CHAPTER 17 Local and Humoral Control of Tissue Blood Flow
Local Tissues Autoregulate Blood Flow in Response to Their Individual Needs
In most tissues, blood flow is autoregulated, which means that the tissue regulates its own blood flow. This is beneficial to the tissue because it allows the delivery of oxygen and nutrients and removal of waste products to parallel the rate of tissue activity. Autoregulation permits blood flow from one tissue to be regulated independently of flow to another tissue.
In certain organs, blood flow serves purposes other than supplying nutrients and removing waste products. For instance, blood flow to the skin influences heat loss from the body and in this way helps control body temperature. Delivery of adequate quantities of blood to the kidneys allows them to excrete rapidly the waste products of the body.
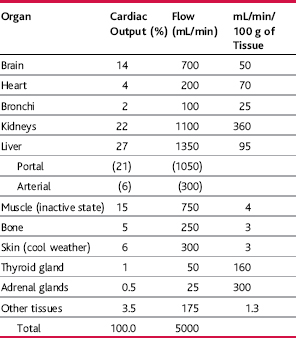
The ability of the tissues to regulate their own flow permits them to maintain adequate nutrition and perform necessary functions to maintain homeostasis. In general, the greater the rate of metabolism in an organ, the greater its blood flow. Table 17–1, for example, shows that there is high blood flow in glandular organs such as the thyroid and adrenal glands, which have a high metabolic rate. In contrast, blood flow in resting skeletal muscles is low because metabolic activity of the muscle is also low in the resting state; however, during heavy exercise skeletal muscle metabolic activity can increase by more than 60-fold and the blood flow can increase by as much as 20-fold.
Mechanisms of Local Blood Flow Control (p. 191)
Local tissue blood flow control can be divided into two phases: (1) acute control and (2) long-term control. Acute control occurs within seconds to minutes via constriction or dilation of arterioles, metarterioles, and precapillary sphincters. Long-term control occurs over a period of days, weeks, or even months and, in general, provides even better control of flow in proportion to the needs of the tissues. Long-term control occurs mainly as a result of increases or decreases in the physical size and number of blood vessels supplying the tissues.
Acute Control of Local Blood Flow (p. 192)
Increased Tissue Metabolic Rate Usually Increases Local Blood Flow
In many tissues, such as skeletal muscle, increases in metabolism up to eight times normal acutely increase the blood flow about fourfold. Initially, the rise in flow is less than that of the metabolism, but once the metabolism increases sufficiently to remove most of the nutrients from the blood a further rise in metabolism can occur only with a concomitant increase in blood flow to supply the required nutrients.
Decreased Oxygen Availability Increases Tissue Blood Flow
One of the required nutrients for tissue metabolism is oxygen. Whenever the availability of oxygen in the tissues decreases, such as at high altitude, in the presence of pneumonia, or with carbon monoxide poisoning (which inhibits the ability of hemoglobin to transport oxygen), the tissue blood flow increases markedly. Cyanide poisoning, for instance, which reduces the ability of the tissues to utilize oxygen, can increase tissue blood flow by as much as sevenfold.
Increased Demand for Oxygen and Nutrients Increases Tissue Blood Flow
In the absence of an adequate supply of oxygen and nutrients as a result of either increased tissue metabolism, the arterioles, metarterioles, and precapillary sphincters relax, thereby decreasing vascular resistance and allowing more flow to the tissues. The relaxation of precapillary sphincters allows flow to occur more often in capillaries that are closed because of periodic contraction of precapillary sphincters (vasomotion).
Accumulation of Vasodilator Metabolites Increases Tissue Blood Flow
The greater the rate of metabolism in the tissue, the greater is the rate of production of tissue metabolites, such as adenosine, adenosine phosphate compounds, carbon dioxide, lactic acid, potassium ions, and hydrogen ions. Each of these substances has been suggested to act as a vasodilator that contributes to increased blood flow associated with stimulation of tissue metabolism.
Lack of Other Nutrients May Cause Vasodilation
For example, a deficiency of glucose, amino acids, or fatty acids may contribute to local vasodilation, although this has not been proven. Vasodilation occurs with beriberi, in which the patient usually has a deficiency of the vitamin B substances thiamine, niacin, and riboflavin. Because these vitamins are all involved in the oxidative phosphorylation mechanism for generating adenosine triphosphate (ATP), a deficiency of these vitamins may lead to diminished ability of the smooth muscle to contract, thereby causing local vasodilation.
Special Examples of Local Blood Flow Control (p.194)
“Reactive Hyperemia” Occurs after the Blood Supply to a Tissue Is Blocked for a Short Time
If blood flow is blocked for a few seconds to several hours and then unblocked, flow to the tissue usually increases to four to seven times normal; the increased flow continues for a few seconds or much longer if the flow has been stopped for 1 hour or longer. This phenomenon is called reactive hyperemia and appears to be a manifestation of local “metabolic” blood flow regulation mechanisms. After vascular occlusion, there is an accumulation of tissue vasodilator metabolites and the development of oxygen deficiency in the tissues. The extra blood flow during reactive hyperemia lasts long enough to almost exactly repay the tissue oxygen deficiency and to wash out the accumulated vasodilator metabolites.
“Active Hyperemia” Occurs When the Tissue Metabolic Rate Increases
When a tissue becomes highly active, such as muscle during exercise or even the brain during increased mental activity, blood flow to the tissue increases. Again, this appears to be related to increases in local tissue metabolism that cause accumulation of vasodilator substances and possibly a slight oxygen deficit. The dilation of local blood vessels helps the tissue receive the additional nutrients required to sustain its new level of function.
Tissue Blood Flow Is “Autoregulated” during Changes in Arterial Pressure
In any tissue of the body, acute increases in arterial pressure cause an immediate increase in blood flow. Within less than 1 minute, however, the blood flow in many tissues returns toward the normal level even though the arterial pressure remains elevated. This is called “autoregulation of blood flow.”
The relative importance of these two mechanisms for autoregulation of blood flow is still debated by physiologists. It seems likely that both mechanisms contribute to maintaining a relatively stable blood flow during variations in arterial pressure.
Additional Mechanisms for Blood Flow Control in Specific Tissues
Although the general mechanisms for local blood flow control discussed thus far are present in most tissues of the body, there are special mechanisms that control blood flow in special areas. These mechanisms are discussed in relation to specific organs, but the following two are notable:
The local mechanisms for controlling tissue blood flow act mainly on the very small microvessels of the tissues because local feedback by vasodilator substances or oxygen deficiency can reach only these vessels, not the larger arteries upstream. When blood flow through the microvascular portion of the circulation increases, however, the endothelial cells lining the larger vessels release a vasodilator substance called endothelium-derived relaxing factor, which appears to be mainly nitric oxide. This release of nitric oxide is caused, in part, by increased shear stress on the endothelial walls, which occurs as blood flows more rapidly through the larger vessels. The release of nitric oxide then relaxes the larger vessels, causing them to dilate. Without the dilation of larger vessels, the effectiveness of local blood flow would be compromised because a significant part of the resistance in blood flow is in the upstream arterioles and small arteries.
Endothelial Cells Also Release Vasoconstrictor Substances
The most important of these is endothelin, a peptide that is released by when blood vessels are injured. The usual stimulus for release is damage to the endothelium, such as that caused by crushing the tissues or injecting a traumatizing chemical into the blood vessel. After severe blood vessel damage, release of local endothelin and subsequent vasoconstriction helps to prevent extensive bleeding from arteries.
Long-Term Blood Flow Regulation (p. 196)
Most of the mechanisms that have been discussed thus far act within a few seconds to a few minutes after the local tissue conditions have changed. Even with full function of these acute mechanisms, blood flow usually is adjusted only about three fourths of the way back to the exact requirements of the tissues. Over a period of hours, days, and weeks, long-term local blood flow regulation develops that helps adjust the blood flow so it matches precisely the metabolic needs of the tissues.
Changes in Tissue Vascularity Contribute to Long-Term Regulation of Blood Flow
If metabolism of a tissue is increased for prolonged periods of time, the physical size of the vessels in a tissue increases; under some conditions, the number of blood vessels also increases. One of the major factors that stimulate this increased vascularity is low oxygen concentration in the tissues. Animals that live at high altitudes, for instance, have increased vascularity. Likewise, fetal chicks hatched at low oxygen levels have up to twice as much vascular conductivity as in normal fetal chicks. This growth of new vessels is called angiogenesis.
Angiogenesis occurs mainly in response to the presence of angiogenic factors released from (1) ischemic tissues, (2) tissues that are growing rapidly, and (3) tissues that have excessively high metabolic rates.
Many Angiogenic Factors Are Small Peptides
Three of the best characterized angiogenic factors are vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and angiogenin, each of which has been isolated from tumors or other tissues that are rapidly growing or have inadequate blood supply.
Essentially all angiogenic factors promote new vessel growth by causing the vessels to sprout from small venules or, occasionally, capillaries. The basement membrane of the endothelial cells is dissolved, followed by the rapid production of new endothelial cells that stream out of the vessel in extended cords directed toward the source of the angiogenic factor. The cells continue to divide and eventually fold over into a tube. The tube then connects with another tube budding from another donor vessel and forms a capillary loop through which blood begins to flow. If the flow is sufficient, smooth muscle cells eventually invade the wall so that some of these vessels grow to be small arterioles and/or perhaps even larger vessels.
Collateral Blood Vessels Develop When an Artery or a Vein Is Blocked
New vascular channels usually develop around a blocked artery or vein and allow the affected tissue to be at least partially resupplied with blood. An important example is the development of collateral blood vessels after thrombosis of one of the coronary arteries. In many people over age 60, there is blockage of at least one of the smaller coronary vessels; yet most people do not know that it has happened because collateral blood vessels have gradually developed as the vessels have begun to close, thereby providing blood flow to the tissue sufficient to prevent myocardial damage. It is in instances in which thrombosis occurs too rapidly for the development of collaterals that serious heart attacks occur.
Humoral Control of the Circulation (p. 199)
Several hormones are secreted into the circulation and transported in the blood throughout the entire body. Some of these hormones have important effects on circulatory function.
Ions and Other Chemical Factors Can Also Alter Local Blood Flow
Many ions and chemical factors can either dilate or constrict local blood vessels. Their specific effects are as follows: