Inflammation of Bone
Infectious Inflammation of Bone
Inflammation of bone is termed osteitis. Periostitis occurs if the periosteum is involved, and osteomyelitis is the appropriate term if the medullary cavity of the bone is involved (Table 16-2). These conditions generally occur together and may be life threatening, requiring early diagnosis and vigorous treatment. They often involve the simultaneous necrosis and removal of bone and the compensatory production of new bone, occurring over a prolonged period. Infectious inflammation of bone in animals is usually caused by bacteria. Hematogenous bacterial osteomyelitis is uncommon in dogs and cats, but it is common in neonatal foals and animals used for food and fiber production. A wide range of Gram-positive and Gram-negative bacteria is responsible for hematogenous osteomyelitis in calves and foals. Arcanobacterium pyogenes and other pyogenic bacteria (e.g., Streptococcus spp., Staphylococcus spp.) and Salmonella spp., Escherichia coli, and other coliforms are among the most common microbes that cause hematogenous osteomyelitis. Staphylococcus intermedius is the most common cause of hematogenous osteomyelitis in dogs.
Fungi, viruses, and protozoa also can cause bony lesions. Mycotic agents, such as Coccidioides immitis and Blastomyces dermatitidis, frequently spread hematogenously to bone to produce granulomatous or pyogranulomatous osteomyelitis, with bone lysis and irregular new bone formation. The viruses of hog cholera and infectious canine hepatitis can cause endothelial damage, resulting in metaphyseal hemorrhage, necrosis, and acute inflammation. Osseous localization of the distemper virus injures osteoclasts, disrupting metaphyseal modeling and producing a growth retardation lattice (described earlier), and a variant of the feline leukemia virus (FeLV) has been associated with myelosclerosis (increased density of medullary bone) in cats; however, these latter two viruses do not cause inflammation of bone.
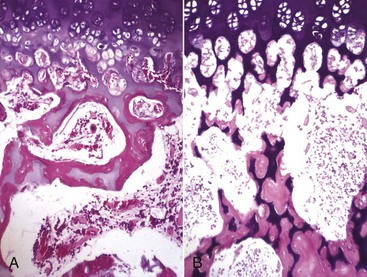
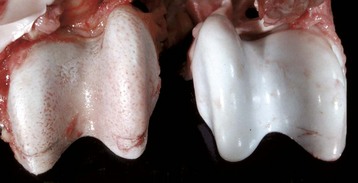
Growth plate: Epiphyseal cartilage (Fig. 16-56) (cartilage of the epiphysis that has yet to undergo endochondral ossification) and physeal cartilage can be eroded by invasion of inflammation from adjacent bone or may undergo direct bacterial embolization via cartilage canal blood vessels or in the blind-loop vessels at sites of endochondral ossification (see the section on Portals of Entry of Bone). Growth cartilage may appear thickened secondary to osteomyelitis because of disruption of endochondral ossification by the inflammatory process and the resulting failure to replace cartilage with bone (see Fig. 16-35). In growing animals, articular cartilage can undergo lysis by extension of osteomyelitis from the subjacent AEC, a lesion that may be mistaken for primary arthritis (see Fig. 16-36).
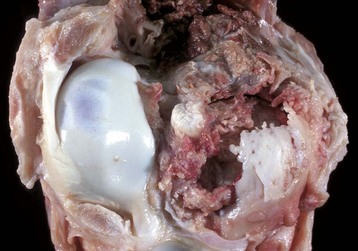
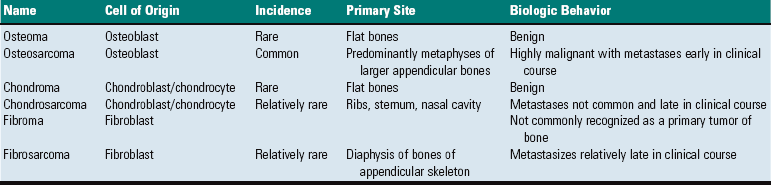
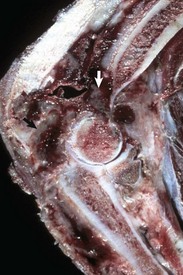
Fig. 16-56 Emboli (suppurative) epiphysitis, bone, distal femur, foal.
Bacterial emboli in the articular-epiphyseal complex (AEC) have produced suppurative inflammation that has destroyed both the subchondral bone and overlying articular cartilage of the condyle (right). (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
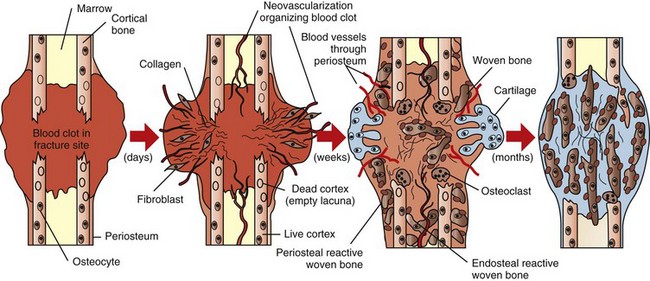
Trabecular bone: The composition of the exudate in metaphyseal osteomyelitis is determined by the infectious agent and typically is purulent in bacterial infections in domestic animals. Exudate in the medullary cavity increases the intramedullary pressure and can cause compression of vessels resulting in thrombosis and infarction of intramedullary fat, hematopoietic marrow, and bone. In areas of inflammation, bone resorption is mediated primarily by osteoclasts that are stimulated by prostaglandins and cytokines released by local tissue and inflammatory cells. Reduced blood flow through large vessels also promotes osteoclastic bone resorption, possibly by altering electrostatic charges in bone. Proteolytic enzymes released by inflammatory cells and activation of matrix metalloproteinases by the acid environment of inflammation also assist in resorbing matrix. Lack of drainage and persistence of the offending agent in areas of necrotic bone account for the chronicity of the process, which may continue for years. Inflammation in the medullary cavity also may penetrate into and through cortical bone and undermine the periosteum, where it can further disrupt the blood supply to the bone at the nutrient foramen and nutrient canal.
Cortical bone: The lesions involving cortical bone that occur with infectious osteomyelitis may vary based on the route of entry of the organism and the nature of the exudates (Fig. 16-57). Bone lysis is expected with suppurative inflammation and is subperiosteal for bacteria induced traumatically via the periosteum and endosteal in cases of embolic osteomyelitis. Lysis within the cortex begins within existing vascular channels and can occur with either route of entry. Periostitis can develop by direct inoculation from trauma (e.g., puncture wounds) or by centripetal spread of inflammation from the marrow cavity and through the cortex. Chronic bacterial periostitis is characterized by multiple coalescing pockets of exudate and areas of irregular periosteal new bone formation and cortical lysis (Fig. 16-58). Additional sequelae of osteomyelitis include extension of inflammation to adjacent bone, hematogenous spread to other bones and to soft tissues, pathologic fractures, and development of sinus tracts that penetrate cortical bone and drain to the exterior. Occasionally, fragments of dead bone become isolated from their blood supply and surrounded by exudate (bone sequestrum). Sequestra can form when bone fragments are contaminated at the site of a compound fracture, when the fragments at a fracture site become infected hematogenously, or when fragments of necrotic bone become isolated (and thus avascular) in osteomyelitis (Fig. 16-59). These sequestra and associated exudates can become surrounded by a dense collar of reactive bone, which is termed the involucrum. Extracellular matrix is not living tissue; therefore it cannot be resorbed in an area in which the cells (bone or marrow) are dead. For this reason, relatively large sequestra can persist for long periods and may interfere with repair. Grossly, they often become pale and chalky and lack the glistening appearance of normal bone.
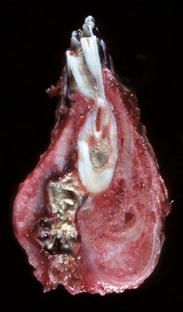
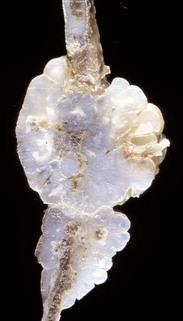
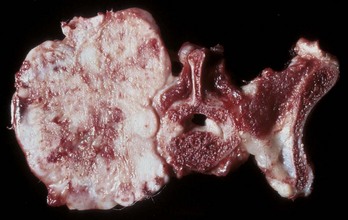
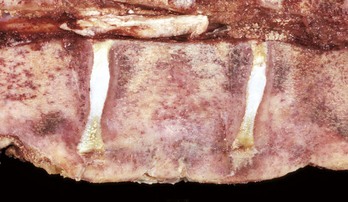
Fig. 16-57 Chronic (suppurative) osteomyelitis, bone, mandible, transverse section, sheep.
Chronic suppurative osteomyelitis has caused a fistulous tract that penetrates through the full dorsoventral thickness of the mandible. This lesion likely began as a periodontal bacterial infection. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
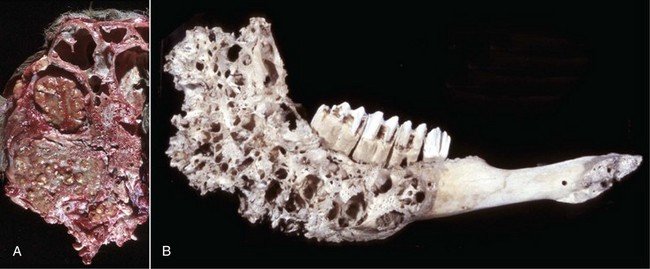
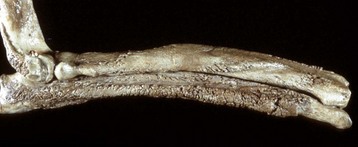
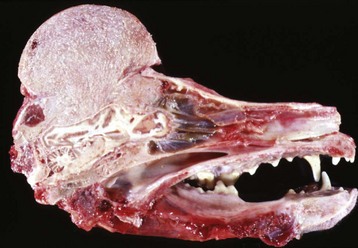
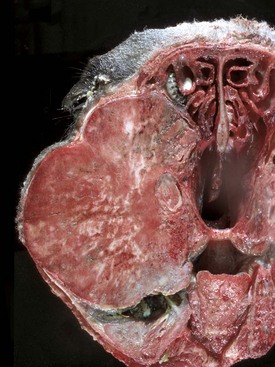
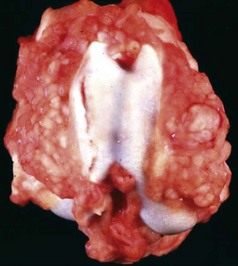
Fig. 16-58 Chronic (pyogranulomatous) osteomyelitis and sinusitis, actinomycosis (Actinomyces bovis), maxillary sinus, cow.
A, Transverse section of the maxillae. The nodules apparent within the masses in the sinuses and maxilla represent pockets of pyogranulomatous inflammation that are surrounded by fibrous tissue and woven bone. B, Macerated and bleached specimen of the mandible. Note the spicules of woven bone radiating from the mandible. Within the spaces formed by this reactive bone were nodules of pyogranulomatous inflammation and colonies of Actinomyces bovis. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
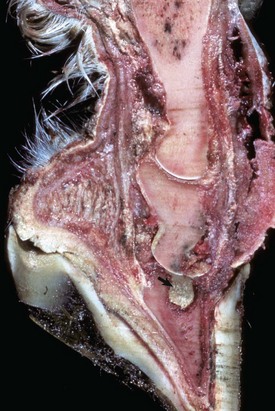
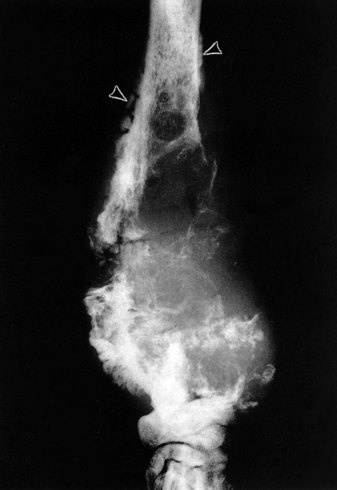
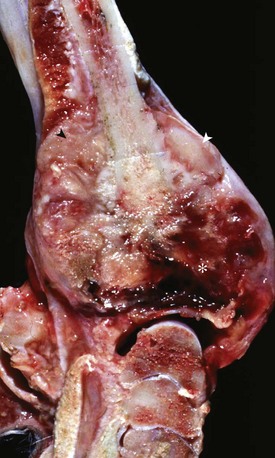
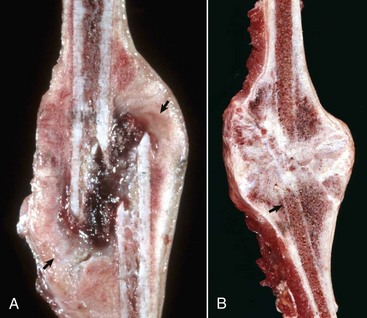
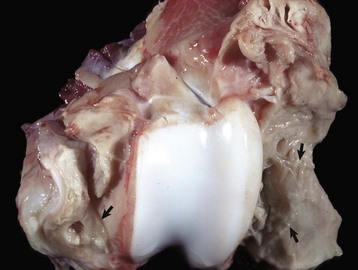
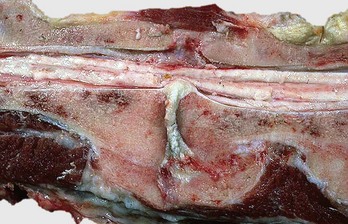
Fig. 16-59 Suppurative periostitis and osteomyelitis, phalanges, horse.
Trauma to the dorsal aspect of the hoof inoculated bacteria into the subcutis, causing suppurative cellulitis and periostitis and, subsequently, cortical osteolysis of the dorsal aspect of the distal first phalanx and the entire dorsal surface of the second phalanx. From there, the infection spread to the distal interphalangeal joint and then to the third phalanx, where it caused suppurative osteomyelitis, loss of articular cartilage, and formation of a sequestrum in the proximal portion of the third phalanx (arrow). The viable tissue immediately adjacent to the sequestrum is not different from that which is more distant, implying that in this case an involucrum (reactive bone surrounding the exudate around a sequestrum) was not formed. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
Noninfectious Inflammation of Bone
Metaphyseal Osteopathy: Metaphyseal osteopathy, previously termed hypertrophic osteodystrophy, is a disease of young (usually 3 to 6 months of age), growing dogs of the large and giant breeds. Both names, unfortunately, are misleading, since the initial lesion is a suppurative and fibrinous osteomyelitis of the trabecular bone of the metaphysis, which is replaced in the chronic stages of the disease by the formation of abundant periosteal new bone. Remissions/exacerbations may occur over weeks to months, but most cases resolve completely. The cause and pathogenesis are unknown; infectious agents have not been isolated. There are reports of metaphyseal osteopathy in litters of Weimaraners, in which granulocytopathies have been suspected, and in littermates of Irish setters, in which canine leukocyte adhesion deficiency was confirmed. The lack of CD18 expression on the neutrophil surface in the affected Irish setters results in the failure of neutrophils to marginate or extravasate, as well as a failure of these cells to phagocytose by CD18. Affected dogs have an underlying genetic defect that is expressed clinically to various degrees, ranging from normal to the development of severe, repeated infections. Interestingly, 75% to 85% of these dogs develop metaphyseal osteopathy by 10 to 12 weeks of age, and hematopoietic stem cell transplants lead to resolution of all lesions. Based on these findings, it is possible that neutrophil entrapment at the chondro-osseous junction in the metaphysis (same location that is predisposed to the development of osteomyelitis in young animals; see section on Portals of Entry into Bone) results in autoinflammation and necrosis, with the periosteal proliferation occurring as a secondary event. Clinically, metaphyseal osteopathy is characterized by lameness, fever, and swollen, painful metaphyses in multiple long bones.
Growth plate: Lesions in the growth plate are not expected in metaphyseal osteopathy.
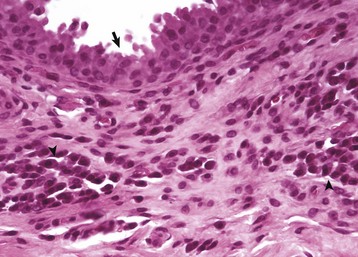
Trabecular bone: Lesions are usually bilaterally symmetric. Radiographically, alternating metaphyseal zones of increased lucency and increased density are present parallel to the physes (Fig. 16-60, A and B). Microscopically, the lucent areas represent suppurative and fibrinous inflammation and necrosis of the metaphyseal marrow and bone (Fig. 16-60, C). The death of osteoblasts results in primary trabeculae that are not reinforced by apposition of bone matrix. These trabeculae collapse and fracture without external distortion of the bone (infractions) and appear radiographically as relatively dense regions.
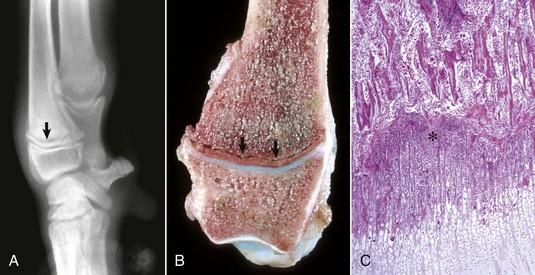
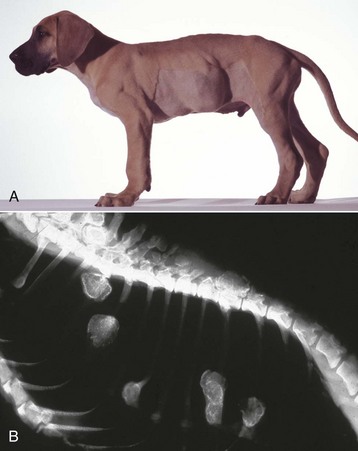
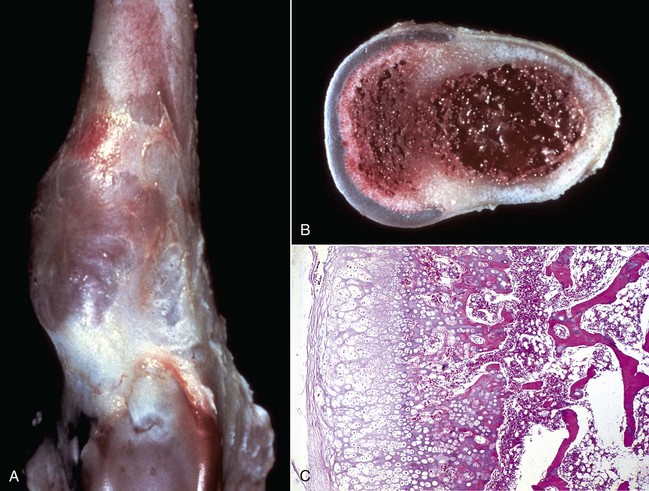
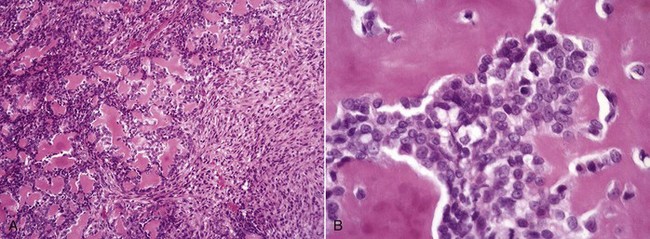
Fig. 16-60 Metaphyseal osteopathy, bone, distal radius, dog.
A, Radiograph. The radiolucent line in the metaphysis (arrow), parallel with growth plate, is characteristic of metaphyseal osteopathy. B, Grossly, this line appears to be a fracture (arrows) within the metaphysis. C, Histologically, this line is a hypercellular band (asterisk) of neutrophils between the primary and secondary trabeculae. (Courtesy Dr. S.E. Weisbrode, College of Veterinary Medicine, The Ohio State University.)
Cortical bone: The inflammation can extend from the medulla through the cutback zone into the periosteum. This periosteal inflammation, along with mechanical instability caused by the metaphyseal infractions, can cause notable new metaphyseal periosteal bone formation in chronic cases.
Eosinophilic Panosteitis: Eosinophilic panosteitis is another canine bone disease with an unfortunate name, since the lesion is neither inflammatory nor eosinophilic. The cause is unknown, and the disease is almost always self-limiting. It occurs in growing (commonly large-breed) dogs, usually at 5 to 12 months of age, and is painful. German shepherd dogs appear to be predisposed. Morphologic studies are few because the disease is easily recognized clinically and resolves spontaneously so that biopsy evaluation is rarely necessary. Radiographically, the lesions are recognized as increased densities in the medullary cavity in the diaphysis, usually beginning near the nutrient foramen. Increased densities can also be present in the periosteum. These densities are the result of proliferation of well-differentiated woven bone and fibrous tissue. No inflammation is present. The cause of the lameness is presumed to be pressure on nerves by the proliferating woven bone both within the medullary cavity and on the periosteum.