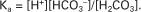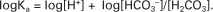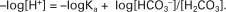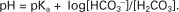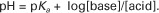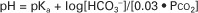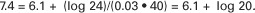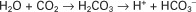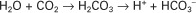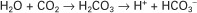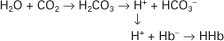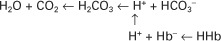Chapter 52 Acid-Base Homeostasis
1. Relative constancy of the body’s pH is essential because metabolism requires enzymes that operate at an optimal pH.
2. Hydrogen ion concentration is measured as pH.
3. An acid can donate a hydrogen ion, and a base can accept a hydrogen ion.
4. Buffers are combinations of salts and weak acids that prevent major changes in pH.
5. Hemoglobin and bicarbonate are the most important blood buffers.
6. The first defense against a change in blood pH is provided by the blood buffers, but the lungs and kidneys must ultimately correct the hydrogen ion load.
7. Changes in ventilation can rapidly change carbon dioxide tension and therefore alter pH.
8. Metabolic production of fixed acids requires that the kidneys eliminate hydrogen ions and conserve bicarbonate.
1. Acid-base abnormalities accompany many diseases, and the restoration of normal blood pH should be a consideration in the treatment of any disease.
2. Respiratory acidosis is caused by the accumulation of carbon dioxide, which decreases blood pH.
3. Respiratory alkalosis is caused by the loss of carbon dioxide, which increases blood pH.
4. Metabolic acidosis is caused by the accumulation of fixed acids or the loss of buffer base, which decreases blood pH.
5. Metabolic alkalosis is caused by the excessive elimination of hydrogen ions or by the intake of base, such as bicarbonate, which increases blood pH.
6. Respiratory compensations for acid-base abnormalities occur rapidly; renal compensations occur over several hours.
7. Hydrogen and potassium ions are interrelated in acid-base homeostasis.
8. The diagnosis of acid-base disturbances depends on interpretation of measurements of arterial blood pH and carbon dioxide tension, from which bicarbonate concentration and total buffer base are calculated.
9. Over the years, many terms have been used to explain acid-base balance.
ACID-BASE REGULATION
Relative Constancy of the Body’s pH Is Essential Because Metabolism Requires Enzymes That Operate at an Optimal pH
For optimal functioning of the cells constituting the animal, the ionic composition of body fluids is maintained within fairly narrow limits. Regulation of hydrogen ion (proton) concentration is extremely important because it determines the acidity or alkalinity, or pH, of the body fluids. Serious deviations of pH outside the normal range can drastically disrupt cell metabolism and therefore body function. For example, the activity of the sodium-potassium (Na+-K+) pump decreases by half when pH falls by one unit, and the activity of phosphofructokinase decreases by 90% when pH decreases by only 0.1.
When veterinarians use the terms acidosis and alkalosis, they are comparing the pH of an animal’s arterial blood with the normal value of 7.4. A pH below 7.4 is referred to as acidosis; a pH above 7.4 is referred to as alkalosis. The range of pH compatible with life is 6.85 to 7.8, but rarely are these extremes approached. The body buffers, lungs, and kidneys all defend the body from onslaught of hydrogen ions (H+) from a variety of sources.
The largest daily load of H+ (protons) arises during the transport of carbon dioxide (CO2) from the tissues to the lungs. If the lungs eliminate CO2 as fast as it is produced in the tissues, there is no net H+ gain by the body. However, the balance between CO2 production and CO2 elimination may be disturbed during exercise or in respiratory disease, thus threatening the acid-base homeostasis of the body.
Hydrogen ions also are a product of protein metabolism, which produces sulfuric and phosphoric acids, fat metabolism, and the incomplete oxidation of glucose to lactic acid. Although H+ ions from these sources are few compared with those produced in CO2 transport, the kidneys must eliminate them continually. In a disease state the H+ load imposed on the body is frequently increased because of an increase in tissue breakdown (catabolism) or because the kidneys fail to eliminate H+. In less common situations, such as vomiting, H+ is lost from the body.
To understand how the body regulates pH and how acid-base disorders are diagnosed, it is necessary to first review acids, bases, and buffering.
Hydrogen Ion Concentration Is Measured as pH
Only 1 in 550 million molecules of water is ionized; thus the concentration of hydrogen and hydroxyl ions in water is 1 × 10−7 mol/L. The chemical potential of H+ is known as the acidity and is expressed in pH units. pH is the negative logarithm of H+ concentration. Water with 1 × 10−7 mol/L of hydrogen ion and an equal concentration of hydroxyl ions has a pH of 7.0, that is, a neutral pH. A decrease in pH indicates increasing acidity, caused by an increase in H+ concentration. For example, a decrease of 1.0 pH unit represents a 10-fold increase in H+ concentration. Doubling H+ concentration decreases pH by only 0.3 unit.
The normal range of blood pH, 6.85 to 7.80, represents an H+ concentration of 1.4 × 10−7 to 1.6 × 10−8 Eq/L. Thus, although the hydrogen ion concentration is regulated, changes up to 10-fold in magnitude can occur, much greater than the fluctuations observed in the concentration of other ions, such as sodium or potassium.
An Acid Can Donate a Hydrogen Ion, and a Base Can Accept a Hydrogen Ion
Hydrochloric acid (HCl) is a strong acid because it dissociates completely in water into H+ and Cl−. Chloride ion is a base because it has the potential to accept a hydrogen ion, but it is a weak base because HCl dissociates so completely. Carbonic acid (H2CO3), in contrast, is a weak acid because it dissociates incompletely in solution into hydrogen and bicarbonate ions. Bicarbonate (HCO3−), however, is a relatively strong base that can accept a hydrogen ion and form undissociated carbonic acid. The latter reaction removes hydrogen ions from solution, and the concentration of free H+ decreases, causing the pH to rise. Bases do not have to be ions; for example, ammonia (NH3) is a base because it can accept a proton and become ammonium ion (NH4+). This reaction is of little importance in the blood, but it is important in the renal collecting duct. In addition, proteins also act as buffers by virtue of the terminal carboxyl and amino groups, which can donate (R-COOH → R-COO− + H+) or accept (R-NH2 + H+ → R-NH3+) protons, respectively.
Buffers Are Combinations of Salts and Weak Acids That Prevent Major Changes in pH
Buffers “soak up” free hydrogen ions and prevent their accumulation in body fluids. By so doing, buffers prevent drastic changes in pH. Buffers are mixtures of weak acids and their salts. For example, sodium bicarbonate dissociates completely into sodium and bicarbonate ions; carbonic acid dissociates incompletely into hydrogen and bicarbonate ions. Thus, in a solution containing sodium bicarbonate and carbonic acid, there are sodium, hydrogen, and bicarbonate ions and undissociated carbonic acid. If a strong acid such as hydrochloric acid is added to the solution, the added hydrogen ions upset the dissociation equilibrium of carbonic acid. Hydrogen ions combine with bicarbonate ion to form carbonic acid, thus reducing the concentration of hydrogen ions, that is, preventing a major change in pH.
If, in contrast, sodium hydroxide is added to the solution, the hydroxyl ions, formed by dissociation of sodium hydroxide, combine with hydrogen ion to form water. The decrease in hydrogen ion causes dissociation of more carbonic acid and liberation of hydrogen ions, again preventing a large change in pH.
The dissociation of a weak acid, and therefore the concentration of H+, base, and undissociated acid, is determined by the dissociation constant (Ka) and can be described by the law of mass action. For carbonic acid:
Taking logarithms of both sides of this equation results in:
Rearrangement of this equation yields:
However, −log[H+] is pH and −logKa is called pKa; therefore:
This is the Henderson-Hasselbalch equation written for the bicarbonate–carbonic acid system. It can be written for any buffering system in the generic form:
This equation shows that the pH of a solution is determined by the ratio of the concentration of base (the H+ acceptor) to that of undissociated acid (the H+ donor) and by the pKa of the buffering system.
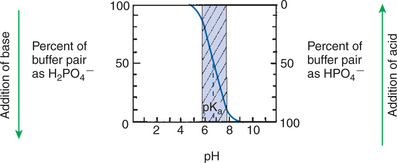
Figure 52-1 shows the change in pH that results when acid is added to a phosphate buffer with a pKa of 6.8. This is a graphic presentation of the Henderson-Hasselbalch equation. Initially, as acid is added, there is a large decrease in pH. As considerably more acid is added to the solution, the pH changes little. H+ ions combine with HPO42− and form H2PO4−. Finally, the pH decreases considerably. The zone over which the pH changes little as acid is added (i.e., where buffering capacity is optimal) is within ±1 pH unit of the pKa. Note that when the pH equals the pKa, 50% of the buffer has been consumed. From this buffer curve, it is obvious that an effective buffer must have a pKa within ±1 pH unit of the solution in which it operates. Thus the optimal blood buffers should have a pKa between 6.4 and 8.4. In addition, buffers must be sufficiently plentiful to be effective.
Hemoglobin and Bicarbonate Are the Most Important Blood Buffers
Hemoglobin is an important blood buffer because it is plentiful and because the imidazole residues of globin histidine have a pKa close to the blood pH. In actuality, the pKa of hemoglobin changes with the degree of oxygenation. Because deoxyhemoglobin has a pKa (7.93) closer to blood pH than does oxyhemoglobin (pKa = 6.68), deoxyhemoglobin provides more buffering capacity. When arterial blood enters the tissue capillaries, oxygen leaves hemoglobin, so the resulting deoxyhemoglobin is an excellent buffer for the H+ produced when CO2 is added to the blood.
The other blood buffer with an optimal pKa is the HPO42−/H2PO4− system, with a pKa of 6.8 (see Figure 52-1). The normally low phosphate concentration in the blood makes this buffering system quantitatively unimportant; however, it is important in the renal tubules, where phosphate is concentrated. Plasma proteins also provide a small amount of blood buffering.
Although a pKa of 6.1 seems to make the HCO3−/H2CO3 buffer unimportant for blood buffering, this is not so for two reasons. First, there is a large amount (24 mEq/L) of HCO3− in the blood, making it readily available for buffering. Second, the kidneys can regulate the concentration of HCO3−, and the lungs can regulate the concentration of H2CO3. Because the base and acid concentration can be regulated, the HCO3−/H2CO3 system is said to be an open system.
Figure 52-2 shows the value of this open system in maintaining body pH. It should be noted that the concentration of H2CO3 ([H2CO3]) in solution is directly proportional to the carbon dioxide tension (Pco2); 1 molecule of H2CO3 is in equilibrium with 340 molecules of CO2. Therefore, [H2CO3] is calculated as 0.03 · Pco2. In Figure 52-2, A and B, top panels, 5 mmol of hydrochloric acid is added to plasma. Figure 52-2, A, shows what happens if the Pco2 and thus [H2CO3] is held constant. In such a closed system, the 5 mmol of HCl reacts with 5 mmol of HCO3− to form 5 mmol of H2CO3. As a consequence of this reaction, [HCO3−] decreases from 24 to 19 mmol/L, [H2CO3] increases from 1.2 to 6.2 mmol/L, and Pco2 increases from 40 to 206 mm Hg. Using the law of mass action, one can calculate that [H+] increases from 40 to 257 nEq/L or, stated in other terms, pH decreases from 7.4 to 6.5. If the system is open, however, as it is in the top panel of Figure 52-2, B, carbon dioxide evolves to the environment as fast as it is produced so that Pco2 and therefore [H2CO3] remain constant, then [H+] increases to only 50 nEq/L and the pH decreases only to 7.3. The lower panel in Figure 52-2, B, shows similar advantages to the open system when a strong base is added to the buffer system. Under most conditions, the body functions as an open system with regard to the HCO3−/H2CO3 system so that pH changes are minimized. When tissues are ischemic, however, they have no connection to the lungs, so CO2 cannot be eliminated. The ischemic tissue then functions as a closed system, and pH changes within the tissue can therefore be drastic.
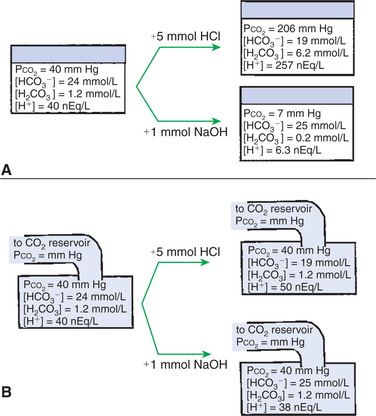
FIGURE 52-2 Buffer function of the carbonic acid–bicarbonate system under closed and open conditions. A, Under closed conditions, the total quantity of the buffer (acid plus base components) remains constant. B, Under open conditions, the carbon dioxide tension (Pco2) of the system, and thus the concentration of carbonic acid (H2CO3), is maintained at a fixed level by continuous equilibration of the liquid phase with a gas reservoir of constant Pco2. The term [H2CO3] denotes the combined concentration of carbonic acid and dissolved carbon dioxide.
(From Madias NE, Cohen JJ: Acid-base chemistry and buffering. In Cohen JJ, Kassirer JP, editors: Acid-base, Boston 1982, Little, Brown.)
The HCO3−/H2CO3 buffering system is of great value to clinicians because its components can be readily measured in the clinical laboratory and thereby used to diagnose acid-base disturbances. It is not necessary to measure the components of every buffering system to diagnose acid-base disturbances. If one system is known, changes in other systems can be predicted. It is standard to measure pH and Pco2 and to use the Henderson-Hasselbalch equation to derive [HCO3−]. In actual practice, these calculations are now done for the clinician by computers in the blood gas machine.
For clinical use, the Henderson-Hasselbalch equation for the HCO3−/H2CO3 system is written as follows:
Under normal conditions, the pH of arterial blood is 7.4, the concentration of HCO3− is 24 mEq/L, and the arterial carbon dioxide tension (Paco2) is equal to 40 mm Hg:
This equation demonstrates that a normal blood pH requires an [HCO3−]/[0.03 · Pco2] ratio of 20:1. An increase or decrease in this ratio increases or decreases pH, respectively.
The First Defense against a Change in Blood pH Is Provided by the Blood Buffers, but the Lungs and Kidneys Must Ultimately Correct the Hydrogen Ion Load
When body pH is threatened by a change in the production or elimination of H+, the first line of defense is provided by buffers within the blood and tissues. However, buffers only prevent drastic changes in pH; they cannot correct the problem by increasing or decreasing the elimination of H+ or by replacing lost buffering capacity. Ultimately, pH must be corrected by adjustments in ventilation or by changes in renal function. Because the lungs can alter Paco2 and the kidneys can regulate the concentration of HCO3−, the Henderson-Hasselbalch equation has been written as follows:
Changes in Ventilation Can Rapidly Change Carbon Dioxide Tension and Therefore Alter pH
As blood flows through the tissues, CO2 diffuses into the plasma and the erythrocytes (red blood cells), where carbonic acid forms and then dissociates into hydrogen and bicarbonate ions:
Because the initial concentration of HCO3− in the blood is greater than that of H2CO3, the relative increase in the concentration of H2CO3 is greater than the increase in the concentration of HCO3−, and thus the [HCO3−]/[H2CO3] ratio (i.e., [HCO3−/0.03 · Pco2]) is decreased; consequently, pH decreases. In the lungs, CO2 leaves the blood, and the pH increases again. For these reasons, venous blood is more acidic than arterial blood. Normally, the lungs eliminate CO2 as fast as the tissues produce it, so the Paco2 and pH of arterial blood remain relatively constant.
The lungs can cause rapid changes in blood pH by increasing or decreasing the elimination of CO2. When ventilation increases in relation to CO2 production (hyperventilation), Paco2 decreases, the [HCO3−/0.03 · Paco2] ratio increases, and pH increases. Conversely, when ventilation decreases in relation to CO2 production (hypoventilation), PaCO2 increases, [HCO3−/0.03 · Paco2] decreases, and pH decreases. Figure 52-3 shows how the concentration of HCO3− and the pH change as the Pco2 of the blood increases or decreases.
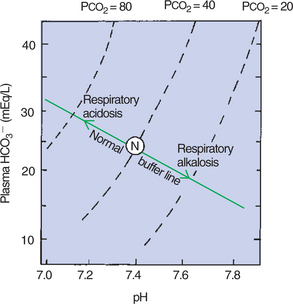
FIGURE 52-3 A pH-bicarbonate diagram showing the effect of increasing and decreasing carbon dioxide tension (Pco2) on pH and bicarbonate (HCO3−) concentration. As Pco2 increases or decreases, the changes in pH and bicarbonate concentration are predicted by the normal buffer line. N, Normal arterial blood composition.
Metabolic Production of Fixed Acids Requires That the Kidneys Eliminate Hydrogen Ions and Conserve Bicarbonate
When fixed acids are added to the blood, for example, from protein metabolism, the hydrogen ions are buffered in part by HCO3−. Buffering results in the conversion of HCO3− to H2CO3 and CO2, which is eliminated from the lungs. Fixed acids are produced continuously and would consume the body’s HCO3− if the kidneys were not continually regenerating HCO3−.
The role of the kidneys in acid-base balance is described in Chapter 44. Large amounts of HCO3− are filtered daily through the glomerulus and subsequently reabsorbed in the renal tubule. The amount of HCO3− reabsorbed depends on the amount filtered, which is determined by the plasma concentration of HCO3−, the glomerular filtration rate, and the rate of H+ secretion by renal tubular cells. This last rate is controlled in part by the acid-base status of the body.
When Pco2 is high, the reaction:
within the renal tubules is driven to the right, producing more H+ for secretion into the tubular lumen and HCO3− for return to the blood. When Pco2 is low, H+ elimination and therefore HCO3− reabsorption decrease.
Ammonia, an important buffer in the distal renal tubule, is produced by the action of glutaminase on glutamine. In acidosis, the activity of glutaminase increases, resulting in increased ammonia production, an increased buffering capacity of the renal tubular fluid and, therefore, increased ability to eliminate hydrogen ions.
Intracellular pH Is Regulated by Buffers and Ion Pumps
Whereas the hemoglobin and bicarbonate provide the most immediately available source of buffers to prevent drastic changes in blood pH, intracellular buffers within the body tissues, other than blood, provide another large reserve of buffering capacity. In order to enter cells, hydrogen ion must be exchanged with other cations, such as sodium or potassium. Once inside the cell, hydrogen ion is buffered by amino acids, peptides, proteins, and organic phosphates. These buffers provide approximately five times the buffering capacity of the extracellular fluid. As a general rule, intracellular pH follows extracellular pH but because of the large intracellular buffering capacity, the pH changes are less dramatic in the cytosol.
Two acid-base ion pumps play a major role in regulation of intracellular pH. The Na+/H+ exchanger uses the energy derived from the extracellular to intracellular Na+ gradient to move H+ out of the cell and the Cl−/HCO3− exchanger uses a similar mechanism to move HCO3− out of the cell.
When H+ concentration within the cell increases, the activity of the Na+-H+ pump increases so that more H+ is extruded. At the same time, the activity of the Cl−-HCO3− pump is inhibited so that HCO3− accumulates within the cell. These two mechanisms can restore intracellular pH to normal as long as extracellular pH also is normal. If however, extracellular pH is low, it is more difficult for the Na+-H+ pump to extrude H+, and thus intracellular pH tends also to be acidic. If the cytosol becomes alkaline, the activity of the Cl−-HCO3− pump is facilitated, and the Na+-H+ pump is inhibited to restore pH. This corrective action is inhibited if extracellular pH also is alkaline.
ACID-BASE DISTURBANCES
Acid-Base Abnormalities Accompany Many Diseases, and Restoration of Normal Blood pH Should Be a Consideration in the Treatment of Any Disease
In most diseases the buffering systems, lungs, and kidneys keep pH within tolerable limits, but in severe disease these homeostatic mechanisms may be inadequate, and life-threatening changes in pH can occur. In the diagnosis and treatment of acid-base abnormalities, it is important to realize that a primary abnormality causes the change in blood pH, which is followed by compensatory changes. Because of the body’s attempt to correct the abnormality, the clinician often must disentangle the data to differentiate the primary cause of the problem from the compensatory changes. The primary problems are excessive accumulation or elimination of carbon dioxide (respiratory abnormalities) or the excessive accumulation or elimination of fixed acids (metabolic abnormalities).
Respiratory Acidosis Is Caused by the Accumulation of Carbon Dioxide, Which Decreases Blood pH
Respiratory acidosis is caused by alveolar hypoventilation, which can result from damage to or depression of the respiratory control centers, injury to the respiratory pump (e.g., fractured ribs, bloated abdomen), or severe respiratory disease that either obstructs the airways or excessively stiffens the lungs. When alveolar hypoventilation occurs, Pco2 increases because carbon dioxide is incompletely eliminated by the lungs. The reaction:
is driven to the right by the accumulating CO2; H+ accumulates, and pH decreases. Bicarbonate accumulates simultaneously, but the amount is too small to keep the [HCO3−/0.03 · PCO2] ratio at a normal value of 20:1.
In the blood, other nonbicarbonate buffers not only take up H+ produced by the accumulation of CO2 but also assist in the accumulation of HCO3−, as follows:
By buffering H+, hemoglobin (Hb−) pulls the first reaction to the right and produces HCO3−. This accumulation of bicarbonate, shown on the normal buffer line in the pH-HCO3− diagram (see Figure 52-3), is still insufficient to maintain a normal [HCO3−/0.03 · Pco2] ratio, and therefore the pH decreases. As a result of these various reactions, the characteristic findings in acute respiratory acidosis are an elevated Paco2, a decreased pH, and a minor increase in [HCO3−].
To facilitate the clinical interpretation of acid-base status, clinicians use the terms total buffer base, base excess, and base deficit. Total buffer base is the sum of the concentrations of available blood buffers. Base excess and base deficit refer to an increase or decrease, respectively, in total buffer base. In acute respiratory acidosis, total buffer base does not change because the accumulation of HCO3− is accompanied by an equivalent decrease in the concentration of other buffers, such as Hb−. Therefore, there is no base excess or base deficit.
The ideal way to correct respiratory acidosis is to restore alveolar ventilation. However, because disease processes that impede ventilation cause the respiratory acidosis, this option is not available to the animal, and other means to correct pH, primarily renal mechanisms, must be used. The elevated Pco2 and decreased pH increase H+ and NH3 production in the kidney. This increases the elimination of H+ in the urine and generates new HCO3−, and as the plasma [HCO3−] increases, the [HCO3−/0.03 · Pco2] ratio and pH are adjusted toward normal. The newly generated HCO3− adds to the total buffer base and therefore causes a base excess. Figure 52-4 shows how this accumulating HCO3− adjusts the pH toward normal during respiratory acidosis, even though Pco2 remains constant.
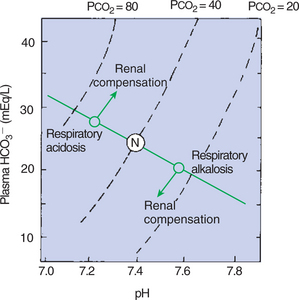
FIGURE 52-4 A pH-bicarbonate diagram showing the effects of respiratory acidosis and alkalosis on pH, bicarbonate (HCO3−) concentration, and carbon dioxide tension (Pco2) of arterial blood. In acute respiratory acidosis, as Pco2 increases, the changes in pH and bicarbonate concentration are predicted by the normal buffer line. Renal compensation leads to an accumulation of HCO3−, which increases the pH, whereas Pco2 remains constant. In respiratory alkalosis, Paco2 and HCO3− decrease and pH increases. The kidneys compensate by increasing HCO3− resorption, which decreases pH. N, Normal arterial blood composition.
Respiratory Alkalosis Is Caused by the Loss of Carbon Dioxide, Which Increases Blood pH
Respiratory alkalosis is caused by alveolar hyperventilation, which results from stimulation of the chemoreceptors by hypoxia or from stimulation of intrapulmonary receptors by lung injury or inflammation. Overly vigorous use of a ventilator also can cause hyperventilation in an anesthetized animal. Carbon dioxide is eliminated faster than the tissues produce it, and so blood Pco2 decreases. The changes in blood chemistry are the inverse of those in respiratory acidosis:
As CO2 is eliminated, H2CO3 is formed from H+ and HCO3−, and thus the pH increases and [HCO3−] decreases. Hydrogen ion is supplied by release from nonbicarbonate buffers, such as hemoglobin. The net result of these processes is that Paco2 decreases, the pH increases, and [HCO3−] decreases and is replaced by other buffers. There is no change in total buffer base. The increase in the [HCO3−/0.03 · Pco2] ratio increases pH.
Figure 52-4 shows the increase in pH and decrease in [HCO3−] as Pco2 decreases. To adjust the pH toward normal, hyperventilation must be stopped, or the kidneys must eliminate HCO3−. The latter occurs because the low Pco2 and alkalosis reduce H+ and NH3 production by the kidney. When H+ is not produced in sufficient amounts to capture all the filtered HCO3−, the latter spills into the urine and is lost from the body.
Metabolic Acidosis Is Caused by the Accumulation of Fixed Acids or the Loss of Buffer Base, Which Decreases Blood pH
Metabolic acidosis is the most common acid-base abnormality. During metabolism, there is a continuous production of fixed acids. An increase in their production or a failure of H+ elimination by the kidneys is the cause of metabolic acidosis. Increased production of fixed acids results from protein catabolism or ketone production during starvation or from anaerobic metabolism that leads to lactic acidosis. Diarrhea can also cause metabolic acidosis because excessive amounts of HCO3− (buffer) are lost in the feces. In ruminants, excessive feeding of carbohydrates can lead to increased H+ production in the rumen (rumen acidosis). The H+ ions that are absorbed cause metabolic acidosis.
The accumulation of H+ in the blood is combined with HCO3− and other buffers. The CO2 produced by the combination of H+ and HCO3− is lost through the lungs. The loss of buffer base gives rise to a base deficit, and the HCO3− depletion decreases the [HCO3−/0.03 · Pco2] ratio; therefore the pH decreases (Figure 52-5).
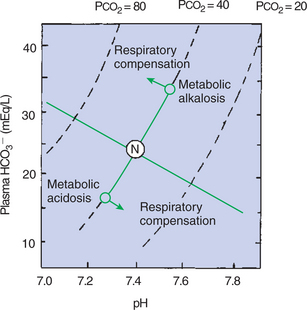
FIGURE 52-5 A pH-bicarbonate diagram showing the effects of metabolic acidosis and alkalosis on pH, bicarbonate (HCO3−) concentration, and carbon dioxide tension (Pco2) of arterial blood. In uncompensated metabolic acidosis, there is a decrease in the bicarbonate concentration, which leads to a decrease in pH, whereas Pco2 remains constant. Respiratory compensation results in a decrease in Pco2, with a subsequent increase in pH and movement of data points parallel to the normal buffer line. In metabolic alkalosis, HCO3− increases and causes an increase in pH. Respiratory compensation is by alveolar hypoventilation, which leads to an increase in Paco2 and a return of pH toward normal. N, Normal arterial blood composition.
The decrease in pH accompanying metabolic acidosis stimulates ventilation. This increase in alveolar ventilation has a compensatory effect on metabolic acidosis by eliminating CO2, leading to a decreased Pco2, which ultimately adjusts the [HCO3−/0.03 · Pco2] ratio and pH toward normal. This sequence is shown in Figure 52-5. As the Pco2 decreases, [HCO3−] decreases along a line that parallels the normal buffer line. This decrease in [HCO3−] is accompanied by a decrease in pH. Complete restoration of normal acid-base balance requires the restoration of the depleted base by the kidney or by therapy with intravenous fluid that contains buffers, such as bicarbonate.
Metabolic Alkalosis Is Caused by the Excessive Elimination of Hydrogen Ions or by the Intake of Base, Such as Bicarbonate, Which Increases Blood pH
The most common cause of metabolic alkalosis is vomiting, during which H+-rich gastric contents are lost from the body. In ruminants, torsion and dilation of the abomasum cause metabolic alkalosis because H+ ions are trapped in the abomasum. Metabolic alkalosis can also result from potassium (K+) depletion because low concentrations of K+ in the blood (hypokalemia) result in increased H+ excretion by the kidney (see later discussion).
The loss of H+ from the body frees buffer, and thus the plasma [HCO3−] and total buffer base increase. The [HCO3−/0.03 · Pco2] ratio, pH, and base excess all increase (see Figure 52-5). The increase in pH reduces the drive to ventilate; alveolar ventilation decreases, and so Pco2 increases. This adjusts the [HCO3−/0.03 · Pco2] ratio toward normal, and thus the pH decreases toward normal (see Figure 52-5).
Respiratory Compensations for Acid-Base Abnormalities Occur Rapidly; Renal Compensations Occur over Several Hours
This discussion of acid-base disturbances shows that the lungs compensate for metabolic problems, and the kidneys compensate for respiratory problems. Because the chemoreceptors respond almost immediately to changes in blood pH, and because changes in ventilation rapidly change Pco2, respiratory compensation for metabolic acid-base problems occurs almost immediately. For this reason, it is rare to observe “pure” metabolic acidosis or alkalosis without a respiratory compensation. The response of the kidneys to a respiratory acid-base disturbance is less rapid, and changes in NH3 and HCO3− production occur over about 24 hours.
As compensatory mechanisms adjust the pH toward normal, there is less “error signal” to drive the compensation; thus these mechanisms alone rarely return the pH to normal. In metabolic acidosis, for example, the low pH drives ventilation to decrease Pco2. As the pH returns to normal, however, the respiratory drive to compensate is reduced; therefore, restoration of normal pH is rarely complete.
Hydrogen and Potassium Ions Are Interrelated in Acid-Base Homeostasis
Acid-base disturbances affect the distribution of potassium within the body. A decrease in blood pH leads to an increase in the plasma K+ concentration [K+] (hyperkalemia), whereas a blood alkalosis leads to a decrease in plasma [K+] (hypokalemia). The hyperkalemia accompanying blood acidosis is a consequence of reduced activity of the Na+-K+ pump and the Na+/K+/Cl− co-transporter, both of which normally transport K+ back into the cell. In addition, intracellular acidosis liberates K+ from nondiffusible intracellular anions so that more K+ is free to diffuse out of the cell. In the case of blood alkalosis, high [HCO3−] can stimulate K+ uptake into cells.
Just as changes in blood pH affect [K+], the converse also is true. Hypokalemia is frequently associated with metabolic alkalosis, and hyperkalemia with metabolic acidosis. These changes are a consequence of the actions of K+ on the renal tubule. Depletion of K+ increases H+ elimination by (1) increasing tubular Na+/H+ exchange and basolateral Na+/HCO3− co-transport; (2) increasing NH3 synthesis and NH4+ excretion; and (3) stimulating K+/H+ exchange in the collecting tubules. Increases in K+ cause metabolic acidosis by inhibition of NH3 synthesis and NH4+ excretion.
The Diagnosis of Acid-Base Disturbances Depends on Interpretation of Measurements of Arterial Blood pH and Carbon Dioxide Tension, from Which Bicarbonate Concentration and Total Buffer Base Are Calculated
Arterial samples must be used to determine the respiratory component of an acid-base abnormality, and samples must be obtained anaerobically to prevent the loss of CO2 from the blood. The Paco2 and pH are measured with specific electrodes in an arterial blood gas (ABG) analyzer. The plasma [HCO3−] and total buffer base are determined from nomograms or frequently from a built-in program in the ABG analyzer.
When ABG data are analyzed, it is useful to ask the following questions:
1. Is the sample acidotic (pH <7.4) or alkalotic (pH >7.4)?
2. What is the respiratory component (is Paco2 high, low, or normal), and will it explain the pH?
3. What is the metabolic component (is there a base excess or deficit), and will it explain the pH?
4. How can items 1, 2, and 3 be combined to explain the data, in view of the fact that compensations rarely return the pH toward normal?
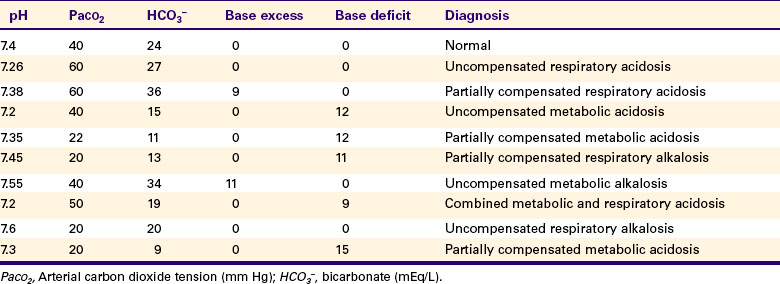
Examples are provided in Table 52-1.
Over the Years, Many Terms Have Been Used to Explain Acid-Base Balance
The terms used to explain acid-base balance include the following:
Anion gap: In the blood the total cation concentration (concentration of Na+ + K+ + Mg2+ + Ca2+) should approximately equal the total anion concentration (concentration of HCO3− + Cl−). Usually, the total cations exceed the total anions; the difference is called the anion gap. This gap results from the presence of unaccounted-for anions from fixed acids, such as lactate. In metabolic acidosis the anion gap increases because of increased production of fixed acids.
Standard bicarbonate: The plasma [HCO3−] when Pco2 = 40 mm Hg is known as “standard” bicarbonate. The plasma [HCO3−] can change as a result of respiratory and metabolic disturbances. An increase or decrease in [HCO3−], measured when Pco2 is normal (i.e., 40 mm Hg), results only from metabolic disturbances.
Total carbon dioxide (Tco2): Carbon dioxide is present in the blood in solution and as carbamino compounds, but largely as HCO3−. Tco2 can be measured by adding an acid to the blood and collecting the evolved CO2, which comes primarily from HCO3−. Changes in Tco2 should be interpreted as changes in plasma [HCO3−].
CLINICAL CORRELATIONS
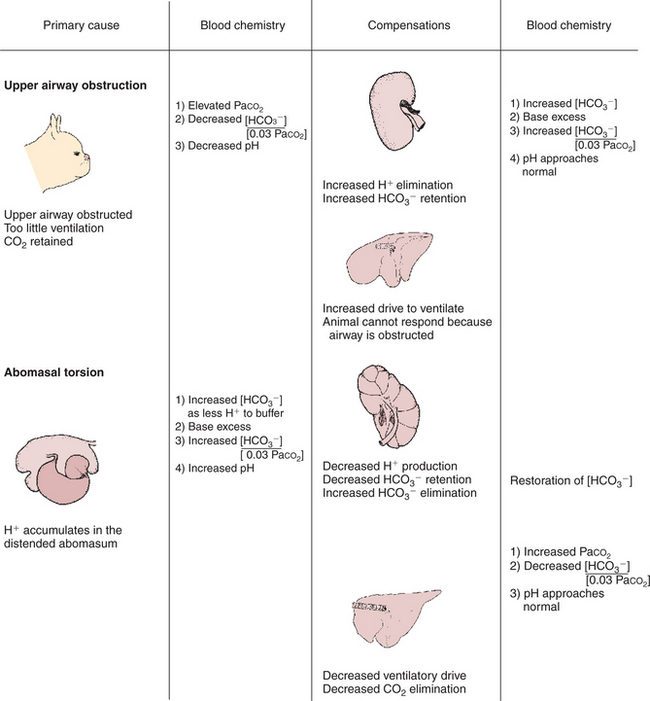
Upper Airway Obstruction in a Boston Terrier
History.
A Boston terrier exhibits signs of severe respiratory distress. It has difficulty inhaling and makes a snoring sound during inhalation. The effort of walking magnifies the distress. An arterial blood gas (ABG) sample reveals that Paco2 is 80 mm Hg (normal, 40 mm Hg), pH is 7.3, [HCO3−] is 39 mEq/L, and base excess is 10 mEq/L (Figure 52-6).
Clinical Examination.
Examination reveals excessively narrowed (stenotic) nares and excessive folds of tissue in the soft palate, the latter occluding the glottis. The larynx and trachea appear normal.
Treatment.
Reconstructive surgery is performed on the dog to enlarge the nares and remove the excessive tissues from the palate. Two weeks after surgery, respiratory distress is greatly reduced. ABG analysis reveals that Paco2 is 45 mm Hg, pH is 7.39, [HCO3−] is 27 mEq/L, and base excess is 2 mEq/L.
Comment.
Before surgery the animal is acidotic with an elevated Paco2 and base excess. Only the high Paco2 explains the acidosis; therefore the dog has respiratory acidosis. The increase in [HCO3−] (normal, 24 mEq/L) is caused primarily by creation of new HCO3− (a base excess) by the kidneys and indicates the condition is of at least several days’ duration. Respiratory acidosis is caused by alveolar hypoventilation resulting from the upper airway obstruction. Surgery corrects the obstruction and alleviates the hypoventilation. This returns the pH to a more normal value. Two weeks after surgery the base excess has been virtually eliminated.
Torsion of the Abomasum in a Cow
History.
A Holstein cow gave birth 2 weeks ago and became inappetent 2 days ago. Over the past 12 hours she has become lethargic, and her right flank is distended. Examination shows she is depressed and dehydrated. Her extremities are cold. Rectal examination as well as percussion reveals a large, fluid-filled organ between the rumen and the right abdominal wall. A fluid sample obtained percutaneously from the distended organ is chloride rich and very acidic. An ABG sample shows that Paco2 is 50 mm Hg, pH is 7.6, [HCO3−] is 50 mEq/L, and base excess is 24 mEq/L (Figure 52-6).
Comment.
The history and physical findings are typical of a dilation or torsion of the abomasum. This condition occasionally occurs shortly after parturition in dairy cows fed high levels of concentrates and chopped feeds. The abomasum distends and may rotate, and thus its inlet and outlet are obstructed. Fluid rich in chloride and H+ continues to be secreted into and is trapped within the abomasum. The loss of H+ from the blood results in a base excess and causes the metabolic alkalosis. The alkalosis depresses ventilation, which elevates Paco2. This is a compensation to restore pH toward normal.
Treatment.
The torsion of the abomasum must be surgically corrected. However, the metabolic alkalosis and any fluid deficits should be treated concurrently to provide the best chance of recovery. The alkalosis is enhanced by loss of Cl− into the abomasum along with H+. Repletion of Cl− allows the kidneys to eliminate the excess bicarbonate and restores normal pH. In practical terms, this is accomplished by treating the cow intravenously with large volumes of 0.9% NaCl solution.
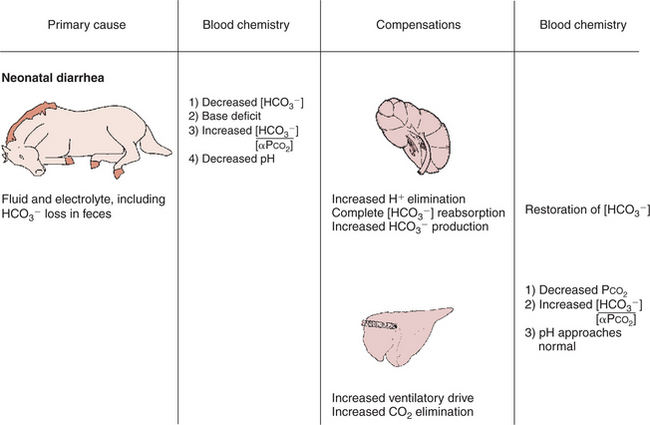
Neonatal Diarrhea in a Foal
History.
A 2-week-old foal has profuse diarrhea. It is lethargic and cold to the touch, its eyes are sunken and dull, and it lies in a pool of feces. The foal’s hematocrit is 65 mL/dL (normal, 45), pH is 7.2 (normal, 7.4), Paco2 is 30 mm Hg (normal, 40), [HCO3−] is 12 mEq/L (normal, 24), and base deficit is 15 mEq/L (see Figure 52-6).
Comment.
The foal shows typical clinical signs of severe dehydration as a result of excessive fluid loss in the feces. Fluid loss from the intravascular compartment reduces blood volume and cardiac output. To maintain blood pressure, vasoconstriction occurs in the extremities, which therefore have less blood flow and become cold. The loss of fluid from the interstitial space causes the dryness of the eyes and muzzle, the sunken appearance of the eyes, and inelastic skin. The increased hematocrit of 65 mL/dL (normal, 45) confirms the dehydration.
Feces contain HCO3−, and its excessive loss causes a base deficit and a decrease in pH. In addition, poor tissue perfusion results in lactic acidosis. The acidosis results from loss of buffer base and accumulation of lactic acid; it is a metabolic acidosis. The acidosis stimulates ventilation, which reduces Paco2 in an attempt to correct pH. The foal has a metabolic acidosis that is partially corrected by the reduction in Paco2.
Treatment.
This foal needs fluid replacement to increase plasma volume, raise cardiac output, and restore circulatory perfusion. The fluid should contain electrolytes, to replace those lost in diarrhea, and a source of buffer, such as bicarbonate. A good choice would be lactated Ringer’s solution supplemented with additional bicarbonate. If the foal’s respiratory and acid-base homeostasis can be maintained until the diarrhea ceases, the foal has a moderate chance of recovery. A serious concern is that this 2-week-old foal could have an infectious cause of diarrhea. The foal could become septic from the infection, or the foal could have an initial septicemia, which is now manifested as diarrhea. In either situation, antibiotics are also warranted in most cases to treat the infection.
Boron WF. Acid-base physiology. In: Boron WF, Boulpaep EL. Medical physiology: a cellular and molecular approach, updated edition. Philadelphia: Saunders, 2005.
Cohen JJ, Kassirer JP. Acid-base. Boston: Little, Brown, 1982.
Davenport HW. The ABC of acid-base chemistry, ed 6. Chicago: University of Chicago Press, 1974.
Gamble JL. Acid-base physiology: a direct approach. Baltimore: Johns Hopkins University Press, 1982.
Hlastala MP, Berger AJ. Physiology of respiration, ed 2. New York: Oxford University Press, 2001.
PRACTICE QUESTIONS
1. Elevated Paco2, low pH, and no base excess or deficit are characteristic of:
2. Elevated Paco2, alkaline pH, and base excess are characteristic of:
3. Low Paco2, acid pH, and base deficit are characteristic of:
4. The most likely acid-base disturbance to be found in a dog at the top of Mount McKinley (Denali) in Alaska (height, 20,320 ft, or 6353 m) is:
5. The distal tubule of the kidney affects acid-base balance by:
6. Which of the following buffers will be most effective in blood (pH = 7.4)?