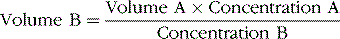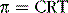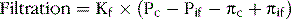CHAPTER 25 The Body Fluid Compartments
Extracellular and Intracellular Fluids; Edema
The total amount and composition of the body fluids are maintained relatively constant under most physiological conditions, as required for homeostasis. Some of the most important problems in clinical medicine, however, arise because of abnormalities in the control systems that maintain this constancy. In this section, we discuss the overall regulation of body fluid volume, control of the constituents of the extracellular fluid, regulation of the fluid exchange between the extracellular and intracellular compartments, and regulation of the acid-base balance.
Fluid Intake and Output Are Balanced During Steady-State Conditions (p. 285)
The total intakes of water and electrolytes must be carefully matched by equal outputs from the body to prevent fluid volumes and electrolyte concentrations from increasing or decreasing. Table 25–1 shows the routes of daily water intake and output from the body. Under most conditions, the primary means of regulating output is by altering renal excretion. Urine volume can be as low as 0.5 L/day in a dehydrated person or as high as 20 L/day in a person who has been drinking large amounts of fluids. This ability of the kidneys to adjust the output to such an extreme to match intake also occurs for the electrolytes of the body such as sodium, chloride, and potassium.
Table 25–1 Daily Intake and Output of Water
| Parameter | Normal (mL/day) | With Prolonged Heavy Exercise (mL/day) |
|---|---|---|
| Intake | ||
| Fluids ingested | 2100 | ? |
| From metabolism | 200 | 200 |
| Total intake | 2300 | ? |
| Output | ||
| Insensible skin | 350 | 350 |
| Insensible lungs | 350 | 650 |
| Sweat | 100 | 5000 |
| Feces | 100 | 100 |
| Urine | 1400 | 500 |
| Total output | 2300 | 6600 |
Total Body Fluid Is Distributed between the Extracellular Fluid and the Intracellular Fluid (p. 286)
The total amount of body water averages about 60% of the body weight, or about 42 L in a 70-kg adult man. Because women normally have more body fat than men, their total body water averages about 50% of the body weight. In premature and newborn babies, the total body water ranges from 70% to 75% of body weight. Therefore, when discussing the “average” body fluid compartments, we should realize that variations exist, depending on age, gender, and percentage of body fat.
Total body fluid is distributed into two main compartments: (1) the intracellular fluid, which is about 40% of body weight or 28 L, and (2) the extracellular fluid, which is about 20% of body weight, or 14 L in a 70-kg person.
The two main compartments of the extracellular fluid are the interstitial fluid, which makes up about three fourths of the extracellular fluid, and the plasma, which makes up about one fourth of the extracellular fluid, or about 3 L. The plasma is the noncellular portion of the blood that mixes continuously with interstitial fluid through the pores of the capillary membranes.
Blood Contains Extracellular and Intracellular Fluids
The average blood volume in a normal adult human is 8% of the body weight, or about 5 L. About 60% of the blood is plasma, and about 40% is red blood cells. The hematocrit, the fraction of blood that is composed of red blood cells, is normally about 0.42 in men and about 0.38 in women. With severe anemia, the hematocrit may fall to as low as 0.10, which is barely sufficient to sustain life. When there is excessive production of red blood cells, resulting in polycythemia, the hematocrit can rise to as high as 0.65.
The Constituents of Extracellular and Intracellular Fluids Differ
Table 25–2 compares the compositions of the intracellular and extracellular fluids.
Table 25–2 Chemical Compositions of Extracellular and Intracellular Fluids
| Chemical | Intracellular Fluid | Extracellular Fluid |
|---|---|---|
| Na+ (mmol/L) | 10 | 142 |
| K+ (mmol/L) | 140 | 4 |
| Cl− (mmol/L) | 4 | 108 |
| HCO3− (mmol/L) | 10 | 24 |
| Ca2+ (mmol/L) | 0.0001 | 2.4 |
| Mg2+ (mmol/L) | 58 | 1.2 |
| SO42− (mmol/L) | 2 | 1 |
| Phosphates (mmol/L) | 75 | 4 |
| Glucose (mg/dL) | 0–20 | 90 |
| Amino acids (mg/dL) | 200? | 30 |
| Protein (g/dL) | 16 | 2 |
The plasma and interstitial fluid of the extracellular compartment are separated by highly permeable capillary membranes, so their ionic compositions are similar. The most important difference between these two compartments is that the plasma has a higher protein concentration. The capillaries have low permeability to proteins and therefore leak only small amounts of protein into the interstitial spaces in most tissues.
The intracellular fluid is separated from the extracellular fluid by a highly selective cell membrane that is permeable to water but not to most electrolytes found in the body. For this reason, the concentration of water and the osmolarity of intracellular and extracellular fluids are approximately equal under steady-state conditions, although the concentrations of various solutes are markedly different in these fluid compartments.
The Indicator-Dilution Principle Can Be Used to Measure Volumes of Body Fluid Compartments (p. 287)
The volume of a fluid in a compartment in the body can be estimated by injecting a substance into the compartment, allowing it to disperse evenly, and then analyzing the extent to which the substance has become diluted. This method is based on the assumption that the total amount of substance remaining in the fluid compartment after dispersion is the same as the total amount that was injected into the compartment. Thus when a small amount of substance contained in syringe A is injected into compartment B and the substance is allowed to disperse throughout the compartment until it becomes mixed in equal concentrations in all areas, the following relation can be expressed:
This method can be used to measure the volume of virtually any compartment in the body if (1) the amount of indicator injected into the compartment (the numerator of the equation) is known, (2) the concentration of the indicator in the compartment is known, (3) the indicator disperses evenly throughout the compartment, and (4) the indicator disperses only in the compartment that is being measured.
Table 25–3 shows some of the indicators that can be used to measure the fluid volumes of the body compartments. The volumes of two of the compartments, the intracellular and extracellular interstitial fluids, cannot be measured directly but, instead, are calculated from the values for other body fluid volumes.
Table 25–3 Measurement of Body Fluid Volume
| Volume | Indicators |
|---|---|
| Total body water | 3H2O, 2H2O, antipyrine |
| Extracellular fluid | 22Na, 125I-iothalamate, inulin |
| Intracellular fluid | Calculated as: Total Body Water – Extracellular Fluid Volume |
| Plasma volume | 125I-albumin, Evans blue dye (T-1824) |
| Blood volume | 51Cr-labeled red blood cells; calculated as: Blood Volume = Plasma Volume/(1 − Hematocrit) |
| Interstitial fluid | Calculated as: Extracellular Fluid Volume – Plasma Volume |
Intracellular and Extracellular Fluid Distribution Is Determined Mainly by the Osmotic Effect of Electrolytes Acting across the Cell Membrane (p. 290)
Because the cell membrane is highly permeable to water but relatively impermeable to even small ions, such as sodium and chloride, the distribution of fluid between the intracellular and extracellular compartments is determined mainly by the osmotic effects of these ions. The basic principles of osmosis and osmotic pressure are presented in Chapter 4. Therefore, only the most important principles as they apply to volume regulation are discussed in this section.
Osmosis Is the Net Diffusion of Water across a Selectively Permeable Membrane from a Region of High Water Concentration to One of Lower Water Concentration
The addition of a solute to pure water reduces the water concentration and causes water to move toward the region of high solute concentration. The concentration term used to measure the total number of solute particles in solution is the osmole: 1 osmole is equal to 1 mole (6.02 × 1023) of solute particles. For biological solutions, the term milliosmole (mOsm), which equals 1/1000 osmole, is commonly used.
The osmolar concentration of a solution is called its osmolality when the concentration is expressed as osmoles per kilogram of water and osmolarity when it is expressed as osmoles per liter of solution. The amount of pressure required to prevent osmosis of water through a semipermeable membrane is called the osmotic pressure. Expressed mathematically, the osmotic pressure (π) is directly proportional to the concentration of osmotically active particles in that solution.
where C is the concentration of solutes in osmoles per liter, R is the ideal gas constant, and T is the absolute temperature in degrees Kelvin. If π is expressed in millimeters of mercury (the unit of pressure commonly used for biologic fluids), π calculates to be about 19.3 mm Hg for a solution with an osmolarity of 1 mOsm/L. Thus, for each milliosmole concentration gradient across the cell membrane, 19.3 mm Hg of force is required to prevent water diffusion across the membrane. Very small differences in solute concentration across the cell membrane can therefore cause rapid osmosis of water.
Isotonic, Hypotonic, and Hypertonic Fluids
A solution is said to be isotonic if no osmotic force develops across the cell membrane when a normal cell is placed in the solution. An isotonic solution has the same osmolarity as the cell, and the cells do not shrink or swell if placed in the solution. Examples of isotonic solutions include a 0.9% sodium chloride solution and a 5% glucose solution.
A solution is said to be hypertonic when it contains a higher concentration of osmotic substances than does the cell. In this case, an osmotic force develops that causes water to flow out of the cell into the solution, thereby reducing the intracellular fluid volume and increasing the intracellular fluid concentration.
A solution is said to be hypotonic if the osmotic concentration of substances in the solution is less than the concentration of the cell. The osmotic force develops immediately when the cell is exposed to the solution, causing water to flow by osmosis into the cell until the intracellular fluid has about the same concentration as the extracellular fluid or until the cell bursts as a result of excessive swelling.
Volumes and Osmolarities of Extracellular and Intracellular Fluids in Abnormal States (p. 292)
Some of the factors that can cause extracellular and intracellular volumes to change markedly are ingestion of large amounts of water, dehydration, intravenous infusion of various solutions, loss of large amounts of fluid from the gastrointestinal tract, and loss of abnormal amounts of fluid via sweating or from the kidneys.
One can approximate the changes in intracellular and extracellular fluid volumes and the therapy that must be instituted if the following basic principles are kept in mind:
Effect of Adding Isotonic, Hypertonic, and Hypotonic Saline Solutions to Extracellular Fluid
If an isotonic solution is added to the extracellular fluid compartment, the osmolarity of the extracellular fluid does not change, and there is no osmosis through the cell membranes. The only effect is an increase in the extracellular fluid volume (Fig. 25–1). Sodium and chloride mainly remain in the extracellular fluid because the cell membrane behaves as though it were virtually impermeable to sodium chloride.
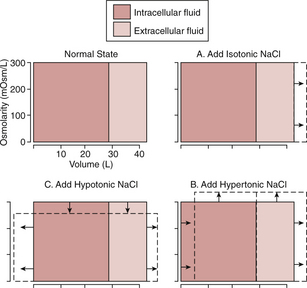
Figure 25–1 Effect of adding isotonic, hypertonic, and hypotonic solutions to extracellular fluid after osmotic equilibrium. The normal state is indicated by the solid lines, and the shifts from normal are shown by the dashed lines. The volumes of intracellular and extracellular fluid compartments are shown on the abscissa of each diagram, and the osmolarities of these compartments are shown on the ordinates.
If a hypertonic solution is added to the extracellular fluid, the extracellular fluid osmolarity increases and causes osmosis of water out of the cells into the extracellular compartment. The net effect is an increase in extracellular volume (greater than the volume of fluid that was added), a decrease in intracellular fluid volume, and an increase in the osmolarity of both compartments.
If a hypotonic solution is added to the extracellular fluid, the osmolarity of the extracellular fluid decreases, and some of the extracellular water diffuses into the cells until the intracellular and extracellular compartments have the same osmolarity. Both the intracellular and extracellular volumes are increased by addition of hypotonic fluid, although the intracellular volume is increased to a greater extent.
Edema: Excess Fluid in the Tissues (p. 296)
Intracellular Edema: Increased Intracellular Fluid
Three conditions especially likely to cause intracellular swelling are (1) hyponatremia, (2) depression of the metabolic systems of the tissues, and (3) lack of adequate nutrition to the cells. When the cell’s metabolic systems are depressed or they receive inadequate nutrition, sodium ions that normally leak into the interior of the cells can no longer be effectively pumped out of the cells, and the excess sodium ions cause osmosis of water into the cells.
Intracellular edema can also occur in inflamed tissues. Inflammation usually has a direct effect on the cell membranes to increase their permeability, allowing sodium and other ions to diffuse into the interior of the cells with subsequent osmosis of water into the cells.
Extracellular Edema: Increased Fluid in Interstitial Spaces
The two general causes of extracellular edema are (1) abnormal leakage of fluid from the plasma to the interstitial spaces across the capillaries and (2) failure of the lymphatics to return fluid from the interstitium to the blood, often called lymphedema.
Factors Can Increase Capillary Filtration and Cause Interstitial Fluid Edema
To understand the causes of excessive capillary filtration, it is useful to review the determinants of capillary filtration discussed in Chapter 16 as shown in the following equation:
where Kf is the capillary filtration coefficient (the product of the permeability and surface area of the capillaries), Pif is the interstitial fluid hydrostatic pressure, πc is the capillary plasma colloid osmotic pressure, and πif is the interstitial fluid colloid osmotic pressure. Thus, any of the following changes can increase the capillary filtration rate:
Lymphatic Blockage Causes Edema
When lymphatic blockage occurs, edema can become especially severe because plasma proteins that leak into the interstitium have no other way to be returned to the plasma. The rise in protein concentration increases the colloid osmotic pressure of the interstitial fluid, which draws even more fluid out of the capillaries.
Blockage of lymph flow can be especially severe with infections of the lymph nodes, such as occurs with infection by filarial nematodes. Lymph vessels may also be blocked with certain types of cancer or after surgery in which the lymph vessels are removed or obstructed.
Safety Factors That Normally Prevent Edema
Although many abnormalities can cause fluid accumulation in interstitial spaces, the disturbances must be substantial before clinically significant edema develops. Three major safety factors normally prevent fluid accumulation in the interstitial spaces:
Combining all of the safety factors, the total safety factor that protects against edema is about 17 mm Hg. Capillary pressure in peripheral tissues could therefore theoretically rise 17 mm Hg before significant interstitial edema would occur.