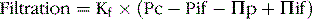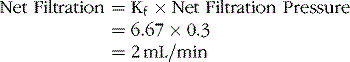CHAPTER 16 The Microcirculation and Lymphatic System
Capillary Fluid Exchange, Interstitial Fluid, and Lymph Flow
A primary function of the circulation—to transport nutrients to the tissues and remove waste products—occurs in the capillaries. The capillaries have only a single layer of highly permeable endothelial cells, permitting rapid interchange of nutrients and cellular waste products between the tissues and circulating blood. About 10 billion capillaries, which have a total surface area of 500 to 700 square meters (about one eighth the size of a football field) provide this function for the body.
Structure of the Microcirculation (p. 177)
Blood Enters the Capillaries through an Arteriole and Leaves through a Venule
Blood from the arteriole passes into a series of metarterioles, which have structures midway between those of arterioles and capillaries. Arterioles are highly muscular and play a major role in controlling blood flow to the tissues. The metarterioles do not have a continuous smooth muscle coat, but smooth muscle fibers encircle the vessel at intermittent points called precapillary sphincters. Contraction of the muscle in these sphincters can open and close the entrance to the capillary.
This arrangement of the microcirculation is not found in all parts of the body, but similar arrangements serve the same purposes. Both the metarterioles and arterioles are in close contact with the tissues they serve, and local conditions, such as changes in the concentration of nutrients or waste products of metabolism, can have direct effects on these vessels in controlling the local blood flow.
The Thin Capillary Wall Consists of a Single Layer of Endothelial Cells
Capillaries are also very porous, with several million slits, or pores, between the cells that make up their walls to each square centimeter of capillary surface. Because of the high permeability of the capillaries for most solutes and the high surface area, as blood flows through the capillaries large amounts of dissolved substances diffuse in both directions through these pores. In this way, almost all dissolved substances in the plasma, except the plasma proteins, continually mix with the interstitial fluid.
Blood Flows Intermittently through Capillaries, a Phenomenon Called “Vasomotion.”
In many tissues, blood flow through capillaries is not continuous but, instead, turns on and off every few seconds. The cause of this intermittence is contraction of the metarterioles and precapillary sphincters, which are influenced mainly by oxygen and waste products of tissue metabolism. When oxygen concentrations of the tissue are reduced (e.g., because of increased oxygen utilization), the periods of blood flow occur more often and last longer, thereby allowing the blood to carry increased quantities of oxygen and other nutrients to the tissues.
Exchange of Nutrients and Other Substances between Blood and Interstitial Fluid (p. 179)
Diffusion Is the Most Important Means for Transferring Substances between Plasma and Interstitial Fluid
As blood traverses the capillary, tremendous numbers of water molecules and dissolved substances diffuse back and forth through the capillary wall, providing continual mixture of the interstitial fluid and plasma. Lipid-soluble substances, such as oxygen and carbon dioxide, can diffuse directly through the cell membranes without having to go through the pores. Water-soluble substances, such as glucose and electrolytes, diffuse only through intercellular pores in the capillary membrane. The rate of diffusion for most solutes is so great that cells as far as 50 μm away from the capillaries can receive adequate quantities of nutrients.
The primary factors that affect the rate of diffusion across the capillary walls are as follows:
The Interstitium and Interstitial Fluid (p. 180)
About one sixth of the body consists of spaces between cells, which collectively are called the interstitium. The fluid in these spaces is the interstitial fluid. The interstitium has two major types of solid structures: (1) collagen fiber bundles and (2) proteoglycan filaments. The collagen provides most of the tensional strength of the tissues, whereas the proteoglycan filaments, composed mainly of hyaluronic acid, are very thin and form a filler of fine reticular filaments, often described as a “brush pile.”
“Gel” in the Interstitium Consists of Proteoglycan Filaments and Entrapped Fluid
Fluid in the interstitium is derived by filtration and diffusion from the capillaries and has almost the same constituency as plasma except with lower concentrations of protein. The interstitial fluid is mainly entrapped in the minute spaces among the proteoglycan filaments and has the characteristics of a gel.
Because of the large number of proteoglycan filaments, fluid and solutes do not flow easily through the tissue gel. Instead, solutes mainly diffuse through the gel. This diffusion occurs about 95% to 99% as rapidly as it does through free fluid.
Capillary Fluid Filtration Is Determined by Hydrostatic and Colloid Osmotic Pressures, and Capillary Filtration Coefficient (p. 181)
Although the exchange of nutrients, oxygen, and metabolic waste products across the capillaries occurs almost entirely by diffusion, the distribution of fluid across the capillaries is determined by another process—the bulk flow or ultrafiltration of protein-free plasma. As discussed previously, capillary walls are highly permeable to water and most plasma solutes, except plasma proteins; therefore, hydrostatic pressure differences across the capillary wall push protein-free plasma (ultrafiltrate) through the capillary wall into the interstitium. In contrast, osmotic pressure caused by the plasma proteins (called colloid osmotic pressure) tends to produce fluid movement by osmosis from the interstitial spaces into the blood. Interstitial fluid hydrostatic and colloid osmotic pressures also influence fluid filtration across the capillary wall.
The rate at which ultrafiltration occurs across the capillary depends on the difference in hydrostatic and colloid osmotic pressures of the capillary and interstitial fluid. These forces are often called Starling forces in honor of Ernest Starling, the physiologist who described their functional significance more than a century ago.
Four Forces Determine Fluid Filtration through the Capillary Membrane
The four primary forces that determine fluid movement across the capillaries are shown in Figure 16–1; the forces are as follows:
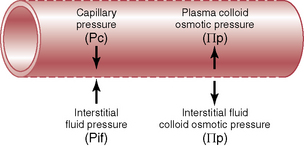
Figure 16–1 Forces operative at the capillary membrane tend to move fluid outward or inward through the membrane pores.
The net rate of filtration out of the capillary is determined by the balance of these forces as well as by the capillary filtration coefficient (Kf) as follows:
Functional Capillary Hydrostatic Pressure Averages about 17 mm Hg in Many Tissues
When blood is flowing through many capillaries, the pressure averages 30 to 40 mm Hg on the arterial ends and 10 to 15 mm Hg on the venous ends, or about 25 mm Hg in the middle. When the capillaries are closed, the pressure in the capillaries beyond the closure is about equal to the pressure at the venous ends of the capillaries (10 mm Hg). When averaged over a period of time, including the periods of opening and closure of the capillaries, the functional mean capillary pressure is closer to the pressure in the venous ends of the capillaries than to the pressure in the arteriole ends; it averages about 17 mm Hg in many tissues. In some tissues, such as the kidneys, capillary hydrostatic pressure may be as high as 60 to 65 mm Hg (see Chapter 26).
Interstitial Fluid Hydrostatic Pressure Is Subatmospheric (Negative Pressure) in Loose Subcutaneous Tissue and Positive in Tightly Encased Tissues
Measurements of interstitial fluid hydrostatic pressure have yielded an average value of about –3 mm Hg in loose subcutaneous tissue. One of the basic reasons for this negative pressure is the lymphatic pumping system (discussed later). When fluid enters the lymphatic capillaries, any movement of the tissue propels the fluid forward through the lymphatic system and eventually back into the circulation. In this way, free fluid that accumulates in the tissue is pumped away as a consequence of tissue movement. This pumping action of lymphatic capillaries appears to account for the slight intermittent negative pressure that occurs in the tissues at rest.
In Tissues Surrounded by Tight Encasements, Such as the Brain, Kidneys, and Skeletal Muscle (Surrounded by Fibrous Sheaths), Interstitial Fluid Hydrostatic Pressures Are Usually Positive
For instance, the brain interstitial fluid hydrostatic pressure averages about +4 to +16 mm Hg. In the kidneys, interstitial fluid hydrostatic pressure averages about +6 mm Hg.
Plasma Colloid Osmotic Pressure Averages about 28 mm Hg
The proteins are the only dissolved substances in the plasma that do not readily pass through the capillary membrane. These substances exert an osmotic pressure referred to as the colloid osmotic pressure. The normal concentration of plasma protein averages about 7.3 g/dL. About 19 mm Hg of the colloid osmotic pressure is due to the dissolved protein, but an additional 9 mm Hg is due to the positively charged cations, mainly sodium ions, that bind to the negatively charged plasma proteins. This is called the Donnan equilibrium effect, which causes the colloid osmotic pressure in the plasma to be about 50% greater than that produced by the proteins alone.
The plasma proteins are mainly a mixture of albumin, globulin, and fibrinogen. About 80% of the total colloid osmotic pressure of the plasma results from the albumin fraction, 20% from the globulin, and only a tiny amount from the fibrinogen.
Interstitial Fluid Colloid Osmotic Pressure Averages about 8 mm Hg
Although the size of the usual capillary pore is smaller than the molecular size of the plasma protein, this is not true of all pores; therefore, small amounts of plasma protein leak through the pores into the interstitial spaces. The average protein concentration of the interstitial fluid is around 40% of that in the plasma, or about 3 g/dL, giving a colloid osmotic pressure of about 8 mm Hg. In some tissues, such as the liver, the interstitial fluid colloid osmotic pressure is much greater because the capillaries are much more permeable to plasma proteins.
Summary of Fluid Volume Exchange through the Capillary Membrane
The average capillary pressure at the arteriolar ends of the capillaries is 15 to 25 mm Hg greater than at the venular ends. Because of this difference, fluid filters out of the capillaries at the arteriolar ends, and fluid is reabsorbed back into the capillaries at their venular ends. A small amount of fluid flows through the tissues from the arteriolar ends of the capillaries to the venular ends.
Under normal conditions, however, a state of near-equilibrium exists between the amount of fluid filtering outward at the arteriolar ends of the capillaries and the amount of fluid returned to the circulation by absorption at the venular ends of the capillaries. There is a slight disequilibrium that occurs, and a small amount of fluid is filtered in excess of that reabsorbed. This fluid is eventually returned to the circulation by way of the lymphatics. Table 16–1 shows the average forces that exist across the entire capillaries and illustrates the principles of this equilibrium. The pressures in the arterial and venous capillaries in Table 16–1 are averaged to calculate the mean functional capillary pressure, which is about 17.3 mm Hg.
Table 16–1 Equilibrium of Forces across Capillaries
| Forces | mm Hg |
|---|---|
| Mean forces tending to move fluid outward | |
| Mean capillary hydrostatic pressure | 17.3 |
| Negative interstitial free fluid pressure | 3.0 |
| Interstitial fluid colloid osmotic pressure | 8.0 |
| Total outward force | 28.3 |
| Mean force tending to move fluid inward | |
| Plasma colloid osmotic pressure | 28.0 |
| Total inward force | 28.0 |
| Summation of mean forces | |
| Outward | 28.3 |
| Inward | –28.0 |
| Net outward force | 0.3 |
The small imbalance of forces, 0.3 mm Hg, causes slightly more filtration than reabsorption of fluid into the interstitial spaces.
The Rate of Filtration in the Capillaries Is Also Determined by the Capillary Filtration Coefficient (Kf)
The filtration coefficient in an average tissue is about 0.01 mL of fluid per minute per millimeter of mercury per 100 g of tissue. For the entire body, the capillary filtration coefficient is about 6.67 mL of fluid per minute per millimeter of mercury. Thus the net rate of capillary filtration for the entire body is expressed as follows:
Because of the extreme differences in the permeabilities and surface areas of the capillary systems in different tissues, the capillary filtration coefficient may vary more than 100-fold among tissues. For example, the capillary filtration coefficient in the kidneys is about 4.2 mL/min per millimeter of mercury per 100 g of kidney weight, a value almost 400 times as great as the Kf of many other tissues. This obviously causes a much greater rate of filtration in the glomerular capillaries of the kidney.
An Abnormal Imbalance of Pressures in the Capillary Can Cause Edema
If the mean capillary hydrostatic pressure rises above the normal 17 mm Hg, the net pressure causing filtration of fluid into the tissue spaces also rises. A rise in mean capillary pressure of 20 mm Hg causes an increase in the net filtration pressure from 0.3 mm Hg to 20.3 mm Hg, which results in 68 times as much net filtration of fluid into the interstitial spaces as normally occurs. Prevention of accumulation of excess fluid in the spaces would require 68 times the normal flow of fluid into the lymphatic system, an amount that is too great for the lymphatics to carry away. As a result, large increases in capillary pressure can cause accumulation of fluid in the interstitial spaces, a condition referred to as edema.
Similarly, a decrease in plasma colloid osmotic pressure increases the net filtration force and therefore the net filtration rate of fluid into the tissues.
The Lymphatic System (p. 186)
The lymphatic system carries fluid from tissue spaces into the blood. Importantly, the lymphatics also carry away proteins and large particulate matter from the tissue spaces, neither of which can be removed through absorption directly into the blood capillary.
Almost all tissues of the body have lymphatic channels. Most of the lymph from the lower part of the body flows up the thoracic duct and empties into the venous system at the juncture of the left interior jugular vein and subclavian vein. Lymph from the left side of the head, left arm, and parts of the chest region also enters the thoracic duct before it empties into the veins. Lymph from the right side of the neck and head, right arm, and parts of the thorax enter the right lymph duct, which then empties into the venous system at the juncture of the right subclavian vein and internal jugular vein.
Lymph Is Derived from Interstitial Fluid
As lymph first flows from the tissue, it has almost the same composition as the interstitial fluid. In many tissues, the protein concentration averages about 2 g/dL, but in other tissues such as the liver the protein concentration may be as high as 6 g/dL.
In addition to carrying fluid and protein from the interstitial spaces to the circulation, the lymphatic system is one of the major routes for absorption of nutrients from the gastrointestinal tract, as discussed in Chapter 65. After a fatty meal, for instance, thoracic duct lymph sometimes contains as much as 1% to 2% fat.
The Rate of Lymph Flow Is Determined by Interstitial Fluid Hydrostatic Pressure and the Lymphatic Pump
The total rate of lymph flow is approximately 120 mL/hr, or 2 to 3 L per day. This rate of formation can change dramatically, however, in certain pathological conditions associated with excessive fluid filtration from the capillaries into the interstitium.
The Lymphatic System Is Important as an “Overflow Mechanism” That Returns to the Circulation Excess Proteins and Fluid Volume That Enter the Tissue Spaces
When the lymphatic system fails, as occurs with blockade of a major lymphatic vessel, proteins and fluid accumulate in the interstitium, causing edema. The accumulation of protein in the interstitium is especially important in causing edema because the lymphatics provide the only mechanism for proteins that leak out of the capillaries to re-enter the circulation in significant quantities. When protein accumulates in the interstitial spaces owing to lymphatic failure, there is an increase in colloid osmotic pressure of the interstitial fluid that tends to allow more fluid filtration into the interstitium. As a result, complete blockade of the lymphatic vessels results in severe edema.
Bacteria and Debris from the Tissues Are Removed by the Lymphatic System at Lymph Nodes
Because of the very high permeability of the lymphatic capillaries, bacteria and other small particulate matter in the tissues can pass into the lymph. The lymph passes through a series of nodes on its way out to the blood. It is in these nodes that bacteria and other debris are filtered out, phagocytized by macrophages in the nodes, and finally digested and converted to amino acids, glucose, fatty acids, and other low-molecular-weight substances before being released into the blood.