CHAPTER 18 Nervous Regulation of the Circulation, and Rapid Control of Arterial Pressure
Except for certain tissues, such as skin, blood flow regulation is mainly a function of local control mechanisms. Nervous control mainly affects more global functions, such as redistributing blood flow to various parts of the body, increasing the pumping activity of the heart, and providing rapid control of arterial pressure. This control of the circulation by the nervous system is exerted almost entirely through the autonomic nervous system.
Autonomic Nervous System (p. 201)
The two components of the autonomic nervous system are the sympathetic nervous system, which is most important for controlling the circulation, and the parasympathetic nervous system, which contributes to the regulation of heart function.
Sympathetic Stimulation Causes Vasoconstriction, and Increases Heart Rate and Cardiac Contractility
Sympathetic vasomotor fibers exit the spinal cord through all of the thoracic and the first one or two lumbar spinal nerves. They pass into the sympathetic chain and then go via two routes to the circulation: (1) through specific sympathetic nerves that innervate mainly the vasculature of the internal viscera and heart and (2) through spinal nerves that innervate mainly the vasculature of the peripheral areas. Almost all of the blood vessels, except the capillaries, are innervated by sympathetic nerve fibers. Sympathetic stimulation of the small arteries and arterioles increases the vascular resistance and decreases the rate of blood flow through the tissues. Innervation of large vessels, especially the veins, makes it possible for sympathetic stimulation to decrease the volume of the vessels.
Sympathetic fibers also go to the heart and stimulate its activity, increasing both the rate and strength of pumping.
Parasympathetic Stimulation Decreases Heart Rate and Cardiac Contractility
Although the parasympathetic system plays an important role in controlling many other autonomic functions of the body, its main role in controlling the circulation is to decrease the heart rate markedly and slightly decrease heart muscle contractility.
Control of the Sympathetic Vasoconstrictor System by the Central Nervous System (p. 201)
The sympathetic nerves carry large numbers of vasoconstrictor nerve fibers and only a few vasodilator fibers. The vasoconstrictor fibers are distributed to almost all segments of the circulation. Their distribution is greater in some tissues, such as skin, gut, and spleen.
Vasomotor Centers of the Brain Control the Sympathetic Vasoconstrictor System
Located bilaterally in the reticular substance of the medulla and lower third of the pons is an area called the vasomotor center, which transmits parasympathetic impulses through the vagus nerves to the heart and sympathetic impulses through the cord and peripheral sympathetic nerves to almost all blood vessels of the body (Figure 18–1).
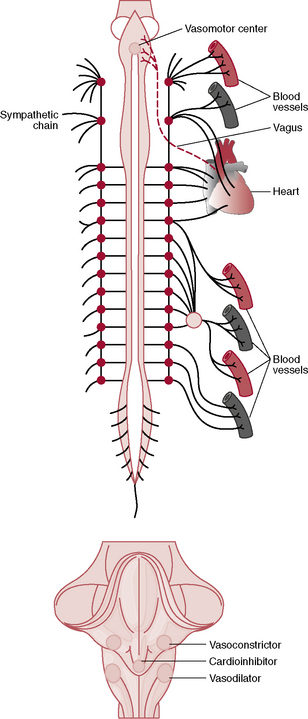
Figure 18–1 Anatomy of sympathetic nervous control of the circulation. Also shown by the dashed red line, a vagus nerve that carries parasympathetic signals to the heart.
Although the organization of the vasomotor centers is not completely understood, certain areas appear to be especially important.
Continuous Sympathetic Vasoconstrictor Tone Causes Partial Constriction of Most Blood Vessels
Normally, the vasoconstrictor area of the vasomotor center transmits signals continuously to the sympathetic vasoconstrictor nerve fibers over the entire body, causing slow firing of these fibers at a rate of about one impulse per second. This sympathetic vasoconstrictor tone maintains a partial state of contraction of the blood vessels. When this tone is blocked (e.g., by spinal anesthesia), the blood vessels throughout the body dilate, and arterial pressure may fall to as low as 50 mm Hg.
The Vasomotor System Is Influenced by Higher Nervous Centers
Large numbers of areas throughout the reticular substance of the pons, mesencephalon, and diencephalon can either excite or inhibit the vasomotor center.
The hypothalamus plays a special role in controlling the vasoconstrictor system and can exert powerful excitatory or inhibitory effects on the vasomotor center.
Many parts of the cerebral cortex can also excite or inhibit the vasomotor center; for example, stimulation of the motor cortex excites the vasomotor center. Many areas of the brain can have profound effects on cardiovascular function.
Norepinephrine Is the Neurotransmitter of the Sympathetic Vasoconstriction System
Norepinephrine, which is secreted at the endings of the vasoconstrictor nerves, acts directly on α-adrenergic receptors of vascular smooth muscle to cause vasoconstriction.
The Adrenal Medulla Releases Norepinephrine and Epinephrine During Sympathetic Stimulation
Sympathetic impulses are usually transmitted to the adrenal medullae at the same time they are transmitted to the blood vessels, stimulating release of epinephrine and norepinephrine into the circulating blood. These two hormones are carried in the bloodstream to all parts of the body, where they act directly on the blood vessels to cause vasoconstriction through stimulation of α-adrenergic receptors. Epinephrine, however, also has potent β-adrenergic effects, which cause vasodilation in certain tissues, such as skeletal muscle.
Role of the Nervous System in Rapid Control of Arterial Pressure (p. 204)
One of the most important functions of the sympathetic nervous system is to provide rapid control of arterial pressure by causing vasoconstriction and stimulation of the heart. At the same time that sympathetic activity is increased, there often is reciprocal inhibition of parasympathetic vagal signals to the heart that also contribute to a greater heart rate. As a consequence, there are three major changes that take place to increase arterial pressure through stimulation of the autonomic nervous system.
An important characteristic of nervous control is that it is rapid, beginning within seconds. Conversely, sudden inhibition of nervous stimulation can decrease the arterial pressure within seconds.
The Autonomic Nervous System Contributes to Increased Arterial Pressure During Muscle Exercise
During heavy exercise, the muscles require greatly increased blood flow. Part of this increase results from local vasodilation, but additional increase in flow results from simultaneous elevation of arterial pressure during exercise. During heavy exercise arterial pressure may rise as much as 30% to 40%.
The rise in arterial pressure during exercise is believed to result mainly from the following effect: At the same time the motor areas of the nervous system become activated to cause exercise, most of the reticular activating system in the brain is also activated, which greatly increases stimulation of the vasoconstrictor and cardioaccelerator areas of the vasomotor center. These effects increase the arterial pressure instantly to keep pace with increased muscle activity. Vasodilation of the muscle, however, is maintained despite increased sympathetic activity because of the overriding effect of local control mechanisms in the muscle.
The Autonomic Nervous System Increases Arterial Pressure During the “Alarm Reaction.”
For instance, during extreme fright, the arterial pressure often rises to as high as 200 mm Hg within a few seconds. This alarm reaction provides the necessary increase in arterial pressure that can immediately supply blood to any of the muscles of the body that might need to respond instantly to flee from the perceived danger.
Reflex Mechanisms Help Maintain Normal Arterial Pressure (p. 205)
Aside from special circumstances such as stress and exercise, the autonomic nervous system operates to maintain the arterial pressure at or near its normal level through negative feedback reflex mechanisms.
The Arterial Baroreceptor Reflex Control System
This reflex is initiated by stretch receptors, called baroreceptors, that are located in the walls of large systemic arteries, particularly in the walls of the carotid sinus and the aortic arch. Signals from the carotid sinus receptors are transmitted through Herring’s nerve to the glossopharyngeal nerve and then to the tractus solitarius in the medullary area of the brain stem. Signals from the aortic arch are transmitted through the vagus nerves to the same area of the medulla. The baroreceptors control arterial pressure as follows:
The Baroreceptors Function as a “Buffer” to Maintain Arterial Pressure Relatively Constant During Changes in Body Posture and Other Daily Activities
When a person stands up after lying down, the arterial pressure in the head and upper parts of the body tends to fall. The reduction in pressure decreases the signals sent from the baroreceptors to the vasomotor centers, eliciting a strong sympathetic discharge that minimizes the reduction in arterial pressure. In the absence of functional baroreceptors, marked reductions in arterial pressure can decrease cerebral blood flow so low that consciousness is lost.
Daily activities that tend to increase blood pressure, such as eating, excitement, defecation, and so forth, can cause extreme increases in blood pressure in the absence of normal baroreceptor reflexes. A primary purpose of the arterial baroreceptor system is to reduce the daily variation in arterial pressure to about one half to one third of the pressure that would occur if the baroreceptor system were not present.
Are the Baroreceptors Important in Long-Term Regulation of Arterial Pressure?
The arterial baroreceptors provide powerful moment-to-moment control of arterial pressure, but their importance in long-term blood pressure regulation is still uncertain because they tend to reset within 1 to 2 days to the blood pressure to which they are exposed. If, for example, the arterial pressure rises from the normal value of 100 mm Hg to a high 160 mm Hg, very high rates of baroreceptor impulses are at first transmitted. However, the rate of baroreceptor firing returns to nearly normal over a period of 1 to 2 days, even when the mean arterial pressure remains at 160 mm Hg.
This “resetting” of the baroreceptors may attenuate their potency for correcting disturbances that tend to change arterial pressure for longer than a few days. Experimental studies, however, have suggested that the baroreceptors do not completely reset and may therefore contribute to long-term blood pressure regulation, especially by influencing sympathetic nerve activity of the kidneys (see Chapters 19 and 29).
Control of Arterial Pressure by the Carotid and Aortic Chemoreceptors—Effect of Oxygen Lack on Arterial Pressure
Closely associated with the baroreceptor control system is a chemoreceptor reflex that operates in much the same way as the baroreceptor reflex, except that chemoreceptors, instead of stretch receptors, initiate the response.
Chemoreceptors Are Sensitive to Oxygen Lack, Carbon Dioxide Excess, or Hydrogen Ion Excess
Chemoreceptors are located in two carotid bodies, one of which lies in the bifurcation of each common carotid artery, and in several aortic bodies adjacent to the aorta. Whenever the arterial pressure falls below a critical level, the chemoreceptors become stimulated because of diminished blood flow to the bodies and the resulting diminished availability of oxygen and excess buildup of carbon dioxide and hydrogen ions that are not removed by the slow blood flow. Signals transmitted from the chemoreceptors into the vasomotor center excite the vasomotor center, which in turn elevates the arterial pressure.
Cardiopulmonary Reflexes Help Regulate Arterial Pressure
Both atria and pulmonary arteries have in their walls stretch receptors called cardiopulmonary receptors or low-pressure receptors that are similar to the baroreceptor stretch receptors of the systemic arteries. These low-pressure receptors play an important role in minimizing arterial pressure changes in response to blood volume changes. Although the low-pressure receptors do not directly detect systemic arterial pressure, they detect increases in pressure in the heart and pulmonary circulation caused by changes in volume, and they elicit reflexes that parallel the baroreceptor reflexes to make the total reflex system more potent for controlling arterial pressure.
Increased stretch of the atria causes reflex decreases in sympathetic activity to the kidney, which causes vasodilation of the afferent arterioles and increases in the glomerular filtration rate as well as decreases in tubular reabsorption of sodium. These changes cause the kidney to excrete more sodium and water, thereby ridding the body of excess volume.
The Central Nervous System Ischemic Response Raises Arterial Pressure in Response to Diminished Blood Flow in the Brain’s Vasomotor Center (p. 209)
When blood flow to the vasomotor center in the lower brain stem becomes sufficiently decreased to cause cerebral ischemia (i.e., nutritional deficiency), the neurons of the vasomotor center become strongly excited. When this occurs, the systemic arterial pressure often rises to a level as high as the heart can pump. This may be due to the effect of low blood flow, which causes buildup of carbon dioxide in the vasomotor centers. Increased carbon dioxide concentration is a potent agent for stimulating the sympathetic nervous control areas of the medulla of the brain. Other factors, such as build up of lactic acid, may also contribute to marked stimulation of the vasomotor center and increased arterial pressure.
This arterial pressure elevation in response to cerebral ischemia is known as the central nervous system ischemic response. This response is an emergency control system that acts rapidly and powerfully to prevent further decline in arterial pressure when blood flow to the brain becomes dangerously decreased; it is sometimes called the “last ditch” mechanism for blood pressure control.
The Cushing Reaction Is a Central Nervous System Ischemic Response That Results from Increased Pressure in the Cranial Vault
When cerebrospinal fluid pressure rises to equal the arterial pressure, a central nervous system ischemic response is initiated that can raise the arterial pressure to as high as 250 mm Hg. This response helps protect the vital centers of the brain from loss of nutrition, which could occur if pressure in the cranial vault exceeds the normal arterial pressure and compresses blood vessels supplying the brain.
If cerebral ischemia becomes so severe that a maximal increase in arterial pressure still cannot relieve the ischemia, the neuronal cells begin to suffer metabolically, and within 3 to 10 minutes they become inactive. This causes the arterial pressure to decrease.