Chapter 15 Cerebrospinal Fluid and the Blood-Brain Barrier
1. Cerebrospinal fluid has many functions.
2. Most cerebrospinal fluid is formed at the choroid plexus of the ventricles.
3. Cerebrospinal fluid flows down a pressure gradient through the ventricular system into the subarachnoid space.
4. Cerebrospinal fluid is absorbed into the venous system.
5. Hydrocephalus is an increased volume of cerebrospinal fluid in the skull.
Cerebrospinal fluid (CSF) is a clear fluid present in the ventricles (core cavities) of the brain, in the central canal that runs through the core of the spinal cord, and in the subarachnoid space that surrounds the entire outer surface of the brain and spinal cord (Figure 15-1). The CSF contains almost no blood cells and little protein. Its rates of formation, flow, and absorption are sufficiently high to cause its replacement several times daily. Sampling its pressure, cell count, and levels of various biochemical constituents is a common diagnostic procedure called a spinal tap. Injecting radiopaque dyes into the CSF of the subarachnoid space is the basis of a common neuroradiographic technique called myelography that can assess the integrity of the spinal canal. Obstruction of the flow of CSF produces a condition called hydrocephalus. An understanding of the formation, flow, and absorption of CSF is essential for understanding these diagnostic procedures and the pathophysiology of hydrocephalus.
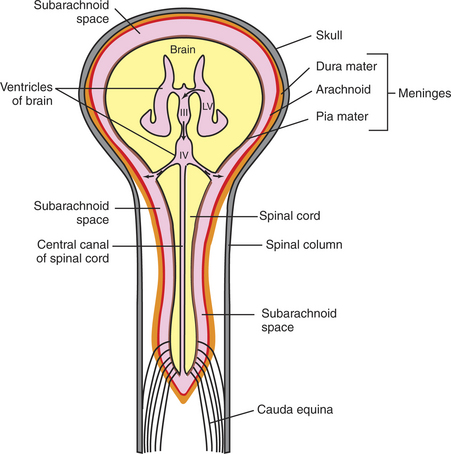
FIGURE 15-1 Schematic diagram of the relationships among the central nervous system, ventricles, cerebrospinal fluid (CSF), and meninges. The CSF is colored pink. LV, lateral ventricle; III, third ventricle; IV, fourth ventricle; solid curved arrow, interventricular foramen; solid straight arrow, cerebral aqueduct; dashed curved arrow, lateral aperture of the fourth ventricle.
(Modified from Behan M: Organization of the nervous system. In Reece WO, editor: Duke’s physiology of domestic animals, ed 12, Ithaca, NY, 2004, Comstock Cornell University Press.)
The blood-brain barrier refers to the selective nature of central nervous system (CNS) blood vessels with respect to the materials that can move across their walls, compared with blood vessels in other parts of the body. Understanding the blood-brain barrier helps clarify why it is difficult to deliver certain drugs effectively to the brain.
Cerebrospinal Fluid Has Many Functions
To work properly, the CNS needs protection not only from physical injury but also from significant variation in the local environment of its neurons. A buildup of toxins or a significant change in ionic concentration in this neuronal microenvironment could result in pathological changes in neuronal physiology.
One of the most important functions of CSF is to cushion the brain, protecting it against blows to the head. Because the specific gravities of the brain and CSF are similar, the brain floats in the fluid. Thus the force of a blow to the head is buffered by the CSF instead of being transferred directly to brain tissue.
Because the composition of the CSF is tightly controlled and it is in equilibrium with the extracellular fluid of the brain and spinal cord, the CSF also helps maintain a consistent extracellular microenvironment for the neurons and glia of the CNS. This diffusional equilibrium between the CSF and the extracellular fluid, in conjunction with the flow and multiple daily turnover of the CSF, also makes the CSF an effective waste control system that can remove potentially harmful cellular metabolites. Evidence indicates that these properties may also allow the CSF to function as a brain distribution system for some polypeptide hormones and growth factors that are secreted into the CSF.
Most Cerebrospinal Fluid Is Formed at the Choroid Plexus of the Ventricles
The ventricles are a series of interconnected cavities in the core of the brain that have an ependymal cell lining and are filled with CSF (Figure 15-2). The lateral ventricles are respectively located in the two cerebral hemispheres, the third ventricle is found at the midline of the diencephalon, and the fourth ventricle is located between the cerebellum and the dorsal surface of the hindbrain (pons and medulla) (Figure 15-3).
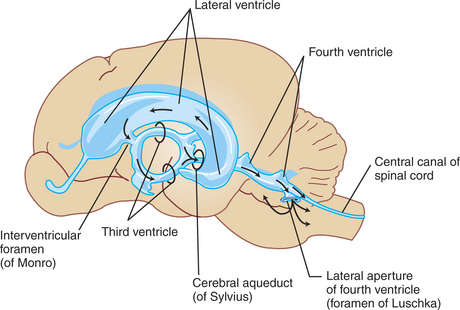
FIGURE 15-2 Lateral view of the ventricular cavities and their approximate spatial position within the brain.
(Modified from Fletcher TF: Spinal cord and meninges. In Evans HE, editor: Miller’s anatomy of the dog, ed 3, Philadelphia, 1993, Saunders.)
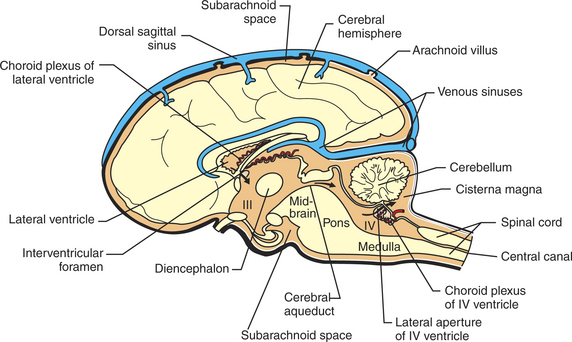
FIGURE 15-3 Midsagittal section of the brain showing portions of the ventricles and subarachnoid space, the choroid plexuses that produce CSF, and the dorsal sagittal sinus into which CSF is absorbed. The cisterna magna is a common location for the sampling of CSF. The CSF is colored light orange. III, third ventricle; IV, fourth ventricle.
(Modified from Fletcher TF: Spinal cord and meninges. In Evans HE, editor: Miller’s anatomy of the dog, ed 3, Philadelphia, 1993, Saunders.)
The majority of CSF is formed by a choroid plexus located in each of the four ventricles. These are small, cauliflower-like growths of clustered villi that form a portion of the floor or roof of each ventricle (Figure 15-3). The plexuses consist of tufts of capillaries covered by a layer of ependymal cells. These ependymal cells, unlike the cells lining the rest of the ventricle, form a selective, tight-junction barrier to the secretions of the leaky capillaries and to other surrounding fluids (e.g., CSF, extracellular fluid). Membrane transporters and selective channels regulate the passage of ions and molecules across the ependymal cell barrier, effectively controlling the composition of the CSF being synthesized in the ventricle. The active transport of sodium ions (Na+) contributes to a net movement of sodium chloride (NaCl) into the ventricles. This osmotic gradient regulates the water content of the CSF as water follows the NaCl passively into the ventricle. There is evidence that some potentially harmful metabolic waste products deposited in the CSF can actually be absorbed and removed by the choroid plexus.
It is important to note that CSF is formed at an almost constant rate, independent of either CSF pressure or blood pressure. Therefore, if CSF pressure or general intracranial pressure were to rise as a result of an obstruction to CSF flow or a space-occupying mass, CSF formation would continue.
Cerebrospinal Fluid Flows down a Pressure Gradient Through the Ventricular System into the Subarachnoid Space
CSF flows, by bulk, down a pressure gradient from its site of formation at the choroid plexuses through the ventricular system and subarachnoid space into the venous system. Fluid formed in the lateral ventricles passes into the third ventricle through the interventricular foramina (foramina of Monro) (see Figures 15-1, 15-2, and 15-3). The fluid mixes with fluid formed in the third ventricle and from there passes through the cerebral aqueduct (aqueduct of Sylvius) of the midbrain into the fourth ventricle. Fluid in the fourth ventricle passes into the subarachnoid space through two lateral apertures or foramina of Luschka. Some mammals have a third, medially located passageway from fourth ventricle to subarachnoid space (foramen of Magendie).
Recall that the brain and spinal cord are encased in bone (the skull and spinal canal, respectively) and covered by a series of three membranes called the meninges (see Chapter 3). From outer to inner, these membranes are the dura, arachnoid, and pia (see Figure 15-1). The subarachnoid space lies between the arachnoid and pia, and when the CSF exits the brain through the apertures (foramina) of the fourth ventricle, the CSF fills this space, spreading out over the entire outer surface of the brain and spinal cord. Thus the entire CNS is essentially floating in a fluid-filled, membranous bag. As the CSF circulates up over the dorsal convexity of the brain, it is absorbed into the venous system near the midline.
The pressure, cell count, and chemical constituents of CSF can be sampled by placing a styletted spinal needle into the subarachnoid space. Anatomically, the most convenient place to perform this varies with species. In humans it is usually performed in the lumbar spinal column because the human spinal cord tapers to a cone (conus medullaris) near the first lumbar vertebra (humans have five lumbar vertebrae) as the dura and arachnoid continue down to around the second sacral vertebra. This provides a relatively large subarachnoid space in the human midlumbar spinal column from which to sample. In most veterinary species, however, the conus medullaris extends to about the sixth or seventh lumbar vertebra, leaving only a small subarachnoid space. Therefore, most veterinary spinal taps are performed by sampling from the subarachnoid space between the skull and the first cervical vertebra in anesthetized animals. In this location the subarachnoid space, formed as the arachnoid stretches from the caudal cerebellar surface to the dorsal surface of the medulla, is called the cisterna magna (“big cistern”) and is much deeper than other portions of the subarachnoid space (see Figure 15-3). Spinal taps provide valuable information about such neuropathological lesions as intracranial space-occupying masses and inflammation.
Cerebrospinal Fluid Is Absorbed into the Venous System
CSF is absorbed into the venous system, principally into a dura-lined venous sinus (dorsal sagittal sinus) that lies between the dorsal surfaces of the cerebral hemispheres (see Figure 15-3). Most of the fluid is absorbed from the subarachnoid space into the dural sinus through arachnoid villi (Figure 15-4). These are small, fingerlike projections of the arachnoid membrane that poke through the walls of the sinus. Absorption appears to be pressure dependent and is unidirectional; CSF can flow from subarachnoid space to venous sinus, but venous blood cannot normally move from the sinus back into the subarachnoid space. The movement of CSF into the venous sinus is sometimes referred to as a “bulk flow” because all constituents of the fluid, including waste products and other foreign matter (e.g., red blood cells) move into the sinus. Whether materials cross the cells of the arachnoid villi by vesicular transport or by the formation and movement of giant, fluid-filled vacuoles is still a subject of debate. The entire volume of CSF is replaced approximately six times per day in species such as sheep and goat.
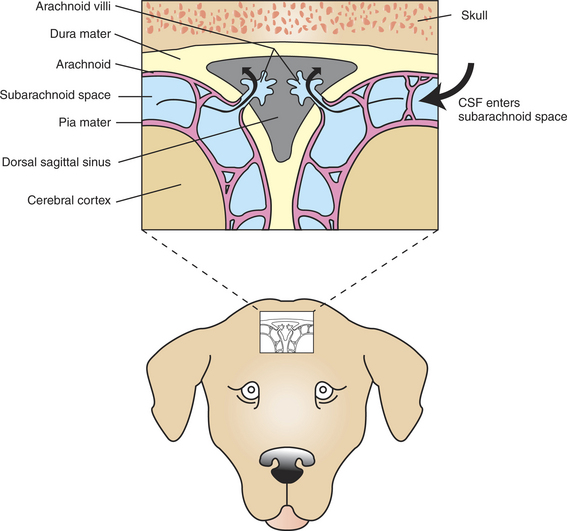
FIGURE 15-4 Transverse (coronal) section through the dorsal midline of the brain showing the absorption of CSF into the dorsal sagittal sinus, through arachnoid villi. The CSF is colored light blue. The small window on the dog’s head shows the approximate dorsoventral position of the sinus.
(Modified from Oliver JE, Lorenz MD: Handbook of veterinary neurology, ed 2, Philadelphia, 1993, Elsevier-Saunders.)
In normal animals, CSF pressure is regulated primarily by its absorption at the arachnoid villi because the rate of absorption can respond to changes in CSF pressure, whereas its formation is fairly constant and independent of changes in pressure. Therefore, any obstruction of CSF absorption into the venous sinus causes CSF pressure to rise almost immediately. In some pathological conditions, such as brain tumors or meningitis, CSF pressure can increase dramatically.
Hydrocephalus Is an Increased Volume of Cerebrospinal Fluid in the Skull
Hydrocephalus is defined as an increased CSF volume in the skull, often associated with an increased ventricular volume and increased intracranial pressure. In theory, hydrocephalus could be caused by too much fluid production at the choroid plexuses, obstruction to its flow through the ventricular system or subarachnoid space, or impaired absorption at the arachnoid villi. In practice, overproduction seems rare, whereas obstruction to flow seems more common, particularly at such vulnerable sites as the narrow cerebral aqueduct (connecting the third and fourth ventricles) and the exits from the fourth ventricle. Such blockages in the ventricular system produce a noncommunicating hydrocephalus that results in a buildup of freshly produced CSF in portions of the ventricular system behind the blockage. This causes the ventricular regions inside the brain to expand at the expense of the surrounding brain tissue, and intracranial pressure rises.
Impairment of absorption (a type of communicating hydrocephalus) can be secondary to meningitis or hemorrhage, presumably as a result of cellular debris that obstructs the transfer of CSF from subarachnoid space to venous sinus at the arachnoid villi. This can increase CSF volume in the subarachnoid space, which increases pressure on the outside surface of the brain and increases intracranial pressure.
The pathogenesis of many cases of hydrocephalus is not known. A common form of treatment in humans is surgical implantation of a tube that shunts CSF into the atria of the heart or into the peritoneal cavity, thus relieving episodes of increased CSF pressure and preventing further brain damage.
Permeability Barriers Exist Between Blood and Brain
Many dyes, once injected into the blood, stain other tissues of the body but not the brain. This suggests that the brain’s blood vessels have the ability to restrict certain substances from accessing brain tissue. This physiological property of CNS blood vessels is referred to as the blood-brain barrier (BBB). The BBB contributes to a stable environment for the neurons and glial cells of the CNS. As Claude Bernard noted more than a century ago, homeostasis, or constancy of the internal environment, is a precondition for autonomous life. It is not surprising that the brain, the “master choreographer of autonomous life,” should have special mechanisms for protecting its internal environment. Such protection from direct exposure to the blood supply is necessary because the composition of blood can significantly vary with factors such as diet, exercise, metabolic activity, illness, age, and exposure to environmental toxins. Many of the varying blood-borne nutrients, metabolites, and toxins are neuroactive and capable of affecting membrane receptors, transporters, or ion channels. In the absence of a BBB, these substances could result in unregulated and undesirable changes in neural activity and behavior.
In most capillaries, water-soluble compounds leave through open clefts between capillary endothelial cells, and exchange is relatively unrestricted. In brain capillaries, however, passage through intercellular clefts is blocked by tight junctions, and exchange of blood solutes is highly selective (Figure 15-5). As a general rule, molecules that are small, uncharged, lipid soluble, and unbound to plasma proteins (e.g., O2, CO2, ethanol, nicotine) can easily pass across the capillary endothelium of the BBB. Some molecules that do not fit this profile (e.g., glucose, some amino acids) are able to pass through the BBB by specific, carrier-mediated transport mechanisms. Brain capillaries have a greater number of mitochondria, which reflects the operation of such transporters. Certain degradative enzymes expressed by brain capillary endothelium (e.g., monoamine oxidase) provide a further restriction on substances that can pass the BBB. A notable feature of brain capillaries is that they are surrounded by a layer of glial astrocytic “end-feet.” these end-feet are thought to play a role in formation of the capillary endothelial tight junctions during development. evidence also indicates that the end-feet interact with the BBB-forming capillary endothelial cells and neurons to facilitate molecular homeostasis of the brain.
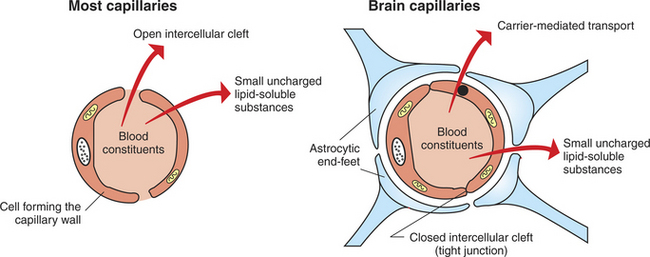
FIGURE 15-5 Blood-brain barrier (BBB). Unlike most capillaries of the body, cells of brain capillary walls are joined by tight junctions that restrict the passage of material between the cells. Materials leaving brain capillaries must pass through the cells forming the capillary wall. Substances that are not small, uncharged, and lipophilic must be carried across the cells by selective transport mechanisms. Astrocytic end-feet are thought to contribute to the tight-junction organization during development.
Unfortunately, for many patients, the protection afforded to the CNS by the BBB often prevents many antibiotics and other drugs from reaching the brain, particularly drugs with low lipid solubility or drugs bound to plasma proteins. Attempts to circumvent this problem have focused on temporary disruption of the BBB, direct delivery to the brain, “hitching a ride” on BBB membrane transporters, and increasing the lipid solubility of drugs.
In some parts of the brain known as the circumventricular organs, which include the hypothalamus, the brain capillaries do not form tight junctions, and the BBB is apparently not effective. This is significant because these brain regions are involved in functions such as the control of serum osmolality and glucose levels, hormonal communication, feeding, drinking, and vomiting, and therefore they need to detect the levels of many serum solutes.
CLINICAL CORRELATIONS
Increased Intracranial Pressure
History.
You examine a 9-year-old female boxer dog. The owner states that recently the dog has seemed more drowsy than usual and had what you recognize to be a generalized tonic-clonic seizure the preceding night.
Clinical Examination.
Physical examination of the dog reveals a hard, nodular mass of the mammary gland. Other deficits are referable to the nervous system and are characterized by apparent drowsiness and confusion and by a deficit of the proprioceptive positioning reaction of the right front and right rear legs. Lateral radiographs of the chest reveal metastatic, neoplastic lesions in the lungs. The CSF pressure, as measured with a manometer through a needle placed in the cisterna magna, is 310 mm CSF. (The normal CSF pressure in dogs is less than 180 mm CSF.)
Comment.
This is a typical case of a dog with a neoplasm of the mammary gland that has spread first to the lungs, which contain the first capillary bed filter encountered by tumor cells as they invade the venous system, and then to the brain. As the tumor mass increases within the fixed encasement of the cranial vault, CSF and other fluid volumes are displaced. Some loss of myelin may compensate temporarily for the expanding intracranial mass, but eventually the expanding tumor, encased in the skull, causes an increase in intracranial pressure, which is reflected in an increased CSF pressure in the cisterna magna. In measuring this pressure, the dog is anesthetized, and a styletted spinal needle is placed in the cisterna magna. The stylet is removed, and a rigid glass or plastic tube (manometer) is attached using a right-angle, three-way valve. CSF rises up the manometer to a height proportional to intracranial pressure. Its height is measured off the millimeter graduations marked on the tube.
The proprioceptive deficits of the right front and right rear legs result from a focal, asymmetric lesion of the left cerebral cortex. The seizure also resulted from this mass. With the mammary mass, metastatic lesions in the lungs, asymmetric neurological signs, seizures, and elevated CSF pressure, it is reasonable to conclude that this dog has an intracranial neoplasm that probably spread from the mammary gland to the lungs and the brain. Computed tomography (CT) or magnetic resonance imaging (MRI) is warranted to further define the tumor in the brain.
Seizures in a Foal
History.
A 2-day-old Arab colt from an unobserved foaling displays lethargy and inability to rise, and he started to go into seizure in the last hour. The foal stood, but it took longer than normal. The foal has been nursing, but the mare does not appear to have much milk; this is the mare’s first foal. The foal seems less active than normal foals and has become more lethargic as the day progressed. Finally, it seemed as though he would not stand, and he had a seizure while traveling to the clinic.
Clinical Examination.
The foal has a fever, and pulse and respiration are increased. Mucous membranes are darker red than normal, the membranes are dry (dehydration), and the capillary refill time is prolonged (poor perfusion). Auscultation reveals harsh lung sounds and crackles. The umbilicus is thickened and wet. There is petechiation (petechial hemorrhages) inside the ears and in the sclera. There appears to be signs of uveitis in the eyes. The foal is not responsive to manipulations and thrashes, although not in seizure, while being examined. The foal also lacks a suckle reflex.
Comment.
Although many possibilities exist for the cause of seizures in this foal, the two most likely reasons are low glucose level (hypoglycemia) and infection (meningitis). The blood glucose level is high in this foal, so meningitis (inflammation of the meninges) with septicemia is most likely. This foal probably has become septic (blood-borne infection) based on the history and clinical signs, which support that he likely did not receive enough colostrum and may not be receiving enough milk from the mare. Without adequate colostrum and nutrition, the foal’s immune system is more susceptible to infection. With the thickened umbilicus, harsh lung sounds, fever, petechiation, uveitis, and seizures, the signs are consistent with septicemia, which is manifesting in different regions of the body. The umbilicus could be infected (omphalophlebitis), the lung sounds are consistent with infection (pneumonia), and seizures are consistent with meningitis.
Complete blood count, chemistry, blood gases, and blood culture are warranted to determine the overall status of the foal. In many cases, these tests will be sufficient to make a diagnosis and determine treatment. In some cases, to make a definitive diagnosis, an atlanto-occipital (A/O) CSF tap (which samples from the cisterna magna) is best because it is closest to the site of the lesion, compared with a lumbosacral tap. The tap also allows a culture to be submitted so that the foal can be treated with the most efficacious antibiotics. In performing the CSF tap, the foal can be sedated with diazepam (Valium). The CSF is submitted for protein, glucose, cytology, and culture. Typically, the protein level is high with meningitis, and cytology shows an increased number of leukocytes (neutrophils). there is always the potential for a false-negative result with the culture.
Treatment.
Prognosis for a septic foal with seizures is poor, with many factors to consider. In regard to the meningitis, treatment consists of antibiotics, antiinflammatory agents, and anticonvulsants as needed. Seizures can cause hypoxia to the affected area, which can result in permanent damage. Besides meningitis, other problems include the umbilical infection, respiratory infection, and uveitis. With septicemia, other organs often become infected (joints, gastrointestinal tract, renal system). Additional concerns are potential renal insult caused by dehydration or complications associated with some antibiotics. Supportive care is another consideration. Managing a recumbent foal is challenging, not only for the reasons already listed, but also because of other factors, including additional infection, aspiration, and nutritional support.
Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41.
Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach, updated edition. Philadelphia: Saunders, 2005.
Brodal P. The central nervous system: structure and function, ed 3. New York: Oxford University Press, 2004.
Fletcher TF. Spinal cord and meninges. Evans HE, ed. Miller’s anatomy of the dog, ed 3, Philadelphia: Saunders, 1993.
Guyton AC, Hall JE. Textbook of medical physiology, ed 11. Philadelphia: Saunders, 2006.
Kandel ER, Schwartz JH, Jessell TM. Principles of neural science, ed 4, New York: McGraw-Hill, 2000.
Mollanji R, Papaiconomou C, Boulton M, et al. Comparison of cerebrospinal fluid transport in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol. 2001;281(4):R1215.
Purves D, Augustine G, Fitzpatrick D, et al. Neuroscience, ed 3. Sunderland, Mass: Sinauer Associates, 2004.
Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877.
PRACTICE QUESTIONS
1. Obstruction of cerebrospinal fluid (CSF) flow at the cerebral aqueduct (aqueduct of Sylvius) would lead to dilation (enlargement) of the:
2. CSF is principally formed at the:
3. You are performing a spinal tap on an anesthetized horse and measuring CSF pressure. Cellular debris has obstructed the arachnoid villi following meningitis. What would you expect regarding CSF pressure?
4. For many veterinary species, diagnostic sampling of CSF is often performed by placing a sampling needle in the:
5. Which two of the following are false regarding the blood-brain barrier (BBB)?