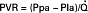Chapter 46 Pulmonary Blood Flow
1. The structure of the small pulmonary arteries varies among species.
2. Functionally, pulmonary blood vessels can be classified as alveolar and extra-alveolar vessels.
3. The pulmonary blood vessels offer a low resistance to flow.
4. The distribution of pulmonary blood flow within the lung is influenced by several factors.
5. Passive changes in vascular resistance result from changes in vascular transmural pressure.
6. Neural and humoral factors cause contraction of the muscular pulmonary arteries.
7. Alveolar hypoxia is a potent constrictor of small pulmonary arteries.
8. During exercise the pulmonary circulation must accommodate a large increase in blood flow.
The lung receives blood flow from two circulatory systems: the pulmonary circulation and the bronchial circulation. The pulmonary circulation receives the total output of the right ventricle, perfuses the alveolar capillaries, and participates in gas exchange. The bronchial circulation, a branch of the systemic circulation, provides a nutritional blood supply to airways and other structures within the lung.
PULMONARY CIRCULATION
The pulmonary circulation differs from the systemic circulation in that all the blood passes through only one organ: the lung. When cardiac output increases, as occurs during exercise, the pulmonary circulation must be able to accommodate this increase in blood flow without a large increase in the work of the right ventricle. In addition, control mechanisms must exist to regulate the distribution of blood within the lung so that blood preferentially perfuses the well-oxygenated regions of the lung. The ability to regulate blood flow depends on the presence of smooth muscle in the walls of small pulmonary arteries. The quantity of smooth muscle varies among species.
The Structure of the Pulmonary Arteries Varies Among Species
The main pulmonary arteries that accompany the bronchi are elastic, but the smaller arteries adjacent to the bronchioles and the alveolar ducts are muscular. The adult pig and the cow have a thick medial muscle layer in the smaller pulmonary arteries; the horse has less muscle; and the sheep and dog have only a thin muscle layer. The amount of smooth muscle in the media of pulmonary arteries determines the reactivity of the vasculature to alveolar hypoxia and other neural and humoral stimuli (see later discussion).
The small pulmonary arteries lead into pulmonary capillaries, which form an extensive branching network of vessels within the alveolar septum, almost covering the alveolar surface. Not all capillaries are perfused in the resting animal. As a result, vessels that are unperfused in the resting animal can be recruited when pulmonary blood flow increases (e.g., during exercise). Pulmonary veins with thin walls conduct blood from capillaries to the left atrium and also form a reservoir of blood for the left ventricle. The reservoir of blood in the pulmonary veins can be used to initiate a change in cardiac output (e.g., at the start of a sudden burst of exercise).
Functionally, Pulmonary Blood Vessels Can Be Classified as Alveolar and Extra-Alveolar Vessels
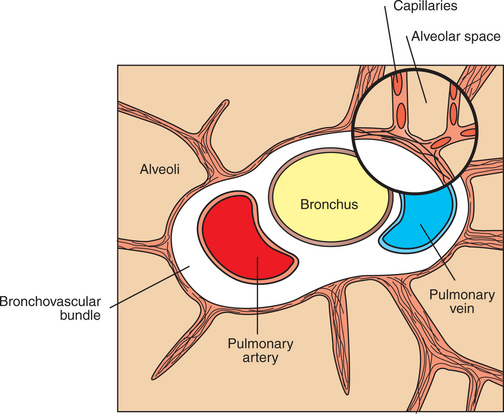
Alveolar vessels are the thin-walled capillaries that perfuse the alveolar septum (Figure 46-1). They are exposed almost directly to the pressure changes that occur in the alveoli during breathing. Extra-alveolar vessels include the pulmonary arteries, veins, arterioles, and venules. They generally occur together with bronchi in a loose connective tissue sheath called the bronchovascular bundle. This bundle is bounded by a limiting membrane to which alveolar septa are attached (Figure 46-2). The behavior of extra-alveolar vessels is determined by pressure changes within the connective tissue space of the bronchovascular bundle rather than by changes in alveolar pressure.
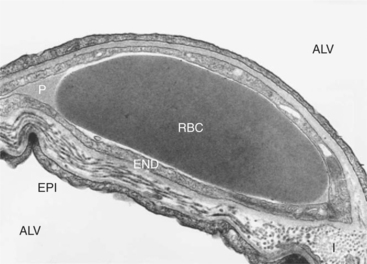
FIGURE 46-1 Transmission electron micrograph of capillary in alveolar septum of horse lung. A red blood cell (RBC) is shown bathed by plasma (P) in a capillary surrounded by endothelium (END). Alveoli (ALV) are on both sides of the septum and separated from the capillary by the epithelium (EPI) and a layer of interstitium (I). The interstitium is much thicker on one side of the capillary than on the other. Fluid exchange between the capillary and the interstitium occurs primarily on the thicker side.
(Courtesy WS Tyler, Department of Anatomy, University of California–Davis.)
The Pulmonary Blood Vessels Offer a Low Resistance to Flow
Pulmonary vascular pressures can be measured by advancing a catheter through the jugular vein into the right ventricle and pulmonary artery. Even though the pulmonary circulation receives the total output of the right ventricle, pulmonary arterial pressures are much less than systemic pressures. Pulmonary arterial systolic, diastolic, and mean pressures average 25, 10, and 15 mm Hg, respectively, in mammals at sea level. If the catheter is advanced until it becomes wedged in a pulmonary artery, the occluded vessel becomes an extension of the catheter, allowing estimation of pulmonary venous pressure, also known as pulmonary wedge pressure. Pulmonary wedge pressure (average, 5 mm Hg) is only slightly greater than left atrial pressure (average, 3-4 mm Hg). The small difference in pressure between the pulmonary artery and left atrium indicates that the pulmonary circulation offers little vascular resistance to blood flow. Pulmonary vascular resistance (PVR) is calculated as follows:
where Ppa is mean pulmonary arterial pressure, Pla is left atrial pressure, and 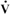 is cardiac output.
is cardiac output.
Although PVR is low in the normal resting animal, it decreases even further when pulmonary blood flow or pulmonary arterial pressure increases, as occurs during exercise. Recruitment of previously unperfused vessels and distention of all vessels cause the PVR decrease.
Micropuncture studies have shown that approximately half the vascular resistance in the pulmonary circulation is precapillary, and that the capillaries themselves provide a considerable portion of resistance to blood flow (Figure 46-3). Unlike the arterioles in the systemic circulation, the small arteries in the pulmonary circulation neither provide large resistance nor dampen the arterial pulsations; consequently, pulmonary capillary blood flow is pulsatile. The pulmonary veins provide little resistance to blood flow.
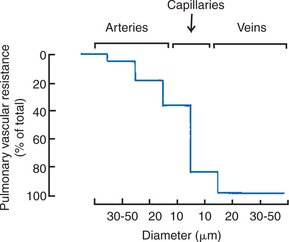
FIGURE 46-3 Distribution of vascular resistance in the pulmonary circulation, as determined by micropuncture studies. Unlike the resistance in the systemic circulation, a major portion of the resistance to blood flow in the pulmonary circulation is in the capillary bed.
(From Bhattacharya J, Staub NC: Direct measurement of microvascular pressures in the isolated perfused dog lung, Science 210:327, 1980. Copyright © 1980 by the American Association for the Advancement of Science.)
The Distribution of Pulmonary Blood Flow Within the Lung Is Influenced by Several Factors
The understanding of the distribution of blood flow within the lung was based for many years on experiments performed on isolated, perfused dog lungs suspended vertically to mimic the position of lungs in humans. Such experiments and measurements in humans indicated that there is a vertical gradient of perfusion, with blood flow per unit lung volume increasing from the top to the bottom of the lung. Elegant models that considered pulmonary arterial, pulmonary venous, and alveolar pressures were proposed to explain gravity-dependent distribution of blood flow.
Although this description of gravitational zones has traditionally provided a good theoretical basis for understanding the effects of pressures on blood flow, its applicability to quadrupeds is now in doubt. Gravity is probably not the major factor determining distribution of blood flow in quadrupeds, particularly during exercise. There appears to be gravity-independent, preferential distribution of blood flow to the dorsocaudal region of the lung of standing quadrupeds (Figure 46-4). This distribution is accentuated by exercise and may even persist in animals under anesthesia. It is most likely that the branching pattern of pulmonary arteries and arterioles and the relative resistances of each vessel are the major determinants of blood flow distribution.
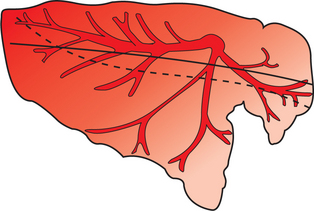
FIGURE 46-4 Graphic representation of the distribution of pulmonary blood flow in the horse’s lung. Relative blood flow is indicated by the intensity of the red shading. Blood flow distribution in a dorsal-caudal direction at rest and during exercise is shown by the solid and broken lines, respectively.
(Compiled from data in Hlastala MP, Bernard SL, Erikson HH, et al: Pulmonary blood flow distribution in standing horses is not dominated by gravity, J Appl Physiol 81:1051, 1996.)
Passive Changes in Vascular Resistance Result from Changes in Vascular Transmural Pressure
The diameter of blood vessels is a function of the pressure difference between the inside and the outside of the vessel, called the transmural pressure. When the pulmonary blood vessels contain an increased volume of blood, such as during exercise, pressure within the vessels increases. This leads to an increase in transmural pressure, which causes the vessels to dilate. Transmural pressure can also increase if the pressure surrounding the vessel decreases. This occurs in large pulmonary arteries and veins as the lung inflates. These vessels are contained in the bronchovascular bundle, which is enlarged by the traction of the surrounding alveolar septa during lung inflation (see Chapter 45). Consequently, pressure in the perivascular connective tissue of the bronchovascular bundle decreases. This leads to an increase in transmural pressure, and these extra-alveolar vessels therefore dilate.
The overall changes in PVR during lung inflation and deflation reflect opposing effects on alveolar and extra-alveolar vessels. When the lung deflates to residual volume, PVR is high because extra-alveolar vessels are narrowed. As the lung inflates to functional residual capacity, resistance decreases, primarily because of dilation of extra-alveolar vessels. Further inflation above functional residual capacity increases PVR, primarily because alveolar capillaries are flattened by the high tension in the stretched alveolar septa (Figure 46-5). Capillaries become progressively more elliptical and therefore offer more resistance to flow.
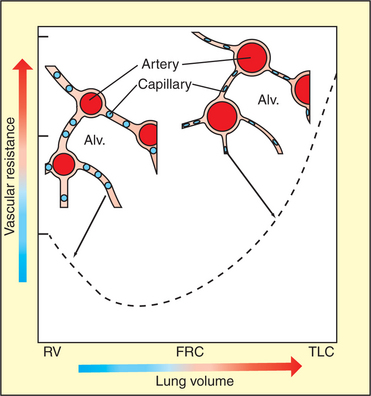
FIGURE 46-5 Change in vascular resistance that occurs with an increase in lung volume. The diagram shows alveolar capillaries (blue) and extra-alveolar vessels, in this case arteries (red). At residual volume (RV) the arteries are narrowed, but the capillaries are distended. At total lung capacity (TLC), the arteries are distended, but the capillaries are flattened because of the tension in the alveolar septum. Vascular resistance, which is the sum of the resistance provided by extra-alveolar vessels and capillaries, is minimal close to functional residual capacity (FRC). Alv., Alveolus.
Neural and Humoral Factors Cause Contraction of the Muscular Pulmonary Arteries
A variety of neural and humoral factors can contract or relax pulmonary vascular smooth muscle and thereby alter the resistance to blood flow. The magnitude of the response of vessels to these stimuli is largely determined by the amount of smooth muscle in the small pulmonary arteries, which varies with species (Figure 46-6). The increase in pulmonary vascular pressure in response to alveolar hypoxia and other stimuli is greater in calves than in sheep because of the greater amount of smooth muscle in calf pulmonary arteries.
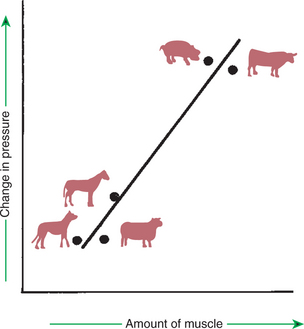
FIGURE 46-6 Relationship between the amount of muscle in the media of small pulmonary arteries and the change in pulmonary arterial pressure when animals are exposed to a hypoxic environment. Animals with thicker muscle layers, such as the cow and pig, have a greater vascular response to hypoxia than do animals with a small amount of muscle in the small pulmonary arteries, such as the dog and sheep. The horse has an intermediate response.
Although pulmonary arteries have both sympathetic and parasympathetic innervation, the functional role of this autonomic innervation is unclear. Table 46-1 lists the responses of the pulmonary vasculature to a variety of chemical mediators. Responses may vary among species and with the initial degree of vascular tone. Some mediators, such as acetylcholine and bradykinin, relax smooth muscle and cause vasodilation by releasing nitric oxide (NO) or vasodilator prostaglandins from the endothelium. Release of NO also occurs in response to the increased shear stress across the endothelium when blood flow increases. The increased NO release may be partly responsible for the dilation of the pulmonary circulation during exercise. Catecholamines, bradykinin, and prostaglandins are metabolized by the vascular endothelium, so their effects may be modified by endothelial damage.
Table 46-1 Pulmonary Vascular Response to Chemical Mediators
| Agent | Action |
|---|---|
| Angiotensin II | Vasoconstriction |
| Histamine (H1, H2) | Usually vasoconstriction through H1 receptors; if vascular tone elevated, vasodilation through H2 receptors; acts on both arteries and veins |
| Serotonin (5-HT2) | Vasoconstriction though 5-HT2 receptors; action restricted to pulmonary arteries |
| Norepinephrine, phenylephrine | Vasoconstriction through α1- and α2-adrenergic receptors |
| Epinephrine | Vasoconstriction or vasodilation, depending on resting vascular tone and predominance of α or β-adrenergic receptors. |
| Isoproterenol | Vasodilation through b receptors |
| Acetylcholine | Vasodilation through release of nitric oxide when endothelium intact. |
| Vasoconstriction when endothelium removed. | |
| Bradykinin | Variable; usually vasodilation; acts through release of vasoactive prostaglandins and nitric oxide |
| Arachidonic acid | Usually vasoconstriction |
| Prostacyclin* | Vasodilation |
| Thromboxane | Vasoconstriction |
| Leukotrienes | Usually vasoconstriction |
Alveolar Hypoxia Is a Potent Constrictor of Small Pulmonary Arteries
The air in poorly ventilated alveoli has a low partial pressure of oxygen, and it is of limited benefit to the animal to keep sending blood to such alveoli. To correct this problem, alveolar hypoxia results in vasoconstriction of pulmonary arteries. This hypoxic vasoconstriction reduces blood flow to poorly ventilated alveoli and redistributes pulmonary blood flow toward better-ventilated regions of lung. Although the vasoconstrictor response to hypoxia is present in all species, the magnitude of the response varies greatly. Among domestic mammals, the response is most vigorous in cattle and pigs, less vigorous in horses, and trivial in sheep and dogs (see Figure 46-6). The response to hypoxia is also minimal in the llama, which normally lives under hypoxic conditions at high altitude.
The ability of local alveolar hypoxia to cause a local reduction in blood flow has been clearly demonstrated in several species. Under conditions of atelectasis, when there is no ventilation to the collapsed region of lung, local blood flow is greatly reduced by a combination of vessel closure as the lung collapses and vasoconstriction in response to the local hypoxia.
Hypoxic vasoconstriction is beneficial when there is localized alveolar hypoxia, but when hypoxia is generalized, as occurs when animals live at high altitude or have diffuse lung disease, the vasoconstriction can have serious consequences. In cattle grazing at high altitude, the hypoxia of altitude causes vigorous, generalized pulmonary hypoxic vasoconstriction (Figure 46-7). This leads to an increase in pulmonary arterial pressure, which increases the work of the right ventricle and leads to right-sided heart failure. The clinical syndrome is known as brisket disease because edematous fluid accumulates in the brisket. There are genetically determined differences in the response to hypoxia; Holsteins respond most vigorously and are therefore highly susceptible to brisket disease. In these species, in which the acute hypoxic constrictor response is most vigorous, chronic hypoxia results in sustained pulmonary hypertension. This is caused by an increase in the quantity of smooth muscle in the media of the small pulmonary arteries. When animals have generalized hypoxic vasoconstriction as a result of lung disease, the resultant right-sided heart failure is known as cor pulmonale.
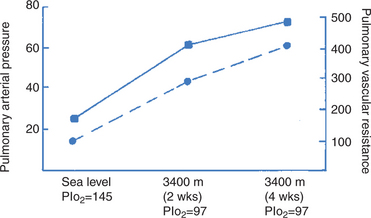
FIGURE 46-7 Change in mean pulmonary arterial pressure (squares, solid line) and pulmonary vascular resistance (circles, broken line) in calves transported from sea level to 3400 m for a 4-week sojourn. Both vascular resistance and arterial pressure increase when the calves are exposed to the hypoxia of altitude. Pressure and resistance continue to increase while at this altitude because of the proliferation of smooth muscle in the small pulmonary arteries. Pressure units are in millimeters of mercury (mm Hg); resistance units in dyne-sec/cm5; inspired oxygen tension (PIo2) units in mm Hg.
(From data in Ruiz AV, Bisgard GE, Will JA: Hemodynamic response to hypoxia and hyperoxia in calves and at sea level and altitude, Pflugers Arch 344:275, 1973.)
Hypoxic vasoconstriction can be demonstrated in isolated, perfused lungs and therefore does not require intact innervation. In the smooth muscle of pulmonary arteries, hypoxia closes voltage-gated potassium channels. This increases positivity within the cell and promotes depolarization. The depolarization leads to an influx of calcium, which causes smooth muscle contraction. Many vasoactive agents, such as histamine, catecholamines, angiotensin, and arachidonic acid metabolites, were previously suggested as being involved in hypoxic vasoconstriction. Their role appears to be modulation of the degree of constriction.
During Exercise the Pulmonary Circulation Must Accommodate a Large Increase in Blood Flow
To transport the extra oxygen required for muscular effort, cardiac output increases sixfold to eightfold during strenuous exercise. This increase in blood flow must pass through the pulmonary circulation, where it collects oxygen. To accommodate the increase in blood flow during exercise, the pulmonary blood vessels dilate; that is, PVR decreases. This dilation is in part passive as a result of the increase in intravascular pressure, which is a result of the increased blood flow. In addition, flow-induced release of NO from the endothelium also causes relaxation of smooth muscle and vessel dilation.
In most species, pulmonary arterial pressure during exercise is about 35 mm Hg, but in the horse it increases to more than 90 mm Hg. The latter increase is attributable in large part to a very high left atrial pressure (50 mm Hg), which is probably necessary for rapid left ventricular filling when the heart rate exceeds 200 beats/min. These high exercise-associated intravascular pressures cause leakage of erythrocytes from the pulmonary capillaries when horses exercise strenuously, a phenomenon known as exercise-induced pulmonary hemorrhage.
BRONCHIAL CIRCULATION
The Bronchial Circulation Provides a Blood Supply to Airways, Large Vessels, and in Some Species the Visceral Pleura
The bronchial circulation, which receives approximately 2% of the output of the left ventricle, originates from two sources: the bronchoesophageal artery and a branch of the bicarotid trunk, the right apical bronchial artery. The former supplies the airways and the interlobular septa of most of the lung; the latter supplies the airways of the right apical lobe. Bronchial arteries follow the tracheobronchial tree to the terminal bronchioles and form a peribronchial plexus in the connective tissue along the length of the airways. Branches from this plexus penetrate the smooth muscle layer of the bronchial wall and form a subepithelial vascular plexus that serves to warm inhaled air. Branches are also given off to form the vasa vasorum (nutrient blood vessels) of pulmonary vessels. At the level of the terminal bronchiole, bronchial vessels anastomose with the pulmonary circulation. There are few anastomoses between bronchial and pulmonary arteries; most anastomoses are present at the capillary or venular level.
The extensiveness of the bronchial blood supply to the pleura varies among species. In cattle, sheep, pigs, and horses, the bronchial artery provides blood flow to the visceral pleura; in dogs, cats, and monkeys, it does not. The bronchial blood flow to the large extrapulmonary airways drains into the azygos vein; venous drainage of the intrapulmonary bronchial circulation enters the pulmonary circulation.
Although the bronchial circulation provides nutrient blood flow to many lung structures, the lung does not die if the bronchial circulation is obstructed. The extensive anastomoses between bronchial and pulmonary vessels provide pulmonary blood flow to bronchial vessels. Similarly, when the pulmonary circulation is obstructed, the bronchial circulation proliferates and maintains blood flow to the lung. The bronchial circulation also proliferates when the airways are inflamed.
Inflow pressure to the bronchial circulation is systemic arterial pressure, but outflow pressure varies, depending on whether venous drainage is through the azygos vein or pulmonary circulation. Changes in pressure in both the systemic and the pulmonary vascular bed affect the magnitude of bronchial blood flow. Increasing systemic pressure increases flow, but increasing pulmonary vascular pressures (downstream pressure) reduces and may even reverse flow. Under hypoxic conditions, bronchial arteries dilate; in contrast, pulmonary arteries constrict under these conditions.
CLINICAL CORRELATIONS
Brisket Disease in a Heifer
History.
A 2-year-old Hereford heifer was kept during the winter on a farm in the foothills of the Rocky Mountains outside Denver. In the late spring the heifer was transported to Climax, Colorado (altitude, 3400 m), for summer grazing. After 6 weeks the owners noticed that the animal was having some difficulty breathing, was reluctant to move around the pasture, and had developed an enlarged pendulous brisket and some swelling between the jaws.
Clinical Examination.
Inspection of the heifer reveals a lethargic animal in poor condition. The respiratory and heart rates are elevated, and air seems to be moving well through the nostrils. The most noticeable observation is an enlarged and pendulous brisket. The swelling extends up the neck, and there is also a pendulous area between the jaws. The jugular veins are distended.
Palpation of the swollen brisket reveals that it is heavy; when it is squeezed, the imprints of the fingers remain for some time. The swelling between the mandibles behaves in a similar manner when palpated. The mucous membranes of the heifer are a normal color, and the lung sounds are unremarkable.
Comment.
The swelling in the brisket and between the mandibles, which pits on palpation, is evidence of accumulation of interstitial edema in the dependent areas of the heifer, in which there is loose connective tissue. Accumulation of edema in these regions is an indication of the increase in systemic venous pressure, which is also causing jugular distention. Both are caused by right-sided heart failure.
The most likely cause of right-sided heart failure in a heifer grazing at high altitude is diffuse vasoconstriction of the pulmonary circulation as a result of chronic hypoxia (inspired oxygen tension at 3400-m elevation is 97 mm Hg, compared with 150 mm Hg at sea level). The smooth muscle in the pulmonary arteries contracts in response to hypoxia; if this response is maintained for several weeks, the amount of smooth muscle in the pulmonary arteries increases. Furthermore, the animal produces extra erythrocytes in an attempt to transport more oxygen. These extra erythrocytes increase the hematocrit and make the blood more viscous and difficult to pump through the lung. Maintenance of cardiac output in the presence of the elevated pulmonary vascular resistance and increased blood viscosity requires an increase in pulmonary arterial and right ventricular pressure. Persistently working against the increased pressure causes right-sided heart failure.
Treatment.
If this animal is returned to lowland pasture, it will recover. The vasospasm in the pulmonary circulation and the hematocrit diminish once the hypoxic stimulus is removed. For immediate treatment, this animal should be moved to an area of lower altitude and could be given oxygen to relieve the hypoxic stimulus. This would cause a reduction in pulmonary arterial pressure, which would cause some relief. However, pulmonary arterial pressure would not decrease to normal levels, because of the increased amount of smooth muscle now present in the pulmonary arteries. In early disease, this process may be reversible. Once cardiac signs develop, however, the prognosis is guarded. Additional treatments include digoxin and diuretics, if desired.
Boron WF. Ventilation and perfusion of the lungs. In: Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach, updated edition. Philadelphia: Saunders, 2005.
Deffebach ME, Charan NB, Lakshminaryan S, et al. State of art. The bronchial circulation: small, but a vital attribute of the lung. Am Rev Respir Dis. 1987;135:463.
Hlastala MP, Berger AJ. Physiology of respiration, ed 2. New York: Oxford University Press, 2001.
Leff AR, Schumacker PT. Respiratory physiology: basics and applications. Philadelphia: Saunders, 1993.
Lekeux P, Art T. The respiratory system: anatomy, physiology and adaptations to exercise and training. In: Hodgson DR, Rose RJ. The athletic horse. Philadelphia: Saunders, 1994.
Robinson NE. Some functional consequences of species differences in lung anatomy. Adv Vet Sci Comp Med. 1982;26:1.
West JB. Respiratory physiology: the essentials, ed 7. Baltimore: Lippincott Williams & Wilkins, 2005.
PRACTICE QUESTIONS
1. Which of the following statements accurately describes the pulmonary circulation?
2. During exercise, cardiac output can increase fivefold, but pulmonary arterial pressure may not even double. This occurs because:
3. Which of the following will cause the greatest increase in pulmonary arterial pressure?