6 Oxygen supplementation
Clinical Tip
Indications for Oxygen Supplementation
Oxygenation may be impaired via a number of mechanisms (ventilation–perfusion mismatch, hypoventilation, diffusion impairment, intrapulmonary or cardiovascular shunt); more than one mechanism can, and often does, occur in the same patient at the same time. Oxygenation may be compromised in a large variety of disorders affecting the respiratory, cardiovascular and neuromuscular systems in particular. Oxygen supplementation is extremely unlikely to cause any harm (unless high concentrations are provided for a sustained period of at least 24 hours), and the author would strongly encourage a liberal approach to its use (bearing potential financial constraints in mind).
In essence, a positive response to oxygen supplementation confirms its requirement (i.e. trial therapy). However, animals in need of oxygen supplementation may be identified and monitored more objectively through the use of pulse oximetry (or preferably arterial blood gas analysis if available). Pulse oximetry measures saturation of arterial haemoglobin with oxygen (SpO2) which should be more than 95% in normal dogs and cats on room air.
Methods of Oxygen Supplementation
A number of oxygen supplementation techniques have been widely described that differ in terms of their cost, technical ease and efficiency. The method used is determined by both patient-related (level and duration of supplementation required, compliance, size) and nonpatient-related (available facilities, clinical expertise, financial constraints) factors. Methods of supplementation include:
Flow-by oxygen
Flow-by oxygen supplementation is extremely easy to provide and is typically the first technique used in emergency patients while major body system examination is performed, an intravenous catheter is placed and other urgent interventions carried out. However, it is noteworthy that some animals, cats in particular, do better if placed in an oxygen cage first instead (depends on underlying disorder). Flow-by oxygen supplementation is usually only used short-term as it is wasteful. Some animals are intolerant of flow-by oxygen supplementation, even with minimal restraint, and it is clearly important not to stress these patients further.
Mask supplementation
Oxygen supplementation using a mask is another readily available technique that allows access to the patient. This technique is typically only used short-term and many of the same comments apply as for flow-by supplementation. In particular, some animals are very intolerant of having a mask placed over their muzzle and this should never be forced upon them. In addition, it must be remembered that panting is the major means of cooling in dogs. Placing a mask over a panting dog’s mouth can result in a marked increase in humidity and lead to severe hyperthermia with potentially disastrous consequences. Although it reduces the inhaled oxygen concentration that can be achieved, masks may therefore need to be only loosely applied in some cases.
Oxygen collar
An oxygen collar is one means of providing more long-term oxygen supplementation, especially to cats and small dogs. A rigid Elizabethan collar is applied and the front of the collar is covered with cling film or a similar transparent wrap that is taped in place; it is important not to cover the whole of the front to allow the collar to vent and prevent the animal from overheating. Oxygen tubing is then passed into the collar from the rear and the tubing is taped to the inside of the collar to secure it in place.
Humidified oxygen is delivered at a higher flow rate initially (e.g. 2 l/min) and then reduced (e.g. to 1 l/min) once the collar has been filled. The author has achieved good results with this technique, which also allows on-going access to the patient.
Nasal cannulae (prongs)
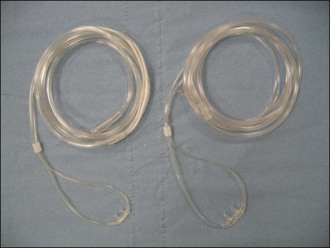
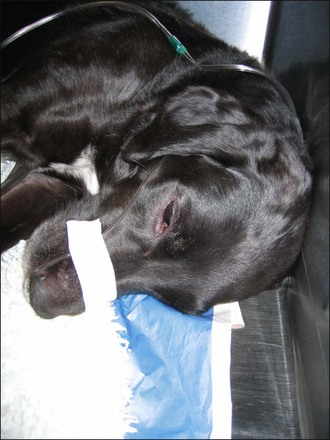
Nasal cannulae (nasal prongs) for human use (e.g. Venticaire®, Flexicare Medical Ltd, Mountain Ash, UK) are available in a variety of sizes (Figure 6.1) that work well for some dogs. They are generally tolerated sufficiently well following application of local anaesthetic drops to the nostrils (Figure 6.2). Humidified oxygen is provided at an initial rate of 50–150 ml/kg/min. Nasal cannulae are less effective in dogs that are panting. Although easy to apply, nasal prongs must be checked regularly as they are very easily dislodged.
Nasal/nasopharyngeal catheters
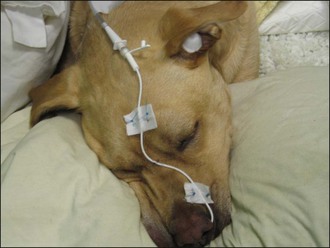
Unilateral or bilateral indwelling nasal catheters (Figure 6.3) are used less frequently nowadays with the availability of nasal cannulae but they remain invaluable in a proportion of patients. In general the author reserves nasal catheters for dogs that are poorly tolerant of nasal prongs and sometimes dogs in need of long-term supplementation that are being sedated or anaesthetized for another procedure (i.e. so as not to waste the episode of chemical restraint). Nasal catheter placement is a relatively simple procedure (see p. 291); some dogs are poorly compliant and mild sedation may be required.
Oxygen cage
Big sophisticated oxygen cages that allow the percentage of oxygen supplementation as well as environmental temperature and humidity to be controlled are found in a small number of referral centres. However, oxygen cages can be improvised and are used predominantly for long-term oxygen supplementation and to provide higher levels of inspired oxygen concentration than can be achieved with other methods. A potential disadvantage of oxygen cages is the inability to interact with and examine the patient regularly without causing repeated decreases in the environmental oxygen concentration. On the other hand, in some cases this lack of interaction is in fact desirable as long as the patient can be monitored closely by other means (e.g. observational, ideally using monitoring equipment as well). Oxygen cages are also not typically appropriate for larger dogs and hyperthermia is a potential complication in general.
Oxygen cages offer an ideal way of providing oxygen supplementation for initial stabilization to some animals presenting in respiratory distress without the need for manual restraint. This is especially the case for dyspnoeic cats and the author routinely leaves appropriate cases in an oxygen cage for 20–30 minutes following presentation prior to further intervention, with close monitoring throughout. It must be remembered that some causes of respiratory distress will be minimally or inadequately responsive to oxygen therapy and the above approach is not appropriate in all cases. The decision is made on an individual case basis and other interventions such as thoracocentesis or sedation may be more helpful.
Improvised oxygen cages can be created using a human neonatal incubator or by covering the front of a normal kennel with cling film and piping oxygen into the kennel; alternatively, a cat carrier or small puppy crate can be wrapped in cling film. Another technique is to place a cat carrier or small puppy crate inside a polythene or similar garbage bag, tie the bag, and pipe oxygen through a hole cut into the side. However, the author would not recommend the use of this technique unless a (relatively) transparent bag can be used as the patient is otherwise completely out of sight.
More recently the Buster ICU cage (Kruuse UK Ltd, Leeds, UK) has become available for cats and small dogs (Figure 6.4). Amongst other facilities, this cage allows oxygen supplementation with some degree of regulation, although overheating can be a problem.
Clinical Tip
Animals that remain severely dyspnoeic or have an SpO2 of less than 90% despite aggressive oxygen supplementation are candidates for anaesthesia, endotracheal intubation and ventilation. However, in the absence of facilities to provide and maintain mechanical ventilation, the practical implications of this intervention need to be carefully considered. If affordable, referral should be considered but transportation should only be undertaken following as much stabilization as possible and preferably once arrangements to provide oxygen supplementation en route have been made.