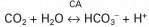Chapter 44 Acid-Base Balance
1. Buffers, lungs, and kidneys together maintain acid-base balance.
2. Acid excretion is achieved by proton secretion by tubule epithelial cells and buffering in the tubule fluid.
3. The kidneys generate new bicarbonate and excrete ammonium ion.
4. The proximal tubule has a high capacity for H+ secretion and bicarbonate reabsorption.
5. The collecting duct determines the final urine pH.
6. The collecting duct can secrete protons and generate acidic urine.
7. The collecting duct can secrete bicarbonate and generate alkaline urine.
Buffers, Lungs, and Kidneys Together Maintain Acid-Base Balance
Normal blood pH is approximately 7.4; normal cellular function requires a pH close to this value. Three systems maintain acid-base homeostasis: (1) intracellular and extracellular buffers, (2) the lungs, and (3) the kidneys. The first two make rapid corrections of blood pH, whereas the kidneys more slowly control acid-base homeostasis, and they excrete excess hydrogen ion (H+).
Usually maintaining acid-base balance requires preventing excess acid in the body. Acid is constantly produced in the body as a byproduct of metabolism. The amount of acid produced varies depending on changes in diet, exercise, other organ functions, and in birds the phases of the egg-laying cycle. Therefore the systems that maintain acid-base homeostasis must adapt to changes in the acid load. Less often, there is an excess base load, which also must be eliminated.
Several intracellular and extracellular buffers titrate H+ to maintain a physiological pH. These include hemoglobin and other proteins, carbonate in bone, phosphate, and bicarbonate (HCO3−). These buffers rapidly normalize the pH after acute alterations in the acid load, unless the buffering capacity is exceeded. In addition, during chronic metabolic acidosis, bone provides a reservoir of buffer that contributes to maintenance of systemic pH. In this condition, excess H+ and low HCO3− in the extracellular fluid promote physicochemical as well as osteoclast-mediated dissolution of bone, releasing carbonate, which buffers H+.
The respiratory system also responds rapidly to maintain normal blood pH by altering the rate of removal of carbon dioxide (CO2) from the blood. The enzyme carbonic anhydrase (CA), present in red blood cells and many other cells, catalyzes the following reaction:
Removal of CO2 from the blood by respiration shifts this reaction to the left, and the concentration of H+ is consequently reduced (pH is raised). Thus the lung is an important avenue for stabilizing blood pH, particularly in response to rapid changes in the acid load.
The kidney is the third line of defense of acid-base balance. Although the buffering and respiratory systems are able to stabilize the blood pH, the kidneys are responsible for the actual excretion of most excess H+.
Acid Excretion Is Achieved by Proton Secretion by Tubule Epithelial Cells and Buffering in the Tubule Fluid
The kidney excretes acid efficiently by the combined effects of (1) enzymes that make protons and bicarbonate available for transport, (2) transporters that move H+ from the epithelial cells into the tubule fluid and bicarbonate into the interstitial fluid, and (3) buffers that minimize increases in H+ concentration in the tubule fluid.
The kidneys excrete acid by tubular secretion of H+, primarily in the proximal tubule and the collecting duct. These two segments use different mechanisms to excrete excess acid and to control blood pH precisely. The proximal tubule secretes the majority of excess acid, whereas the collecting duct controls net acid excretion and the final urine pH.
Most of the secreted H+ is transported across the apical plasma membrane by the following three transporters: (1) a sodium ion (Na+)/H+ exchanger, (2) an electrogenic H+-adenosinetriphosphatase (ATPase) pump, and an H+,potassium ion (K+)–ATPase pump. The Na+/H+ exchanger exchanges luminal Na+ for intracellular H+. The Na+ gradient generated by basolateral Na+,K+-ATPase drives Na+/H+ exchange (secondary active transport). Na+/H+ exchange is the main route of H+ secretion in the proximal tubule. The electrogenic H+-ATPase pump actively transports intracellular H+ across the apical plasma membrane and contributes a net positive charge to the tubule fluid. The H+,K+-ATPase pump, which is similar to the gastric proton pump, actively secretes acid by electrically neutral exchange of intracellular H+ for K+ in the tubule fluid. Although the H+-ATPase pump is responsible for most H+ secretion by the collecting duct, the H+,K+-ATPase pump may contribute equally or exceed the acid secretion rate of the H+-ATPase pump under some conditions.
Buffering of the tubule fluid is necessary for efficient acid excretion. Buffers accept secreted H+ and minimize the decrease in tubule fluid pH that would otherwise follow rapid H+ secretion by the epithelial cells. In mammals the most important buffers are bicarbonate and phosphate. In birds, urates also contribute to titration of secreted acid. Figure 44-1 illustrates the removal of acid by intraluminal buffers.
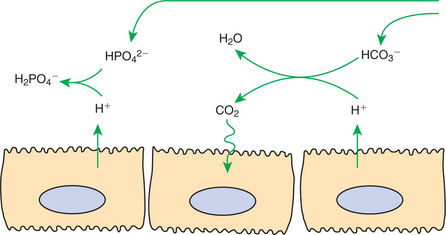
FIGURE 44-1 Schematic illustration of buffer mechanisms at work in tubule fluid. In the proximal tubule, buffering by filtered bicarbonate (HCO3−) predominates because of the relatively high concentration of HCO3−. In the cortical collecting duct, buffering by filtered, nonbicarbonate buffers, such as HPO42−, predominates.
In the proximal tubule, HCO3− is the most important intraluminal buffer, for two main reasons. First, the concentration of HCO3− in the tubule fluid is high. Although large amounts of HCO3− are reabsorbed in the proximal tubule, roughly proportional amounts of H2O are reabsorbed, and the HCO3− concentration remains similar to that of the glomerular filtrate. Second, under the influence of apical plasma membrane– associated carbonic anhydrase, secreted H+ combines with luminal HCO3− to form H2O and CO2. The CO2 freely crosses lipid membranes, disperses rapidly, and is converted to HCO3− in the cell, catalyzed by intracellular carbonic anhydrase.
Filtered phosphate also buffers the tubule fluid. Secreted H+ titrates HPO42− to form H2PO4−. Because the titrated form is a charged molecule, it is lipid insoluble. Furthermore, there is little transport of monovalent Pi (H2PO4−) on the NaPi-2 transporter. Thus the secreted acid is retained in the tubule fluid. Similarly in birds, titration of luminal urate forms uric acid, which traps secreted protons in the tubule fluid. Besides being lipid insoluble, uric acid also has a low aqueous permeability, and thus a significant portion of acid is removed as uric acid precipitates.
The Kidneys Generate New Bicarbonate and Excrete Ammonium Ion
Renal generation and excretion of ammonium ion (NH4+) is a major component in the maintenance of acid-base balance; renal NH4+ excretion is illustrated in Figure 44-2. In proximal tubule cells the amino acid glutamine is metabolized to produce NH4+. This process is called ammoniagenesis. The intracellular NH4+ enters the tubule fluid through secondary active transport by substitution for H+ on the Na+/H+ exchanger. Glutamine metabolism also produces new bicarbonate anions. Thus the net result of the renal generation and excretion of NH44+ is acid excretion and bicarbonate production. Renal ammoniagenesis is enhanced by acidosis and is an important renal response to an increase in the acid load.
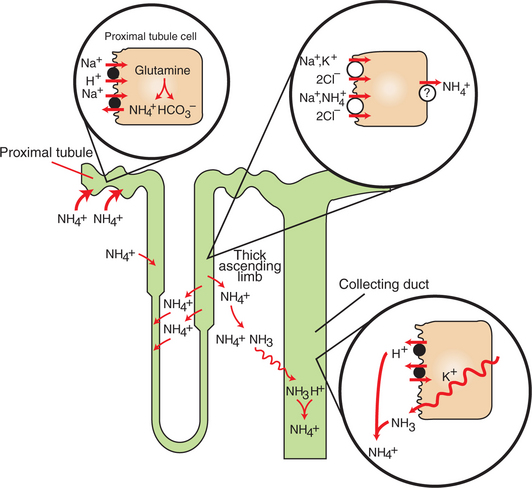
FIGURE 44-2 Schematic illustration of the roles of various nephron segments in ammonium excretion. In the proximal tubule, glutamine is catabolized to generate ammonium ion (NH4+) and bicarbonate (HCO3−). NH4+ is secreted into the lumen by substitution for H+ on the Na+/H+ exchanger in the apical plasma membrane. Ammonium ion recycles in the loop of Henle by reabsorption by the thick ascending limb, in which NH4+ is reabsorbed by substitution for K+ on the Na+/K+,2Cl− co-transporter in the apical plasma membrane, which is followed by some form of facilitated transport across the basolateral plasma membrane. The elevation of the interstitial NH4+ concentration results in movement into the descending thin limbs of Henle’s loop and subsequent return to the thick ascending limb. This medullary recycling results in a high concentration of ammonia (NH3) and NH4+ in the medullary interstitium and prevents its return to the cortex, where it would be reabsorbed into the blood. Ammonia readily diffuses into the collecting duct, where it is rapidly protonated, trapped in the lumen, and then excreted in the urine.
In the thick ascending limb of Henle’s loop, luminal NH4+ is reabsorbed by substitution for K+ on the Na+,K+,2Cl− co-transporter. The NH4+ reabsorption in this segment reduces the amount of ammonia species delivered to the late distal tubule and increases ammonia (NH3) in the medullary interstitium.
High NH3/NH4+ concentrations are enhanced and maintained in the medullary interstitium by a countercurrent multiplication system in the loops of Henle, similar to that described in Chapter 43. This creates a steep concentration gradient for NH3, which favors its movement into the medullary collecting duct. The established paradigm is that ammonia diffuses across plasma membranes and into the luminal fluid, where it acts as a buffer and combines with H+ to form NH4+. Because NH4+ is lipid insoluble, it cannot diffuse back across the apical plasma membrane and is trapped within the tubule fluid.
The formation of NH4+ from intraluminal NH3 and H+ lowers the concentration of both NH3 and H+ in the tubule fluid. This contributes to the maintenance of a favorable gradient for the diffusion of NH3 into the tubule fluid and reduces the electrochemical gradient for H+ that is created by active proton secretion in the collecting duct.
Until recently, NH3 diffusion and trapping in the collecting duct was believed to be the only significant mechanism of NH4+ excretion. However, several studies have demonstrated transporter-mediated NH4+ secretion in the collecting duct. Recently, members of the Rh glycoprotein family, which transport ammonium in other systems, have been found in the mammalian collecting duct. Although it remains to be proven that these transporters mediate ammonium secretion in the native collecting duct, in vitro studies suggest such a role for them.
Finally, the comparative aspects of ammonia excretion are intriguing. It is known that ammoniagenesis and ammonia excretion is an important mechanism controlling acid-base homeostasis in mice, rats, dogs, chickens, and humans. In these species, ammonia excretion accounts for up to 60% of net acid excretion in basal conditions and can increase to 90% of net acid excretion in models of metabolic acidosis. However, the findings in these animals cannot be applied generally to other species. Rabbits have low urinary ammonia excretion rates and do not increase ammonia excretion during metabolic acidosis. Some might not consider this surprising because rabbits normally excrete neutral or alkaline urine and might not be expected to have a full complement of mechanisms to excrete acid urine. However, domestic cats, which usually excrete acid urine, also do not increase renal ammonium excretion in response to a model of metabolic acidosis.
The Proximal Tubule Has a High Capacity for H+ Secretion and Bicarbonate Reabsorption
As described in Chapter 42, the proximal tubule normally reabsorbs the majority of the filtered HCO3−. The mechanism of bicarbonate reabsorption in the proximal tubule is illustrated in Figure 42-7. In brief, apical membrane-bound carbonic anhydrase catalyzes the formation of H2O and CO2 from filtered HCO3− and secreted H+. The CO2 diffuses into the epithelial cell and combines with intracellular H2O under the influence of cytoplasmic carbonic anhydrase to form HCO3− and H+. HCO3− is transported across the basolateral plasma membrane by the Na+(HCO3−)3 co-transporter, driven by the electrical gradient for anions, and is reabsorbed into the blood. Concurrently, H+ is transported into the lumen, primarily by the Na+/H+ antiporter, but also by the H+-ATPase pump, which may transport up to 35% of the total H+ secreted by the proximal tubule. Thus, net HCO3− reabsorption and net H+ secretion are essentially equivalent terms in this system. The rate of bicarbonate reabsorption/acid secretion in the proximal tubule is increased by angiotensin II stimulation of the basolateral Na+(HCO3−)3 co-transporter, the Na+/H+ exchanger, and the vacuolar H+-ATPase.
Although the proximal tubule has a large capacity for H+ secretion (HCO3− reabsorption) and reabsorbs 80% to 90% of the filtered HCO3−, it cannot maintain a large pH gradient across the apical plasma membrane. The net secretion of H+ in this segment is particularly dependent on the intraluminal buffers discussed earlier, which combine with secreted H+ and prevent the concentration of H+ in the tubule fluid from rising significantly. As a result, although the majority of renal acid secretion (HCO3− reabsorption) occurs in the proximal tubule, the pH of the tubule fluid when it leaves this segment is similar to that of the glomerular filtrate.
The Collecting Duct Determines the Final Urine pH
The rate of acid secretion by the collecting duct determines the final urine pH and the net acid excretion by the kidney. Despite robust acid secretion in the proximal tubule, because of luminal buffering the pH of the tubule fluid is virtually unchanged by this segment. The segments intervening between the proximal tubule and the connecting segment have minimal acid-secreting ability, and thus the pH of the tubule fluid that reaches the connecting segment is still similar to that of the glomerular filtrate, pH approximately 7.4. However, the normal urine pH of carnivores ranges from 5.5 to 7.5, that of ruminants ranges from 6 to 9, and even greater extremes of pH in response to acidosis and alkalosis are possible. The collecting duct is responsible for this ability to excrete urine with a pH extremely different from that of plasma.
The Collecting Duct Can Secrete Protons and Generate Acidic Urine
In contrast to the proximal tubule, which is a high-capacity, low-gradient system of H+ secretion, the collecting duct has a lower capacity for H+ secretion but can generate a steep H+ concentration gradient.
Acid secretion in most of the collecting duct system is a function of a specialized group of cells, the intercalated cells (see Figure 42-12). Intercalated cells are present in the connecting segment, cortical collecting duct, and medullary collecting duct. Intercalated cells contain abundant cytoplasmic carbonic anhydrase, which catalyzes the formation of intracellular H+ and HCO3− from intracellular H2O and CO2. H+ is secreted into the tubule fluid across the apical plasma membrane of intercalated cells by the electrogenic proton pump, H+-ATPase, or by the electrically neutral H+,K+-ATPase pump. HCO3− is transported across the basolateral plasma membrane to the blood side of the cell by a Cl−/HCO3− exchanger similar to the Cl−/HCO3− exchanger in red blood cell membranes (Figure 44-3). The acid-secreting intercalated cells alter the rate of H+ secretion by altering the numbers of proton pumps in the apical plasma membrane. The insertion or removal of proton pump–containing membrane vesicles causes structural changes that reflect the physiological response (Figure 44-4). Furthermore, in some species the Cl−/HCO3− exchanger is translocated from intracellular compartments to the basolateral plasma membrane in acidosis. In this way, the acid-secreting intercalated cells respond to changes in the acid load and alter acid secretion accordingly.
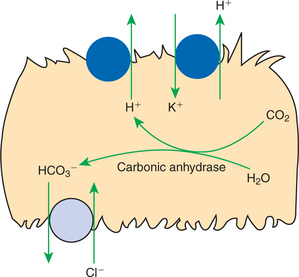
FIGURE 44-3 Schematic illustration of the mechanisms of H+ secretion and HCO3− reabsorption in the acid-secreting intercalated cells of the collecting duct. Two means of active transport of H+ across the apical plasma membrane are present: the electrogenic proton pump, H+-ATPase, and the electrically neutral H+,K+-ATPase pump. The intracellular formation of H+ and HCO3− from CO2 and H2O is catalyzed by the enzyme cytoplasmic carbonic anhydrase. The basolateral plasma membrane contains a Cl−/HCO3− exchanger that allows HCO3− reabsorption.
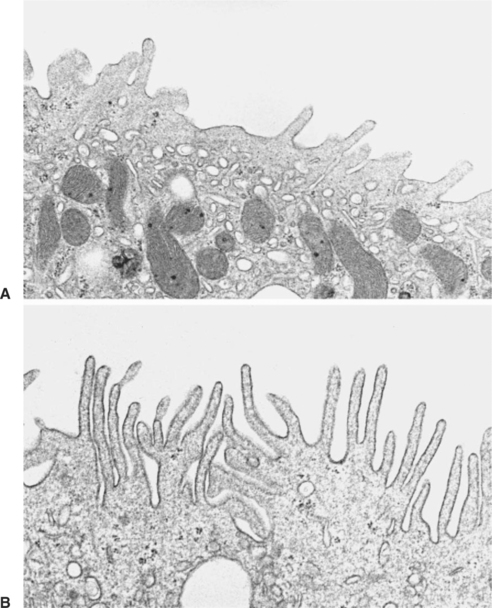
FIGURE 44-4 Transmission electron micrographs of an acid-secreting (type A) intercalated cell from rat cortical collecting duct. A, In a control animal, the apical plasma membrane contains few small membranous projections, and the apical cytoplasm is filled with numerous membrane vesicles. B, In a rat with acute respiratory acidosis, the apical surface is covered with numerous long, membranous projections, and the number of apical cytoplasmic vesicular profiles is greatly reduced. This is the result of the insertion of membrane vesicles containing H+ transporters into the apical plasma membrane in response to acidosis, thus enhancing the acid-secreting capacity of the cell. (Magnification × 11,300.)
The activity of the H+,K+-ATPase pump is enhanced by hypokalemia (low serum K+ level), and thus the contribution of the H+,K+-ATPase pump to renal acidification is augmented during potassium restriction. Mineralocorticoid hormones, such as aldosterone, also enhance acidification in the collecting duct. It has been postulated that this increase in H+ secretion results from an increase in the numbers of proton transporters—either H+-ATPase or H+,K+-ATPase, or both—in the apical plasma membrane of the acid-secreting intercalated cells, which is similar to the adaptive response seen in acidosis.
The terminal segments of the inner medullary collecting duct, where there are few or no intercalated cells, also can secrete acid. An Na+/H+ exchanger, an electrogenic proton pump, a H+,K+-ATPase pump, and substitution of NH4+ for K+ on the basolateral Na+,K+-ATPase all may participate in acid secretion in this region, but the relative importance of these mechanisms is currently unclear.
The Collecting Duct Can Secrete Bicarbonate and Generate Alkaline Urine
The proximal tubule reabsorbs HCO3− and secretes H+, regardless of the plasma HCO3− concentration and the blood pH. In fact, as the plasma HCO3− concentration increases, the concentration of HCO3− in the glomerular filtrate increases, and the amount of HCO3− reabsorption by the proximal tubule epithelium also increases. The amount of H+ secretion and HCO3− reabsorption in this segment is generally determined by the concentration of intraluminal buffers rather than by the need for conserving or excreting acid or base.
However, the collecting duct is capable of net HCO3− secretion in response to alkalosis. Net HCO3− secretion is a function of the connecting segment and the cortical collecting duct in mice, rats, and rabbits. A distinct subset of intercalated cells (type B intercalated cells) is present in these segments (Figure 44-5). These cells are rich in carbonic anhydrase, secrete HCO3− by an apical Cl−/HCO3− exchanger, and have a basolateral electrogenic proton pump. Although the apical Cl−/HCO3− exchanger is distinct from the basolateral Cl−/HCO3− exchanger in acid-secreting intercalated cells, bicarbonate-secreting cells functionally represent a mirror image of acid-secreting cells, with active H+ reabsorption and exchange of Cl− in the tubule fluid for intracellular HCO3− (Figure 44-6).
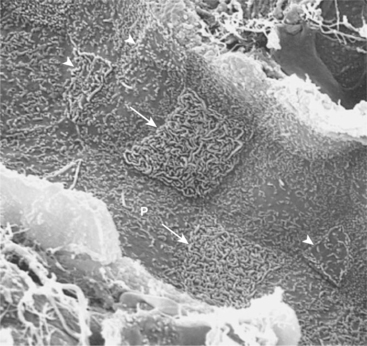
FIGURE 44-5 Scanning electron micrograph of rat cortical collecting duct viewed from the tubule lumen. Three cell types are evident. The principal cells (P) are large, with a single central cilium and few apical surface microprojections. The type A (acid-secreting) intercalated cells (arrows) have a large apical surface covered with extensive membrane folds (microplicae). The type B (bicarbonate-secreting) intercalated cells (arrowheads) have a small apical surface area covered with sparse microprojections, either microvilli or a mixture of microvilli and microplicae. (Magnification × 4000.)
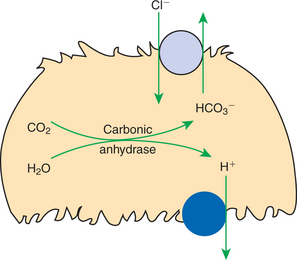
FIGURE 44-6 Schematic illustration of the proposed mechanism of HCO3− secretion (H+ reabsorption) in the type B intercalated cell of the cortical collecting duct. These cells contain H+-ATPase in the basolateral plasma membrane and are rich in cytoplasmic carbonic anhydrase. Functional immunocytochemical evidence indicates that a Cl−/HCO3− exchanger is present in the apical plasma membrane.
The regulation of bicarbonate secretion is an area of active research. Bicarbonate secretion is stimulated not only by models of alkalosis, but also by aldosterone analogues and by dietary Cl− restriction. In Cl− depletion, however, the amount of Cl− delivered to the type B intercalated cells may fall so low that bicarbonate secretion is blocked because not enough Cl− is available for exchange with intracellular HCO3−. For this reason, Cl− depletion may produce metabolic alkalosis.
Little is known about the comparative physiology of renal control of acid-base balance in domestic animals, although proximal tubules and collecting ducts exist in all mammals examined to date, and intercalated cells have been observed at least in cat, dog, and horse collecting ducts. However, it is likely that considerable anatomical and functional differences exist among species, particularly between carnivores, which usually excrete acid urine, and ruminants, which usually excrete neutral or alkaline urine.
CLINICAL CORRELATIONS
Respiratory Acidosis with Renal Compensation
History.
A 6-year-old male German shepherd is brought to you with complaints of weakness, exercise intolerance, and inappetence that have progressively worsened over the past 6 weeks.
Clinical Examination.
The dog is recumbent and anxious. Respiration is labored, and the heart rate is rapid, but pulses are strong and regular. Crackles are heard over all lung fields. Thoracic radiographs reveal a diffuse, severe pulmonary interstitial and alveolar infiltrate with enlargement of the hilar lymph nodes. You obtain samples of blood and urine for a complete blood cell count (CBC), serum chemistry panel, urinalysis, and arterial blood gas (ABG) measurement. The urine pH is 5.0, and the ABG results are as follows: pH, 7.37 (normal, 7.45); Po2, 58 mm Hg (normal, 80-100 mm Hg); Pco2, 70 mm Hg (normal, 31-35 mm Hg); and HCO3−, 37 mEq/L (normal, 18-24 mEq/L).
Comment.
The dog has chronic respiratory acidosis caused by severe pulmonary infiltrates. The lung is unable to ventilate adequately, and the blood level of CO2 rises. The elevated CO2 favors the production of carbonic acid, which releases H+ and lowers the blood pH. Although the increased CO2 level contributes to an increase in blood HCO3− levels, the marked increase in the blood HCO3− levels in this case results from enhanced renal retention of HCO3− and secretion of H+. Acidemia also enhances ammoniagenesis in the proximal tubule, thereby enhancing the generation of new bicarbonate and acid excretion in the form of ammonium ion. Respiratory acidosis activates the acid-secreting intercalated cells in the collecting duct, where HCO3− is reabsorbed and H+ is secreted. The blood HCO3− concentration rises and helps return the blood pH toward normal. A steep H+ gradient is established in the collecting ducts, and acid urine is excreted.
Treatment.
Diagnose and correct the pulmonary disease, if possible. Bicarbonate therapy is not indicated, because the blood bicarbonate level is already high, and the blood pH is partially corrected. Oxygen therapy may improve the Pao2 and help support the animal until specific treatment is instituted.
Metabolic Alkalosis with Paradoxical Aciduria
History.
You examine a 3-year-old Holstein-Friesian cow that has been inappetent for 2 to 3 days. The cow recently calved and freshened normally, but milk production has dropped in the last 2 days, and the feces are loose.
Clinical Examination.
Physical examination reveals dehydration and an elevated heart rate. Percussion of the abdomen reveals an area of high-pitched resonance on the right side. A distended abomasum is palpable on rectal examination. You diagnose a right displaced abomasum and suspect abomasal torsion. Attempts to correct the displacement by rolling the cow fail. The cow is transported to your clinic for surgery, and samples are obtained for a CBC, serum chemistry profile, and urinalysis. The serum K+ level is 2.7 mEq/L (normal, 4.0–5.1 mEq/L), serum Cl− level is 77 mEq/L (normal, 85–103 mEq/L), and total CO2 concentration (approximately the same as serum HCO3− concentration) is 35 mEq/L (normal, 24–27 mEq/L). The urine pH is 6.0.
Comment.
The cow has hypokalemic, hypochloremic, metabolic alkalosis secondary to abomasal displacement. The alkalosis was initiated by continued secretion of HCl by the abomasum and blunted HCO3− secretion by the intestine after the gastrointestinal obstruction. The hypokalemia is a result largely of intracellular movement of K+ secondary to alkalosis and may not reflect a decrease in total body K+ levels.
The expected renal response to alkalosis is the excretion of alkaline urine. However, in this case the volume contraction and hypochloremia prevent the formation of alkaline urine, and the result is paradoxical aciduria. As mentioned, the proximal tubule reabsorbs the filtered HCO3−, regardless of the plasma pH or serum HCO3− concentration. The volume depletion enhances Na+ reabsorption, primarily through the action of aldosterone, and Cl− and H2O reabsorption are enhanced as a secondary reaction to the increased Na+ uptake.
Renal secretion of HCO3− occurs by apical exchange of Cl- in the tubule fluid for intracellular HCO3− in type B intercalated cells in the collecting duct. Because NaCl is avidly reabsorbed to combat volume depletion, little Cl− remains for exchange with HCO3−, and net HCO3− secretion does not occur. Acid secretion in the collecting duct is known to increase in response to aldosterone and may be enhanced in this volume-depleted animal. Hypokalemia activates the acid-secreting intercalated cells in the collecting duct and thus may contribute to the excretion of acid urine in this case.
Brenner BM, ed. Brenner & Rector’s the kidney, ed 7, E-dition, Philadelphia: Saunders, 2004.
Seldin SW, Giebish G. The kidney: physiology and pathophysiology, ed 3, Philadelphia: Lippincott Williams & Wilkins, 2000.
Wagner CA, Finberg KE, Breton S, et al. Renal vacuolar H+-ATPase. Physiol Rev. 2004;84:1263.
Wall SM. Recent advances in our understanding of intercalated cells. Curr Opin Nephrol Hypertens. 2005;14:480.
Weiner ID. The Rh gene family and renal ammonium transport. Curr Opin Nephrol Hypertens. 2004;13:533.
PRACTICE QUESTIONS
1. In carnivores, the usual role of the kidney in maintaining acid-base homeostasis is to:
2. The bulk of acid secretion (bicarbonate reabsorption) is accomplished by which renal tubule segment?
3. Which of the following factors does not contribute to efficient acid excretion (bicarbonate reabsorption) by the renal tubules?
4. Which of the following statements regarding mechanisms of acid-base regulation by the collecting duct is false?