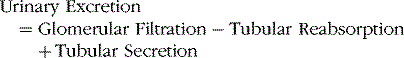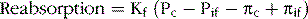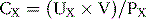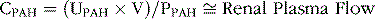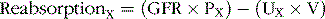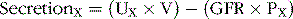CHAPTER 27 Urine Formation by the Kidneys
II. Tubular Reabsorption and Secretion
Renal Tubular Reabsorption and Secretion (p. 323)
After the glomerular filtrate enters the renal tubules, it flows sequentially through the proximal tubules, loops of Henle, distal tubules, collecting tubules, and collecting ducts before it is excreted as urine. Along this course, some substances are reabsorbed from the tubules into the peritubular capillary blood, whereas others are secreted from the blood into the tubules. The urine that is formed and all of the substances in the urine represent the sum of three basic renal processes.
Tubular Secretion—The Net Movement of Solutes from Peritubular Capillaries into the Tubules
Some substances enter the tubules not only by glomerular filtration but also by secretion from the peritubular capillaries into the tubules via two steps: (1) simple diffusion of the substance from the peritubular capillaries into the renal interstitium and (2) movement of the substance across the tubular epithelium into the lumen through active or passive transport. Substances that are actively secreted into the tubules include potassium and hydrogen ions as well as certain organic acids and organic bases.
Reabsorption of Solutes and Water from the Tubules into the Peritubular Capillaries
For a substance to be reabsorbed, it must first be transported across the renal tubular epithelial membrane into the interstitial fluid and then through the peritubular capillary membrane back into the blood. Solutes can be transported either through the cell membranes (transcellular route) by active or passive transport or through the junctional spaces between the cells (paracellular route) by passive transport; water is transported through and between the epithelial cells by osmosis.
After absorption into the interstitial fluids, water and solutes are transported through the peritubular capillary walls by ultrafiltration (bulk flow), which is mediated by hydrostatic and colloid osmotic forces. In contrast to the glomerular capillaries, which filter large amounts of fluid and solutes, the peritubular capillaries have a large reabsorptive force that rapidly moves fluid and solutes from the interstitium into the blood.
Reabsorption Rates for Substances Are Selective and Highly Variable
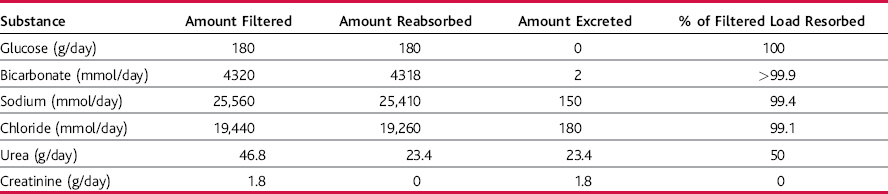
Some substances that are filtered, such as glucose and amino acids, are almost completely reabsorbed by the tubules, so the urinary excretion rate is essentially zero (Table 27–1).
Most of the ions in the plasma, such as sodium, chloride, and bicarbonate, are also highly reabsorbed from the tubules, but their rates of reabsorption and urinary excretion vary depending on the needs of the body. The metabolic waste products, such as urea and creatinine, are poorly reabsorbed and are excreted in relatively large amounts. Tubular reabsorption is highly selective, allowing the kidneys to regulate excretion of substances independent of one another.
Active Transport Requires Energy and Can Move Solutes against an Electrochemical Gradient
Transport directly coupled to an energy source, such as hydrolysis of adenosine triphosphate (ATP), is termed primary active transport. A good example is the sodium-potassium ATPase pump, which plays a major role in reabsorption of sodium ions in many parts of the nephron. On the basal and lateral sides of the tubular epithelial cells, the basolateral membrane has an extensive sodium-potassium ATPase system that hydrolyzes ATP and uses the released energy to transport sodium ions out of the cell into the interstitium. At the same time, potassium is transported from the interstitium to the inside of the cell. This pumping of sodium out of the cell across the basolateral membrane favors passive diffusion of sodium into the cell across the luminal membrane (the side that faces the tubular lumen) and passive diffusion of potassium out of the cell into the tubular lumen.
In certain parts of the nephron there are additional mechanisms for moving large amounts of sodium into the cell. In the proximal tubules, there is an extensive brush border on the luminal side of the membrane that multiplies the surface by 20-fold. There also are sodium carrier proteins that bind sodium ions on the luminal surface of the membrane and release them inside the cell, providing facilitated diffusion of sodium through the membrane into the cell. These sodium carrier proteins are also important for secondary active transport of other substances, such as glucose and amino acids.
Secondary Active Reabsorption of Glucose and Amino Acids Occurs through the Renal Tubular Membrane
During secondary active transport, two or more substances interact with a specific membrane protein and are co-transported together across the membrane. As one of the substances (e.g., sodium) diffuses down its electrochemical gradient, the energy released is used to drive another substance (e.g., glucose) against its electrochemical gradient. Secondary active transport does not require energy directly from ATP or other high-energy phosphate sources; rather, the source of the energy is that liberated by simultaneous facilitated diffusion of another transported substance down its own electrochemical gradient.
Transport Maximums Are Often Displayed for Actively Transported Substances
Many of the nutrients, such as glucose and amino acids, are reabsorbed through secondary active transport with sodium. In most instances, reabsorption of these substances displays a transport maximum, which refers to the maximum rate of reabsorption. When the filtered load of these substances exceeds the transport maximum, the excess amount is excreted. The threshold is the tubular load at which the transport maximum is exceeded in one or more nephrons, resulting in the appearance of that solute in the urine. The threshold usually occurs at a slightly lower tubular load than the transport maximum because not all nephrons have the same transport maximum and some nephrons excrete glucose before others have reached their transport maximum.
Passive Water Reabsorption by Osmosis Is Coupled to Sodium Reabsorption
When solutes are transported out of the tubule via primary or secondary active transport, their concentrations decrease in the tubule and increase in the interstitium. This creates a concentration difference that causes osmosis of water in the same direction as that in which the solutes are transported—from the tubular lumen to the interstitium. Some parts of the renal tubule, especially the proximal tubules, are highly permeable to water, and reabsorption occurs so rapidly that there is only a small concentration gradient across the membrane. In the ascending loops of Henle, however, water permeability is always low, so almost no water is reabsorbed despite a large osmotic gradient. In the distal tubules, collecting tubules, and collecting ducts, water permeability depends on the presence or absence of antidiuretic hormone (ADH). In the presence of ADH, these sections of the renal tubule are highly permeable to water.
Some Solutes Are Reabsorbed by Passive Diffusion
When sodium, a positive ion, is reabsorbed through the tubular cell, negative ions such as chloride also tend to diffuse passively through the paracellular pathway (between the cells). Additional reabsorption of chloride also occurs because of a concentration gradient that develops when water is reabsorbed from the tubule by osmosis, thereby concentrating the chloride ions in the tubular lumen.
Noncharged substances, such as urea, are also passively reabsorbed from the tubule because osmotic reabsorption of water tends to concentrate these solutes in the tubular lumen, favoring their diffusion into the renal interstitium. Urea and many other waste products do not permeate the tubule nearly as rapidly as water, allowing large amounts of these substances to be excreted in urine.
Reabsorption and Secretion Along Various Parts of the Nephron (p. 329)
Proximal Tubules Have a High Capacity for Reabsorption
Approximately 65% of the filtered load of water, sodium, chloride, potassium, and several other electrolytes is reabsorbed in the proximal tubules. One important function of the proximal tubules therefore is to conserve substances that are needed by the body, such as glucose, amino acids, proteins, water, and electrolytes. In contrast, the proximal tubules are not as permeable to waste products of the body and reabsorb a much smaller percentage of the filtered load of the substances.
The Loop of Henle Has Three Functionally Distinct Segments: Descending Thin Segment, Ascending Thin Segment, and Ascending Thick Segment
The loop of Henle dips into the inner part of the kidney, the renal medulla, and plays an important role in allowing the kidney to form concentrated urine. The descending thin loop of Henle is highly permeable to water, which is rapidly reabsorbed from the tubular fluid into the hyperosmotic interstitium (osmolarity rises to 1200–1400 mOsm/L in the inner renal medulla); approximately 20% of the glomerular filtrate volume is reabsorbed in the thin descending loop of Henle, causing the tubular fluid to become hyperosmotic as it moves toward the inner renal medulla.
In the thin and thick segments of the ascending loop of Henle, water permeability is virtually zero, but large amounts of sodium, chloride, and potassium are reabsorbed, causing the tubular fluid to become dilute (hypotonic) as it moves back toward the cortex. At the same time, active transport of sodium chloride out of the thick ascending loop of Henle into the interstitium causes a very high concentration of these ions in the interstitial fluid of the renal medulla. As in the proximal tubule, reabsorption of sodium chloride in the loop of Henle is closely linked to activity of the sodium-potassium ATPase pump in the basolateral membrane. In addition, sodium chloride is rapidly transported across the luminal membrane by a 1-sodium, 2-chloride, 1-potassium co-transporter. About 25% of the filtered loads of sodium, chloride, and potassium are reabsorbed in the loop of Henle, mostly in the thick ascending limb. Considerable amounts of other ions, such as calcium, bicarbonate, and magnesium, are also reabsorbed in the thick ascending loop of Henle.
The thick ascending limb of the loop of Henle is the site of action of the powerful “loop diuretics” furosemide (Lasix), ethacrynic acid, and bumetanide, all of which inhibit the 1-sodium, 2-chloride, 1-potassium co-transporter.
The Early Distal Tubule Dilutes the Tubular Fluid
The thick segment of the ascending limb empties into the distal tubule. The first portion of the distal tubule forms part of the juxtaglomerular complex, which provides feedback control of the glomerular filtration rate (GFR) and blood flow in the same nephron, as described in Chapter 26. The next early portion of the distal tubule has many of the same characteristics as the ascending loop of Henle and avidly reabsorbs most of the ions; however, it is virtually impermeable to water and urea. For this reason, it is referred to as the diluting segment; it also dilutes the tubular fluid. Fluid leaving this part of the nephron usually has an osmolarity of only about 100 mOsm/L.
A sodium chloride co-transporter moves sodium chloride from the lumen into the epithelial cells of the early distal tubule. The thiazide diuretics, used to treat disorders such as hypertension and heart failure, inhibit the sodium chloride co-transporter.
The Late Distal Tubule and Cortical Collecting Tubule Are Similar
The second half of the distal tubules and the cortical collecting tubules have similar functional characteristics. Anatomically, they are composed of two distinct cell types: the principal cells, which absorb sodium and water from the lumen and secrete potassium into the lumen, and the intercalated cells, which absorb potassium ions and secrete hydrogen ions into the tubular lumen.
The tubular membranes of both segments are almost completely impermeable to urea, and their permeability to water is controlled by the ADH concentration. With high levels of ADH, these segments are highly permeable to water. The reabsorption of sodium and secretion of potassium by the principal cells are controlled by the hormone aldosterone. Secretion of hydrogen ions by the intercalated cells plays an important role in acid-base regulation of the body fluids (discussed later).
The principal cells are the main sites of action of potassium-sparing diuretics, including spironolactone and eplerenone (antagonists of aldosterone’s effects of the mineralocorticoid receptor) and amiloride (a sodium channel blocker).
Medullary Collecting Ducts Are the Final Sites for Processing the Urine
Although the medullary collecting ducts reabsorb less than 10% of the filtered water and sodium, they are extremely important when determining the final urine output of water and solutes. Some special characteristics of this tubular segment are as follows:
Regulation of Tubular Reabsorption (p. 334)
Because it is essential to maintain precise balance between tubular reabsorption and glomerular filtration, multiple nervous, hormonal, and local control mechanisms regulate the tubular reabsorption rate as well as the GFR. An important feature of tubular reabsorption is that excretion of water and solutes can be independently regulated, especially through hormonal control.
Glomerulotubular Balance—The Ability of the Tubule to Increase its Reabsorption Rate in Response to a Greater Tubular Load
If the GFR is increased, the absolute rate of tubular reabsorption is increased approximately in proportion to the rise in GFR. Glomerulotubular balance helps prevent overloading of the more distal parts of the renal tubule when the GFR increases; however, glomerulotubular balance does not completely prevent changes in the GFR from altering urinary excretion.
Peritubular Capillary and Renal Interstitial Fluid Physical Forces Influence Tubular Reabsorption
As the glomerular filtrate passes through the renal tubules, more than 99% of the water and most of the solutes are reabsorbed—first into the renal interstitium and then into the peritubular capillaries. Of the fluid that is normally filtered by the glomerular capillaries (125 mL/min), approximately 124 mL/min is reabsorbed into the peritubular capillaries.
Peritubular capillary reabsorption is regulated by hydrostatic and colloid osmotic pressures acting across the capillaries and by the capillary filtration coefficient (Kf), as shown in the following relation:
where Pc is the peritubular capillary hydrostatic pressure, Pif is the interstitial fluid hydrostatic pressure, πc is the colloid osmotic pressure of the peritubular capillary plasma proteins, and πif is the colloid osmotic pressure of proteins in the renal interstitium. The two primary determinants of peritubular capillary reabsorption that are directly influenced by renal hemodynamic changes are the hydrostatic and colloid osmotic pressures of the peritubular capillaries. The peritubular capillary hydrostatic pressure is, in turn, influenced by (1) the arterial pressure and (2) the resistance of the afferent and efferent arterioles (Table 27–2).
Table 27–2 Factors That Can Influence Peritubular Capillary Reabsorption
| ↑Pc → ↓ Reabsorption |
| ↓RA → ↑Pc |
| ↓RE → ↑Pc |
| ↑Arterial pressure → ↑Pc |
| ↑πc → ↑Reabsorption |
| ↑πA → ↑πc |
| ↑FF → ↑πc |
| ↑Capillary filtration coefficient → ↑Reabsorption |
πc, peritubular capillary colloid osmotic pressure; πA, systemic plasma colloid osmotic pressure; FF, filtration fraction; Pc, peritubular capillary hydrostatic pressure; RA and RE, afferent and efferent arteriolar resistances, respectively.
The peritubular capillary colloid osmotic pressure is influenced by (1) the systemic plasma colloid osmotic pressure and (2) the filtration fraction, which is the GFR/renal plasma flow ratio. The higher the filtration fraction, the greater is the fraction of plasma that is filtered through the glomerular capillaries; consequently, the more concentrated become the proteins in the plasma that remains behind. An increase in filtration fraction therefore tends to increase the peritubular capillary reabsorption rate.
Increased Arterial Pressure Reduces Tubular Reabsorption
Even small increases in arterial pressure can increase the urinary excretion rates of sodium and water, phenomena referred to as pressure natriuresis and pressure diuresis, respectively. There are three primary mechanisms by which increased arterial pressure increases urinary excretion:
Aldosterone Increases Sodium Reabsorption and Potassium Secretion
Aldosterone, which is secreted by the adrenal cortex, acts on mineralocorticoid receptors mainly on the principal cells of the cortical collecting tubule to stimulate the sodium-potassium ATPase pump, which increases sodium reabsorption from the tubule and potassium secretion into the tubule. In the absence of aldosterone, as occurs with destruction or malfunction of the adrenals (Addison’s disease), there is marked loss of sodium from the body and accumulation of potassium. Conversely, excess aldosterone secretion, as occurs in patients with adrenal tumors (Conn’s syndrome), is associated with sodium retention and potassium depletion.
Angiotensin II Increases Sodium and Water Reabsorption
Angiotensin II, the most powerful sodium-retaining hormone of the body, increases sodium and water reabsorption through three main effects:
These multiple actions of angiotensin II cause marked sodium and water retention by the kidneys in circumstances associated with low blood pressure, low extracellular fluid volume, or both, such as during hemorrhage or loss of salt and water from body fluids.
ADH Increases Water Reabsorption
ADH, secreted by the posterior pituitary gland, increases water permeability of the distal tubules, collecting tubules, and collecting ducts. These portions of the nephron then reabsorb water avidly and form highly concentrated urine. These effects help the body conserve water during circumstances such as dehydration, which greatly stimulates ADH secretion. In the absence of ADH, these portions of the nephrons are virtually impermeable to water, causing the kidneys to excrete large amounts of dilute urine.
Atrial Natriuretic Peptide Decreases Sodium and Water Reabsorption
Specific cells of the cardiac atria, when distended as a result of plasma volume expansion, secrete a peptide called atrial natriuretic peptide. Greater levels of this peptide inhibit reabsorption of sodium and water by the renal tubules, thereby increasing the excretion of sodium and water.
Parathyroid Hormone Increases Calcium Reabsorption and Decreases Phosphate Reabsorption
Parathyroid hormone is one of the most important calcium- and phosphate-regulating hormones of the body. Its principal action in the kidneys is to increase reabsorption of calcium, especially in the distal tubules. Another action of parathyroid hormone is inhibition of phosphate reabsorption by the proximal tubule.
Sympathetic Nervous System Activation Increases Sodium Reabsorption
Stimulation of the sympathetic nervous system constricts the afferent and efferent arterioles, thereby decreasing the GFR. At the same time, sympathetic activation directly increases sodium reabsorption in the proximal tubule, ascending loop of Henle, and distal tubule while stimulating renin release and angiotensin II formation.
Use of Clearance Methods to Quantify Kidney Function (p. 340)
Renal Clearance Is the Volume of Plasma That Is Completely Cleared of a Substance Each Minute
For a given substance X, renal clearance is defined as the ratio of the excretion rate of substance X to its concentration in the plasma, as shown by the following relation:
where CX is renal clearance in milliliters per minute, UX × V is the excretion rate of substance X (UX is the concentration of X in the urine, and V is urine flow rate in milliliters per minute), and PX is the plasma concentration of X. Renal clearances can be used to quantify several aspects of kidney functions, including the rates of glomerular filtration, tubular reabsorption, and tubular secretion of various substances.
Renal Clearance of Creatinine or Inulin Can Be Used to Estimate the GFR
Creatinine, a byproduct of skeletal muscle metabolism, is filtered at the glomerulus but is not reabsorbed or secreted appreciably by the tubules; therefore the entire 125 mL of plasma that filters into the tubules each minute (GFR) is cleared of creatinine. This means that creatinine clearance is approximately equal to the GFR. For this reason, creatinine clearance is often used as an index of the GFR. An even more accurate measure of GFR is the clearance of inulin, a polysaccharide that is not reabsorbed or secreted by the renal tubules.
Renal Clearance of Para-aminohippuric Acid (PAH) Can Be Used to Estimate Renal Plasma Flow
Some substances, such as PAH, are freely filtered and not reabsorbed by the tubules but are secreted into the tubules; therefore, the renal clearance of these substances is greater than the GFR. In fact, about 90% of the plasma flowing through the kidney is completely cleared of PAH, and renal clearance of PAH (CPAH) can be used to estimate the renal plasma flow, as follows:
where UPAH and PPAH are urine and plasma concentrations of PAH, respectively, and V is the urine flow rate.
The filtration fraction is the GFR/renal plasma flow ratio. If renal plasma flow is 650 mL/min and the GFR is 125 mL/min, the filtration fraction is 125/650, or 0.19.
Tubular Reabsorption or Secretion Can Be Calculated from Renal Clearances
For substances that are completely reabsorbed from the tubules (e.g., amino acids, glucose), the clearance rate is zero because the urinary secretion rate is zero. For substances that are highly reabsorbed (e.g., sodium), the clearance rate is usually less than 1% of the GFR, or less than 1 mL/min. In general, waste products of metabolism, such as urea, are poorly reabsorbed and have relatively high clearance rates.
The tubular reabsorption rate is calculated as the difference between the rate of filtration of the substance (GFR × PX) and the urinary excretion rate (UX × V), as follows:
If the excretion rate of a substance is greater than the filtered load, the rate at which it appears in the urine represents the sum of the rate of glomerular filtration plus tubular secretion; the secretion rate is therefore the difference between the rate of urinary excretion of a substance and the rate at which it is filtered, as follows: