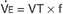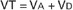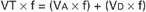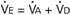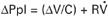Chapter 45 Overview of Respiratory Function: Ventilation of the Lung
1. The respiratory system’s primary function is the transport of oxygen and carbon dioxide between the environment and the tissues.
1. Ventilation is the movement of gas into and out of the lung.
2. Ventilation requires muscular energy.
3. The respiratory muscles generate work to stretch the lung and overcome the frictional resistance to airflow.
4. Lung elasticity results from tissue and surface tension forces.
5. The lung is mechanically connected to the thoracic cage by the pleural liquid.
6. Airflow is opposed by frictional resistance in the airways.
7. Smooth muscle contraction affects the diameters of the trachea, bronchi, and bronchioles.
8. Dynamic compression can narrow the airways and limit airflow.
9. The distribution of air depends on the local mechanical properties of the lung.
10. In some species, air travels between adjacent regions of lung through collateral pathways.
RESPIRATORY FUNCTION
The Respiratory System’s Primary Function Is the Transport of Oxygen and Carbon Dioxide Between the Environment and the Tissues
The respiratory system provides oxygen (O2) to support tissue metabolism and removes carbon dioxide (CO2). Oxygen consumption and carbon dioxide production vary with the metabolic rate, which is dependent on the animal’s level of activity. Basal metabolism, the metabolism of the resting animal, is a function of metabolic body weight (M0.75). Therefore, smaller species consume more oxygen per kilogram of body weight than do larger species. When animals exercise, their muscles need more oxygen, which leads to an increase in oxygen consumption. Oxygen consumption can increase up to a maximum known as 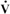 o2max. Although
o2max. Although 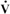 o2max generally increases with body size, there are some interesting deviations from this general relationship. Maximal oxygen consumption in the horse is threefold greater than that in a cow of similar body weight, and dogs have higher
o2max generally increases with body size, there are some interesting deviations from this general relationship. Maximal oxygen consumption in the horse is threefold greater than that in a cow of similar body weight, and dogs have higher 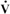 o2max than similarly sized goats. The more aerobic species, such as the dog and horse, have a higher
o2max than similarly sized goats. The more aerobic species, such as the dog and horse, have a higher 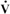 o2max per kilogram because their skeletal muscle mitochondrial density is greater than that of the less aerobic species.
o2max per kilogram because their skeletal muscle mitochondrial density is greater than that of the less aerobic species.
Gas exchange requirements vary with metabolism and may increase up to 30 times during strenuous exercise (Figure 45-1). Surprisingly, these variations are normally accomplished with only a small energy cost. In animals with respiratory disease, the energy cost of breathing can increase. This results in less energy available for exercise or weight gain, and the owner notices the animal’s poor performance. The respiratory system is also important in thermoregulation; metabolism of endogenous and exogenous substances; and protection of the animal against inhaled dusts, toxic gases, and infectious agents.
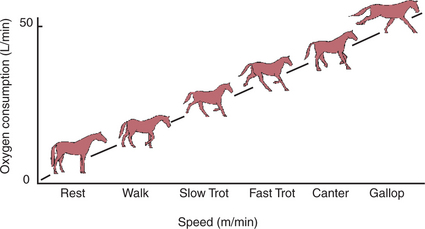
FIGURE 45-1 Effect of exercise on oxygen consumption in the horse. Oxygen consumption increases in a linear manner as the horse increases speed; the total increase is approximately 30-fold.
(Modified from Hornicke H, Meixner R, Pollman U: Equine exercise physiology, Cambridge, UK, 1983, Granta Editions.)
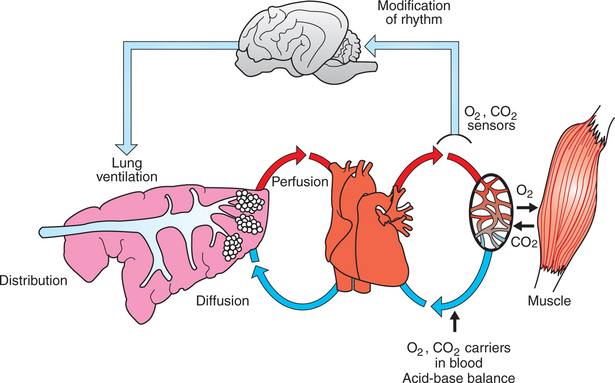
Figure 45-2 shows the processes involved in gas exchange, including ventilation; distribution of gas within the lung; diffusion at the alveolocapillary membrane; transport of O2 in the blood from the lungs to the tissue capillaries and of CO2 in the reverse direction; and diffusion of gases between blood and tissues.
VENTILATION
Ventilation Is the Movement of Gas into and out of the Lung
The oxygen needs of metabolism require that an animal take a certain volume of air into its lungs, especially its alveoli, each minute. The total volume of air breathed per minute, or minute ventilation ( E), is determined by the volume of each breath, known as the tidal volume (VT), and the number of breaths per minute, known as respiratory frequency (f), as clarified next from the following equation:
E), is determined by the volume of each breath, known as the tidal volume (VT), and the number of breaths per minute, known as respiratory frequency (f), as clarified next from the following equation:
The increase in  E, which must occur when an increase in metabolic rate demands more oxygen, can be brought about through an increase in VT, f, or both.
E, which must occur when an increase in metabolic rate demands more oxygen, can be brought about through an increase in VT, f, or both.
Air flows into the alveoli through the nares, nasal cavity, pharynx, larynx, trachea, bronchi, and bronchioles. These structures constitute the conducting airways. Because gas exchange does not occur in these pathways, they are also known as the anatomic dead-space (Figure 45-3). Dead-space can also occur within the alveoli. This alveolar dead-space is caused by alveoli that are poorly perfused with blood, so that gas exchange cannot occur optimally (see Chapter 47). Physiologic dead-space is the sum of the anatomic and the alveolar dead-space. Let us define the portion of each VT enters the alveoli as VA and the part that enters the dead-space as VD. Then:
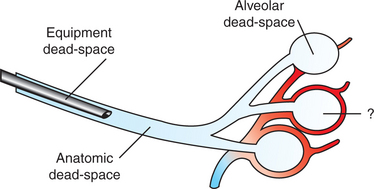
FIGURE 45-3 Types of dead-space. The volume of the trachea and bronchi constitutes the anatomic dead-space; equipment dead-space is created by an endotracheal tube; and alveolar dead-space is the volume of air ventilating poorly perfused alveoli. Top, An unperfused alveolus is shown; bottom, a normally perfused alveolus; middle, an alveolus with less-than-optimal perfusion for the amount of ventilation received.
If each side of this equation is multiplied by respiratory frequency (f) as follows:
Therefore, minute ventilation ( E) is the sum of alveolar ventilation (
E) is the sum of alveolar ventilation (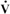 A), which is essential for gas exchange, and dead-space ventilation (
A), which is essential for gas exchange, and dead-space ventilation (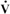 d), which is wasted ventilation.
d), which is wasted ventilation.
Alveolar ventilation is regulated by control mechanisms to match the O2 uptake and CO2 elimination necessitated by metabolism. Thus, when an animal exercises, its alveolar ventilation increases to take in more O2 and eliminate more CO2.
The fraction of each breath ventilating the dead-space is known as the dead-space/tidal volume ratio (VD/VT). The VD/VT varies considerably among species. In smaller species, such as dogs, it approximates 33%, whereas in some larger species, such as cattle and horses, it approximates 50% to 75%.
Because the volume of the anatomic dead-space is relatively constant, changes in VT, f, or both can alter the relative amounts of air that ventilate the alveoli and the dead-space. These changes in VT and f occur in animals during exercise and thermoregulation. For example, the small VT and high f characteristic of panting in dogs cause more air to ventilate the dead-space in order to cause water evaporation and heat loss. Cattle, pigs, and mules subjected to heat stress also increase respiratory rate and dead-space ventilation when trying to lose heat. In contrast to the effects of heat stress, cold-stressed animals have a higher metabolic rate, which is necessary to maintain body temperature in cold conditions. This leads to an increase in both O2 consumption and CO2 production, making it necessary for the animal to increase alveolar ventilation and decrease dead-space ventilation. Reducing the f and increasing VT accomplish the latter adaptations.
The veterinarian needs to ensure that equipment used for anesthesia or respiratory therapy does not increase the dead-space. Excessively long endotracheal tubes or overly large face masks create a large amount of equipment dead-space. The consequence of this is that the animal must take in a large VT to obtain adequate alveolar ventilation.
Ventilation Requires Muscular Energy
Energy provided by muscles causes air to enter the lungs during inhalation. During exhalation, much of the energy causing air to leave the lungs is provided by the elastic energy stored in the stretched lung and thorax. Therefore, in most animals at rest, inhalation is an active process, whereas exhalation is passive. Horses are an exception to this general rule because they have an active phase to exhalation even at rest. During exercise or in the presence of respiratory disease, exhalation often is assisted by muscle contraction in most species.
The primary inspiratory muscle is the diaphragm, which is a domed musculotendinous sheet separating the abdomen and thorax and innervated by the phrenic nerve. The diaphragm consists of a costal portion, arising from the xiphoid process and the costochondral junctions of the 8th to 12th ribs (8th to 14th ribs in Equidae), and a crural portion, arising from the ventral surface of the first three to four lumbar vertebrae and extending toward the tendinous center of the diaphragm. The apex of the dome of the diaphragm extends rostrally to the seventh or eighth intercostal space at the level of the base of the heart. During contraction of the diaphragm the dome is pulled caudally and thereby enlarges the thoracic cavity. The tendinous center pushes against the abdominal contents, elevating intraabdominal pressure, which displaces the abdominal wall and caudal ribs outward, thus also tending to enlarge the thorax. It is the enlargement of the thorax that creates the negative (subatmospheric) pressure necessary to make air flow into the lungs during inhalation.
The external intercostal muscles also are active during inhalation. The fibers of these muscles are directed caudoventrally, from the caudal border of one rib to the cranial border of the next, so that muscle contraction moves the ribs rostrally and outward. The relative contributions of diaphragmatic and costal movement to ventilation under different metabolic demands are not well defined in animals. Because the cranial ribs support the forelimbs in quadrupeds, they participate less in ventilation than do the more caudal ribs. Other inspiratory muscles include those connecting the sternum and head. These muscles contract during strenuous breathing and move the sternum rostrally.
The subatmospheric pressure generated within the respiratory tract during inhalation tends to collapse the external nares, pharynx, and larynx. Contraction of abductor muscles attached to these structures is essential for preventing collapse. Abductor muscle contraction during inhalation can be observed as dilation of the external nares. Laryngeal hemiplegia in horses is a condition in which the abductor muscles on the left side of the larynx fail to contract during inhalation. During exercise these horses exhibit a sound known as “roaring.” Roaring occurs as the result of the turbulent airflow that is generated on inhalation as the vocal fold on the paralyzed side is sucked into the lumen of the larynx by the subatmospheric pressure.
The principal expiratory muscles are the abdominal muscles and internal intercostal muscles. Contraction of the abdominal muscles increases abdominal pressure, which forces the relaxed diaphragm forward and reduces the size of the thorax. The fibers of the internal intercostal muscles are directed cranioventrally, from the cranial border of one rib to the caudal border of the next cranial rib, so that their contraction decreases the size of the thorax by moving the ribs caudally and ventrally. As the thorax becomes smaller, the thoracic pressure increases and forces air out of the lungs.
During exercise, respiratory muscle activity increases in order to generate the increase in 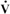 E. In cursorial (running) mammals, ventilation is synchronized with gait in the canter and gallop, but not in the trot (Figure 45-4). Inhalation occurs as the forelimbs are extended and the hind limbs are accelerating the animal forward. Exhalation occurs when the forelimbs are in contact with the ground. In the galloping horse and perhaps in other galloping quadrupeds, much of the increase in size of the thorax during inhalation is a consequence of elongation of the trunk rather than an increase in the diameter of the thorax.
E. In cursorial (running) mammals, ventilation is synchronized with gait in the canter and gallop, but not in the trot (Figure 45-4). Inhalation occurs as the forelimbs are extended and the hind limbs are accelerating the animal forward. Exhalation occurs when the forelimbs are in contact with the ground. In the galloping horse and perhaps in other galloping quadrupeds, much of the increase in size of the thorax during inhalation is a consequence of elongation of the trunk rather than an increase in the diameter of the thorax.
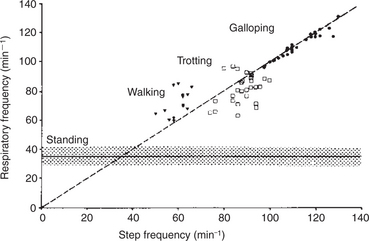
FIGURE 45-4 Relationship between gait and respiration in the horse. In the walk and trot, step and respiratory frequency are not correlated. At the gallop (and canter), respiratory and step frequency bear a 1:1 relationship.
(Modified from Hornicke H, Meixner R, Pollman U: Equine exercise physiology, Cambridge, UK, 1983, Granta Editions.)
The Respiratory Muscles Generate Work to Stretch the Lung and Overcome the Frictional Resistance to Airflow
At the end of a normal exhalation, some air (∼45 mL/kg) remains in the lung. This air volume is known as functional residual capacity (FRC). At FRC, the pressure in the pleural cavity (Ppl) that surrounds the lung is approximately 5 cm H2O below atmospheric pressure (–5 cm H2O). As previously mentioned, Ppl decreases during inhalation as the thorax enlarges and the respiratory muscles perform work to stretch the elastic lung and thorax and to generate airflow against the frictional resistance of the air passages (Figure 45-5). Lung compliance is a measure of the elastic properties of the lungs, and airway resistance is a measure of the frictional resistance of the airways. The magnitude of the change in pleural pressure (ΔPpl) during each breath is determined by the change in lung volume (ΔV), by lung compliance (C), by airflow rate (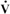 ), and by airway resistance (R), as follows:
), and by airway resistance (R), as follows:
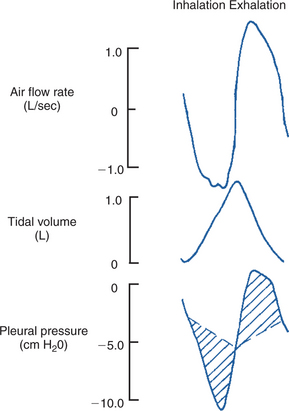
FIGURE 45-5 Airflow rate, tidal volume, and pleural pressure during inhalation (left) and exhalation (right). During inhalation, pleural pressure decreases as the thorax enlarges, air flow rates increase, and the volume of air in the lung increases. At the end of inhalation, flow rates return to zero, and pleural pressure becomes less negative. During exhalation, pleural pressure increases as the thorax decreases in size, flow rates increase to a peak and then decrease again, and the volume of air in the lung decreases. The broken line shows the change in pleural pressure necessary to overcome the frictional resistance of the airways. The peaks of pleural pressure on both inhalation and exhalation coincide with peaks of flow.
Resting animals breathe slowly and have low flow rates. In this situation the primary work of the respiratory muscles is against the compliance of the lung. When the respiratory rate increases (e.g., during exercise), flow rates increase, and more energy is used to generate flow against the frictional resistance of the airways.
Lung Elasticity Results from Tissue and Surface Tension Forces
At FRC, the slightly subatmospheric pressure in the pleural cavity keeps the lung inflated. If the thorax is opened and the lungs are exposed to atmospheric pressure, the lungs collapse to their minimal volume. At this volume, some air remains trapped within the alveoli behind closed bronchioles. This trapped gas causes collapsed normal lungs to float in water. The collapse of the lung when the thorax is opened is a result of the lung’s inherent elasticity, which is generated by both elastic and collagen tissue and by surface tension forces.
The importance of surface tension can be demonstrated experimentally by inflating the lung with air or saline while concurrently measuring the pressure required for inflation, also known as transpulmonary pressure (PL). Figure 45-6 represents the resulting lung pressure-volume curve and demonstrates the following important points:
1. A high pressure is required initially to inflate the lung with air from the gas-free state. This is because high pressure is required for the initial opening of the bronchioles. In life, the lung is gas free only in the fetus and for a few seconds after birth until the first breath is taken. Usually, tidal breathing is initiated from FRC. At this lung volume, the bronchioles are open.
2. The lung reaches its elastic limits at PL of approximately 30 cm H2O. The volume of air in the lung at this point is known as the total lung capacity.
3. The lung’s elastic properties differ during inflation and deflation; less pressure is necessary to maintain a given volume during deflation than during inflation. This phenomenon, known as pressure-volume hysteresis, is a result of changes in surface tension forces, the same forces that made it necessary for the high pressure initially to inflate the lung.
4. When saline is used instead of air to inflate the lung, less pressure is required for inflation, and the pressure-volume hysteresis is abolished. These two phenomena occur because when the lung is inflated with saline, the air-liquid interface of the fluid film overlying the alveolar epithelium is abolished. Because surface tension forces arise from this interface, they too are eliminated when the lung is inflated with saline. A comparison of the pressure-volume curves when the lung is inflated with air and with saline (see Figure 45-6) shows that surface forces are responsible for a considerable part of the elastic recoil of the air-filled lung.
FIGURE 45-6 Pressure-volume curve of the lung during inflation with saline and with air. The pressure gradient across the lung (transpulmonary pressure) is shown on the abscissa and lung volume on the ordinate. During air inflation the inflation and deflation curves are separated; that is, there is pressure-volume hysteresis. Hysteresis is abolished by inflating the lung with saline. The lung inflates more easily (i.e., it takes less pressure for a given volume) with saline than with air. Numbers correspond to numbers in the section Lung Elasticity Results from Tissue and Surface Tension Forces.
Surface tension forces continually try to collapse the alveoli. Alveolar stability is a consequence of the presence of a pulmonary surfactant that reduces the surface tension of the alveolar lining. Pulmonary surfactant is a mixture of lipids and proteins. The most plentiful lipid component, dipalmitoyl phosphatidylcholine, is responsible for the surface tension reduction. Surfactant is produced in type II alveolar cells, and its hydrophilic and hydrophobic portions cause it to seek the surface of the alveolar lining (Figure 45-7). As lung volume decreases and the alveolar surface area shrinks, surfactant molecules become concentrated on the alveolar surface, reducing surface tension and promoting alveolar stability.
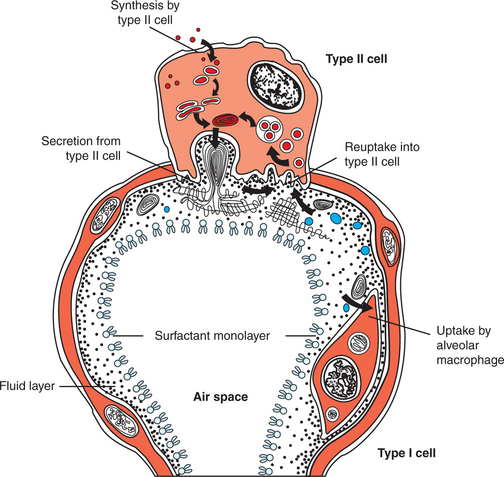
FIGURE 45-7 Diagram of an alveolus to show the movement of surfactant components through the type II cell and the alveolar liquid.
There are four important surfactant apoproteins. Two of these, surfactant proteins B and C, are hydrophobic and aid in surfactant secretion, maintenance of surface films, and reuptake of surfactant into type II cells. The other two apoproteins, surfactant proteins A and D, are hydrophilic and have antimicrobial properties.
Pulmonary surfactant is released into the alveolar spaces and tracheal fluid late in gestation (85% of the length of gestation in the sheep). Its appearance correlates with the rise in fetal plasma cortisol levels. Animals born prematurely have difficulty inflating their lungs because of inadequate surfactant. Synthetic surfactants can be used to treat premature newborns that lack adequate surfactant.
Lung compliance is the slope of the lung-pressure volume curve (see Figure 45-6). Because the pressure-volume curve is not linear, compliance obviously varies with the state of lung inflation. It is usually measured over the range of VT and, when adjusted for differences in lung size, does not vary greatly among adult mammals. Consequently, most mammals generate similar changes in pleural pressure during breathing. Anesthesiologists frequently refer to lung compliance when trying to artificially ventilate an animal. A “compliant lung” is easy to inflate. A “lung with low compliance,” as occurs in some diseases, is difficult to inflate.
The Lung Is Mechanically Connected to the Thoracic Cage by the Pleural Liquid
The lung is covered by the visceral pleura, and the thorax is lined by the parietal pleura. These two pleural surfaces are maintained in close apposition by a thin layer of pleural fluid. It is useful to liken the pleural fluid to a thin layer of water between two pieces of glass (the pleural surfaces). The liquid allows the pieces of glass to slide over one another but makes them difficult to separate. In the thorax, therefore, the pleural fluid mechanically links the lungs to the thorax so that the respiratory system behaves as a single unit. When the thorax expands during inhalation, for example, the lungs must expand as well. Similarly, when an animal exhales below FRC, the stiff thorax increasingly resists deformation, so that residual volume, the volume of air in the lung at the end of a maximal exhalation, is determined by the limits to which the rib cage can be compressed.
The thorax is generally stiffer—that is, it is less compliant—in large animals than small animals; the stiff chest wall of the horse and cow is in contrast to the very compliant chest wall of small rodents. Neonates need to have a compliant chest to pass through the birth canal. Lung collapse, also known as atelectasis, is more likely to occur in species with compliant thoraces because the thorax cannot adequately support the lung to prevent its collapse. This is one reason that atelectasis is more common in newborn animals than in adults.
Airflow Is Opposed by Frictional Resistance in the Airways
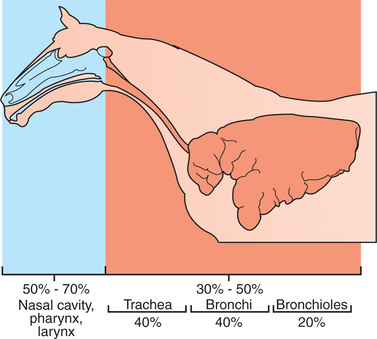
During breathing, air flows through the tubes of the upper airway (i.e., nose, pharynx, and larynx) and the tracheobronchial tree, which present frictional resistance to the movement of air. In the resting animal the nasal cavity, pharynx, and larynx, which warm and humidify the air, provide approximately 60% of the frictional resistance to breathing (Figure 45-8). Nasal resistance can be decreased (e.g., during exercise) by dilation of the external nares and by vasoconstriction of the extensive vascular tissue in the nose. Vasoconstriction reduces the volume of blood in the vascular sinuses within the nasal mucosa and, as a consequence, the mucosal thickness decreases and the space available for air within the nose increases. When airflow rates increase during exercise, or when the nasal cavity is obstructed, some species, such as the cow and dog, breathe through the mouth to bypass the high-resistance nasal cavity. Other species, such as the horse, are obligate nose breathers and are solely dependent on a decrease in nasal resistance to keep the work of breathing at a reasonable level. The horse accomplishes this by flaring its nostrils and by constricting blood vessels to shrink the nasal mucosa.
The tracheobronchial tree is a branching system that delivers air to the alveoli. The number of branches depends on the animal’s size. Humans have 24 branches, mice about 10, and horses 40 or more. The tracheobronchial tree is lined by a secretory, ciliated epithelium. The larger airways—trachea and bronchi—are supported by cartilage and supplied with bronchial glands and goblet cells, the secretions of which contribute to the mucous lining of the airways. The smaller airways, known as bronchioles, lack cartilage, glands, and goblet cells. With the exception of the trachea and the cranial part of the mainstem bronchi, the airways are intrapulmonary. Alveolar septa attach to the outer layers of the airways so that the tension within the septa pulls the airways open and helps to maintain their patency.
The lungs of most species have a total of six lobes, each supplied by a lobar bronchus, which gives rise to daughter bronchi. Even in species such as the horse that lack lobation, the same pattern of six lobar bronchi is still present. At each division of a parent bronchus, the diameters of the daughter airways are not equal. One daughter airway is much narrower than the parent, whereas the diameter of the other is similar to that of the parent. This monopodial system of branching continues through at least the first six generations of bronchi. At the level of the bronchioles, the diameters of parent and daughter bronchioles are the same. As a result of this branching pattern, the total cross-sectional area of the tracheobronchial tree through which air flows increases only slightly between the trachea and the first four generations of bronchi, but it doubles at each division of the peripheral airways. Because the total cross-sectional area increases dramatically toward the periphery of the lung, the velocity of airflow diminishes progressively from the trachea toward the bronchioles. The high-velocity turbulent airflow in the trachea and bronchi produces the lung sounds heard through a stethoscope in a normal animal. Laminar airflow (low-velocity flow) in the bronchioles produces no sound. Also as a result of the branching pattern of the tracheobronchial tree, airways larger than 2 to 5 mm in diameter contribute up to 80% of the frictional resistance to breathing in the tracheobronchial tree; bronchioles contribute as little as 20%.
Resistance to airflow is determined by the radius and length of the airways. Airway length changes minimally, but radius can be altered by several passive and active forces. As the lung inflates, airways dilate passively. This occurs because the alveolar septa are attached to the airways, and as the alveoli inflate, tension increases in their septa, causing the attached airways to dilate (Figure 45-9). Contraction of bronchial smooth muscle is the other major factor determining airway caliber.
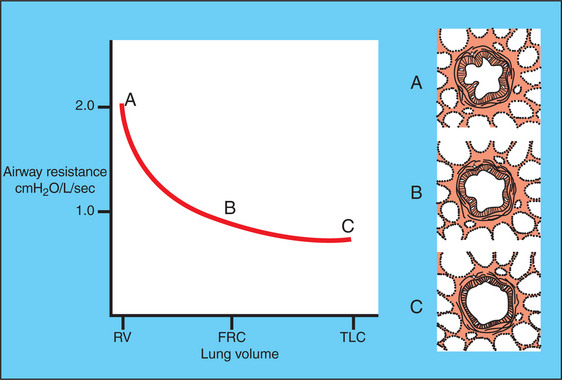
FIGURE 45-9 Effect of change in lung volume on airway resistance. The airway is represented in the diagrams on the right side of the figure by the large “circle”, to which are attached alveoli, the septa of which link the airway wall to the pleural surface. As lung volume increases, the alveolar septa become stretched and thus dilate the airway and reduce resistance. FRC, Functional residual capacity; RV, residual volume; TLC, total lung capacity.
Smooth Muscle Contraction Affects the Diameters of the Trachea, Bronchi, and Bronchioles
There is smooth muscle in the walls of the airways from the trachea to the alveolar ducts. In the trachea, it forms the trachealis muscle, which connects the ends of tracheal cartilages. In the bronchi and the bronchioles, smooth muscle encircles the airways. Smooth muscle actively regulates airway diameter in response to neural and other stimuli. The parasympathetic nervous system supplies airway smooth muscle through the vagus nerve (Figure 45-10). Activation of this system causes the release of acetylcholine, which binds to muscarinic receptors on airway smooth muscle, leading to muscle contraction. This narrows the trachea, bronchi, and bronchioles, a phenomenon known as bronchoconstriction. When irritating materials such as dusts are inhaled, tracheobronchial irritant receptors are stimulated, which in turn activates the parasympathetic system, resulting in reflexbronchoconstriction.
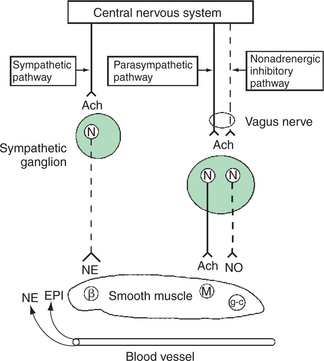
FIGURE 45-10 Diagrammatic representation of efferent autonomic innervation of tracheobronchial tree. Smooth muscle β2-adrenergic receptors (β) are activated by circulating catecholamines such as epinephrine (EPI) or, in a few species, by release of norepinephrine (NE) from sympathetic nerves. Muscarinic receptors (M) are activated by acetylcholine (Ach) released from postganglionic parasympathetic nerve terminals. The nonadrenergic inhibitory nervous system that travels in the vagus nerve releases nitric oxide (NO) that activates guanyl cyclase (g-c) in the smooth muscle. N, Neuron.
(From Nadel JA, Barnes PJ, Holtzman MJ: Autonomic factors in hyperreactivity of airway smooth muscle. In Fishman AP, Macklem PT, Mead J, et al, editors: Handbook of physiology, section 3, vol 3, part 2, Bethesda, Md, 1986, American Physiology Society.)
Airway smooth muscle also contracts in response to many of the inflammatory mediators, particularly histamine and the leukotrienes, that are released from mast cells during an allergic reaction. Some inflammatory mediators act directly on the smooth muscle; others act in a reflexive manner through the parasympathetic system. They are responsible for the airway obstruction that occurs in diseases such as heaves in horses and asthma in cats.
Relaxation of smooth muscle, and therefore dilation of the airways, occurs during activation of β2-adrenergic receptors by circulating catecholamines released from the adrenal medulla. Norepinephrine released from the sympathetic nervous system also causes airway dilation through β2-adrenergic receptors, but to a lesser extent. Another bronchodilator system, the nonadrenergic noncholinergic inhibitory nervous system, exists in some species. The efferent fibers are in the vagus nerve, and neurotransmission involves nitric oxide.
Dynamic Compression Can Narrow the Airways and Limit Airflow
The walls of the airway are not rigid, and therefore the airways can be compressed or expanded by the pressure gradient across their walls. Understanding when dynamic compression is most likely to occur in different parts of the airways can provide diagnostic clues to the location of an airway obstruction. In the nasal cavity, pharynx, and larynx, dynamic compression of the airway occurs during inhalation. These extrapulmonary airways are surrounded by atmospheric pressure, whereas the pressure within the airways is subatmospheric during inhalation. The resulting negative transmural pressure therefore tends to make the airways collapse. Because of its bony support, the nasal cavity is not prone to compression, but the less-well-supported nares, pharynx, and larynx are prone to compression. Normally, contraction of the abductor muscles of the nares, pharynx, and larynx during inhalation prevents collapse of these regions.
Laryngeal hemiplegia provides an excellent example of dynamic collapse of the extrapulmonary airway during inhalation. In this disease the intrinsic muscles on the left side of the larynx lose their nerve supply and undergo atrophy. As previously mentioned, when the abductor muscles of the larynx fail to contract during inhalation, the left vocal fold is sucked into the lumen of the airway, producing the inspiratory noise known as “roaring.” In addition, the vocal fold provides an obstruction to airflow that leads to poor performance when affected horses perform strenuous exercise. Dynamic collapse of the extrapulmonary airways does not occur during exhalation because the pressure within the airways is greater than atmospheric pressure, and the resulting positive transmural pressure keeps the airway open.
In the intrathoracic airways, dynamic collapse occurs during forced exhalation because intrapleural pressure exceeds pressures within the intrathoracic airway lumen. Cough is a forced exhalation during which dynamic collapse narrows the airways. The high air velocity through the narrowed portion of the airway facilitates removal of foreign material. Toy breeds of dogs have a high incidence of collapsing trachea. In this disease the weakened intrathoracic trachea is dynamically collapsed during the forceful ventilation of exercise. Affected dogs make a “honking” expiratory noise as air is forced past the collapsed intrathoracic portion of the trachea.
The Distribution of Air Depends on the Local Mechanical Properties of the Lung
Optimal gas exchange requires bringing together air and blood at the alveolus, that is, the matching of ventilation and blood flow. Obviously, gas exchange cannot occur if an alveolus receives blood but no ventilation, or vice versa. Ideally, each region of lung should receive approximately equal amounts of ventilation, but this never occurs in either animals or people. Distribution of ventilation is always uneven to some degree and becomes more so in disease. Uneven distribution of ventilation can be caused by local decreases in lung compliance (e.g., in pneumonia) or local airway obstructions (e.g., by mucus or bronchospasm) (Figure 45-11).
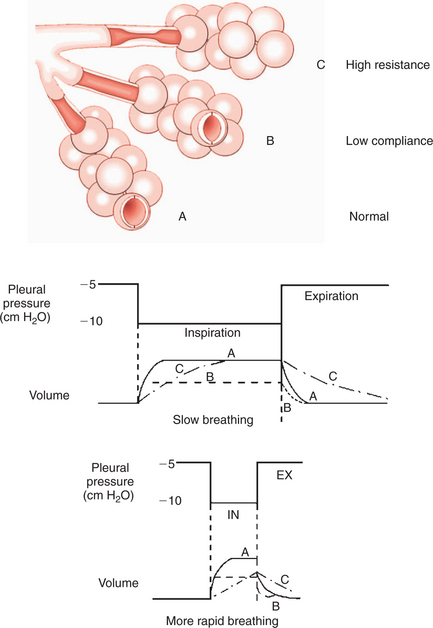
FIGURE 45-11 Effects of mechanical properties of the lung on airway resistance. Alveolus A is normal, alveolus B has low compliance, and the airway supplying alveolus C has high resistance as a result of a partial obstruction. Step changes in pleural pressure are applied to these three schematic alveoli, and the changes in volume are shown during slow breathing and during more rapid breathing. During rapid breathing, alveolus C does not have time to fill, so ventilation becomes more unevenly distributed.
The distribution of ventilation is very uneven in recumbent large animals, especially in the supine and laterally recumbent positions. This is because the lowermost regions of the lung are compressed to such an extent that they receive little or no ventilation. This can cause severe derangements of gas exchange in anesthetized horses.
In Some Species, Air Travels Between Adjacent Regions of Lung Through Collateral Pathways
The lungs of mammalian species differ in the degree to which they are subdivided by connective tissue into secondary lobules. In the lungs of pigs and cattle, there is complete separation of lobules, and in dogs and cats there is no separation. In horses and sheep there is partial separation. The connective tissue septa prevent collateral ventilation (i.e., movement of air between adjacent lobules) in cattle and pigs. Collateral ventilation is extensive in dogs and intermediate in horses. Collateral ventilation provides air to alveoli when their main parent bronchus is obstructed. The differences in collateral ventilation mean that gas exchange abnormalities that follow airway obstruction are more serious in pigs and cattle than in dogs.
CLINICAL CORRELATIONS
Lung Fibrosis in the Dog
History.
A 3-year-old English setter in respiratory distress is presented at a teaching hospital. The owner first noticed reluctance of the dog to exercise 3 weeks ago. Since that time, the animal has had progressive difficulty in breathing. It appears hungry but cannot eat because it “gets out of breath.”
Clinical Examination.
Inspection reveals a thin dog breathing through its mouth. The respiratory rate is elevated, but the dog seems to be moving little air despite strong inspiratory efforts during which the intercostal spaces sink. Exhalation presents no difficulty; the ribs collapse rapidly, and there is no accentuated abdominal effort.
Examination reveals slightly blue-colored mucous membranes. Lung sounds are not remarkable. All other systems are normal.
Radiographs of the thorax show diffuse miliary density (whiteness) over the parts of the lung that are normally air filled. The bronchi are normal. An elevated change in pleural pressure during breathing, normal airway resistance, and decreased static lung compliance are the key findings on lung function testing. Tidal volume (VT) is greatly reduced.
Comment.
The history and clinical signs indicate a respiratory problem. The elevated change in pleural pressure during breathing confirms the increased effort necessary to breathe. This could be caused by (1) increased air movement resulting from an increased metabolic rate, (2) airway obstruction, or (3) a decrease in lung compliance (stiffening of the lung). The increased density in the normally air-filled elastic part of the lungs, coupled with the normal air passages, suggests a decrease in lung compliance rather than airway obstruction. This was confirmed when measurements of lung function revealed a normal airway resistance and decreased lung compliance.
The retraction of intercostal spaces indicates that the stiff lung is resisting expansion. Exhalation is not a problem because the lung has an increased tendency to collapse, and the airways are normal.
This dog has a diffuse disease of the exchange area of the lung, which, by decreasing compliance, increases the work of breathing. The blue tinge to the mucous membranes indicates desaturation of hemoglobin as a result of impaired oxygen exchange in the diseased lung. A biopsy reveals diffuse fibrosis around mineral particles in the walls of the alveoli. The prognosis for the dog is poor.
Chronic Airway Disease in the Horse
History.
A 10-year-old horse is presented with a 2-year history of coughing and a progressive loss of exercise tolerance. Recently, the horse’s problem has become so severe that it has difficulty breathing while resting in its stall. The cough is frequent and is usually worse when the horse is kept inside. The horse has a normal appetite; however, it is losing weight even though the teeth are normal and it is on a good parasite control program.
Clinical Examination.
Inspection reveals a thin horse with flared nostrils and an anxious expression. The respiratory rate is elevated, and respiratory movements are accentuated. During inhalation the intercostal spaces are pulled in between the ribs. The initial part of exhalation is characterized by a rapid relaxation of the rib cage. This is followed by a prolonged contraction of the abdominal muscles, which is terminated immediately before the next inhalation. During the prolonged contraction of the abdominal muscles, wheezes can be heard when you place your ear close to the nostrils.
The horse has an elevated pulse rate. The mucous membranes of the gums have a bluish tinge. Auscultation of the thorax reveals increased breath sounds over all the lung fields and musical wheezes audible at end exhalation. Excessive mucus pooled in the airway is viewed through an endoscope advanced into the trachea.
Because the horse is being examined at a teaching hospital, there are facilities for measurement of lung function. The change in pleural pressure (ΔPpl) during each breath is 25 cm H2O (normal, 5 cm H2O), and airway resistance is 3 cm H2O/L/sec (normal, 1 cm H2O/L/sec). Administration of atropine intravenously decreases ΔPpl to 7 cm H2O and airway resistance to 1.5 cm H2O/L/sec. The horse looks less distressed, and wheezes are reduced after atropine therapy.
Comment.
The respiratory distress, cough, and lack of exercise tolerance indicate a respiratory problem. The increased effort of breathing documented by the elevated ΔPpl could be caused by airway obstruction, a decrease in lung compliance, or increased breathing resulting from increased metabolic rate. The latter cause is eliminated because the horse is resting in the clinic. The mucus in the airway and elevated airway resistance confirm airway obstruction. Musical wheezes at the end of exhalation typify airway disease and result from increased air turbulence or vibration of mucus and the airway walls. Airway obstruction is caused in part by bronchospasm resulting from parasympathetic activity, because it is reversed by atropine, a parasympathetic antagonist. Atropine does not return resistance to normal, so there is also considerable obstruction by mucus and swelling of the airway wall.
The flared nostrils are an effort to reduce the work of breathing by dilating the upper airway. The blue-tinged mucous membranes indicate desaturation of hemoglobin because of impaired oxygen uptake in the diseased lungs.
Retraction of the intercostal muscles during inhalation indicates a major decrease in pleural pressure as the respiratory muscles work to inflate the lung and pull air through the obstructed airways. The prolonged contraction of abdominal muscles, or heaving, represents an effort by the horse to force air out through obstructed airways. Weight loss is probably a result of the increased work of breathing. Coughing is an effort by the horse to expel the excessive mucus.
Treatment.
This horse has heaves (also known as recurrent airway obstruction [RAO]), a problem exacerbated by stabling in a dusty barn and eating poorly cured, moldy hay. Heaves is the result of an allergic response to particles, antigens, and endotoxin in the hay and barn dust. The best treatment for the horse is to keep it out at pasture and supplement its diet with pelleted feed rather than hay. In many cases, including this horse, additional treatments are needed when the horse endures a crisis. Treatment is aimed at dilating the airways (bronchodilator such as clenbuterol), oxygen therapy when warranted, and corticosteroids (inhalation or systemic) when severely affected. With good management control, some horses do not need constant treatment. In advanced stages and when treating some performance horses, however, treatment such as bronchodilators and inhaled steroids may be necessary.
Boron WF. Mechanics of respiration. Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach, updated edition, Philadelphia: Saunders, 2005.
Hlastala MP, Berger AJ. Physiology of respiration, ed 2. New York: Oxford University Press, 2001.
Leff AR, Schumacker PT. Respiratory physiology: basics and applications. Philadelphia: Saunders, 1993.
Leith DE. Comparative mammalian respiratory mechanics. Physiologist. 1976;19:485.
Lekeux P, Art T. The respiratory system: anatomy, physiology and adaptations to exercise and training. In: Hodgson DR, Rose RJ. The athletic horse. Philadelphia: Saunders, 1994.
Robinson NE. Some functional consequences of species differences in lung anatomy. Adv Vet Sci Comp Med. 1982;26:1.
West JB. Respiratory physiology: the essentials, ed 7. Baltimore: Lippincott Williams & Wilkins, 2005.
PRACTICE QUESTIONS
1. Which of the following lists includes only structures that compose the anatomic dead-space?
2. A horse has a tidal volume (VT) of 5 L, respiratory rate of 12 breaths/min, and VD/VT ratio of 0.5. Calculate minute ventilation (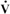 E) and alveolar ventilation (
E) and alveolar ventilation (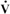 A).
A).
3. Which of the following occur during inhalation?
6. Which of the following increases the frictional resistance to breathing?
7. The distribution of ventilation within the lung is influenced by: