CHAPTER 19 Role of the Kidneys in Long-Term Control of Arterial Pressure and in Hypertension
The Integrated System for Arterial Pressure Regulation
Renal–Body Fluid System for Arterial Pressure Control (p. 213)
The short-term control of arterial pressure by the sympathetic nervous system, discussed in Chapter 18, occurs mainly through changes in vascular resistance and capacitance and cardiac pumping ability. However, the body also has powerful mechanisms for long-term blood pressure regulation that are closely linked to control of body fluid volume by the kidneys, a mechanism known as the renal–body fluid feedback system. When arterial pressure rises too high, the kidneys excrete increased quantities of sodium and water because of pressure natriuresis and pressure diuresis, respectively. As a result of the increased renal excretion, the extracellular fluid volume and blood volume both decrease until blood pressure returns to normal and the kidneys excrete normal amounts of sodium and water.
Conversely, when the arterial pressure falls too low, renal sodium and water excretion are reduced; over a period of hours to days, if the person drinks enough water and eats enough salt to increase the blood volume, the arterial pressure returns to its previous level. This mechanism for blood pressure control is slow to act, sometimes requiring several days, a week, or longer to reach equilibrium; therefore, it is not of major importance in the acute control of arterial pressure. However, it is by far the most potent of all long-term arterial pressure controllers.
Renal Output of Salt and Water Is Balanced with the Intake of Salt and Water under Steady-State Conditions
Figure 19–1 shows the effect of various arterial pressures on urine volume output by an isolated kidney, demonstrating marked increases in the output of volume (pressure diuresis) and sodium (pressure natriuresis) as arterial pressure rises. Note that so long as the arterial pressure is above the normal equilibrium point, renal output exceeds the intake of salt and water, resulting in a progressive decline in extracellular fluid volume. Conversely, if blood pressure falls below the equilibrium point, the renal output of water and salt is lower than the intake, resulting in a progressive increase in extracellular fluid volume. The only point on the curve at which a balance between renal output and intake of salt and water can occur is at the normal arterial pressure (the equilibrium point).
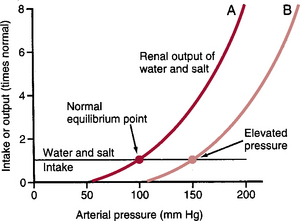
Figure 19–1 Arterial pressure regulation can be analyzed by equating the renal output curve with the salt and water intake curve. The equilibrium point describes the level at which the arterial pressure is regulated. Curve A (red line) shows the normal renal output curve. Curve B (pink line) shows the renal output curve in hypertension.
The Renal–Body Fluid Feedback Mechanism Demonstrates a Near “Infinite Feedback Gain” in Long-Term Blood Pressure Control
To illustrate why this mechanism demonstrates nearly “infinite gain” in controlling blood pressure, let us assume that the arterial pressure rises to 150 mm Hg. At this level, renal output of water and salt is about three times more than the intake. The body loses fluid, blood volume decreases, and arterial pressure decreases. Furthermore, this loss of fluid does not cease until the arterial pressure decreases to the equilibrium point (see Fig. 19–1A). Conversely, if blood pressure falls below the equilibrium point, the kidneys decrease salt and water excretion to a level below intake, causing accumulation of fluid and blood volume until the arterial pressure returns to the equilibrium point. Because there is little or no remaining error in arterial pressure after full correction, this feedback system has nearly infinite gain.
There Are Two Primary Determinants of the Long-Term Arterial Pressure
From the curve shown in Figure 19–1, one can see that two factors determine long-term arterial pressure: (1) the renal output curve for salt and water and (2) the level of salt and water intake. So long as these two factors remain constant, the arterial pressure also remains exactly at the normal level of 100 mm Hg. For arterial pressure to deviate from the normal level for long periods of time, one of these two factors must be altered.
In Figure 19–1, curve B, an abnormality of the kidney has caused the renal output curve to shift 50 mm Hg toward higher blood pressure. This results in a new equilibrium point, and the arterial pressure follows to this new pressure level within a few days. Although greater salt and water intake can theoretically elevate arterial pressure (discussed later), the body has multiple neurohumoral mechanisms that protect against large increases in arterial pressure when salt and water intake is elevated. This is accomplished mainly by decreasing the formation of angiotensin II and aldosterone, which increases the ability of the kidneys to excrete salt and water and results in a steep renal output curve. Therefore, the chronic renal output curve is much steeper than the acute curve shown in Figure 19–1, and in most persons, large increases in salt and water output can be accomplished with minimal increases in arterial pressure.
Increased Total Peripheral Vascular Resistance Cannot Elevate the Long-Term Arterial Pressure if Fluid Intake or Renal Function Does Not Change
When total peripheral vascular resistance is acutely increased, the arterial pressure increases almost immediately. However, if the vascular resistance of the kidneys is not increased and they continue to function normally, the acute rise in arterial pressure is not maintained. The reason is that increasing resistance everywhere in the body except in the kidneys does not change the equilibrium point for blood pressure as dictated by the renal output curve. With increased peripheral resistance and arterial pressure, the kidneys undergo pressure diuresis and pressure natriuresis, causing loss of salt and water from the body. This loss continues until the arterial pressure returns to the normal equilibrium point (see Fig. 19–1, curve A).
In many cases, when total peripheral resistance increases, renal vascular resistance also increases; this causes hypertension by shifting the renal function curve to higher blood pressures. When this shift occurs, it is the increase in renal vascular resistance, not the increase in total peripheral resistance, that causes the long-term increase in arterial pressure.
Increased Fluid Volume Can Elevate Arterial Pressure if Vascular Capacity Does Not Increase
The sequential events that link increased extracellular fluid volume and increased arterial pressure are the following (in order of occurrence):
The increased cardiac output, by itself, tends to elevate the arterial pressure; however, the increased cardiac output also causes excess blood flow in many of the tissues of the body that respond by vasoconstriction, which tends to return the blood flow toward normal. This phenomenon is called autoregulation and tends to raise the total peripheral vascular resistance. With higher extracellular fluid volume, there is an initial rise in cardiac output and a rise in tissue blood flow; but after several days the total peripheral resistance begins to increase because of autoregulation, and cardiac output usually returns toward normal.
If increases in extracellular fluid volume and blood volume are associated with increased vascular capacity, arterial pressure may not increase. For example, in liver cirrhosis there is often a large increase in extracellular fluid volume resulting from decreased liver synthesis of plasma proteins and subsequent leakage of fluid from the blood into the tissues. Fibrous liver tissue may also impeded blood flow through the liver causing high pressures in the portal circulation, distending the veins, and increasing vascular capacity. Likewise with large varicose veins there is increased vascular capacity. In these instances, the kidneys actually retain salt and water and the increases in extracellulary fluid volume and blood volume serve as a compensatory response that helps to prevent blood pressure from decreasing.
Hypertension (High Blood Pressure) (p. 218)
The normal systolic/diastolic arterial pressures are about 120/80 mm Hg, with a mean arterial pressure of 93 mm Hg under resting conditions. Hypertension is said to occur when the diastolic pressure is higher than 90 mm Hg or the systolic pressure is higher than 135 or 140 mm Hg.
Even moderate elevation of the arterial pressure leads to shortened life expectancy by at least three ways:
There are multiple ways by which hypertension can occur. With all types of hypertension studied so far, however, there has been a shift of the renal output curve toward higher blood pressures. Lessons learned from one type of hypertension called volume loading hypertension have been crucial to understanding the role of the renal–body fluid feedback mechanism for arterial pressure regulation.
Sequential Changes That Occur in Circulatory Function During the Development of Volume-Loading Hypertension
In experimental animals in which the kidney mass has been surgically reduced to about 30% of normal, an increase in the salt and water intake causes marked hypertension. Although reduction of the functional kidney mass, by itself, does not cause significant hypertension, it reduces the ability of the kidney to excrete a large load of salt and water effectively. When salt and water intake are increased, the following sequence of events occur:
This sequence illustrates how an initial abnormality of kidney function and excess salt and water intake can cause hypertension and how the volume-loading aspects of hypertension may not be apparent after the kidneys have had sufficient time to re-establish sodium and water balance and after the autoregulatory mechanisms have caused an increase in total peripheral resistance. The following are two clinical examples of volume-loading hypertension:
Renin-Angiotensin System: Its Role in Arterial Pressure Control and Hypertension (p. 220)
In addition to its capability of controlling arterial pressure through changes in extracellular fluid volume, the kidneys control pressure through the renin-angiotensin system. When the arterial pressure falls too low, the kidneys release a protein enzyme, renin, that activates the renin-angiotensin system and helps increase the arterial pressure in several ways, thus helping correct for the initial fall of pressure.
Components of the Renin-Angiotensin System and Role of Angiotensin II in Regulation of Arterial Pressure
The renin-angiotensin system acts in the following manner for acute blood pressure control:
Angiotensin II has two principal effects that can elevate the arterial pressure:
The Effects of Angiotensin II That Cause Renal Retention of Salt and Water Are Especially Important for Long-Term Control of Arterial Pressure
Angiotensin II causes salt and water retention by the kidneys in two ways:
The Renin-Angiotensin System Helps Maintain Normal Arterial Pressure During Wide Variations in Salt Intake
One of the most important functions of the renin-angiotensin system is to allow a person to ingest either a very small or very large amount of salt without causing great changes in either extracellular fluid volume or arterial pressure. For example, when salt intake is increased, there is a tendency for extracellular fluid volume and arterial pressure to increase. This greater arterial pressure also decreases renin secretion and angiotensin II formation, which in turn decreases renal tubular salt and water reabsorption. The reduced tubular reabsorption allows the person to excrete the extra amounts of salt and water with minimal increases in extracellular fluid volume and arterial pressure.
When salt intake is decreased below normal levels, the opposite effects take place. So long as the renin-angiotensin system is fully operative, salt intake can be as low as 1/10 normal or as high as 10 times normal with only a few millimeters of mercury change in arterial pressure. On the other hand, when the renin-angiotensin system is blocked, the same changes in salt intake cause large variations in blood pressure, often as much as 50 mm Hg.
Excessive Angiotensin II Formation Causes Hypertension
Occasionally, a renin-secreting tumor of the juxtaglomerular cells occurs and causes excessive formation of angiotensin II. This almost invariably leads to severe hypertension.
The effect of angiotensin II to increase total peripheral resistance is the primary cause of the rapid rise in blood pressure that occurs when angiotensin II levels are suddenly elevated. The long-term increase in blood pressure associated with excessive angiotensin II formation is due mainly to the various actions of angiotensin II that cause renal salt and water retention.
Impaired Renal Circulation Causes Hypertension (p. 223)
Any condition that seriously reduces the ability of the kidneys to excrete salt and water can cause hypertension. One type of renal dysfunction that can cause severe hypertension is renal vascular damage, such as occurs with (1) stenosis of the renal arteries, (2) constriction of the afferent arterioles, or (3) increased resistance to fluid filtration through the glomerular membrane (i.e., decreased glomerular capillary filtration coefficient). Each of these factors reduces the ability of the kidney to form glomerular filtrate, which in turn causes salt and water retention as well as increased blood volume and increased arterial pressure. The rise in arterial pressure then helps return the glomerular filtration rate toward normal and reduces tubular reabsorption, permitting the kidneys to excrete normal amounts of salt and water despite the vascular disorders.
Constriction of the Renal Arteries Causes Hypertension
When one kidney is removed and a constrictor is placed on the renal artery of the remaining kidney, the immediate effect is greatly reduced pressure in the renal artery beyond the constriction. Within a few minutes, the systemic arterial pressure begins to rise, and it continues to rise for several days until the renal arterial pressure beyond the constriction has returned almost to normal levels. The hypertension produced in this way is called one-kidney Goldblatt hypertension, in honor of Harry Goldblatt, who first described the features of hypertension caused by this method in experimental animals.
The rapid rise in arterial pressure in Goldblatt hypertension is caused by activation of the renin-angiotensin vasoconstrictor mechanism. Because of poor blood flow through the kidney after a reduction of renal artery pressure, large quantities of renin are secreted, causing increased angiotensin II formation and a rapid rise in blood pressure. The more delayed rise in blood pressure, occurring over a period of several days, is caused by fluid retention. The fluid retention and expansion of the extracellular fluid volume continue until the arterial pressure has risen sufficiently to return the renal perfusion pressure to almost normal levels.
Hypertension also occurs when the artery of one kidney is constricted and the artery of the other kidney is normal; this is often called two-kidney Goldblatt hypertension. The constricted kidney retains salt and water because of decreased arterial pressure in this kidney. The “normal” kidney retains salt and water because of the renin produced in the ischemic kidney and the increase in circulating angiotensin II, which causes the opposite kidney to retain salt and water. Both kidneys become salt and water retainers, and hypertension develops.
Coarctation of the Aorta above the Renal Arteries Also Causes Hypertension, with Characteristics Similar to Those Described for One-Kidney Goldblatt Hypertension
Aortic coarctation results in decreased perfusion pressure to both kidneys, stimulating the release of renin and angiotensin II formation as well as salt and water retention by the kidneys. These changes increase the arterial pressure in the upper part of the body above the coarctation, thereby helping to return the perfusion pressure of the kidneys toward normal.
Patchy Ischemia of One or Both Kidneys Can Also Cause Hypertension
When this occurs, the characteristics of the hypertension are almost identical to those of two-kidney Goldblatt hypertension; the patchy ischemic kidney tissue secretes renin, which in turn stimulates formation of angiotensin II, causing the remaining kidney mass to retain salt and water. This type of hypertension is much more common than hypertension caused by constriction of the main renal arteries or aortic coarctation, especially in older patients with atherosclerosis.
Toxemia of Pregnancy (Preeclampsia) Is Also Associated with Hypertension
Although the precise cause of hypertension of this condition is not completely understood, many physiologists believe that it is due to ischemia of the placenta and subsequent release by the placenta of toxic factors that cause many of the manifestations of this disorder, including endothelial dysfunction, impaired renal-pressure natriuresis and hypertension in the mother.
Another pathological factor that may cause hypertension in preeclampsia is thickening of the glomerular membranes perhaps caused by an autoimmune process, which reduces the glomerular capillary filtration coefficient and rate of fluid filtration from the glomeruli into the renal tubules.
The Causes of Human Primary (Essential) Hypertension Are Unknown
Approximately 25% to 30% of adults in industrialized societies have high blood pressure, although the incidence of hypertension is higher among the elderly. The precise cause of hypertension in about 90% of these people is unknown; this type of hypertension is called primary or essential hypertension.
Although the exact causes of primary hypertension are not fully understood, most patients who develop essential hypertension slowly over many years have significant changes in kidney function. Most important, the kidneys cannot excrete adequate quantities of salt and water at normal arterial pressures; instead, they require a high arterial pressure to maintain a normal balance between the intake and output of salt and water unless they are treated with drugs that enhance their ability to excrete salt and water at lower blood pressures.
Abnormal renal excretory capability could be caused by renal vascular disorders that reduce glomerular filtration or tubular disorders that increase reabsorption of salt and water. Because patients with essential hypertension are highly heterogeneous with respect to the characteristics of the hypertension, it seems likely that both disorders contribute to increased blood pressure.
Summary of the Integrated, Multifaceted System for Arterial Pressure Regulation (p. 226)
It is clear that arterial pressure is regulated by several systems, each of which performs a specific function. Some systems are most important for acute regulation of blood pressure and react rapidly, within seconds or minutes. Others respond over a period of minutes or hours. Some provide long-term arterial pressure regulation over days, months, and years.
Nervous System Reflexes Are Rapidly Acting Blood Pressure Control Mechanisms
The three nervous reflexes that act rapidly (within seconds) are (1) the baroreceptor feedback mechanism, (2) the central nervous ischemic mechanism, and (3) the chemoreceptor mechanism. These mechanisms not only act within seconds but also are powerful in preventing acute decreases in blood pressure (e.g., during severe hemorrhage). They also operate to prevent excessive increases in blood pressure, as might occur in response to excessive blood transfusion.
Intermediate Blood Pressure Control Mechanisms That Act after Several Minutes
Three mechanisms that are important in blood pressure control after several minutes of acute pressure change are the (1) renin-angiotensin vasoconstrictor mechanism, (2) stress relaxation of the vasculature, and (3) shift of fluid through the capillary walls in and out of the circulation to readjust the blood volume as needed.
The role of the renin-angiotensin vasoconstrictor mechanism has been described. The stress relaxation mechanism is demonstrated by the following example: When pressure in the blood vessels becomes too high, the vessels become stretched and continue to stretch for minutes or hours. As a result, the pressure in the vessels tends to fall back toward normal.
The capillary fluid shift mechanism means that any time the capillary pressure falls too low fluid is absorbed from the tissue into the capillaries of the circulation, thereby increasing the blood volume and helping to return the blood pressure toward normal. Conversely, when capillary pressure rises too high, fluid is lost out of the circulation, thereby reducing blood volume and arterial pressure.
The Long-Term Mechanism for Arterial Pressure Regulation Involves the Renal–Body Fluid Feedback
The renal–body fluid feedback control mechanism takes several hours to show any significant response, but then it operates powerfully to control arterial pressure over days, weeks, and months. So long as kidney function is unaltered, disturbances that tend to alter arterial pressure, such as increased total peripheral resistance, have minimal effect on blood pressure over long periods of time. Factors that alter the ability of the kidneys to excrete salt and water can cause major long-term changes in arterial pressure. This mechanism, if given sufficient time, controls the arterial pressure at a level that provides normal output of salt and water by the kidneys.
Many factors can affect the renal–body fluid feedback mechanism and therefore long-term blood pressure control. One of the most important factors is the renin-angiotensin system, which allows a person to have very low or very high salt intake with minimal changes in arterial pressure. Thus arterial pressure control begins with lifesaving measures of the nervous reflexes, continues with the sustaining characteristics of the intermediate pressure controls, and finally is stabilized at the long-term pressure level by the renal–body fluid feedback mechanism.