the glomerulus and Bowman’s capsule usually accompany the lesions discussed previously. These changes include adhesions between the epithelial cells of the glomerular tuft and Bowman’s capsule (synechiae; singular = synechia), hypertrophy and hyperplasia of the parietal epithelium lining Bowman’s capsule, deposition of fibrinogen and fibrinous thrombi in glomerular capillaries, secondary to or as a result of the glomerular damage, and dilated renal tubules filled with homogeneous proteinaceous fluid. An increase in mesangial matrix is often also present. If the damage is mild and the cause is removed, glomeruli can heal without obvious or with minimal residual lesions. However, if the lesion is severe and prolonged, subacute to chronic glomerular changes develop. Bowman’s capsule can become thickened, hyalinized, and reduplicated. In severe cases, proliferation of parietal epithelium, an influx of monocytes, and deposition of fibrin can occur within Bowman’s capsule, resulting in the formation of a semicircular, hypercellular, intraglomerular lesion known as a glomerular crescent. The glomerular crescent can also undergo fibrosis, and if Bowman’s capsule ruptures, glomerular fibrosis can become continuous with interstitial fibrosis. Interstitial and periglomerular fibrosis, foci of interstitial lymphocytes, and plasma cells and glomerulosclerosis may be present in chronic GN.
Glomerulosclerosis: In chronic GN, severely affected glomeruli shrink and become hyalinized because of an increase in both fibrous connective tissue and mesangial matrix and a loss of glomerular capillaries (Fig. 11-36, D) and additionally there is periglomerular fibrosis. These glomeruli are hypocellular and essentially nonfunctional. This process is referred to as glomerulosclerosis. There is a specific nomenclature for describing the numbers of glomeruli involved and the location of the lesion in the glomerulus. Glomerulosclerosis can be diffuse, involving all glomeruli, or multifocal. In addition, glomerulosclerosis can involve a whole glomerular tuft (global) or only portions of the tuft (segmental), thus appearing as a nodular or segmental hyalinized thickening in affected glomeruli. Because tubules receive their blood supply from the vasa recta, derived from the glomerular efferent arteriole, glomerulosclerosis reduces the blood flow through the vasa recta, thus decreasing oxygen tension in the tubules. The resulting hypoxia is responsible for tubular epithelial cell death via apoptosis and results because of failure to regenerate to columnar cells. The tubules are lined instead by cuboidal or squamous cells, which lack a brush border and the functions of the normal columnar cells. In addition, chronic proteinuria often accompanies glomerulosclerosis and has been reported to promote tubular epithelial cell loss through apoptosis.
Numerous factors are associated with and accelerate glomerulosclerosis. These factors include the following:
• Unrestricted protein in the diet
• Increased glomerular capillary pressure in the remaining functional glomeruli
These factors have the following effects:
• Alter cellular components of the functional glomerular tufts
• Cause hypertension and transglomerular hyperfiltration with resultant damage to endothelium
• Activate mesangial cells to proliferate
• Increase mesangial matrix production
• Accelerate visceral epithelial cell loss, which allows synechiae (i.e., adhesions between visceral and parietal epithelial cell layers in the glomerulus) to form
Glomerulosclerosis is not only the end-stage of GN but also can develop in any chronic disease in which severe damage to nephrons or loss of nephron function occurs. Mild multifocal glomerulosclerosis of unknown cause is often an incidental finding in aged animals. Glomerulosclerosis has been reported occasionally in animals with hypertension and diabetes mellitus. In these cases, global or nodular eosinophilic glycoprotein material (hyaline material) is deposited in the glomerular mesangium.
Glomerular Amyloidosis: Amyloid, an insoluble fibrillar protein with a β-pleated sheet conformation, is produced after incomplete proteolysis of several soluble amyloidogenic proteins. Amyloid deposits in patients with plasma cell myelomas or other B lymphocyte dyscrasias (called AL amyloidosis) are composed of fragments of the light (λ) chains of immunoglobulins. In domestic animals, spontaneously occurring amyloidosis is usually an example of what is called reactive amyloidosis (AA amyloidosis). This form of the disease is often associated with chronic inflammatory diseases; the amyloid deposits are composed of fragments of a serum acute-phase reactant protein called serum amyloid–associated (SAA) protein. Amyloid fibrils from either source are deposited in tissue along with a glycoprotein called amyloid P component.
Glomeruli are the most common renal sites for deposition of amyloid in most domestic animal species, although the medullary interstitium is a common site in cats, particularly in Abyssinian breeds. Renal amyloidosis commonly occurs in association with other diseases, particularly chronic inflammatory or neoplastic diseases. However, idiopathic renal amyloidosis (i.e., amyloidosis in which an associated disease process is not recognized) is also described in dogs and cats. The underlying pathogenic mechanisms of idiopathic renal amyloidosis are not known. In a recent study, 23% of dogs that presented with proteinuria had renal amyloidosis. A hereditary predisposition for the development of reactive amyloidosis (AA) has been found in Abyssinian cats and Chinese Shar-Pei dogs. A familial tendency is suspected in Siamese cats, English foxhounds, and beagle dogs. In cattle, renal amyloidosis is nearly always due to chronic systemic infectious disease. Glomerular amyloidosis is responsible for many cases of protein-losing nephropathy in animals that have notable proteinuria and uremia. It can, like immune-complex GN, result in the nephrotic syndrome. Long-standing glomerular amyloidosis results in diminished renal blood flow through the glomeruli and the vasa recta. Such reduced renal vascular perfusion can lead to renal tubular atrophy, degeneration, and diffuse fibrosis and in severe cases, renal papillary necrosis. Medullary amyloidosis is usually asymptomatic unless it results in papillary necrosis.
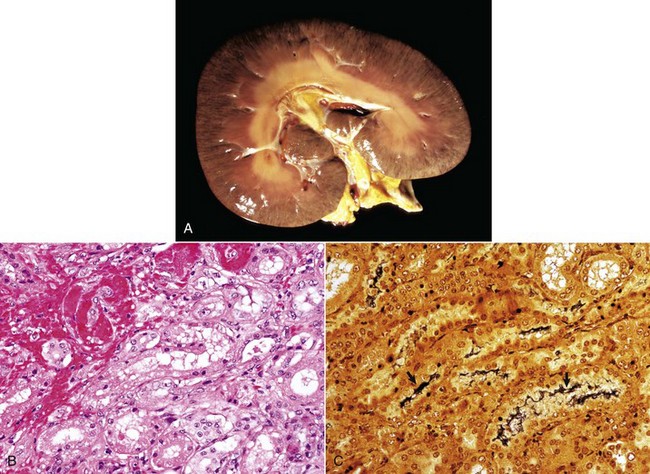
Kidneys affected with glomerular amyloidosis are often enlarged, pale, and increased in consistency, and have a smooth to finely granular capsular surface (Fig. 11-39). Amyloid-laden glomeruli may be visible grossly as fine translucent dots on the capsular surface. Similarly, the cut surface of the cortex can have a finely granular appearance with scattered glistening foci, less than 0.5 mm diameter in the cortex (see Fig. 11-39). Treatment of kidneys with an iodine solution, such as Lugol’s iodine, in many cases results in red-brown staining of glomeruli, which become purple when treated with dilute sulfuric acid (Fig. 11-40). This technique provides a rapid presumptive diagnosis of renal amyloidosis. Medullary amyloidosis is usually not grossly recognizable.

Fig. 11-39 Amyloidosis, kidney, dog.
Grossly, kidneys affected by amyloid deposition are diffusely tan, waxy (firm), and of normal size or slightly enlarged. Affected glomeruli are not grossly visible in this specimen, unlike in advanced cases of glomerular amyloidosis or chronic GN. In advanced cases of amyloidosis, glomeruli may be visible as pinpoint, glistening, round, cortical foci. In cats and Shar-Pei dogs, amyloid is deposited in the medullary interstitium, not in the glomeruli. There are also multiple foci of medullary crest necrosis (yellowish-green [arrows]). (Courtesy Dr. G.K. Saunders, The Virginia-Maryland Regional College of Veterinary Medicine; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
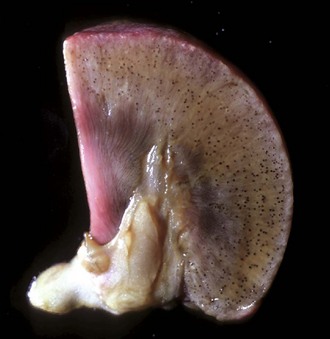
Fig. 11-40 Amyloidosis, kidney, transverse section, dog.
On the cut surface of fresh kidney treated with Lugol’s iodine followed by dilute sulfuric acid, glomeruli containing amyloid are visible as multiple dark blue dots in the cortex. Lugol’s iodine treatment. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Microscopically, glomerular amyloid is deposited in both the mesangium and subendothelial locations. Amyloid is relatively acellular and can accumulate segmentally within glomerular tufts; thus a portion of the normal glomerular architecture is replaced by eosinophilic, homogeneous to slightly fibrillar material (Fig. 11-41, A). When amyloidosis involves the entire glomerular tuft, the glomerulus is enlarged, capillary lumina become obliterated, and the tuft can appear as a large hypocellular eosinophilic hyaline sphere (Fig. 11-41, B). Amyloid can be present in renal tubular basement membranes, and these membranes appear hyalinized and thickened. Additionally, in cases of glomerular amyloid deposition, secondary changes may be present in renal tubules, which are usually markedly dilated, have variably atrophic epithelium and contain proteinaceous and cellular casts. Amyloid is confirmed microscopically by staining with Congo red stain (Fig. 11-41, C). When viewed with polarized light, amyloid has a green birefringence (Fig. 11-41, D). Loss of Congo red staining after treatment of a section of affected kidney with potassium permanganate suggests amyloid is AA (i.e., of acute-phase reactant protein origin).
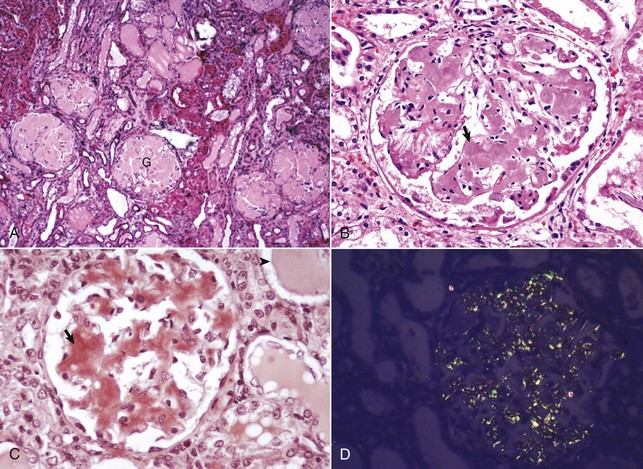
Fig. 11-41 Amyloidosis, glomerulus, kidney, dog.
A, All glomerular tufts (G) are diffusely and notably expanded by amyloid (pale eosinophilic homogeneous deposits), with the result that they are relatively acellular. H&E stain. B, Amyloid, the pale eosinophilic homogeneous hyalinized deposits, expands the mesangium of the glomerulus (arrow). H&E stain. C, Amyloid stains orange with Congo red staining (arrow), a technique used to confirm it. Note the proteinaceous casts in tubular lumina (arrowhead), a consequence of glomerular damage allowing leakage of proteins into the filtrate (protein-losing nephropathy). Congo red stain. D, Congo red–stained amyloid deposits. These deposits have a light-green (often called apple green) birefringence when viewed under polarized light. Polarized light microscopy. (A courtesy Dr. B.C. Ward, College of Veterinary Medicine, Mississippi State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy Dr. S.J. Newman, College of Veterinary Medicine, University of Tennessee. C courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. D courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
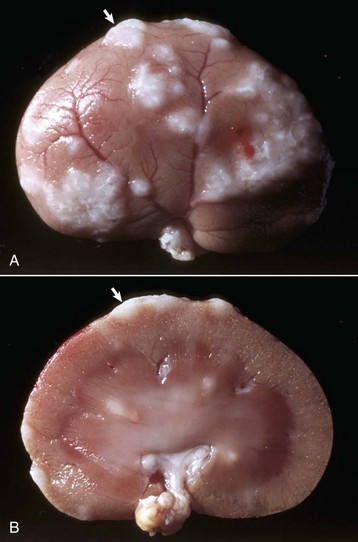
Acute Suppurative Glomerulitis: Bacterial (Embolic) Nephritis: Embolic nephritis, which can also be referred to as acute suppurative glomerulitis, is the result of a bacteremia in which bacteria lodge in random glomerular capillaries and to a lesser extent in interstitial capillaries and cause the formation of multiple foci of inflammation (microabscesses) throughout the renal cortex. While the glomeruli appear targeted, this is really a manifestation of renal vascular disease. A specific example of embolic GN is Actinobacillosis of foals caused by Actinobacillus equuli, although many emboli may also affect vasculature within the interstitium (Fig. 11-42). These foals usually die within a few days of birth and have small abscesses in many visceral organs, especially the renal cortex. Embolic nephritis also occurs commonly in the bacteremia of pigs infected with Erysipelothrix rhusiopathiae or sheep and goats infected with Corynebacterium pseudotuberculosis. Arcanobacterium pyogenes was the most common isolate (26/31) from cases of embolic nephritis in necropsy cattle. Staphylococcus aureus, Mannheimia haemolytica, and Streptococcus bovis were also represented.
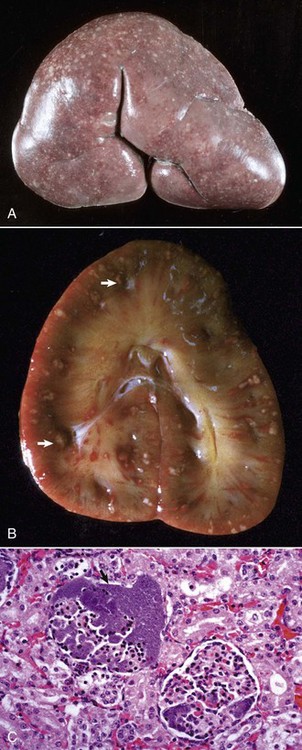
Fig. 11-42 Embolic nephritis (suppurative glomerulitis), kidney, horse.
A, Multiple, small pale white necrotic foci and abscesses are present subcapsularly. B, Dorsal section. Variably sized abscesses are scattered throughout the cortex (arrows). C, Causative bacteria (arrow) enter the kidney via the vasculature (bacteremia) and lodge in the capillaries of glomeruli, where they replicate and induce necrosis and inflammation. H&E stain. (A courtesy Dr. A. Confer, College of Veterinary Medicine, Oklahoma State University. B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. C courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Grossly, multifocal random, raised, tan pinpoint foci are seen subcapsularly and on the cut surface throughout the renal cortex. Microscopically, glomerular capillaries contain numerous bacterial colonies intermixed with necrotic debris and extensive infiltrates of neutrophils that often obliterate the glomerulus. Glomerular or interstitial hemorrhage can occur as well. As with many other inflammatory diseases, if the affected animal survives, the neutrophilic infiltrates either persist as focal residual abscesses or are progressively replaced by increasing numbers of lymphocytes, plasma cells, and macrophages; reactive fibroblasts; and ultimately coalescing scars.
Viral Glomerulitis: Glomerulitis, caused by a direct viral insult to the glomerulus, occurs in acute systemic viral diseases, such as acute infectious canine hepatitis (Fig. 11-43), equine arteritis virus infection, hog cholera, avian Newcastle disease, and neonatal porcine cytomegalovirus infection. The lesions are mild, usually transient, and result from viral replication in capillary endothelium. Acute viral GN produces the following gross lesions:
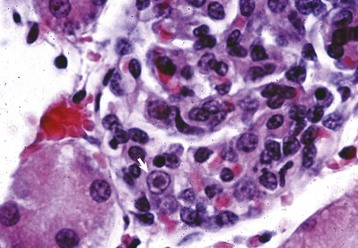
Fig. 11-43 Infectious canine hepatitis, kidney, cortex, dog.
Renal glomerular endothelial cells contain intranuclear inclusion bodies (arrow). H&E stain. (Courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
• Kidneys are often slightly swollen.
• Renal capsular surface is smooth.
• Kidneys are normal color or pale.
• Glomeruli are visible as pinpoint red dots on the cut surface of the cortex.
Viral-induced intranuclear inclusions are present in glomerular capillary endothelium from viremias of infectious canine hepatitis and cytomegalovirus infections. The inclusions of each disease are similar and are usually large, basophilic to magenta, and either fill the nucleus or are separated from the nuclear membrane by a clear halo. In the other diseases (equine arteritis, hog cholera, maedi-visna, porcine circovirus, and avian Newcastle), viral antigens can be demonstrated in endothelium, epithelium, or mesangial cells by immunofluorescence, immunohistochemistry or polymerase chain reaction (PCR). In cases of viral glomerulitis, lesions include endothelial hypertrophy, hemorrhages, necrosis of endothelium, and a thickened and edematous mesangium. Clinically, animals are systemically ill from the viral infection, but the glomerular signs are specifically those of a transient proteinuria.
Chemical Glomerulonephritis: Although much less common than the immune-mediated forms of GN, chemically induced glomerular disease occurs in a variety of different ways. Chemicals typically induce glomerular injury by any of the following:
• Direct injury to glomerular epithelial cells
• Direct injury to endothelial cells of the glomerulus
• Induction of immunologic reactions and inflammatory responses, which may occur with any of the following:
Puromycin aminonucleoside, adriamycin, and histamine-receptor antagonists all induce proteinuria through targeted damage to glomerular epithelial cells. The immunosuppressive drug, cyclosporine A, alters renal perfusion and ultimately the glomerular filtration rate by damaging glomerular endothelial cells. Examples of foreign substances capable of producing immune complexes include injectable hyperimmune serum, gold, and d-penicillamine. Procainamide and hydralazine result in production of antinuclear antibodies, and occupational exposure to hydrocarbon solvents can create anti-GBM antibodies. Often, drug-induced lesions lead to irreversible nephron loss and compensatory cellular and functional hypertrophy of other nephrons. The continuing physical loss of nephrons sets up a cycle for an increase in glomerular hypertension and hyperfiltration, which results in glomerulosclerosis, progressive nephron loss, and interstitial fibrosis.
Miscellaneous Glomerular Lesions:
Glomerular Lipidosis: Glomerular lipidosis, characterized by small aggregates of lipid-laden foamy macrophages in glomerular tufts, is an occasional incidental finding in dogs. A similar but more extensive glomerular lipidosis has been described in cats with inherited hyperlipoproteinemia, which is a generalized disease characterized by hyperchylomicronemia, atherosclerosis, and xanthogranulomas in numerous parenchymatous organs, including the kidneys (see the section on Granulomatous Nephritis). Microscopically, glomeruli contain foamy macrophages, characteristic of glomerular lipidosis, as well as increased mesangium and thickened Bowman’s capsule.
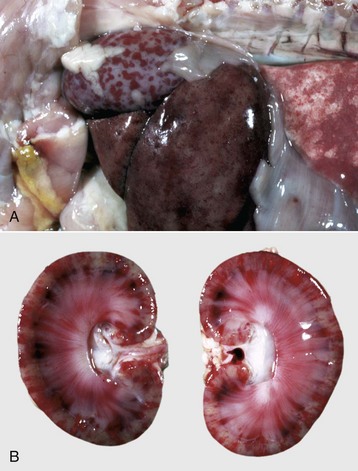
Glomerular Vasculopathy: An idiopathic renal glomerular vasculopathy and cutaneous vasculopathy occurs in greyhounds. The cause of this disease is unknown, but renal lesions are similar to those seen in DIC, thrombotic thrombocytopenic purpura, and hemolytic-uremic syndrome in humans. At necropsy, kidneys from affected dogs are swollen and congested and show cortical petechiae (Fig. 11-44, A). Microscopically, numerous glomeruli have segmental or global fibrinous thrombi, hemorrhage, and necrosis (Fig. 11-44, B). At the glomerular vascular pole, the walls of afferent arterioles have fibrin deposits and foci of necrosis. Affected greyhounds have multifocal erythematous and ulcerated skin lesions and distal limb edema. Variable systemic signs of uremia often accompany the cutaneous lesions.
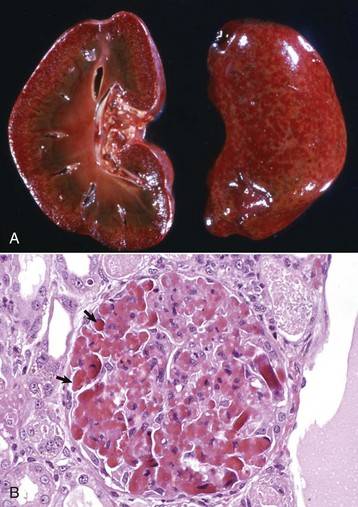
Fig. 11-44 Vasculopathy, renal (and cutaneous) vasculopathy syndrome, glomerulus, kidney, dog, greyhound.
A, The fine white dots in the cortex (both on the capsular and cut surfaces) are glomeruli with extensive glomerular capillary thrombosis. B, Necrotic glomerular endothelial cells and extensive glomerular capillary thrombosis (arrows) are typical of idiopathic glomerular (and cutaneous) vasculopathy syndrome in greyhound dogs. H&E stain. (A courtesy Dr. B. Weeks, College of Veterinary Medicine, Texas A&M University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy Dr. B.W. Fenwick, Virginia Tech.)
Diseases of the Tubules
Inherited Abnormalities in Renal Tubular Function: Inherited abnormalities in tubular metabolism, in transport, or in reabsorption of glucose, amino acids, ions, and proteins have been described in dogs. Primary renal glucosuria, an inherited disorder in Norwegian elkhounds and sporadically occurring in other dog breeds, occurs when the capacity of tubular epithelial cells to reabsorb glucose is significantly reduced. Gross and histologic lesions are not seen because this is a functional disorder. Glucosuria most commonly results from diabetes mellitus, acromegaly, or catecholamine release and predisposes dogs to the following:
• Bacterial infections of the lower urinary tract
• Urinary bladder emphysema, secondary to splitting of glucose molecules by bacteria (principally Escherichia coli, Clostridium perfringens, and rarely with Candida yeasts), with subsequent release of carbon dioxide (CO2) into the bladder lumen and absorption of gas into the bladder lymphatocele (Fig. 11-45).
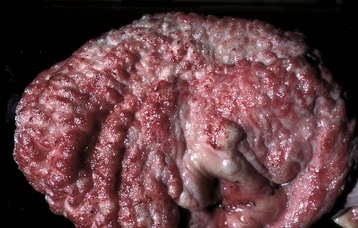
Fig. 11-45 Emphysema, urinary bladder mucosa, cow.
The multiple “nodules” are mucosal gas bubbles that have expanded the mucosa and are secondary to bacterial infections of the lower urinary tract (principally by Escherichia coli, Clostridium perfringens, and rarely Candida yeasts). Microorganisms split glucose molecules to release CO2 into the bladder lumen, from where the gas can be absorbed into bladder lymphatics. This animal was injected with calcium borogluconate as a calcium source to treat milk fever. Following intravenous injection, calcium ions readily dissociate from the parent molecule, and the resulting gluconate provides a sugar source for resident urinary bacteria. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
A hereditary generalized defect in tubular reabsorption similar to the Fanconi syndrome in humans has been described in Basenji dogs. The underlying tubular defect appears to be abnormal membrane structure of the proximal tubular epithelial cell brush borders because of altered lipid content in the cell membrane. Gross lesions are not identifiable in the early stages. Histopathologic changes in the kidneys are initially minimal, consisting of irregularly sized tubular epithelial cells in the convoluted tubules and loops of Henle. With time, dogs with Fanconi syndrome develop progressive renal insufficiency and associated renal fibrosis. Aminoaciduria, glucosuria, proteinuria, increased phosphaturia, metabolic acidosis, and multiple endocrine abnormalities characterize this disease clinically. Transient acquired forms have been noted in association with copper storage hepatopathy.
The excretion of large quantities of cystine in the urine (cystinuria) is a sex-linked inherited tubular dysfunction seen occasionally in purebred and mongrel male dogs. It is important because it predisposes affected dogs to calculus formation and obstruction of the lower urinary tract (see the section on Urolithiasis).
Acute Tubular Necrosis: Acute tubular necrosis, as described in the section on tubular response to injury, can be seen following exposure to any of the following nephrotoxins (Box 11-10):
• Pharmaceutical agents (e.g., chemotherapeutic and antimicrobial agents)
• Aminoglycosides (see the section on Disorders of Dogs)
• Nonsteroidal antiinflammatory drugs
• Pet food contaminants (see the section on Disorders of Dogs)
These nephrotoxins are covered in greater detail in the next section.
Hemoglobinuric nephrosis: A set of events leading to ischemic tubular necrosis frequently occurs in hypoperfused kidneys complicated by hemoglobinuria. Hemoglobinemia results in hemoglobinuria when the renal threshold for resorption is exceeded. Hemoglobinuria may occur in the following:
• Chronic copper toxicity in sheep
• Leptospirosis or babesiosis in cattle
In these diseases, serum concentrations of hemoglobin are increased. Hemoglobin passes into the glomerular filtrate, producing greatly increased intraluminal concentrations that cause hemoglobinuric nephrosis. Hemoglobin attaches to a carrier haptoglobin for transportation, but the latter is too big to pass through the glomerulus. Hemoglobin is not excreted in the urine until supplies of the carrier molecule are depleted and hemoglobin becomes free in the plasma. Hemoglobin is not nephrotoxic itself, and intravenous infusions of hemoglobin into healthy animals produce no recognizable lesions. However, large concentrations of hemoglobin in the glomerular filtrate can increase the tubular necrosis that occurs as a result of renal ischemia, for example, in chronic copper toxicity in sheep, renal ischemia is secondary to hypovolemic shock or severe anemia. Hemoglobinuria can have an additive deleterious affect on tubular epithelium already undergoing ischemic necrosis.
At necropsy, the renal cortices in severe hemoglobinuria are diffusely stained red-brown to blue-black and have intratubular hemoglobin casts (Fig. 11-46, A). These hemoglobin casts appear as a red-black stippling of the capsular surface and continue into the cortex as radially oriented, dark red streaks. The medulla is diffusely dark red or has patchy red streaks. Classically, kidneys from sheep with chronic copper toxicity are diffusely, uniformly, and strikingly blue-black and described as “gunmetal blue.” Microscopically, proximal tubular epithelial degeneration and necrosis are severe and tubular lumens are filled by abundant orange-red granular refractile material, the characteristic appearance of a heme compound (Fig. 11-46, B).
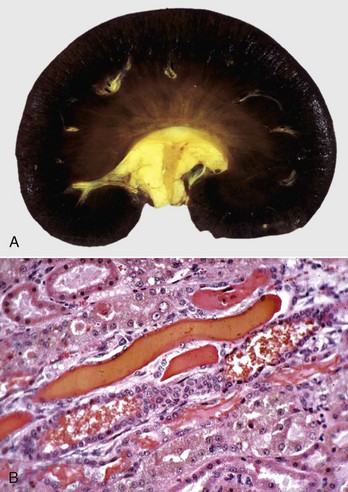
Fig. 11-46 Hemoglobinuric nephrosis, kidney.
A, Dog. Severe diffuse hemoglobin staining of the cortex and medulla is secondary to hemoglobinemia from an acute intravascular hemolytic crisis. Note the yellow staining (jaundice) of the pelvic fat and the intima of cross sections of the arcuate artery at the corticomedullary junction. B, Sheep. Several distal tubules contain hyaline and coarsely granular hemoglobin casts that occurred following intravascular hemolysis (hemoglobinemia) from chronic copper toxicosis. H&E stain. (A courtesy Dr. A. Confer, College of Veterinary Medicine, Oklahoma State University. B courtesy Dr. A.R. Doster, University of Nebraska; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Myoglobinuric nephrosis: Myoglobinuria results from acute and extensive muscle necrosis and occurs in the following:
• Azoturia (“Monday morning disease”) of horses (see the section on Disorders of Horses)
A set of events leading to ischemic tubular necrosis frequently occurs in hypoperfused kidneys complicated by myoglobinuria. In these diseases, serum concentrations of myoglobin are increased, as these products pass into the glomerular filtrate, producing greatly increased intraluminal concentrations that cause myoglobinuric nephrosis. Myoglobin does not use a carrier protein for transportation, and because it is a small molecule, it more freely passes through the glomerulus and is excreted in the urine. Myoglobin is not nephrotoxic in itself, and intravenous infusions into healthy animals produce no recognizable lesions. However, large concentrations of myoglobin in the glomerular filtrate can increase the tubular necrosis that occurs as a result of renal ischemia; for example, in rhabdomyolysis in horses, renal ischemia is secondary to hypovolemic shock or severe anemia. Myoglobinuria can have an additive deleterious effect on tubular epithelium already undergoing ischemic necrosis.
At necropsy, the renal cortices in myoglobinuria are diffusely stained red-brown to blue-black and have intratubular myoglobin casts (Fig. 11-47).
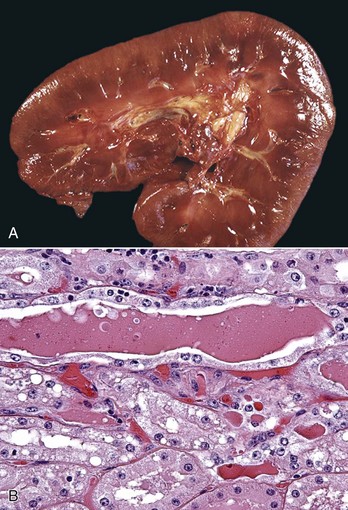
Fig. 11-47 Myoglobinuric nephrosis, kidney, horse.
A, Diffuse myoglobin staining of the cortex and medulla (reddish-brown) is secondary to myoglobinemia from severe rhabdomyolysis. B, Myoglobin casts are present in dilated distal tubules, which are lined by flattened epithelial cells. H&E stain. (A courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy Dr. J. F. Zachary, College of Veterinary Medicine, University of Illinois.)
Cholemic Nephrosis: Increased serum concentrations of bilirubin, as in young lambs, calves, and foals with immature hepatic conjugating mechanisms, can be associated with proximal tubular cellular swelling, degeneration, and yellow-brown-green pigmentation of the proximal tubular epithelial cells. The term cholemic nephrosis has been applied to this lesion; however, its significance is doubtful. Acute tubular necrosis, when seen in association with severe bilirubinemia, the so-called hepatorenal syndrome, probably is not caused by bile acid or bilirubin retention per se but by ischemia from prerenal causes such as constriction of renal vessels related to shock or catecholamine release.
Heavy Metals: Nephrotoxic tubular necrosis is caused by several classes of naturally occurring or synthetic compounds. Inorganic arsenic and certain heavy metals, including inorganic mercury, lead, cadmium, and thallium, are nephrotoxins. Common sources of heavy metals for oral exposure include herbicides (arsenic), old paints (lead), batteries (lead), automobile components (lead), impure petroleum distillates, and other environmental contaminants. Acute tubular necrosis from mercury is caused by the following:
• Damage to membranes of proximal convoluted tubular epithelial cells.
• Mitochondrial damage produced by these toxins; damage is often related to the interaction of these metals with protein sulfhydryl groups.
In mercury toxicosis, mercuric ions enter the proximal tubular cells both from the luminal side because the ions are present in the glomerular filtrate and from the peritubular side, where the mercuric ions diffuse from the capillary blood, traverse the interstitium and tubular basement membrane, and enter the tubular epithelium. Mercuric ions become concentrated in the rough endoplasmic reticulum and cause early tubular changes that include loss of brush border and dispersion of ribosomes. These changes are followed by mitochondrial swelling and cellular death. Recently, cadmium has been reported to cause cell death in proximal convoluted tubules by apoptosis.
The specific metal involved in toxic tubular injury cannot be identified by the renal lesions alone. The exception is lead toxicity, in which the endothelial and epithelial cells of affected glomeruli and proximal tubules, respectively, sometimes have acid-fast intranuclear inclusions composed of a lead-protein complex (Fig. 11-48).
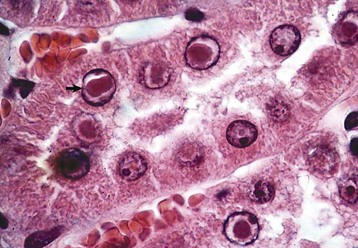
Fig. 11-48 Nephrosis, lead toxicosis, kidney, cortex, rat.
Acid-fast intranuclear inclusion bodies (arrow) present in the proximal convoluted tubular epithelium are diagnostic of lead poisoning. Acid-fast stain with H&E counterstain. (Courtesy Dr. J. King, College of Veterinary Medicine, Cornell University.)
Pharmaceutical Agents: These agents are nephrotoxic and cause acute tubular necrosis when administered at excessive doses or too frequently. Cisplatin, a platinum-containing cancer chemotherapeutic agent, causes tubular necrosis by the following:
• Direct tubular epithelial damage.
• Reducing renal blood flow via vasoconstriction mediated by the renin-angiotensin mechanism.
Oxytetracycline is occasionally nephrotoxic in cattle and dogs. The mechanism of tubular damage has not been determined, but it is known that large concentrations of tetracycline antibiotics are necessary and are thought to inhibit protein synthesis in tubular epithelial cells.
Amphotericin B, an antifungal polyene antibiotic, is nephrotoxic by either vasoconstriction and/or the direct disruption of cellular membranes; this membrane damage interferes with normal cholesterol-lipid interactions and causes potassium ion loss, intracellular hydrogen ion accumulation, acute cellular swelling, and necrosis of proximal and distal tubules. These renal changes are not confined to cases of an overdose of the drug but can occur in animals given the recommended therapeutic dosage.
Sulfonamide-induced tubular necrosis, a common entity in years past, occurs infrequently today because the presently used sulfonamides have greater solubility than those used in the past. Sulfonamides produce tubular epithelial cell necrosis most readily in dehydrated animals. Crystals form in tubules and cause necrosis of the renal tubular epithelium by direct toxicity and by mechanical damage. Fine granular yellow crystalline deposits can be seen grossly in the medullary tubules of affected animals, but the crystalline deposits are dissolved during fixation in aqueous fixatives such as 10% buffered neutral formalin.
Monensin is an ionophore antibiotic used as a feed additive to control coccidiosis and stimulate weight gains in poultry and cattle. Horses are particularly susceptible to toxicosis with monensin. Although necrosis of striated muscle is the major lesion, renal tubular degeneration or necrosis occurs concurrently.
Nonsteroidal Antiinflammatory Drugs: Ingestion of nonsteroidal antiinflammatory drugs (NSAIDs), such as phenylbutazone, aspirin, carprofen, flunixin meglumine, ibuprofen, and naproxen, have been associated with acute renal failure in small animals, especially dogs. The mechanism of acute renal failure is related to NSAIDs decreasing the synthesis of renal prostaglandins. Because prostaglandins are responsible for maintaining normal renal blood flow, NSAID administration results in afferent arteriolar constriction that decreases renal perfusion, resulting in acute tubular degeneration and medullary papillary necrosis and acute renal failure. The overall incidence of NSAID-induced renal failure in small animals is low and is seen most commonly in animals that ingest excessive amounts of the drug or have a concomitant disorder such as dehydration, congestive heart failure, or chronic renal disease.
Fungal Toxins: Naturally occurring nephrotoxins can originate from plants (ochratoxins and citrinins, castor beans) or from fungal organisms (mycotoxins produced by Aspergillus sp. and Penicillium sp). Ochratoxin A is nephrotoxic for monogastric animals, particularly pigs, in which the lesions are tubular degeneration and necrosis. In addition, long-term ingestion results in diffuse renal fibrosis presumably as the result of continual damage to the tubule epithelial cells and thus allowing no time for regeneration.
Plant Toxins: Several species of pigweed, particularly Amaranthus retroflexus, can be responsible for acute tubular necrosis and perirenal edema in pigs and cattle. The toxic principle has not been identified. Oxalate-induced tubular necrosis also occurs in sheep and cattle after ingestion of toxic quantities of oxalates that accumulate in plants of various genera such as Halogeton, Sarcobatus, Rheum, and Rumex. After absorption from the intestine, calcium oxalates precipitate in either vessel lumens or walls or within renal tubules, where they cause obstruction and epithelial cell necrosis. Illness in oxalate poisoning occurs not only because of renal disease but also because of neuromuscular dysfunction, the result of the hypocalcemia produced by chelation of serum calcium by oxalates. Recently, an oxalate-induced nephrosis was described in Tibetan spaniels with an inherited hyperoxaluria. A chronic oxalate nephrosis in Ragdoll cats of unknown inheritance and etiology is also reported.
Tannins: See the section on Disorders of Cattle for a discussion of tannins as a nephrotoxin.
Antifreeze: See the section on Disorders of Dogs for a discussion of antifreeze as a nephrotoxin.
Vitamin D: Vitamin D given as multiple excessive doses (vitamin D intoxication [vitamin D nephropathy]) or by accidental ingestion of calciferol-containing rodenticides can cause nephrosis in dogs and cats. In livestock, chronic ingestion of plants, such as Cestrum diurnum in the southern United States (US) or Solanum sp. or Trisetum sp. in other countries, each of which contains a chemical with vitamin D–like biologic activity, can also cause nephrosis. Ingestion of excessive amounts of vitamin D can induce hypercalcemia. Hypercalcemia results in decreased cAMP formation, which impairs sodium resorption and interferes with ADH receptors. Additionally, if the hypercalcemia persists, progressive mineralization of tubular and GBMs occurs (see Fig. 11-31). Development of lesions depends on the length of time between exposure to rodenticides and death or the duration of continued exposure to vitamin D. In acute cases, the kidneys have a smooth capsular surface. Microscopically, tubular epithelium is necrotic and atrophic with a few calcified deposits in the tubules scattered randomly throughout the cortex. In more chronic cases, the surface of the kidney is finely granular as a result of fibrosis. White, chalky deposits can be seen within the cortex. Interstitial fibrosis, tubular dilation, glomerular atrophy, and extensive calcification of tubular basement membranes are seen microscopically.
Interstitial calcification (hypercalcemic nephropathy): Hypercalcemia from a variety of causes results in inactivation of adenyl cyclase, decreased AMP so that sodium transport is impaired in the ascending limb of the loop of Henle, distal tubule, and collecting ducts. Hypercalcemia interferes with ADH receptors in the collecting ducts, resulting in renal diabetes insipidus. Mineralization of the basement membrane and epithelium initially in the outer zone of the medulla and then involving the interstitium, vessels, and glomeruli is seen when hypercalcemia persists. The leading cause of hypercalcemia in dogs and cats is hypercalcemia of malignancy, a paraneoplastic syndrome. PTH-related peptide (PTHrp), a peptide that resembles PTH, results in bone resorption. It is produced most commonly by lymphosarcomas or carcinomas of the apocrine glands of the anal sac. Additionally, excess vitamin D either from rodenticides or excess dietary sources (toxic plants) can result in a similar syndrome. Less common causes of hypercalcemia include primary hyperparathyroidism and secondary renal hyperparathyroidism.
Bacterial Toxins: Bacterial toxins, such as the epsilon exotoxin, produced after marked enteric proliferation by Clostridium perfringens type D in small ruminants, can result in grossly recognizable bilateral renal lesions termed pulpy kidney (Fig. 11-49, A). The pulpy texture of the kidney is due to acute tubular epithelial degeneration and/or necrosis and interstitial edema and hemorrhage (Fig. 11-49, B). Epsilon toxin binds to receptors on distal renal tubular epithelium causing this degeneration. Autolysis can produce similar changes, and this finding should be interpreted with caution, especially with a long necropsy interval.
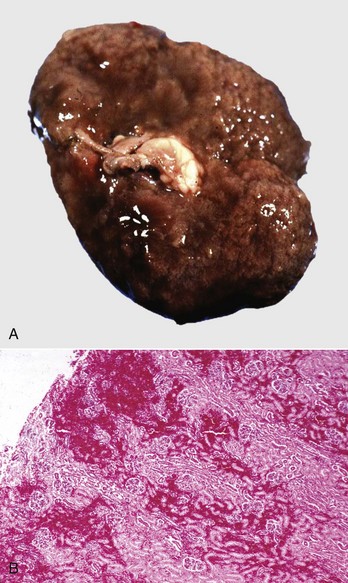
Fig. 11-49 Pulpy kidney disease, Clostridium perfringens type D toxin, kidney, lamb.
A, The Epsilon exotoxin from an enteric overgrowth of Clostridium perfringens type D causes soft, swollen, and pale kidneys, often with hemorrhage, and are termed pulpy kidneys. B, The soft pulpy nature of the kidney is the result of acute tubular epithelial cell degeneration and/or necrosis, interstitial edema, and hemorrhage. H&E stain. (A courtesy Dr. J. King, College of Veterinary Medicine, Cornell University. B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Pet Food Contaminants: See the section on Disorders of Dogs for a discussion of pet food contaminants.
Diseases of the Renal Pelvis
Hydronephrosis: Hydronephrosis refers to dilation of the renal pelvis because of obstruction of urine outflow and is principally caused by a slow or intermittent increase in pelvic pressure. Abrupt increases in pressure, such as those associated with inadvertent surgical ligation of a ureter, more commonly result in a decline in filtration rate in the affected kidney and a lesser propensity to develop hydronephrosis.
Obstruction leading to hydronephrosis can occasionally be caused by congenital malformation of the ureter, vesicoureteral junction, or urethra or from congenitally malpositioned kidneys with secondary kinking of the ureter. The more common causes of hydronephrosis are as follows:
• Ureteral or urethral blockage due to urinary tract calculi (see the section on Lower Urinary Tract)
Hydronephrosis occurs in all domestic animals. Depending on the location of the obstruction, hydronephrosis can be unilateral (ureteral) or bilateral (both ureters, bladder trigone, or urethra). Unilateral hydronephrosis is caused by obstruction of the ureters anywhere throughout its length or at its entrance into the urinary bladder. Bilateral hydronephrosis can be caused by urethral obstruction, bilateral ureteral obstruction, or extensive urinary bladder lesions centered on the trigone. When hydronephrosis is unilateral, pelvic enlargement of the kidney can become extensive, even cystic, before the lesion is recognized clinically. If the obstructive process causes partial or intermittent blockage, bilateral hydronephrosis can become notable because of continual urine production and pooling of urine in the expanding pelvis. When obstruction is complete and bilateral, death as a result of uremia occurs before pelvic enlargement becomes extensive.
When the increase in intrapelvic pressure is substantial and sustained, the following occur:
• Intratubular pressure is increased and results in microscopic renal tubular dilation.
• Glomeruli remain functional, and even with complete obstruction, glomerular filtration does not stop completely and soon overwhelms tubular reabsorption pathways.
• Much of the glomerular filtrate diffuses into the interstitium, where it is initially removed via lymphatic vessels and veins.
• As intrapelvic pressure increases, the interstitial vessels collapse and renal blood flow is reduced, resulting in hypoxia, tubular atrophy, and if the pressure increase is continued, interstitial fibrosis.
• The glomeruli have a relatively normal morphologic appearance for a prolonged period, but they eventually become atrophic and sclerotic.
Early changes of hydronephrosis include dilation of the pelvis and calyces and blunting of the renal crest and papillae (Fig. 11-50). When pelvic dilation is progressive, the kidney silhouette is enlarged and rounder than normal, and the cortex and medulla are progressively thinned (Fig. 11-51). Interstitial vascular obstruction from compression produces an expanding front of medullary and later cortical ischemia and necrosis. The continued pelvic dilation causes loss of tubules by degeneration and atrophy, followed by condensation of interstitial connective tissue and fibrosis of the renal parenchyma. In its most advanced form, the hydronephrotic kidney is a thin-walled (2- to 3-mm), fluid-filled sac. This sac is lined by flattened transitional epithelium, which is spared during lesion development. Occasionally, a severely hydronephrotic kidney becomes contaminated by bacteria and the thin-walled sac becomes filled with pus instead of urine. This lesion, referred to as pyonephrosis, is likely the result of blood-borne bacteria lodging in a hydronephrotic kidney.
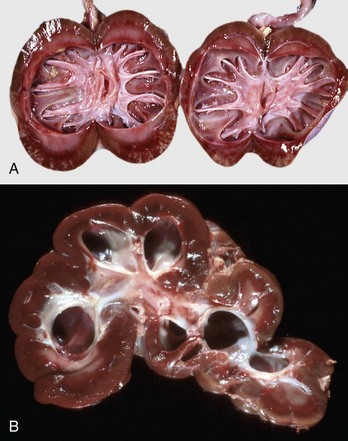
Fig. 11-50 Hydronephrosis, kidney, dorsal section.
A, Sheep. The pelvis of each kidney is markedly dilated. B, Cow. Bovine kidneys are lobulated, and each lobule has its own renal papilla surrounded by a calyx, an extension of the pelvis. Thus in early hydronephrosis, each of these calyces is distended, and these distended calyces should not be confused with the cysts of a cystic or polycystic kidney. (A courtesy Dr. J. King, College of Veterinary Medicine, Cornell University. B courtesy College of Veterinary Medicine, University of Illinois.)
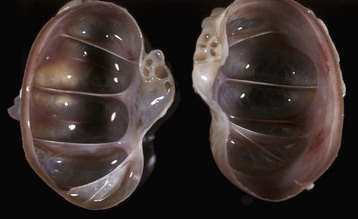
Fig. 11-51 Chronic hydronephrosis, kidney, dorsal section, cat.
Advanced hydronephrosis is characterized by loss of medullary tissue and atrophy or even loss of the entire cortex in response to elevated pelvic fluid pressure. Note that this case was so severe that only the renal capsule, which contains clear yellow fluid, remains. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Pyelonephritis: Bacterial infection of the pelvis with extension into the renal tubules and a concomitant interstitial inflammation is referred to as pyelonephritis. Because of differences in pathogenesis, lesion distribution, and microscopic appearance, pyelonephritis is considered a form of tubulointerstitial nephritis.
Although pyelitis refers to inflammation of the renal pelvis, pyelonephritis is inflammation of both the renal pelvis and renal parenchyma and is an excellent example of suppurative tubulointerstitial disease. The condition usually originates as an extension of a bacterial infection affecting the lower urinary tract that ascends the ureters to the kidneys and establishes an infection in the pelvis and inner medulla (Fig. 11-52). Rarely, pyelonephritis can result from descending bacterial infections, wherein bacterial infection of the kidneys occurs via the hematogenous route (i.e., embolic nephritis). In human pathology, the term pyelonephritis is used to include both ascending and descending infections. Ascending infection, however, is by far the most common cause of pyelonephritis in animals.
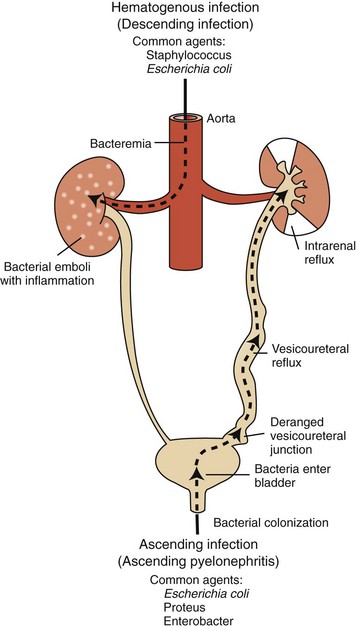
Fig. 11-52 Schematic diagram of the pathways of renal infection.
Hematogenous infection results from bacteremia. More common is ascending infection, which results from a combination of urinary bladder infection, vesicoureteral reflux, and intrarenal reflux. (From Kumar V, Abbas AK, Fausto N: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2010, Saunders.)
The pathogenesis of ascending pyelonephritis depends on the abnormal reflux of bacteria-contaminated urine from the lower tract to the renal pelvis and collecting ducts (vesicoureteral reflux). Normally, little vesicoureteral reflux occurs during micturition. Vesicoureteral reflux occurs more readily when pressure is increased within the urinary bladder, as with urethral obstruction. Most recently, this mechanism has been postulated for end-stage pyelonephritis with mild dysplasia seen in young Boxer dogs in Norway. Bacterial infection of the lower urinary tract can enhance vesicoureteral reflux by several other mechanisms as follows:
• When the bladder wall is inflamed (cystitis), the normal competency of the vesicoureteral valve can be compromised, allowing greater opportunity for urine to reflux.
• Endotoxin, liberated from Gram-negative bacteria infecting the ureter and bladder, can inhibit normal ureteral peristalsis, increasing reflux.
The urinary tract has a number of protective features in place to help prevent bacterial colonization and these include the following:
• Mucoproteins in the surface urothelial mucosal lining that prohibit bacterial adherence
• Desquamation of superficial urothelial cells to minimize surface colonization
Bacteria that colonize the pelvis can readily infect the inner medulla. The medulla is highly susceptible to bacterial infection because of the following:
• Its great interstitial osmolality and/or osmolarity that inhibits neutrophil function
• Its large ammonia concentration that inhibits complement activation
Thus bacteria can infect and ascend collecting ducts, cause tubular epithelial necrosis and hemorrhage, and incite a notable inflammatory response. Bacterial infection can progressively ascend within tubules and the interstitium until the inflammatory lesions extend from pelvis to capsule. Chronic pyelonephritis may result from infection superimposed on conditions that result in recurrent obstructive disease or reflux (reflux nephropathy). Recurrent infections lead to recurrent bouts of inflammation that result in scarring.
Because most occurrences of pyelonephritis are ascending infections and because females are more susceptible to lower urinary tract infections, pyelonephritis occurs more frequently in females. Escherichia coli, especially uropathogenic strains that produce virulence factors such as α-hemolysin, adhesions, and P fimbria, is one of the most common causes of lower urinary tract disease and pyelonephritis. Proteus sp., Klebsiella sp., Staphylococcus sp., Streptococcus sp., and Pseudomonas aeruginosa are also common causes of lower urinary tract infection and pyelonephritis in all species. Corynebacterium renale, Arcanobacterium pyogenes, and Eubacterium (Corynebacterium) suis are specifically pathogenic for the lower urinary tract of cattle and pigs, respectively, and are common causes of pyelonephritis. Recent or multiple catheterizations may be a predisposing factor.
A gross diagnosis of pyelonephritis is accomplished by recognizing the existence of pelvic inflammation with extension into the renal parenchyma (Fig. 11-53, A). Pyelonephritis can be unilateral, but it is often bilateral and most severe at the renal poles. The pelvic and ureteral mucous membranes can be acutely inflamed, thickened, reddened, roughened, or granular and coated with a thin exudate. The pelvis and ureters can be markedly dilated and have purulent exudate in the lumina (Fig. 11-53, B). The medullary crest (papilla) is often ulcerated and necrotic. Renal involvement is notable by irregular, radially oriented, red or gray streaks involving the medulla extending toward and often reaching the renal surface. Occasionally, inflammation extends through the surface of the kidneys to produce extensive subcapsular inflammation and localized peritonitis.
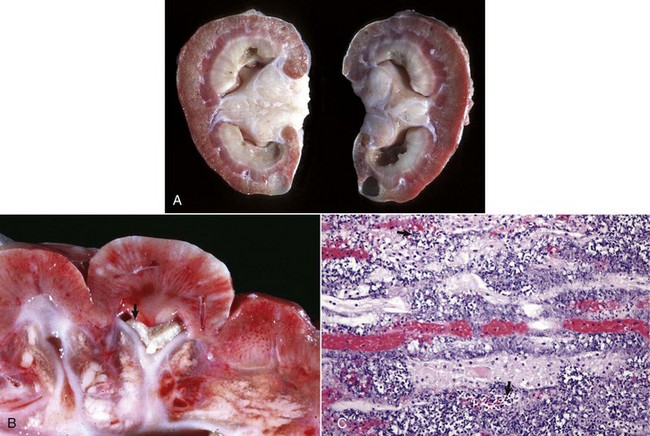
Fig. 11-53 Pyelonephritis, kidney.
A, Dorsal section, dog. Extensive pelvic inflammation has destroyed areas (gray-white) of the inner medulla and extends focally into the outer medulla. B, Dorsal section, cow. Renal calyces in the cow contain suppurative exudate (arrow). C, Dog. There is both intratubular and interstitial inflammation with tubular necrosis, characterized by infiltrates of principally neutrophils (arrows). H&E stain. (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. K. Read, College of Veterinary Medicine, Texas A&M University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. C courtesy Dr. J. F. Zachary, College of Veterinary Medicine, University of Illinois.)
Microscopically, the most severe acute lesions of pyelonephritis are usually in the inner medulla. The transitional epithelium is usually focally or diffusely necrotic and sloughed. Necrotic debris, fibrin, neutrophils, and bacterial colonies can be adherent to the denuded surface. Medullary tubules are notably dilated, and their lumina contain neutrophils and bacterial colonies. Focally the tubular epithelium is necrotic. An intense neutrophilic infiltrate, present in the renal interstitium, can be accompanied by notable interstitial hemorrhages and edema (Fig. 11-53, C). If obstruction of vasa recta has occurred, coagulative necrosis of the inner medulla (papillary necrosis) can be severe. Similar tubular and interstitial lesions, although less severe, extend radially into the cortical tubules and interstitium. When the lesions become subacute, the severity of the neutrophilic infiltrates diminishes, and lymphocytes, plasma cells, and monocytes infiltrate the interstitium. Chronic lesions have severe fibrosis. If active bacterial infection persists or is untreated, an intense infiltrate of all inflammatory cell types interspersed with tubular necrosis and fibrosis can be seen. All stages of disease progression can occur in a single kidney.
The renal lesions of chronic pyelonephritis, in which an active bacterial infection exists, include most of the elements of acute inflammation described previously and extensive necrosis of the medulla, patchy fibrosis in the outer medulla and cortex, and variable amounts of pelvic inflammatory exudates. Chronic pyelonephritis often produces a grossly visible deformity of the renal parenchyma because of extensive interstitial inflammation and scarring (Fig. 11-54). Fibrosis secondary to the tubulointerstitial inflammation of pyelonephritis follows the pattern of the acute disease (targeting the renal poles), and results in irregularly distributed, patchy scarring that is seen as deeply depressed regions on the renal capsular surface and linear areas extending through both the cortex and medulla to the pelvis. Such lesions often resemble chronic polar infarcts.
Fig. 11-54 Chronic pyelonephritis, kidney, dog.
A, Note the two large polar scars visible as large indentations on the capsular surface (arrow). The fine gray spots are regions of chronic inflammatory infiltrates and fibrosis. B, Dorsal section. The cortical scars are localized to the renal poles (arrow), but there is a finely stippled pattern of nodularity and fibrosis in the remaining kidney. This polar pattern of scarring suggests previous bouts of pyelonephritis. (Courtesy Dr. A. Confer, College of Veterinary Medicine, Oklahoma State University.)
Papillary (Medullary Crest) Necrosis: Necrosis of the renal papillae, or their counterpart, the medullary crest, is a response of the inner medulla to ischemia. Papillary necrosis can be a primary or secondary lesion. Papillary necrosis occurs as a primary disease in animals treated with NSAIDs and is analogous to analgesic nephropathy in humans. The primary disease occurs quite frequently in horses treated for prolonged periods with phenylbutazone or flunixin meglumine. It is important in dogs and cats because of accidental ingestion of or treatment with ibuprofen, aspirin, or acetaminophen at excessive dosages. Drugs associated with papillary necrosis are sometimes referred to as papillotoxins. The medullary interstitial cells are the primary targets of papillotoxins. These cells have a key role in synthesis of prostaglandins, antihypertensive factors, and the glycosaminoglycan matrix of the medullary interstitium. Interstitial cell damage decreases prostaglandin synthesis, which reduces normal blood flow and causes ischemia, increases tubular transport, and modifies the interstitial matrix; the net effect is degenerative changes in tubular epithelial cells in the inner medulla. In addition to its inhibition of prostaglandin biosynthesis, acetaminophen also causes direct oxidative damage to medullary tubular epithelium after covalent binding to the cells, further enhancing necrosis of the renal papillae.
Secondary papillary necrosis results from the following:
Reduced blood flow in vasa recta:
Compression of renal papilla by:
The outer medulla and the cells of the thick ascending loop of Henle in particular are the least well perfused of any zones of the kidneys. This is because the medulla has little direct perfusion; instead, most of the medullary blood supply comes from the cortex after passing through the glomeruli and entering the vasa recta. Because of this limited blood flow, in addition to the high metabolic medullary cellular demand, any lesion or disease process that further reduces medullary blood flow can cause ischemic necrosis (infarction) of the papillae. Additionally, the high metabolic demand for cell transport and the maintenance of an ion gradient to enhance urinary concentration makes this area particularly vulnerable. This is most evident after ischemic tubular damage where swollen endothelial and tubular epithelial cells, in conjunction with neutrophil adhesion in small vessels, upset the balance of oxygenation and energy demand by the outer medullary tubular cells. Medullary blood flow is ultimately balanced through levels of vasodilators, such as prostaglandin, nitric oxide, and adenosine, and vasoconstrictors, such as endothelin and angiotensin II.
Typically, acute lesions are irregular, discolored areas of necrotic inner medulla sharply delineated from the surviving medullary tissue (Fig. 11-55). The affected tissue, which initially undergoes coagulation necrosis, is yellow-gray, green, or pink. With time, the necrotic tissue sloughs, resulting in a detached, friable, and discolored tissue fragment in the pelvis. The remaining inner medulla is usually attenuated and on cross-section is narrowed. Overlying cortex can be somewhat shrunken because of atrophy of some of the nephrons caused by blockage of their tubules in the affected medulla. Small pieces of sloughed necrotic medullary tissue pass inconsequentially into the ureter. However, large pieces can obstruct the ureter, causing hydronephrosis, or form a nidus for precipitation of minerals, resulting in the formation of pelvic or ureteral calculi. Papillary necrosis is usually an incidental finding at necropsy and rarely leads to progressive renal damage and failure.
Fig. 11-55 Papillary (medullary crest) necrosis, chronic nonsteroidal antiinflammatory drug treatment, kidney, dorsal section, horse.
Acute coagulation necrosis of the medullary crest and inner medulla (green areas [arrows]). There is also hemorrhage of the outer medulla. The term papillary necrosis is retained for all animals, although only the pig and cow have distinct renal papillae. In other animals, these have fused to form the medullary crest. (Courtesy Dr. A. Confer, College of Veterinary Medicine, Oklahoma State University.)
Diseases of the Interstitium
Granulomatous Nephritis: Granulomatous nephritis is an interstitial disease that often accompanies chronic systemic diseases that are characterized by multiple granulomas in various organs. In domestic animals, granulomatous nephritis has been associated with a variety of infectious agents, including viruses (feline coronavirus [see the section on Disorders of Cats], porcine circovirus), bacteria (mycobacteria), fungi (Aspergillus sp.), and parasites (Toxocara sp. and Angiostrongylus vasorum larvae/eggs). Common to each, however, is the formation of grossly visible granulomas randomly scattered throughout the kidneys, especially in the cortex.
Granulomatous nephritis is also caused by a variety of granuloma-inducing infectious agents, including fungi such as Aspergillus sp., Phycomycetes, or Histoplasma capsulatum; algae, such as Prototheca sp.; Rickettsia such as Ehrlichia canis; protozoa such as Encephalitozoon cuniculi; and bacteria such as Mycobacterium bovis. Small, gray-white, granulomatous foci (2 to 5 mm) or larger nodules (up to 10 cm in diameter) can be scattered randomly throughout the kidneys of animals with granulomatous nephritis. These foci are white to tan, dry, and granular and can have calcified, caseous centers. Microscopically, lesions are characterized by central foci of necrosis surrounded by epithelioid macrophages, variable minerals, and giant cells that contain acid-fast bacteria.
In cattle, granulomatous nephritis is part of the multisystemic granulomatous disease caused by hairy vetch (Vicia villosa) toxicosis (see the section on Disorders of Ruminants). Lesions are characterized by multifocal to coalescing cortical granulomas (Fig. 11-56, A). Microscopically, infiltrates of monocytes, lymphocytes, plasma cells, eosinophils, and multinucleated giant cells are seen primarily within the interstitium of the renal cortex (Fig. 11-56, B).
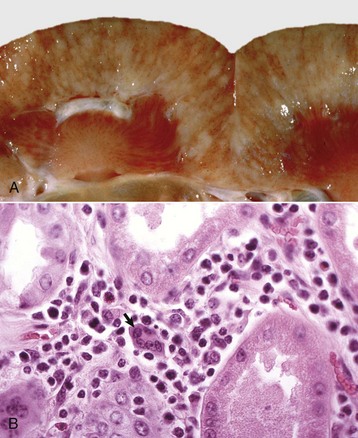
Fig. 11-56 Granulomatous nephritis, hairy vetch toxicosis, kidney, cow.
A, Cortical striations are obliterated by coalescing granulomatous foci associated with hairy vetch toxicosis. B, Cortex. Lesions associated with hairy vetch toxicosis are characterized by a mixed cell interstitial inflammatory infiltrate (macrophages, lymphocytes, and occasional multinucleated giant cell [arrow]) with renal tubular atrophy. It is specifically known as an unusual type of poisoning because of its ability to induce granulomatous inflammation in addition to the necrosis. The kidney is not the primary organ affected. H&E stain. (A courtesy Dr. J. King, College of Veterinary Medicine, Cornell University; and Dr. J. Edwards, College of Veterinary Medicine, Texas A&M University. B courtesy Dr. R. Panciera, College of Veterinary Medicine, Oklahoma State University.)
Migratory Toxocara canis larvae can induce small, gray-to-white granulomas (2 to 3 mm) randomly scattered throughout the subcapsular renal cortex of dogs (Fig. 11-57, A). Such lesions probably are due to cell-mediated foreign body immune response to the migrating larvae and are composed of aggregates of macrophages, lymphocytes, and eosinophils surrounded by fibroblasts within concentrically arranged fibrous connective tissue (Fig. 11-57, B). In recently acquired lesions, nematode larvae can often be seen in the center of these lesions (see Fig. 11-57, B). Following death, the larvae become fragmented and the debris is either phagocytosed and eliminated or less commonly retained with a resultant granulomatous response. Lesions heal by fibrosis, leaving a few finely pitted (contracted) foci on the capsular surface.
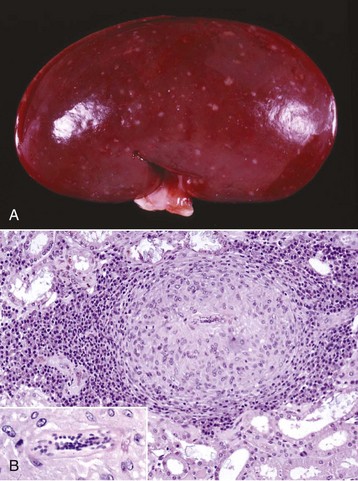
Fig. 11-57 Granulomatous nephritis, kidney, cortex, dog.
A, Multiple subcapsular, cortical, tan, raised granulomas caused by migrating ascarid larvae. B, A mature granuloma composed of a central ascarid larva surrounded by epithelioid macrophages and concentrically arranged fibrous connective tissue and inflammatory cells. H&E stain. Inset, Ascarid larva. (Courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Xanthogranulomas: Cats with inherited hyperlipoproteinemia have xanthogranulomas in various organs, including the kidneys. We have observed similar renal xanthogranulomas in dogs with hypothyroidism and severe atherosclerosis. These lesions are characterized by foamy, lipid-laden macrophages, lymphocytes, plasma cells, and fibrosis interspersed with cleftlike spaces typical of cholesterol deposits (cholesterol clefts).
Renal Interstitial Amyloidosis: Although glomeruli are the most common renal sites for deposition of amyloid in most domestic animal species, the medullary interstitium is a common site in cats, particularly in Abyssinian breeds. Renal amyloidosis commonly occurs in association with other diseases, particularly chronic inflammatory or neoplastic diseases. However, idiopathic renal amyloidosis (i.e., amyloidosis in which an associated disease process is not recognized) is also described in dogs and cats. The underlying pathogenic mechanisms of idiopathic renal amyloidosis are not known. A hereditary predisposition for the development of reactive amyloidosis (AA) has been found in Abyssinian cats and a familial tendency is suspected in Siamese cats. Medullary amyloidosis is usually asymptomatic unless it results in papillary necrosis. Similarly, Shar Pei dogs represent one of the most commonly affected canine breeds to have systemic AA amyloidosis that accumulates preferentially in the renal interstitium, and it is thought to be autosomal recessive in its familial inheritance. The deposition of medullary amyloid may predispose the dog to various aspects of end-stage renal disease, including interstitial fibrosis, lymphoplasmacytic infiltration, tubular atrophy, tubular dilation, mineralization, deposition of oxalate crystals, glomerular atrophy, and glomerulosclerosis.
Neoplasia
The prevalence of primary renal neoplasms in domestic animals is less than 1% of the total neoplasms reported. They are usually unilateral and can be epithelial, mesenchymal, or embryonal in origin. A recent study of canine cases revealed carcinomas (49/82), sarcomas (28/82), and nephroblastomas (5/82) with a 4% bilateral involvement. Median survival for carcinomas was 16 months, for sarcomas was 9 months, and for nephroblastoma 6 months, helping confirm that primary renal tumors are highly malignant and metastatic disease is common (77%). Inappropriate polycythemia is a paraneoplastic condition seen in association with excess erythropoietin production by renal adenocarcinomas.
Epithelial Tumors: Renal adenomas are rare, benign, epithelial neoplasms comprised of proliferations of renal cortical epithelial cells, most often reported in dogs, cats, and horses. They are incidental findings at necropsy and usually appear as a small (1 to 3 cm), white-to-yellow, solitary, well-circumscribed, nonencapsulated mass in the cortex. Microscopically, adenomas are composed of solid sheets, tubules, or papillary proliferations of cuboidal epithelial cells that are uniform in size and have granular eosinophilic cytoplasm and small, round-to-oval nuclei. Mitotic figures, necrosis, and fibrosis are rare. These incidental tumors are clinically asymptomatic.
Oncocytomas are rare benign epithelial tumors that can occur in a variety of tissues. Grossly, renal oncocytomas are tan, homogeneous, well-encapsulated masses composed of oncocytes. Histologically, they are composed of large eosinophilic granular round cells with condensed round nuclei. Ultrastructurally, they are characterized by numerous prominent cytoplasmic mitochondria. Their origin in the kidney is speculated to be from the intercalated cells of the collecting ducts. These tumors are clinically asymptomatic.
Renal carcinomas are the most common primary renal neoplasms and occur most frequently in older dogs. The specific causes of renal adenocarcinomas in humans are well determined compared with those in animal species, but several mechanisms have been proved in natural animal disease or experimental models. These include the following:
• Viruses—herpesvirus oncogenes are known to play a major role in formation of virally induced adenocarcinoma (Lucke’s tumor) in the kidney of frogs, and avian erythroblastosis virus [strain ES4] induces renal adenocarcinomas in chickens.
• Chemical carcinogens—these have been postulated to be causative and typically exert their neoplastic influence by direct DNA damage or inhibition of DNA synthesis or repair.
• Autosomal dominant gene mutations in Eker rats—these predispose these rats to bilateral renal cell carcinoma and a variety of other secondary cancers, resembling the human von Hippel-Lindau disease.
These neoplasms are usually large (up to 20 cm in diameter), spherical to oval, and firm. They often are pale yellow and contain dark areas of hemorrhage and necrosis and foci of cystic degeneration. The masses usually occupy and obliterate one pole of the kidney and grow by expansion, compressing the adjacent normal renal tissue (Fig. 11-58, A and B). Histologic types include papillary, tubular, and solid (Fig. 11-58, C), with solid variants being the most poorly differentiated. Metastasis to the lungs, lymph nodes, liver, and adrenal occur frequently. Renal carcinoma has been associated with paraneoplastic conditions, principally polycythemia. This is because of concurrent overexpression of erythropoietin, which increases bone marrow production of red blood cells.
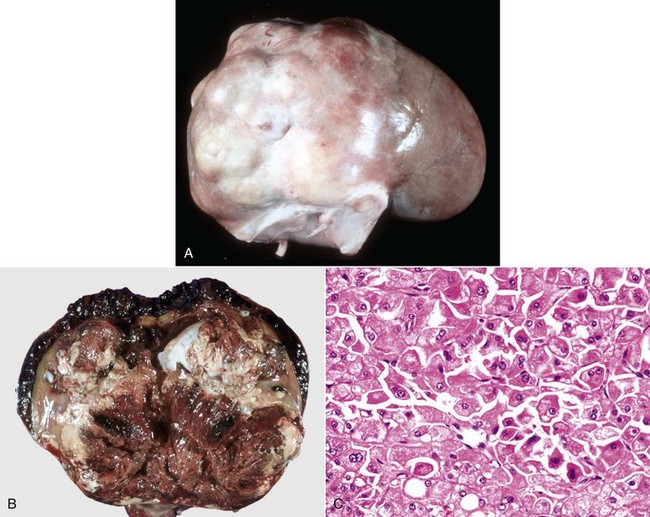
Fig. 11-58 Renal carcinoma, kidney, dog.
A, The neoplasm is pale white with reddish areas, lobulated, and has infiltrated and replaced one pole of the kidney. B, Dorsal section. The normal architecture of the cranial half of the kidney has been obliterated by the tumor, which has hemorrhaged caudally into the adjacent kidney and subcapsularly. C, The tumor consists of anaplastic renal epithelial cells, typical of the solid, more poorly differentiated variant of renal carcinoma. H&E stain. (A and B courtesy College of Veterinary Medicine, University of Illinois. C courtesy Dr. S.J. Newman, College of Veterinary Medicine, University of Tennessee.)
A variant of the typical renal carcinoma has been seen in German shepherd dogs in conjunction with nodular dermatofibrosis. The lesions are hereditary and consist of multifocal, bilateral, renal cystadenomas or cystadenocarcinomas. Grossly, these resemble the adenocarcinomas described previously, but cysts are much more prominent. The neoplastic cells form solid sheets, tubules, or papillary growth patterns, and the cells in the carcinomas are more atypical and anaplastic. Cells vary in shape from cuboidal and columnar to polyhedral, vary in size, and have clear or granular eosinophilic cytoplasm. Nuclei range from small, round, granular, and uniform to large, oval, vesicular, and pleomorphic. Mitotic figures are numerous. These neoplasms have a moderate fibrovascular stroma.
Transitional cell papillomas and transitional cell carcinomas arise in the renal pelvis and lower urinary tract and when large, can obstruct urinary outflow. Such carcinomas can invade into the kidney and typically carry a poor prognosis. The morphologic features of transitional cell neoplasms are discussed later with the urinary bladder neoplasms (see the section on the Lower Urinary System).
Mesenchymal Tumors: Occasionally, fibromas, fibrosarcomas, or hemangiosarcomas originate in the kidneys. Primary renal sarcomas are rare. Microscopically, in hemangiosarcomas (HAS), neoplastic spindle cells are arranged in solid interlacing bundles and whorls or as variable channels lined by neoplastic endothelium. Cellular pleomorphism and mitotic rate are relatively low. Dogs with renal HAS have protracted disease progression with improved 1-year survival rates and longer median survival times compared to dogs with splenic carcinoma or retroperitoneal HAS.
Metastatic Tumors: Carcinomas and sarcomas (metastatic tumors) arising in other organs can metastasize to the kidneys and are characteristically composed of randomly scattered multiple nodules, usually involving both kidneys (Fig. 11-59). Renal lymphosarcoma occurs with some frequency in cattle and cats, particularly as part of generalized or multicentric lymphosarcoma, which is secondary to retrovirus infection. These neoplastic foci appear as single or multiple homogeneous gray-white nodules (Fig. 11-59, B and E) or as diffuse lymphomatous infiltrates that cause uniform enlargement and pale discoloration of the kidney (Fig. 11-59, C). In cats, renal lymphosarcoma must be differentiated histologically from the necrotizing, fibrinous, and granulomatous renal vasculitis of feline infectious peritonitis and less commonly systemic cryptococcosis (Fig. 11-59, E). Microscopically, neoplastic lymphocytes form obliterative sheets of cells within the renal parenchyma, unrelated to the vasculature (Fig. 11-59, F). Neoplastic lymphocytes have distinct cellular borders, moderate amounts of basophilic cytoplasm, and large round vesicular nuclei with variable prominence to the nucleoli. Lymphosarcoma of the kidney can be treated with some success with chemotherapeutics.
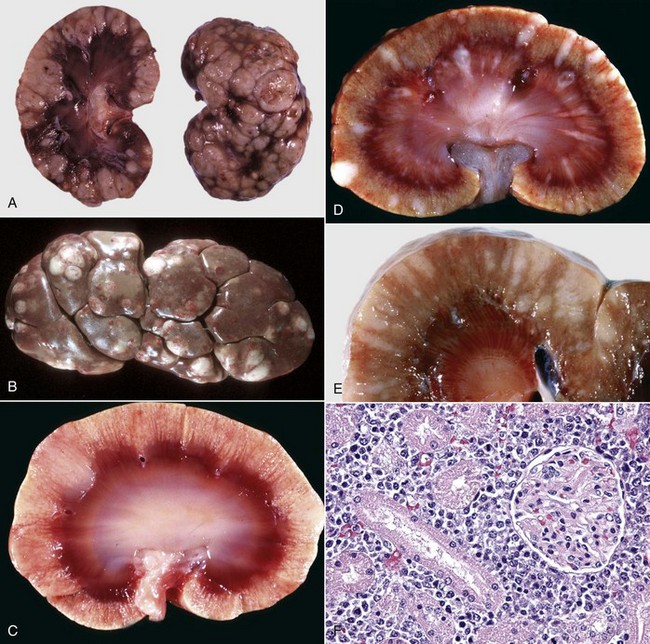
Fig. 11-59 Primary and metastatic renal tumors, kidney.
A, Metastatic mast cell tumor, dorsal section, dog. Multiple pale tan, raised nodules are randomly scattered throughout the renal cortex. B, Lymphoma (lymphosarcoma), cow. Multifocal raised pale white nodules are typical of nodular renal lymphosarcoma. C, Lymphoma (lymphosarcoma), dorsal section, cat. Note the pale white areas in the cortex, which bulge from the surface. This lesion can be confused with the granulomatous vasculitis of renal feline infectious peritonitis, thus warranting histologic evaluation. D, Systemic cryptococcosis (Cryptococcus neoformans), cat. This is not a neoplasm, but the multiple pale, occasionally raised nodules can be confused with the nodular form of lymphoma (C), thus requiring histologic examination. E, Lymphoma (lymphosarcoma), dorsal section, bovine. Multiple coalescing pale white nodules are present throughout the cortex. F, Lymphoma (lymphosarcoma), cow. Neoplastic lymphocytes infiltrate and distend the renal interstitium. H&E stain. (A courtesy Dr. A. Confer, College of Veterinary Medicine, Oklahoma State University. B courtesy College of Veterinary Medicine, University of Illinois. C courtesy Dr. K. Read, College of Veterinary Medicine, Texas A&M University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. D courtesy Dr. S.J. Newman, College of Veterinary Medicine, University of Tennessee. E courtesy Dr. J. King, College of Veterinary Medicine, Cornell University; and Dr. J. Edwards, College of Veterinary Medicine, Texas A&M University. F courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Tumors of Embryonal Origin: Nephroblastomas (embryonal nephroma or Wilms tumor) are common renal neoplasms of pigs and chickens, and are usually recognized as incidental findings at slaughter. They occur in cattle and dogs as well, but less frequently. These neoplasms arise from metanephric blastema and thus occur in young animals. It is speculated that neoplasms result from malignant transformation during normal nephrogenesis or from neoplastic transformation of nests of embryonic tissue that persists in the postnatal kidneys. At necropsy, nephroblastomas can be solitary or multiple masses that often reach a great size and in which recognizable renal tissue can be difficult to detect. They usually are soft to rubbery and gray with foci of hemorrhage. On a cut surface, they are often lobulated. Because nephroblastomas arise from primitive pluripotential tissue, histologic features vary but are morphologically similar to the developmental stages of embryonic kidneys. Characteristically, three components, including primitive, loose myxomatous mesenchymal tissue interspersed with primitive tubules lined by elongated, deeply staining cells and structures that resemble primitive glomeruli, are present. Nests of cells resembling the metanephric blastema can be present. Nephroblastomas also have mesenchymal components such as cartilage, bone, skeletal muscle, and adipose tissue. Clinically, these are usually incidental findings, except in dogs, in which they rarely present as spinal dysfunction, the result of spread to the vertebral canal with secondary spinal cord compression.
Lower Urinary System
Aplasia and Hypoplasia: Ureteral aplasia (agenesis) is the lack of formation of a recognizable ureter, and hypoplasia is the presence of a notably small-diameter ureter. Agenesis of the ureters is the result of failure of the ureteral bud to form and may be unilateral or bilateral. Both conditions are rare. If these defects occur alone, then there is disruption of urinary flow from the kidney to the urinary bladder, resulting in obstructive diseases such as hydronephrosis. If these defects occur with concurrent renal aplasia, then they are clinically silent when unilateral and life-threatening when bilateral.
Ectopic Ureters: Ectopic ureters are ureters that may empty into the urethra, vagina, neck of the bladder, ductus deferens, prostate, or other secondary sex glands. The two possible causes are as follows:
• The ureteral bud arises too far craniad to be incorporated into the urogenital sinus.
• The differential growth of the sinus is abnormal, and the ureter fails to migrate to its usual location.
Ectopic ureters are more subject to obstruction or infection, and thus they predispose animals to pyelitis and pyelonephritis. Otherwise, histologically these are normal. This condition is found most frequently in dogs and certain breeds, especially the Siberian husky, are at greater risk. Affected animals present clinically with urinary incontinence and consequent urine dribbling.
Patent Urachus: The most common malformation of the urinary bladder is patent urachus (pervious urachus). This lesion develops when the fetal urachus fails to close and therefore forms a direct channel between the bladder’s apex and the umbilicus. Failure of the urachal remnant and the umbilical arteries and vein to involute is frequently observed in cases of “neonatal” omphalitis, in which abscess formation may result in a patent urachus. Patent urachus has also occurred because of congenital urethral obstruction. Increased bladder pressure, because of the obstruction, forces urine out into the urachus. Rupture of the urachus causes uroperitoneum. The condition must be differentiated from perinatal rupture of the bladder. Foals are affected most often and animals with this defect dribble urine from the umbilicus. Diverticula of the bladder may be primary or acquired and secondary to partial obstruction of the outflow of urine or the result of pressure changes exerted during normal contractions. Occasionally, during urachal closure, the mucosa closes but closure of the bladder musculature is incomplete. When this occurs, a bladder diverticulum (outpouching) at the apex of the bladder can develop. Urine stasis can occur in the diverticulum, predisposing the animal to cystitis or urinary calculi.
Hydroureter and Hydrourethra
Hydroureter and hydrourethra refer to dilation of the ureter and urethra, respectively, and are caused by obstruction of urine outflow by blockage of the ureter(s) or urethra by calculi, chronic inflammation, or luminal or intramural neoplasia. Hydroureter can be unilateral or bilateral. Depending on the location of the obstruction, hydronephrosis, hydroureter, and hydrourethra can occur concurrently (see the previous section on Hydronephrosis). Histologic findings demonstrate little change except for a greater cross-sectional diameter of the ureter or urethra and compression of their lining epithelium. The clinical signs of these conditions are related to obstruction.
Urolithiasis (Obstructive Disease)
Urolithiasis is a syndrome that occurs when familial, congenital, and pathophysiologic factors occur together and increase the risk of precipitation of excretory metabolites in urine to form stones. Urinary calculi (uroliths) are concretions formed anywhere in the urinary collecting system, and although some clearly originate in the lower urinary tract or as microscopic calculi in the renal collecting tubules, the point of development of most is not known. Uroliths are commonly found in the ureter, followed by any portion of the lower urinary tract and least commonly in the renal pelvis (accounting for 1% to 4% of canine uroliths). The diseases caused by uroliths are among the most important urinary tract problems of domesticated animals, especially cattle, sheep, dogs, and cats, and are of lesser importance in horses and pigs.
Mechanistically, factors that are important either in predisposing to calculus formation or in precipitating disease include the following:
• Calculus precursor material in urine in quantities sufficient to be precipitated.
• Substances metabolized in an unusual way, as is uric acid in Dalmatian dogs.
• Substances processed abnormally by the kidney (hereditary defects), as with cystine or xanthine.
• Substances are encountered in the diet in abnormally high levels such as the following:
• Silicic acid in native pastures
• Phosphate in milo or sorghum products (struvites)
• Estrogen in subterranean clover (clover stones [benzocoumarin] or carbonates)
• Abnormally low levels of a substance are encountered in the diet such as the following:
Regardless of the type of calculus, certain factors are more or less important in calculus formation; these are as follows:
• Urinary pH, in terms of its optimum for solute precipitation (oxalates at acid pH and struvites and carbonates at alkaline pH)
• Reduced water intake, in relation to the degree of urine concentration and mineral supersaturation
• Bacterial infection of the lower urinary tract (struvite calculi in dogs)
• Structural abnormalities of the lower urinary system
• Foreign bodies (suture, grass awn, catheter, or needle), or a conglomerate of bacterial colonies, exfoliated epithelium, or leukocytes, which can serve as a nidus for precipitation of mineral constituents
• Drugs excreted in the urine, which may act as a nidus for calculus formation (e.g., sulfonamides and tetracyclines)
Supersaturation of urine with respect to the components of stone-forming salts is the essential precursor to initiation of urolith formation (nucleation). Supersaturation may be in the unstable range, in which precipitation occurs spontaneously (homogeneous nucleation), or the metastable range, in which precipitation occurs by epitaxy (one type of crystal grows on the surface of another type; heterogeneous nucleation).
Although it was formerly thought that urinary proteins, such as uromucoid, which make up 5% to 20% or more of some calculi, were preeminent initiators of crystal formation in the metastable range, it is now believed that in many cases either co-precipitation of proteins and minerals occurs or that proteins are adsorbed onto formed crystals. It is possible that crystals of one salt, for which urine is supersaturated in the unstable range, cause induction of crystals of another salt for which supersaturation is apparently stable.
Crystals are much more common in urine than are calculi. Even though equine urine is normally supersaturated with calcium carbonate, and crystalluria is normal, horses experience a low prevalence of calculi. The factors, which promote or prevent crystal growth and crystal aggregation, are poorly understood. Experimentally, high concentrations of urinary inorganic pyrophosphate and magnesium are important inhibitors of calcium phosphate and calcium oxalate crystallization. Pyrophosphate also inhibits aggregation of calcium phosphate crystals. Certain urinary macromolecules, probably glycosaminoglycans, are also strong inhibitors of crystal aggregation in experimental systems. Deficiency of inhibitors of crystallization may be important in calcium oxalate and calcium phosphate calculogenesis (crystallization-inhibition theory of urolith initiation).
Macroscopically, calculi are grossly visible aggregations of precipitated urinary solutes, principally mineral admixed with urinary proteins and proteinaceous debris. Calculi typically are hard spheres or ovoids, with a central nidus, surrounded by concentric laminae (“stone”), an outer shell, and surface crystals. Many calculi contain significant quantities of “contaminants” such as calcium oxalates in “silica” calculi; few are relatively pure. Large renal pelvic calculi classically have a “staghorn” appearance because they take the shape of the renal calyces in animal species that have true calyces (Fig. 11-60). These calculi predispose affected animals to pyelitis and pyelonephritis. Urinary bladder calculi can be single or multiple, variable in size (2 to 10 cm), and sometimes are composed of a fine, sandlike material, which causes cloudy urine (Fig. 11-61). Calculi can have smooth or rough surfaces and may be solid, soft, or friable. Calculi vary in color, depending on their composition; however, color is variable among calculi, even of the same composition. The calculi can be white to gray (e.g., struvite and oxalate), yellow (e.g., urate, cystine, benzocoumarin, and xanthine), or brown (e.g., silica, urate, and xanthine), depending on their composition.
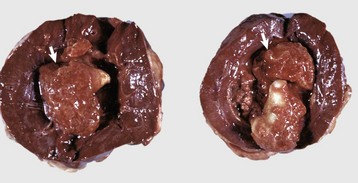
Fig. 11-60 Urolithiasis, kidney, dorsal section, dog.
A calculus fills and distends the renal pelvis (arrows) and has caused pressure atrophy of the medulla. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
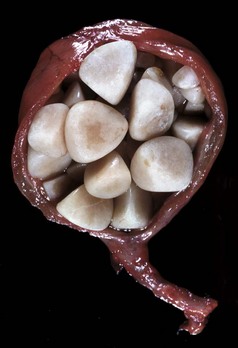
Fig. 11-61 Urolithiasis, urinary bladder, dog.
Multiple smooth calculi are present in the urinary bladder. The bladder wall is diffusely thickened. (Courtesy Dr. A. Confer, College of Veterinary Medicine, Oklahoma State University.)
Small calculi may be voided in the urine, but typically calculi cause urinary obstruction. This is more common in males because of their long and narrow-diameter urethra. The most common sites of lodgement of urethral calculi vary with the animal species. In male cattle, calculi lodge in the urethra at the ischial arch and at the proximal end of the sigmoid flexure; in rams and wethers, the urethral process (vermiform appendage) is the most common site (Fig. 11-62, A); and in dogs, calculi lodge proximal to the base of the os penis (Fig. 11-62, B). At the site in which calculi lodge, there is local pressure necrosis, ulceration of the mucosa, and acute hemorrhagic urethritis. Because the urethral sites are prone to rupture, hydronephrosis after urethral obstruction is less common than with unilateral long-standing ureteral impaction. In cats, fine struvite crystals (sand) in a rubberlike protein matrix can fill the entire urethra, and such calculi are typical for the disease called feline urologic syndrome (Fig. 11-62, C). When obstruction or dysuria occurs in females, calculi are usually large and located in the renal pelvis or urinary bladder.
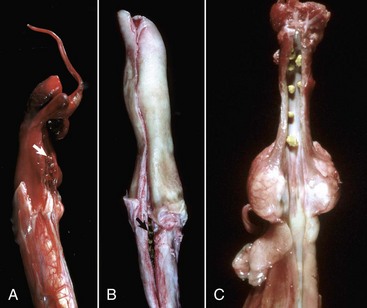
Fig. 11-62 Urolithiasis, penile urethra.
A, Sheep. Multiple calculi are present in the penile urethra (arrow) and the urethral process (vermiform appendage). B, Ventral aspect, dog. Calculi have lodged in the urethra proximal to the caudal end of the os penis (arrow). C, Cat. Calculi are present throughout the penile urethra, several just caudal to the external urethral orifice at tip of the penis. (A and B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. C courtesy College of Veterinary Medicine, University of Illinois.)
At necropsy, animals that have died of urinary obstruction have greatly distended (Fig. 11-63, A), turgid, or ruptured bladders and dilated ureters and renal pelves. The bladder wall is thin and often has mucosal to transmural ecchymoses or diffuse hemorrhage (Fig. 11-63, B). When urine is released from the bladder, either because of rupture or incision at surgery or necropsy, the wall of the bladder is flaccid, the mucosa is often ulcerated, and the urine contains blood clots. Mucosal ulceration, localized lamina propria hemorrhage, and mucosal necrosis are usually present in the ureter, bladder, or urethra adjacent to an obstructive calculus. If the bladder ruptures antemortem, blood clots and fibrin are at the site of rupture, and in some cases there is an acute, localized chemical (urine-induced) peritonitis.
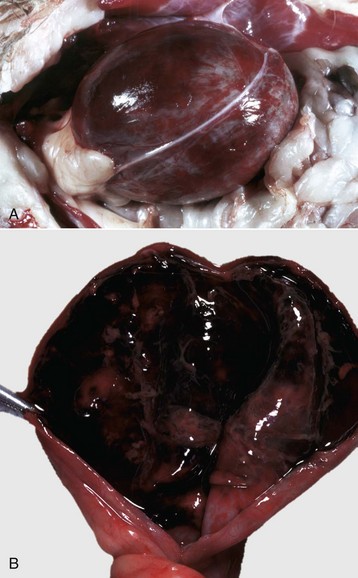
Fig. 11-63 Hemorrhagic urocystitis (feline urologic syndrome) urinary bladder, cat.
A, Obstructive urolithiasis. The bladder is overdistended and turgid as the result of urethral obstruction. Note the serosal and intramuscular ecchymotic and suffusive hemorrhages at the neck and apex of the bladder. B, Urolithiasis, acute hemorrhagic cystitis. The severe diffuse transmural hemorrhage throughout the urinary bladder wall is secondary to blockage of the urethra by calculi and distention of the urinary bladder. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Microscopically, inflammation and hemorrhage are present in the lower urinary tract. Lesions are most severe in cases in which obstruction has been complete. The mucosa is usually ulcerated, and areas of hyperplastic transitional epithelium are interspersed with goblet cells. The lamina propria is usually infiltrated with inflammatory cells. Neutrophils are present at foci of ulceration, and lymphocytes and plasma cells infiltrate perivascularly or uniformly throughout the lamina propria. Hemorrhage is transmural but is most evident in the mucosa and can cause separation of the smooth muscle bundles. Degeneration and necrosis of smooth muscle occurs in severe cases.
Clinically, urolithiasis can cause urinary obstruction or traumatic injury to the urinary bladder mucosa. Lesions of the urinary bladder are manifested clinically as difficult or painful urination (stranguria; dysuria), with or without hematuria. Small calculi may be voided in the urine, but typically calculi cause urinary obstruction. In males, dysuria can result from large calculi, but urinary tract obstruction with uremia most commonly occurs because of obstruction of the urethra with small calculi.
Inflammatory Diseases
Acute Cystitis: Inflammation of the urinary bladder (cystitis) is common in domestic animals. Because inflammation of the ureter (ureteritis) or urethra (urethritis) in the absence of cystitis is rare, this discussion concentrates on cystitis. The causes of acute cystitis are varied; however, for all animal species, bacterial infection is the most common cause. Cystitis may be acute or chronic. Normally, the bladder is resistant to infection, and contaminating bacteria are quickly eliminated by the normal flow of normal urine. Predisposition to urinary tract infection (UTI) occurs when there is stagnation of urine because of obstruction, incomplete voiding at micturition, or urothelial trauma. Other risk factors for UTI include catheterization, vaginoscopy, vaginitis, urinary incontinence, or prolonged administration of medications such as antibiotics that induce bacterial resistance. Bacterial cystitis is more common in females because their relatively short urethra provides a shorter barrier to ascending infections than the longer, narrow diameter of the male urethra. The bacterial species most commonly associated with cystitis are uropathogenic Escherichia coli (α-hemolysin–producing strains) in all animal species; Corynebacterium renale in cattle; Actinobaculum suis (Eubacterium suis) in pigs; Enterococcus faecalis in cats; and Klebsiella sp. in horses. In addition, Proteus sp., Streptococcus sp., and Staphylococcus sp. have been isolated from cases of cystitis in several animal species.
Except for the distal urethra, the lower urinary tract is normally free of bacteria. Sterility of the urinary bladder is maintained through normal repeated voiding of urine and because of the antibacterial properties of urine. These antibacterial properties are attributed to the following:
• The acidic urine of carnivores
• Secreted mucin that inhibits bacterial adhesion
Cystitis occurs when bacteria are able to overcome normal defense mechanisms and adhere to or invade (colonize) the urinary bladder mucosa. Several factors can enhance colonization and predispose animals to cystitis. Colonization is more likely for strains of bacteria that express molecules on their surfaces that enhance adhesion (e.g., the P and type 1 fimbriae of certain strains of Escherichia coli and Actinobaculum suis and the pH-dependent adherence by pili of Corynebacterium renale). Retention of urine as a result of obstruction or neurogenic causes, such as spinal cord diseases, often leads to cystitis.
Trauma to the urinary bladder mucosa as the result of calculi, faulty catheterization, or other causes can cause erosion and hemorrhage and predispose to bacterial invasion of the lamina propria. Hydrolysis of urea by urease-producing bacteria, such as Corynebacterium renale in cattle and Actinobaculum suis in pigs, releases excessive ammonia that can damage the mucosa and result in urine alkalinity. Bacterial growth can be enhanced when glucosuria is present, such as in diabetes mellitus. Compromise of the host immune system as found with infection by the feline immunodeficiency virus (FIV) can increase susceptibility to bacterial cystitis. Other bacterial virulence factors, such as the Escherichia coli hemolysin, enhance pathogenicity and help bacteria overcome antibacterial factors of the urinary bladder and urine.
Once bacteria gain access to the lamina propria, they cause vascular damage and inflammation. Acute cystitis is often grossly described as hemorrhagic, fibrinopurulent, necrotizing, or ulcerative, and these changes are often sequential over time. Vascular damage predisposes to hemorrhage, leakage of fibrin, and if severe, ischemic necrosis of the bladder. This is often accompanied by mucosal ulceration. Neutrophils are present as a component of vascular damage and in any lesion with accompanying bacterial colonization. However, in each case, components of several of these processes are present. The urinary bladder wall often is thickened by edema, an inflammatory cell infiltrate, and is diffusely or focally hemorrhagic. Hemorrhage is most common when obstruction is concurrently present or after direct trauma from catheterization. Urine in such cases is described as cloudy, flocculent, foul smelling, and red-tinged. The mucosa can have foci of erosion or ulceration, patches or sheets of adherent exudate and necrotic debris, or adherent blood clots (Fig. 11-64, A). Corynebacterium urealyticum in dogs and cats and Corynebacterium matruchotii in a horse were implicated in a condition known as encrusted cystitis, in which plaques and accumulation of sediment predominate. Rarely, surgical debridement in addition to appropriate antimicrobial therapy is required.
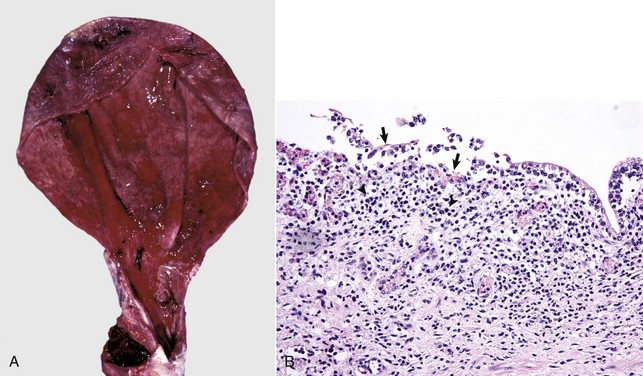
Fig. 11-64 Acute cystitis, urinary bladder.
A, Mucosal and serosal surfaces, calf. Patchy areas of ulcerated mucosa are interspersed with areas of hemorrhagic mucosa. Note the subserosal hemorrhages (top). B, Mucosal and serosal surfaces, dog. The mucosa has been partially denuded of transitional epithelium (arrows). There are mucosal and submucosal infiltrates of neutrophils (arrowheads), which extend into the adjacent tunica muscularis. Note the congested vessels with active hyperemia in the lamina propria. H&E stain. (A courtesy Dr. A. Confer, College of Veterinary Medicine, Oklahoma State University. B courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Microscopically, acute cystitis is characterized by epithelial denudation with bacterial colonies present on the surface. The lamina propria is intensely edematous and has a diffuse neutrophilic infiltrate. Superficial hyperemia and hemorrhage are usually present (Fig. 11-64, B). A mild perivascular leukocytic infiltrate can occur in the tunica muscularis.
Clinically, dysuria, stranguria, and hematuria characterize acute onset of bacterial cystitis. An inflammatory sediment is detected on urinalysis, and bacteria can be grown in pure culture from urine samples.
Viral causes of acute cystitis are relatively rare in veterinary medicine. In cats, a cell-associated herpesvirus has been found in some cases of mild cystitis. Hemorrhagic cystitis sometimes occurs in malignant catarrhal fever in cattle and deer and occasionally is the dominant gross feature in the disease.
Acute cystitis can result from several chemical causes. Active metabolites of cyclophosphamide, a drug used to treat neoplastic and immune-mediated diseases of dogs and cats, can cause a sterile hemorrhagic cystitis. Cantharidin toxicosis in horses results from ingestion of blister beetles (Epicauta spp.) in alfalfa hay, and hemorrhagic and necrotic cystitis develops from cantharidin excreted through the urinary tract. Chronic ingestion of bracken fern (Pteridium aquilinum) by cattle can result in the syndrome of enzootic hematuria, which can be manifested as acute urinary bladder hemorrhage, chronic cystitis, or urinary bladder neoplasia.
Chronic Cystitis: Chronic cystitis presents as several forms based on the pattern and type of inflammatory response noted. These forms include diffuse, follicular, and polypoid variants. Diffuse variants reveal an irregularly reddened and usually thickened mucosa. There is some epithelial desquamation, and the submucosa is heavily infiltrated with mononuclear inflammatory cells; there are few neutrophils. In addition, there is often connective tissue thickening of the submucosa and hypertrophy of the muscularis layer.
Follicular variants reveal disseminated, nodular, submucosal lymphoid proliferations (2 to 4 mm in diameter) so that the mucosa has a cobblestone appearance (follicular cystitis) (Fig. 11-65). This response is particularly common when cystitis is associated with chronic urolithiasis. These white-gray lymphoid foci are often surrounded by a zone of hyperemia. Often, the lesions are a diffusely thickened, hyperplastic mucosa with goblet cell hyperplasia and a chronic lymphoplasmacytic infiltrate and fibrosis in the lamina propria. Hypertrophy of the tunica muscularis can be observed.
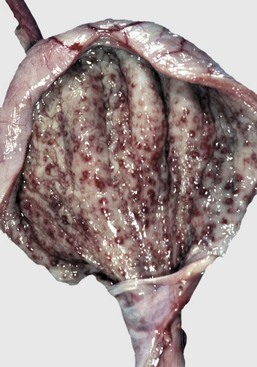
Fig. 11-65 Chronic follicular cystitis, urinary bladder, mucosal surface, dog.
Multiple small raised red nodules are present on the mucosal surface. These nodules are hyperplastic lymphoid cells surrounded by hyperemia and hemorrhage. (Courtesy Dr. A. Confer, College of Veterinary Medicine, Oklahoma State University.)
Polypoid masses (chronic polypoid cystitis), seen predominantly in female dogs, likely develop from inflammatory and hyperplastic responses secondary to chronic irritation, which most often arises from persistent bacterial urinary tract infection or uroliths. These hyperplastic transitional epithelial responses are called cystitis cystica, cystitis glandularis, and Brunn’s epithelial nests. Inflammation, epithelial proliferation, and development of a nonneoplastic mass occur most typically in the cranioventral bladder wall. The mucosa has single or multiple nodular mucosal masses (Fig. 11-66) composed of fibrous connective tissue and infiltrated with neutrophils and mononuclear leukocytes. The masses are broad-based or pedunculated, ulcerated, or covered by hyperplastic epithelium with goblet cell metaplasia. Chronic polypoid cystitis is characterized by clinical hematuria.
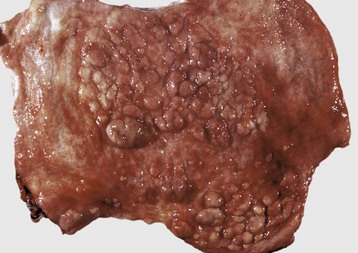
Fig. 11-66 Chronic polypoid cystitis, urinary bladder, mucosal surface, dog.
This type of cystitis is characterized by multiple masses composed of proliferative nodules of connective tissue (polyps) mixed with neutrophils and lymphocytes. (Courtesy Dr. A. Confer, College of Veterinary Medicine, Oklahoma State University.)
Bracken Fern–Induced Hemorrhagic Urocystitis: All parts of the plant are toxic. Bracken fern contains several toxic substances, including a thiaminase, a variety of carcinogens (quercetin, shikimic acid, prunasin, ptaquiloside [braxin C], ptaquiloside Z, aquilide A, and others), a “bleeding factor” of unknown structure, and substances that act as immunosuppressants. After administration of ptaquiloside to guinea pigs, hemorrhagic cystitis results, suggesting that this is one of the toxic principles in bracken fern hematuria.
The extent and persistence with which toxic ferns are grazed probably influences the incidence of bladder lesions. Cattle fed low levels of bracken fern develop microscopic, followed by macroscopic, hematuria. Microhematuria usually is associated with petechial, ecchymotic, or suffusive hemorrhages in the urothelium of the renal calyces, pelvis, ureter, and bladder, and microscopically, ectasia and engorgement of capillaries are present. Altered vessels are prone to hemorrhage into the bladder wall or lumen, and nodular hemangiomatous lesions develop in affected areas. In a few animals, macroscopic hematuria is due solely to these nonneoplastic changes, but usually it is caused by development of tumors, which ulcerate and bleed into the lumen. Additionally, there can be mucosal metaplasia.
Bracken fern–induced neoplasia is discussed later in more detail in the section on Disorders of Ruminants.
Mycotic Cystitis: Mycotic cystitis is occasionally seen in domestic animals when opportunistic fungi, such as Candida albicans or Aspergillus sp., colonize the urinary bladder mucosa. Such fungal infections usually occur secondary to chronic bacterial cystitis, especially when animals are immunosuppressed or subjected to prolonged antibiotic therapy. Occasionally, Blastomyces dermatitidis can produce lower urinary tract lesions in dogs. The urinary bladder is usually ulcerated with proliferation of underlying lamina propria; a generalized thickening of the urinary bladder wall is the result of extensive inflammation consisting of neutrophils, lymphocytes, plasma cells, macrophages and edema, and fibrosis.
Neoplasia
Neoplasms of the lower urinary tract occur predominantly in the urinary bladder. Neoplasms are much more common in the bladder than the urethra and are rare in the ureter. They are observed most frequently in dogs, occasionally in cats, and rarely in other species. Urinary bladder neoplasms comprise less than 1% of total canine neoplasms. Most occur in old dogs, without a sex predisposition.
Risk factors associated with bladder cancer in dogs include the following:
• Exposure to marshes sprayed with chemicals for mosquitoes
Retention of urine in the bladder and longer exposure of the epithelium to carcinogens results in a higher incidence of tumors in the urinary bladder. Many chemicals, including intermediate components of aniline dyes, aromatic hydrocarbons, and tryptophan metabolites, have been found experimentally or epidemiologically to induce urinary bladder neoplasms. Chemically induced and spontaneous tumors progress through a series of histologic stages from hyperplasia, squamous metaplasia, papilloma, adenoma, dysplasia, carcinoma in situ, to carcinoma.
Epithelial Tumors: Epithelial neoplasms are by far the most common of the urinary system and are classified as transitional cell papillomas, transitional cell carcinomas, squamous cell carcinomas, adenocarcinomas, and undifferentiated carcinomas, as follows:
• Papillomas have a papilliferous or pedunculated appearance. Microscopically, they have a papillary growth pattern consisting of multiple papilliferous fibrous cores covered by well-differentiated transitional epithelium.
• Carcinomas are focal, raised nodules or diffuse thickenings of the urinary bladder wall that are most common in the trigone region of the bladder (Fig. 11-67, A). Transitional cell carcinomas are composed of pleomorphic to anaplastic transitional epithelium. Neoplastic transitional cells cover the mucosal surface as irregular layers, readily invade the lamina propria in the form of solid nests and acini, and are found within lymphatic vessels of the submucosal and muscle layers (Fig. 11-67, B). Terriers may be at a slightly elevated risk for development, and there has been an association made between occurrence of these tumors and exposure to lawn pesticides. This neoplasm in cats is rare but aggressive when present. Males are more commonly affected. Unlike dogs, the trigone area is not the predilection site for development.
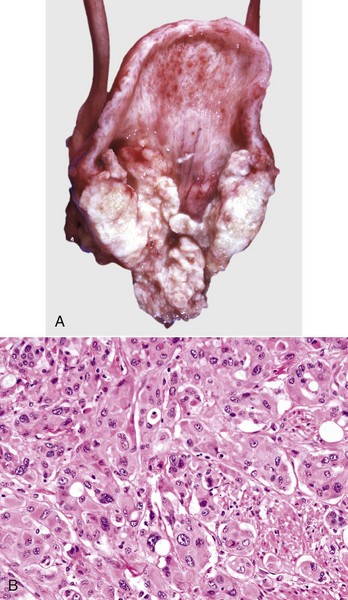
Fig. 11-67 Transitional cell carcinoma, urinary bladder, dog.
A, Transitional cell carcinomas are typically adjacent to the trigone (as here), where they can become large enough to obstruct the opening of a ureter or ureters and result in secondary hydroureter and/or hydronephrosis. B, Lamina propria. The tumor is formed by anaplastic cells grouped in small islands and clusters. Nuclei are vesicular with prominent nucleoli, and some nuclei show remarkable anisokaryosis. H&E stain. (A courtesy Dr. A. Confer, College of Veterinary Medicine, Oklahoma State University. B courtesy Dr. S.J. Newman, College of Veterinary Medicine, University of Tennessee.)
• Squamous cell carcinomas, adenocarcinomas, and unclassified carcinomas most likely arise from transitional epithelium. In the bitch, carcinomas are multicentric in origin, develop not only in the urinary bladder but also in the urothelium of the ureters, urethra, and renal pelvis, and often extend to the vagina and vestibule.
Metastasis of urinary bladder carcinomas is most often first seen in regional lymph nodes adjacent to the aortic bifurcation, including the deep inguinal, medial iliac, and sacral lymph nodes. Other potential sites of metastasis include lungs and kidneys, with metastasis to other parenchymatous organs occurring later.
Mesenchymal Tumors: Mesenchymal tumors, including fibromas, fibrosarcomas, leiomyomas, leiomyosarcomas, rhabdomyosarcomas, lymphosarcomas, hemangiomas, and hemangiosarcomas, occur in the lower tract. Primary fibrosarcomas, leiomyosarcomas, hemangiomas, and hemangiosarcomas are rare. Mesenchymal tumors are classified as follows:
• Leiomyomas are the most common neoplasms and are solitary or multiple, circumscribed, firm, and pale white-to-tan masses in the urinary bladder wall. Leiomyomas have the macroscopic consistency and microscopic appearance of normal smooth muscle. Malignant counterparts (leiomyosarcoma) are much more rarely identified. These are locally infiltrative but only rarely show metastatic spread.
• Fibromas arise from lamina propria connective tissue and project into the bladder lumen as solitary nodules.
• Lymphosarcoma occasionally infiltrates the wall, not only of the bladder but also of the ureters and renal pelves in cattle, pigs, dogs, and/or cats. Common complications include hydronephrosis and hydroureter.
• Rhabdomyosarcomas are rare but occur in the bladder and urethra of young large breed dogs (younger than 18 months old), suggesting an embryonal origin. The cell of origin is speculated to be embryonic myoblasts from the urogenital ridge. These masses are described as botryoid (grapelike) masses (4 to 18 cm in diameter) that protrude into the bladder lumen. Local invasion and occasional metastasis to lymph nodes characterize the typical behavior. Microscopically, the neoplasms are composed of sheets of fusiform cells interspersed with pleomorphic cells. Microscopic demonstration of cross-striations typical of skeletal muscle or immunohistochemical demonstration of the intermediate filament, desmin, are useful to confirm the diagnosis of rhabdomyosarcoma. Clinical presentation includes hematuria, urinary obstruction, hydroureter, hydronephrosis, and hypertrophic osteopathy.