Chapter 51 Fetal and Neonatal Oxygen Transport
1. The fetus depends on the placenta for exchange of gas, nutrients, and metabolic byproducts.
2. The efficiency of gas exchange at the placenta depends on the species-variable arrangement of fetal and maternal blood vessels.
3. The fetal circulation mixes oxygenated and deoxygenated blood at several points, so the fetus exists in a state of hypoxemia.
4. Fetal oxygen transport is assisted by fetal hemoglobin, which has a high affinity for oxygen.
5. The lung develops in three stages, and pulmonary surfactant must be present at birth.
6. At or shortly after birth, umbilical vessels rupture, pulmonary vascular resistance decreases, and the foramen ovale and ductus arteriosus close.
The Fetus Depends on the Placenta for Exchange of Gas, Nutrients, and Metabolic Byproducts
From conception until birth, the embryo and fetus depend on the mother for a supply of oxygen and nutrients and for removal of carbon dioxide and other metabolic byproducts. The embryo exchanges these substances by diffusion through the uterine fluids. As the conceptus increases in size, the specialized exchange organ, known as the placenta, becomes essential. The placenta brings maternal and fetal blood into close apposition over a large surface area that is provided by a network of capillaries.
The gross appearance of the placenta of different species varies widely. In horses and pigs the placenta is diffuse and covers most of the uterine epithelium. In ruminants the placenta has rows of discrete circular-to-oval cotyledons that are attached to approximately 100 highly vascularized caruncles in the uterine epithelium. In dogs the placenta is zonary, forming a circular band around the allantochorion of the puppy. Table 51-1 lists types of placentation for different species.
Table 51-1 Placentation in Domestic Mammals
| Classification | ||
|---|---|---|
| Species | Gross | Histological |
| Horse | Diffuse | Epitheliochorial |
| Pig | Diffuse | Epitheliochorial |
| Cow | Cotyledonary | Epitheliochorial |
| Sheep | Cotyledonary | Epitheliochorial |
| Goat | Cotyledonary | Epitheliochorial |
| Dog | Zonary | Endotheliochorial |
| Cat | Zonary | Endotheliochorial |
| Rabbit | Discoid | Hemochorial |
| Guinea pig | Discoid | Hemochorial |
In addition to differing in the amount of uterine surface to which they are attached, placentas also differ in the number of layers of cells that separate the maternal and fetal blood (see Table 51-1). In horses, pigs, sheep, and cows the fetal chorion is applied to the maternal uterine epithelium (epitheliochorial placentation), whereas in cats and dogs the chorion is applied to the endothelium of maternal vessels (endotheliochorial placentation); in rodents and most primates the chorion invades the uterine mucosa and erodes the maternal capillaries, so it becomes bathed by maternal blood (hemochorial placentation).
The Efficiency of Gas Exchange at the Placenta Depends on the Species-Variable Arrangement of Fetal and Maternal Blood Vessels
The exchange of gases and other substances across the placenta is determined by several factors, including the amount of surface apposition between fetal and maternal tissues and the number of layers of cells separating fetal and maternal blood. However, a major factor determining exchange is the arrangement of fetal and maternal blood vessels within the small, interdigitating villi of the placenta (Figure 51-1). Countercurrent flow of maternal and fetal blood provides the most efficient exchange and allows equilibration of fetal and maternal arterial gas tensions. Concurrent flow of fetal and maternal blood allows fetal vessels to equilibrate with the maternal venous gas tensions. In crosscurrent and pool types of equilibrators, fetal capillaries loop down to maternal vessels or into a pool of maternal blood. No simple model easily describes these types of exchangers. It is likely that several different arrangements of vessels are found in the placentas of all species, but some seem to have more of the characteristics of countercurrent exchangers, and others have those of venous equilibrators.
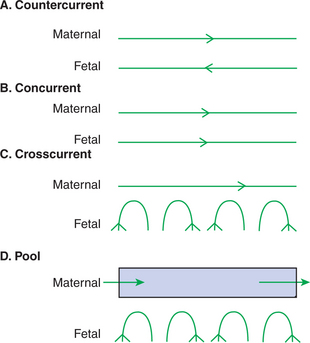
FIGURE 51-1 Schematic representation of possible arrangements of fetal and maternal blood vessels.
(From Dawes GS: Foetal and neonatal physiology, Chicago, 1968, Year Book Medical.)
Figure 51-2 shows the arrangement of vessels in the microcotyledon of the horse, a species in which fetal and maternal blood flow is primarily countercurrent. The cotyledonary placenta of sheep functions as a venous equilibrator, whereas the hemochorial placenta of the rabbit seems to be a countercurrent exchanger.
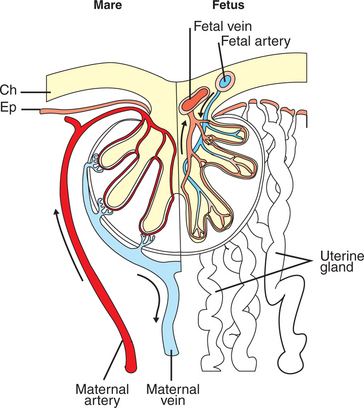
FIGURE 51-2 Diagram showing the arrangement of maternal and fetal blood vessels in the microcotyledons of the equine placenta. Arrows demonstrate the postulated countercurrent directions of maternal and fetal blood flow. Ch, Chorioallantois; Ep, uterine epithelium.
(Based on data in Tsutsumi T: J Agriculture Hokkaide Imperial Univ 52:372, 1962; from Comline KS, Cross GS, Dawes GS, et al, editors: Foetal and neonatal physiology: proceedings of the Sir Joseph Barcroft Centenary Symposium, Cambridge, UK, 1973, Cambridge University Press.)
Placental gas exchange has been best studied in the sheep (Figure 51-3). Maternal blood enters the uterus through the uterine artery with an oxygen tension (Po2) of 80 mm Hg and leaves through the uterine vein with a Po2 of 50 mm Hg. Some of the blood entering the uterus supplies the myometrium and endometrium, but most participates in gas exchange in the cotyledon. Fetal arterial blood reaches the placenta through the umbilical artery and enters the cotyledon with a Po2 of 24 mm Hg. Placental gas exchange occurs, and the blood leaving the placenta in the umbilical veins has a Po2 of only 32 mm Hg. This is because the sheep placenta is a venous equilibrator, so the maximal possible Po2 would be 50 mm Hg. However, this maximum is not reached because venous blood, which has provided nutrient blood flow to the chorion, dilutes the better-oxygenated blood draining from the cotyledon. The countercurrent exchanger of the horse is apparently more efficient because umbilical venous Po2 averages 48 mm Hg.
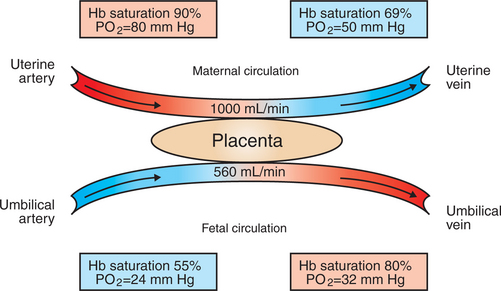
FIGURE 51-3 Placental blood flow, oxygen tension (Po2), and hemoglobin (Hb) saturation in the uterine and umbilical circulation of the sheep.
(From Battaglia FC, Meschia G: An introduction to fetal physiology, Orlando, Fla, 1986, Academic Press.)
The amount of placenta available for exchange partly determines the ultimate size of the fetus. If uterine caruncles are surgically removed from sheep so that there are fewer sites for formation of fetal cotyledons, the full-term weight of lambs is reduced. The diffuse placenta of the horse apparently can support only one full-size fetus. One foal in a set of twins usually dies in utero or is very small. It is rare for horse twins to survive to term and be of equal size.
The Fetal Circulation Mixes Oxygenated and Deoxygenated Blood at Several Points, So the Fetus Exists in a State of Hypoxemia
In the adult the cardiac output of the right and left ventricles is separate and perfuses the pulmonary and systemic circulations, respectively. In the fetus the output of the two sides of the heart mixes at several points, so it is convenient to use the term cardiac output to refer to the combined output of the right and left ventricles. The combined cardiac output averages 500 mL/min/kg in fetal sheep; the output of the right ventricle exceeds that of the left (Figure 51-4).
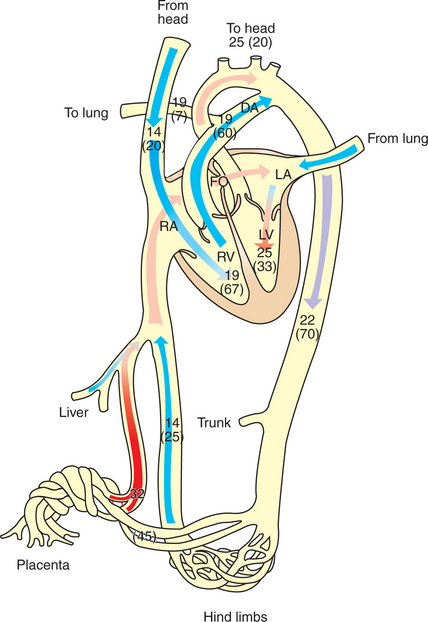
FIGURE 51-4 Diagrammatic representation of the fetal circulation showing oxygen tension (Po2) in millimeters of mercury (mm Hg) and percentage of cardiac output (in parentheses) in different parts of the circulation. DA, Ductus arteriosus; FO, foramen ovale; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The placenta, which has a low vascular resistance, receives 45% of the cardiac output through the umbilical arteries. The umbilical veins drain the placenta toward the liver. In species such as the sheep, most of the umbilical venous blood passes through the liver through a low-resistance channel known as the ductus venosus; in other species, such as the pig and horse, the ductus venosus disappears early in gestation, and umbilical venous blood flows through the liver capillaries. Within the liver, the oxygenated blood from the placenta is mixed with a small amount of more poorly oxygenated blood draining the liver sinusoids. The hepatic venous blood enters the posterior vena cava, where it mixes with poorly oxygenated blood, draining the hind end of the fetus, so the blood returning to the right atrium has a Po2 of 25 mm Hg.
A low-resistance pathway, the foramen ovale, connects the right and left atria, and a structure known as the crista dividens directs the better-oxygenated blood from the posterior vena cava through the foramen ovale to the left atrium. The poorly oxygenated blood returning to the right atrium in the cranial vena cava is directed into the right atrium and right ventricle. Most of the output of the right ventricle does not go through the lungs, however, because fetal lungs have a high vascular resistance. Another low-resistance channel, the ductus arteriosus, connects the pulmonary artery with the aorta and allows blood to bypass the lungs. It is important to note that the arrangement of the fetal circulation allows the better-oxygenated blood to enter the left ventricle, from which it reaches the brachycephalic vessels and the front of the animal. The more poorly oxygenated blood from the ductus arteriosus enters the aorta downstream from the brachycephalic vessels. The tissues of the hind end of the animal and the placenta receive blood with a Po2 of approximately 22 mm Hg.
Flow of blood from the right atrium to the left atrium through the foramen ovale and from the pulmonary artery to the aorta through the ductus arteriosus requires that the pressure in the right side of the fetal circulation be greater than that in the left side. This pressure difference occurs because the left side of the circulation provides most of its output to the low-resistance placenta, whereas the right side of the fetal circulation is opposed by the high-resistance pulmonary circulation. At term, systemic arterial pressure in the lamb is about 42 mm Hg.
The fetal circulation is not a passive system and is capable of considerable regulation, particularly as the fetus matures. Fetal hypoxia can stimulate vasodilation in the heart and brain and vasoconstriction in the gut, kidneys, and skeletal tissues. The fetal pulmonary circulation constricts vigorously when the fetus is hypoxic. This constriction diverts more blood through the ductus arteriosus to the systemic tissues.
Fetal Oxygen Transport Is Assisted by Fetal Hemoglobin, Which Has a High Affinity for Oxygen
Fetal arterial blood has a low Po2 because the placenta is not a highly efficient gas exchanger and because oxygenated blood and venous blood mix at several points in the fetal circulation. The fetus is adapted to this state of chronic hypoxia in two ways. First, it has a high cardiac output that delivers a large volume of blood per minute to the tissues. Second, the fetus produces erythrocytes containing hemoglobin with a high affinity for oxygen.
The production of erythrocytes initially occurs in the yolk sac and in other tissues such as endothelium. These embryonic erythrocytes are nucleated and contain embryonic hemoglobin, the oxygen affinity of which has not been clearly defined. At the termination of the embryonic period, erythrocyte production shifts to the liver and spleen. Depending on the species, fetal erythrocytes contain either fetal or adult hemoglobin (see later discussion). Simultaneously, there are changes in glycolytic enzymes to provide the fetal concentrations of 2,3-diphosphoglycerate (2,3-DPG). Fetal erythrocytes have a higher affinity for oxygen (lower partial pressure [tension] at which hemoglobin is 50% saturated with oxygen [P50]) than do maternal erythrocytes; that is, the fetal blood oxyhemoglobin dissociation curve lies to the left of the adult curve (Figure 51-5). In some species, such as the cat, the difference in the P50 between fetus and adult is small, whereas in ruminants the difference is 10 to 20 mm Hg.
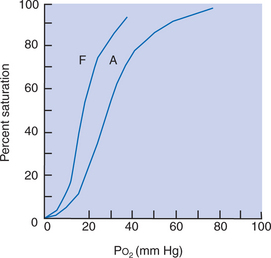
FIGURE 51-5 Oxyhemoglobin dissociation curves of fetal (F) and adult (A) sheep. Po2, Oxygen tension.
Three mechanisms account for the position of the fetal oxyhemoglobin dissociation curve. In ruminants, the higher oxygen affinity results from the synthesis of fetal hemoglobin with a high intrinsic oxygen affinity. Fetal hemoglobin of these species is unresponsive to 2,3-DPG. After birth there is gradual replacement of fetal hemoglobin by adult hemoglobin. In primates, there is little intrinsic difference in the oxygen affinity of fetal and maternal hemoglobin, but fetal hemoglobin has a decreased interaction with 2,3-DPG. In horses and pigs there is no fetal hemoglobin; embryonic hemoglobin is replaced immediately by adult hemoglobin. The fetal erythrocytes of these species have a low concentration of 2,3-DPG. After birth there is an increase in the concentration of 2,3-DPG, which gives the hemoglobin its adult dissociation curve.
The high affinity of fetal hemoglobin for oxygen allows the hemoglobin in the umbilical veins, with a Po2 of 30 mm Hg, to be 80% saturated with oxygen and allows the hemoglobin in the aorta, with a Po2 of 22 mm Hg, to be 56% saturated. The high affinity of fetal hemoglobin for oxygen not only allows oxygen transport at the low Po2 in fetal arteries, but also makes it necessary for fetal tissues to have an extremely low Po2. The low tissue Po2 provides an oxygen concentration gradient to unload oxygen from the fetal hemoglobin. Therefore the fetus exists in a state of tissue hypoxia compared with the adult.
The Lung Develops in Three Stages, and Pulmonary Surfactant Must Be Present at Birth
By the time of birth, the lung must be ready to assume the gas-exchange functions of the placenta. The lung develops in three stages of equivalent duration. Beginning as an outgrowth of the foregut, the lung bud invades the mesenchyme of the thorax and divides into all the major airway branches during the first third of gestation. Because these primordial airways are lined with a cuboidal epithelium and look like a gland in cross section, this stage of development is known as the pseudoglandular stage. In the second phase of development, the bronchioles develop, and the lung is invaded by blood vessels (the canalicular stage). In the final stage, or alveolar sac stage, alveolar sacs, and in some species alveoli, develop. The stage of maturity of the lung at birth in general matches the maturity of the fetus. Lambs and piglets have well-developed alveoli, but humans and especially rodents have thicker-walled alveolar sacs. In these latter species, alveoli develop as the animal grows postnatally.
Pulmonary surfactant is essential if the lung is to remain inflated after birth (see Chapter 45). Beginning at about midgestation, there is an increase in the synthesis of sur-factant components, such as lecithin, within the lung. This increase in lecithin synthesis coincides with the appearance of type II alveolar cells (the source of surfactant) and with an increase in pulmonary blood flow. Some of this lecithin is secreted into the alveolar lumens and appears in the amniotic fluid, where it can be measured as an indicator of the state of lung maturity. Lung maturity co-incides with an increase in serum cortisol levels in the fetus.
Until the time of birth, the vascular resistance of the fetal pulmonary circulation is high, for several reasons. The fetal lung is not inflated; therefore the large vessels are not pulled open by the surrounding alveolar septa. In addiition, the hypoxia of the fetus maintains the pulmonary vascular smooth muscle in a state of contraction that narrows the arteries. The first few breaths alleviate both these conditions.
The fetal lung continuously secretes fluid until about 2 days before birth. This fluid, which is rich in chloride and low in bicarbonate and protein, travels up the trachea and through the fetus’s mouth into the amniotic cavity. The fluid in the alveolar spaces and airways is in part squeezed out of the lung as the thorax is compressed during birth. The majority is reabsorbed into lymphatic and blood vessels shortly after birth.
Beginning at about one third of gestation, the fetus makes breathing movements, although it moves little of the viscous fluid to and fro in the airways. These movements apparently prepare the respiratory muscles for their postnatal function.
At or Shortly after Birth, Umbilical Vessels Rupture, Pulmonary Vascular Resistance Decreases, and the Foramen Ovale and Ductus Arteriosus Close
At term, the fetus is dependent on the placenta and the mother for exchange with the environment, but the lung and other organs must be ready to assume their postnatal functions. During a normal birth, the newborn emerges from the birth canal at about the time the placenta is detaching from the uterine wall. Placental gas exchange probably continues well into third-stage labor. If labor is prolonged, the placenta may detach before the newborn is delivered. This is a medical emergency.
Normally, the newborn takes the first breath immediately after delivery. The stimuli for this include (1) hypoxia and hypercarbia, which result from the loss of the placental gas exchanger; (2) cooling of the fetus as the fetal fluids evaporate from the skin; and (3) a generalized increase in sensory input to the fetus as it is licked and nuzzled by its dam. To expand the lungs initially, the respiratory muscles must create an intrathoracic pressure that is 60 cm H2O less than atmospheric pressure. This is vastly lower than the 5 cm H2O generated by adults breathing tidal volume. The large effort during the first breath is necessary to move viscous fluids down the airways before air can enter the alveoli and to open the fluid-filled alveoli. Not all alveoli may inflate during the first breath, but subsequent inhalations inflate the entire lung and distribute surfactant over the alveolar surface. This surfactant makes the alveoli stable and prevents their collapse so that a stable end-expiratory lung volume, known as functional residual capacity, can be established. After the first few breaths, arterial oxygen tension (Pao2) is much higher than it was before birth, yet breathing continues. It therefore appears that breathing is inhibited in utero, and the chemoreceptors are insensitive to hypoxia. This inhibition is removed after birth.
Inflation and oxygenation of the lung reduce the pulmonary vascular resistance, which leads to a decreased pressure in the pulmonary artery, right ventricle, and right atrium. At about the same time, the umbilical vessels rupture because the animal struggles to stand or the umbilical cord is torn by the mother. Umbilical blood flow is arrested by local vasoconstriction in the umbilical vessels. The loss of the low-resistance placental circulation increases systemic vascular resistance, which results in an increased pressure in the aorta, left ventricle, and left atrium. As a result of these changes, aortic pressure exceeds pulmonary arterial pressure, and left atrial pressure exceeds right atrial pressure. Therefore, blood flow through the ductus arteriosus and foramen ovale reverses. Flow reversal in the foramen ovale causes a flap valve to close and occlude the foramen. Over succeeding days to weeks, this valve becomes adherent to the wall of the atrium, thus permanently closing the foramen.
Reversal of flow in the ductus arteriosus exposes the ductus wall to well-oxygenated blood. This causes constriction of smooth muscle in the wall of the ductus, thus arresting blood flow. Ductus closure involves a decrease in the concentration of vasodilator prostaglandins. Administration of drugs, such as indomethacin, that inhibit prostaglandin synthesis constricts the ductus in fetal sheep, and administration of prostaglandin E2 dilates it. Once the ductus has constricted and flow has been arrested, the ductus is gradually converted into a fibrous band of scar tissue.
The changes just described convert the fetal circulation into the adult circulation, which is able to support the gas-exchange function of the lung. Remarkably, these changes occur routinely and without medical assistance in almost all animal births.
CLINICAL CORRELATIONS
Patent Ductus Arteriosus in a Pomeranian
History.
A 7-week-old female Pomeranian puppy is presented to you because it is not growing as fast as its littermates. The breeder says it is lethargic and prefers to sleep, whereas the other puppies play.
Clinical Examination.
Clinical examination reveals a small puppy with a rapid heart rate. The mucous membranes of its gums are pink, and its temperature is normal. While holding the puppy around the thorax, you notice a vibration in the region of the heart. When you listen with a stethoscope, you hear a loud murmur that is almost continuous through systole and diastole, and you recall that this is called a machinery murmur. It is difficult to listen to the breath sounds because the murmur is audible all over the thorax.
A radiograph reveals a slightly enlarged heart, but the lungs appear normal, although minimally compressed by the heart. An echocardiogram is performed, which better characterizes the suspected patent ductus arteriosus (PDA). The velocity of blood flow through the ductus and its mean diameter indicate that the dog is a candidate for surgical correction of the PDA.
Comment.
The clinical and radiographic findings in a puppy of this age are characteristic of PDA. In some animals the ductus fails to close after birth, and blood continues to flow through it, usually from the aorta to the pulmonary artery. This presents the animal with two problems. First, the left ventricle must increase its output to supply the systemic tissues because so much blood is passing through the ductus. Second, the pulmonary circulation has a volume overload that increases the pressure against which the right ventricle must work. Depending on the magnitude of the PDA, these extra loads result in dilation of the ventricles and sometimes in hypertrophy of the myocardium, which is seen on radiographs as an enlarged heart. The puppy is not growing and is listless because the tissues are not receiving a normal blood flow. Although surgical correction of the PDA allows the puppy to live a normal life, it would be unwise to breed this animal in the future because the condition is inherited.
Treatment.
The patent ductus is closed by surgical ligation, and the puppy is expected to have a normal life.
Recently, use of a transarterial coil occlusion procedure has been successful in the treatment of animals with PDA. The treatment choice is somewhat dictated by the size of the shunt as well as the presence or absence of heart failure.
Battaglia FC, Meschia G. An introduction to fetal physiology. Orlando, Fla: Academic Press, 1986.
Campbell FE, Thomas WP, Miller SJ, et al. Immediate and late outcomes of transarterial coil occlusion of patent ductus arteriosus in dogs. J Vet Intern Med. 2006;20:83.
Dawes GS. Foetal and neonatal physiology. Chicago: Year Book Medical, 1968.
Faber JJ, Thornburg KL. Placental physiology: structure and function of fetomaternal exchange. New York: Raven Press, 1983.
Jones EE, DeCherney AH. Fetal and neonatal physiology. In: Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach, updated edition. Philadelphia: Saunders, 2005.
Leff AR, Schumacker PT. Respiratory physiology: basics and applications. Philadelphia: Saunders, 1993.
Silver M, Steven DH, Comline KS. Placental exchange and morphology in ruminants and the mare. In: Comline KS, Cross KW, Dawes GS, et al. Foetal and neonatal physiology: proceedings of the Sir Joseph Barcroft Centenary Symposium. Cambridge, UK: Cambridge University Press, 1973.
PRACTICE QUESTIONS
1. The vascular channel that allows fetal blood to pass from the pulmonary artery to the aorta is known as the:
2. Which of the following is the correct sequence of events that follow birth?
3. Which of the following fetal blood structures contains blood with the highest Po2?
4. Which of the following statements about the fetal circulation is true?
5. Which of the following does not correctly describe the lung in utero?
6. Fetal oxygen transport is assisted by:
7. Which of the following domestic mammals has a diffuse, epitheliochorial placenta in which fetal and maternal blood flow is countercurrent in the microcotyledons?