Chapter 54 Antigens and Innate Immunity
1. Antigens (or immunogens) stimulate immune cells to induce an immune response.
2. The degree of immune response depends on several characteristics of the antigen.
Body’s Defense Against Invading Antigens
1. Both nonimmune and immune mechanisms defend against invading antigens.
2. A first line of defense includes the skin and certain external and internal body fluids.
3. A second line of defense consists of phagocytic cells of the myeloid and macrophage-monocyte lineages.
4. Macrophage-derived cytokines can induce a variety of physiological processes to help combat infectious antigens.
The immune system performs two vital functions that are critical for the maintenance of homeostasis and survival: (1) inducing an effective and safe response against foreign antigens (infectious and noninfectious) and (2) avoiding a response to components of “self” antigens by enforcing stringent regulatory controls over dangerous self-reactive cells that are capable of mounting devastating immune attacks on “self” tissues. Because the induction of immune responses depends on antigens, this chapter first discusses the nature and characteristics of antigens.
ANTIGENS
Antigens (or Immunogens) Stimulate Immune Cells to Induce an Immune Response
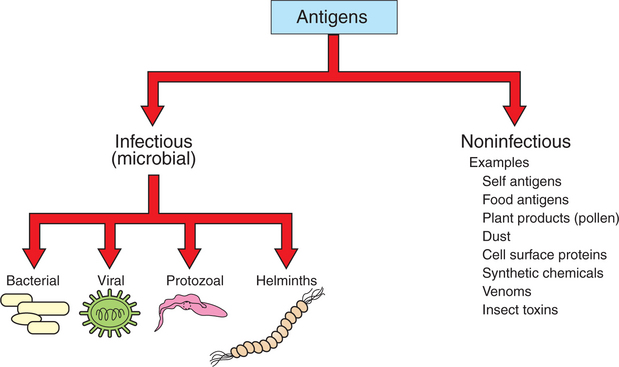
An antigen, or immunogen, is defined as any substance that is capable of stimulating immune cells (T and B cells) to induce an immune response. Antigens can be broadly divided into two large categories: (1) infectious (microbial) and (2) noninfectious (Figure 54-1). Infectious antigens include components that are derived from bacteria, viruses, protozoa, and helminths. Noninfectious antigens include those derived from “self” (autoantigens), food, plants, dust, or insect and animal venoms, as well as synthetic and cell surface proteins.
An antigen is composed of many molecular units to which an antibody binds. These small units on an antigen are called antigenic epitopes, or antigenic determinants. Thus a single antigen may be composed of many antigenic epitopes. In the strictest sense, antibodies bind to an antigenic epitope of an antigen. Some of these antigenic epitopes are shared among different bacteria (e.g., epitopes on Brucella and Yersinia) or between a bacteria and host cells (e.g., Mycobacterium heat shock proteins and synovial tissue; Mycoplasma and lung tissue). These types of antigenic epitopes are called cross-reactive epitopes.
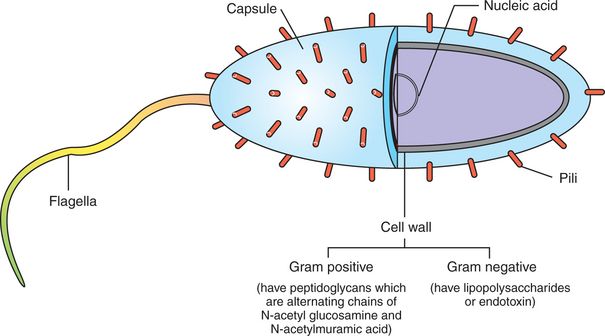
Figure 54-2 shows the following antigenic structures of bacteria:
1. Bacterial cell wall. Cell walls of gram-positive bacteria differ from those of gram-negative bacteria. Gram-positive bacteria are composed of a thick layer of short chains of amino acids or peptides and carbohydrates (peptidoglycans). The cell wall of gram-negative bacteria has a thin layer of peptidoglycan and is largely composed of lipopolysaccharides, which are potent endotoxins.
2. Capsule. Certain bacteria produce a protective outer covering called a capsule, which is composed of polysaccharides.
3. Pili. These small, hairlike protein structures on some bacteria enable the bacteria to adhere to target host cells and transfer genetic information from one bacterium to another.
4. Flagella. Some bacteria possess flagella for mobility. Flagella contain a protein called flagellin, which can be antigenic.
5. Nucleic acids. Nucleic acids, such as bacterial deoxyribonucleic acid (DNA), tend to be antigenic because of differences in methylation compared with mammalian DNA. The antibodies against bacterial DNA tend to cross-react with the host’s DNA.
Viruses have nucleic acids (ribonucleic acid [RNA] or DNA), surrounded by a protein coat called a capsid. Some viruses have an envelope, a lipid membrane–like structure covering the capsid. On the envelope are glycoprotein projections that the viruses use to attach to the host target cells. All these components may be antigenic.
External structures of protozoa and helminths tend to be antigenic. Similarly, fungal spores are antigenic. Pollens, glycoproteins of certain foods, the unique biochemical structure of synthetic chemicals, insect saliva, and venoms are all good antigens. It is beyond the scope of this chapter to discuss each of these antigens in detail.
The immune system is exposed to and tolerates “self” antigens found on all of its own tissues. These antigens can be cell surface antigens (e.g., thyroglobulin, myelin peptides) or internal antigens (e.g., cardiolipin, nucleic acids, histones). In certain individuals who are allergic, antigens derived from food (e.g., peanuts, strawberries, fish) or plants (e.g., pollen, spores) induce an immediate and potent immune reaction. Many synthetic chemicals and drugs are minute in size and tend to be adsorbed onto cell surface antigens to create a new antigenic epitope. With ever-increasing synthesis of chemicals (pesticides, agricultural chemicals, drugs, and consumer products, to name a few), it is likely that synthetic chemicals may become an important class of antigens in the future.
The Degree of Immune Response Depends on Several Characteristics of the Antigen
The degree of immune response induced by an antigen is called antigenicity or immunogenicity. Understanding the characteristics of antigens that provoke a strong or weak immune response provides important insight into the body’s ability to combat invading antigens successfully. Furthermore, this understanding is useful in designing a vaccine preparation with potent antigenicity. Characteristics that contribute to potent antigenicity include the following:
1. Foreign versus self antigens. Antigens that are considered to be foreign to the host tend to be highly antigenic. For example, if a horse is injected separately with antigens that are derived from a dog or from its own tissues, the horse will mount a strong immune response to the dog antigens, but not to its own (self) tissues.
2. Size. The size of an antigen also influences the level of immune response. Large antigens enable better processing by antigen-presenting cells (e.g., macrophages, dendritic cells) and subsequent presentation of antigenic peptides to lymphocytes for induction of an immune response. Examples of large antigens include bacterial and insect toxins, viral capsids, surface proteins on protozoa and helminths, and venoms. At the other extreme, very small antigens (e.g., small synthetic antigens, endogenous hormones, pesticides, etc.) tend to be ineffective in provoking an immune response. Very small antigens are inherently incapable of inducing immune responses; however, when bound to a larger protein, they can be potent antigens. Such small compounds are referred to as haptens. A good example of a hapten is a poison ivy–derived chemical, urishiol, which readily combines with many proteins (e.g., skin proteins) to induce a vigorous immune response.
3. Biochemical structure and complexity. In general, proteins tend to be more antigenic than lipids or carbohydrates. Large size alone is insufficient to provoke a good immune response. For example, many sugars and lipids, even though large in size, are ineffective in inducing an immune response because they consist of simple repeating units (e.g., repeating sugars in starch), which lack complexity. Complex carbohydrates and lipids, on the other hand, as found in many microbes, are strong immunogens. Carbohydrates and lipids, when combined with protein to form glycoproteins and lipoproteins, respectively, have increased complexity and thus are good antigens.
4. Stability and degradability. For immune cells to respond, stability of an antigen is an important feature. Flexible antigens, such as flagellin in a bacterium, are poor immunogens. However, when stabilized and rendered less flexible, as done in vaccine preparations, flagellin tends to be a potent immunogen. For an immune response to be initiated, the antigen ingested by phagocytic cells (e.g., macrophages) must be degraded and broken down into small peptides. Lymphocytes (T cells) will only respond to the peptides and not to large, native molecules. Antigens such as steel pins or plastic heart valves, even though large and complex, are inert and not degradable and thus are not good antigens.
Large, complex proteins (or lipoproteins or glycoproteins) that can be degraded and processed therefore tend to be excellent antigens. Other parameters that influence an individual’s ability to respond to antigens include genetics (e.g., major histocompatibility complex genes), endogenous biomolecules that regulate and modulate immune responses (e.g., hormones, neuropeptides), and the level and route of exposure of antigens.
An antibody that is induced in response to an antigen will specifically bind to the antigen. Any minor alteration of the antigen will negatively impact the antibody’s ability to bind to an antigen. Therefore, invading microbes often alter their antigens to prevent the binding of induced antibodies, thus avoiding immune attack.
BODY’S DEFENSE AGAINST INVADING ANTIGENS
Both Nonimmune and Immune Mechanisms Defend Against Invading Antigens
The body is confronted with literally billions of antigens. Consequently, a unique challenge presented to the immune system is to respond effectively only to foreign antigens while refraining from response to “self” antigens. The induction of immune responses requires energy and protein, and antigens require extensive cellular division (and hence utilization of protein reserves), the body cannot mount immune responses to each of the innumerable antigens it encounters constantly. Instead, the body is well equipped to handle antigens effectively before resorting to a specific immune response.
Initially, most antigens are effectively handled by nonspecific defense mechanisms, such as impervious and formidable physical barriers (e.g., skin and other body surfaces) and antimicrobial body fluids (e.g., lysozymes in tears, saliva, gastric juices). These are considered the first line of defense and are discussed next. Should the antigen survive this “body armor,” phagocytic cells (e.g., neutrophils, macrophages-monocytes) and natural killer (NK, NK-T) cells can effectively eliminate the invading antigens. These cells ingest and destroy a wide range of antigens and thus are non–antigen specific. These cellular defenses constitute a second line of the body’s defense. The body’s initial defense (physical, chemical, and phagocytic, antigen-presenting cells, and natural killer cells) constitute the innate immune system. The antigen-presenting cells closely interact with specific T and B cells to induce a specific immune response. Thus, specific immune responses by the “adaptive” immune system tend to be the last line of the body’s defense (see Chapter 55). Collectively, both nonimmune and immune mechanisms effectively counter invading microbes.
A First Line of Defense Includes the Skin and Certain External and Internal Body Fluids
The physical nonimmune defense barriers include external body surfaces such as skin and internal body surfaces such as the gastrointestinal (GI), reproductive, respiratory, and the urogenital tracts. The skin plays a major role in preventing the entry of organisms through a variety of nonimmunological means, including the secretion of sebum from sebaceous glands, which maintains a low pH, and secretion of enzymes that are not conducive for the invading pathogens. Periodic natural desquamation of the skin also results in sloughing off any invading pathogens. Nonpathogenic bacteria also occupy skin surfaces, thereby preventing the adherence of pathogenic organisms to their target cells, which is prerequisite for entry into the body. Any changes in the skin, such as cuts, burns, and dry or very humid skin, will result in the entry of microbes. In addition to the nonimmunological mechanisms, skin is also rich in dendritic cells (Langerhans cells) and γ-δ T cells that contribute to warding off invading pathogens. The natural flushing action of urine and milk assist in elimination of infectious antigens, as evidenced by the infectious conditions that result from stasis of urine or milk.
Many body fluids are inhospitable to invading pathogens. For example, mucus in the mucosal tissues (respiratory, urogenital, and GI tracts), saliva, tears, gastric juices, and urine are rich in enzymes (e.g., lysozymes) and are low in pH. As with the skin, the GI tract is covered with nonpathogenic bacteria, which prevent the adhesion of pathogenic bacteria to their target cells. Furthermore, resident normal bacterial florae in gastric tissues secrete butyric or lactic acids, which not only maintain a low pH in gastric fluids, but also are bacteriostatic to other microbes. Vaginal epithelium is rich in glycogen and promotes the growth of Lactobacillus, which secretes lactic acids. In the respiratory tract the antigen load is decreased by a variety of mechanisms, including the turbulence created when air is inhaled due to the anatomical construction of the lower respiratory tract, which narrows and branches. Microorganisms in inhaled air are carried by this turbulence and are forced onto the walls of the respiratory tract, which are rich in sticky mucus and bactericidal lysozymes. The ciliary action of the respiratory tract also eliminates antigens effectively.
A Second Line of Defense Consists of Phagocytic Cells of the Myeloid and Macrophage-Monocyte Lineages
Should an antigen survive the first line of the body’s defense (i.e., body surfaces) and penetrate into blood vessels and tissues, the body’s defense relies on cellular response. Key cells involved in cellular defense are phagocytic cells, which are an integral part of innate immunity. These cells, based on their cellular origin, are broadly divided into myeloid and macrophage-monocyte lineages. Included in the myeloid lineage are neutrophils, eosinophils, and basophils. The macrophage-monocyte series includes monocytes and macrophages. Neutrophils constitute the largest percentage of white blood cells in most species (60%-65%), except in ruminants (20%-25%). Neutrophils have a short life span in the blood (half-life, ~12 hours), but in tissues their longevity increases to several days. Neutrophils are approximately 12 μm in diameter, with multilobulated nuclei and a cytoplasm rich in granules. The population of granules is composed of both primary and secondary granules. The primary granules contain important bactericidal enzymes such as myeloperoxidase, lysozymes, acid hydrolases (e.g., β-glucuronidase, cathepsin), and neutral proteases against elastase hydrolases. Neutrophils also have defensins, small proteins that are inserted between the lipid bilayers and disrupt the interactions emanating from lipid membranes. The secondary granules include lysozymes, lactoferrin, and collagenases.
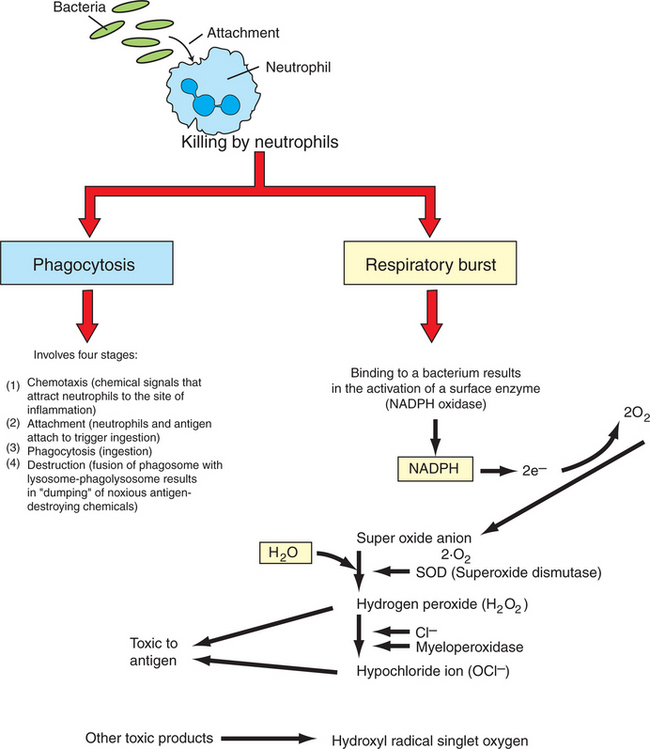
Neutrophils are considered the first responder cells to combat invading antigens. The primary function of neutrophils is to capture and destroy antigens. Neutrophils, unlike monocytes or macrophages, respond rapidly to invading antigens and readily phagocytose antigens. However, neutrophils lack the capacity to present antigens to lymphocytes. Neutrophils destroy antigens by two different but complementary mechanisms: (1) phagocytosis and (2) respiratory burst. Phagocytosis in turn is divided into four arbitrary stages: (a) chemotaxis, (b) adherence or attachment, (c) phagocytosis, and (d) destruction (Figure 54-3).
Neutrophils are attracted to the site of infection in tissues by chemokines and chemical messengers that are released when tissues are damaged. In response to chemical signals and chemokines, vascular endothelial cells induce the expression of adhesion molecules. Neutrophils bind to these cellular adhesion molecules (CAMs) through specific receptors and are triggered to leave the circulation by crossing capillary walls (diapedesis) into tissues. Once neutrophils move out of the circulation, they move toward the antigen. The contact between neutrophils and antigen is greatly facilitated when antigens are coated or bound by a host’s proteins, such as complement or antibodies. These proteins that enhance contact and phagocytosis by neutrophils or other phagocytes are called opsonins. The contact of neutrophils with antigens triggers infolding of the cell membrane (by the action of actin and myosin), and the antigen is trapped in a vacuole called a phagosome. The primary granules move toward the phagosome and fuse their membrane to become phagolysosomes, and in the process the granules release deleterious bacteriostatic and bactericidal biomolecules. Thus, in the contained environment of phagolysosomes, the antigen is destroyed.
A concurrent mechanism by which neutrophils kill invading microbial antigens involves respiratory burst (see Figure 54-3). On contact of neutrophils with an antigen, consumption of oxygen is immediately increased, 70- to 100-fold. This results in activation of an enzyme, NADPH oxidase, which forms an electron transport chain with cytosolic NADPH as an electron donor of oxygen. A molecule of oxygen accepts two donated electrons to result in a superoxide anion (O2−). This O2−, under the influence of the enzyme superoxide dismutase and in the presence of water, will chemically react to yield hydrogen peroxide (H2O2), which is toxic to microbes. This H2O2, under the influence of myeloperoxidase and utilizing chloride ions (Cl−), catalyzes oxidative reactions to form H2O2 and halide ions. All these products are highly toxic to the antigens. Neutrophils are also known to release hydroxyl radical singlet oxygen, which is toxic to bacteria.
Neutrophils have limited energy and a relatively short life. Elastases and collagenases released from these dying neutrophils serve as a powerful chemoattractant for another group of phagocytes called macrophages, and therefore neutrophils are sometimes referred to as “martyrs of the immune system.” Macrophages are attracted by bacterial products as well but also through the chemotactic factors released from damaged tissues. Macrophages differ from neutrophils in several important aspects. Macrophages, even though not rapid-responder cells, have extensive ability to phagocytose antigens repeatedly. These long-lived cells secrete large quantities of cytokines and chemokines that play a key role in regulating immune responses. Some of these cells even have the ability to present antigens to the immune system. Macrophages are present throughout body tissues where entry of antigens is likely.
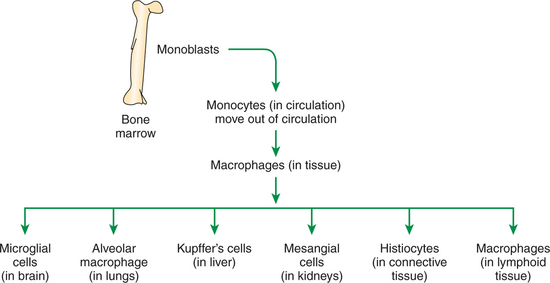
Macrophages are round or elongated cells and express many surface receptors that include major histocompatibility complex antigens I and II (MHC class I and II). MHC classes I and II play a major role in antigen recognition and presentation (see Chapter 55). Macrophages differ in their morphology based on tissues and thus are called by different names. For example, in the lymphoid organs, these cells are macrophages, whereas in the liver they are known as Kupffer’s cells (Figure 54-4). Macrophages are derived from the bone marrow hematopoietic cells and are initially called monoblasts, which mature and move into circulation and are known as monocytes. When monocytes move into the tissues, they are called macrophages. Macrophages are larger than neutrophils and are rich in rough endoplasmic reticulum and Golgi bodies, indicating their extensive ability to produce and secrete immunoregulatory proteins.
Ingestion of antigen will activate the metabolic machinery of macrophages, including increasing lysosomal and bactericidal activity and upregulating the inducible nitric oxide synthase gene (iNOS) that encodes for iNOS protein, which in turn is responsible for enhanced release of potent antimicrobial nitric oxide. Macrophages also release several oxygen free radicals that are also antimicrobial. Thus, macrophages aid in killing of antigens by phagocytosis and by respiratory burst (oxygen and nitrogen free radicals) (see Figure 54-3). Macrophages can potentially secrete more than 100 proteins. Some of these proteins, such as interleukin 1 (IL-1), interleukin-12 (IL-12), tumor necrosis factor alpha (TNF-α), interleukin 18 (IL-18), and interleukin 27 (IL-27), play a central role in activating lymphocytes, especially naïve T lymphocytes (Figure 54-5). After an effective immune response is initiated, macrophages also secrete cytokines such as interleukin 10 (IL-10) and transforming growth factor beta (TGF-β) which downregulate the immune response.
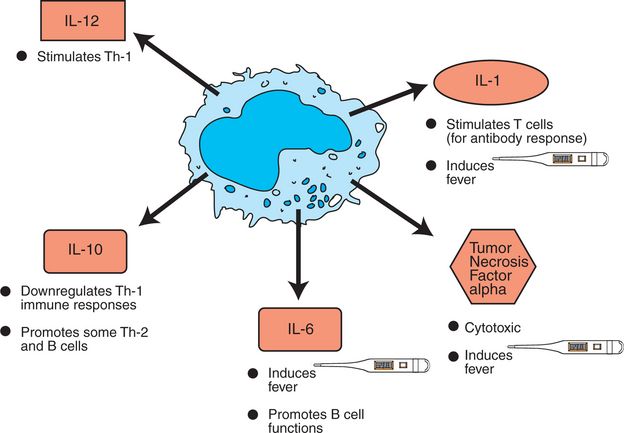
FIGURE 54-5 Macrophage-derived cytokines. IL, Interleukin; Th-1, T-helper cells type 1; Th-2, T-helper cells type 2.
Once the antigen is cleared by macrophages, these cells also play an important role in repairing the damaged tissues. Macrophages secrete angiogenic factors to enhance the blood supply. For example, IL-1 secreted by macrophages stimulates fibroblasts to secrete collagen to rebuild tissues.
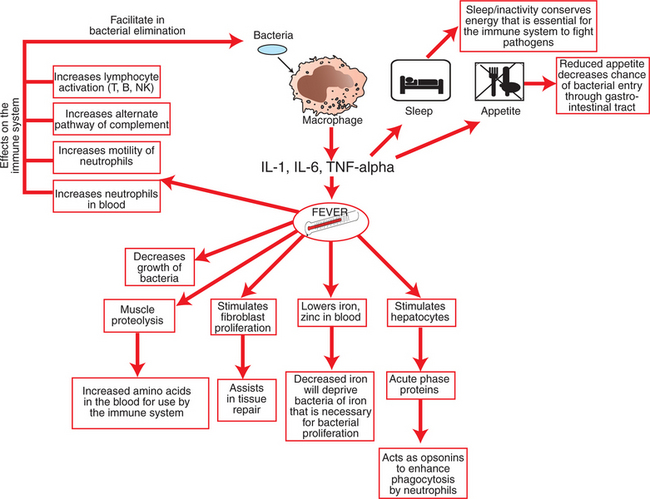
Macrophage-Derived Cytokines Can Induce a Variety of Physiological Processes to Help Combat Infectious Antigens
Physiologically, a key component of combating infectious antigens is the induction of fever, which is mediated by the release of pyrogenic cytokines such as IL-1, IL-6, and TNF-α by macrophages (Figure 54-6). These cytokines act on multiple tissues to mount a coordinated effort aimed at eliminating the invading microbes. For example, they act on thermoregulatory regions in the hypothalamus to induce fever, which is an integral part of this process. Fever accelerates the mobility of neutrophils, enhances their phagocytic ability, and activates lymphocytes and complement proteins while impeding the growth of bacteria. These cytokines also act on the liver to stimulate acute-phase proteins that function as opsonins to promote phagocytosis. Further, these cytokines act on the sleep-associated regions in the hypothalamus to promote sleep in an effort to conserve energy and redirect energy toward the infectious challenge. Because major immunoprotective elements (e.g., antibodies, cytokines, complement) are all proteins, these cytokines increase the amino acid pool by acting on muscles and inducing mild proteolysis and release of amino acids, which are essential for synthesis of various immunoprotective elements.
Another important feature of some macrophages is their ability to present antigens to stimulate T cells in order to start the specific adaptive immune response. Other cells are part of the innate immune system and also serve as antigen-presenting cells (APCs); these include dendritic cells and B cells. Dendritic cells are considered the most potent cells in presenting antigens. These cells have long dendrites that physically allow them to interact simultaneously with many antigens. Dendritic cells are abundant in lymphoid organs, skin, and other tissues that frequently encounter antigens. The role of APCs in the adaptive immune response is discussed in Chapter 55.
CLINICAL CORRELATIONS
Swollen Lymph Nodes in a Colt
History.
A 2-year-old colt who just returned from training has shown marked bilateral nasal discharge for at least 2 days. The colt has not been eating for the last day and appears depressed. The colt also seems to have considerable swelling under the jaw and in the throatlatch area.
Clinical Examination.
On physical examination the colt has a temperature of 39.2° C (102.7° F) but a normal heart rate of 36 beats/min and normal respiratory rate of 12 breaths/min. The horse has bilateral mucopurulent nasal discharge. The lymph nodes underneath the jaw and in the throat area are both greatly swollen. No other abnormalities are present.
Comment.
This colt was vaccinated for Eastern and Western encephalitis as well as rhinopneumonitis, influenza, rabies, tetanus, and West Nile virus. However, the colt was not vaccinated against Streptococcus equi, the causative agent of strangles. Young horses not vaccinated against S. equi that have been traveling are at increased risk for disease. Based on the clinical signs of fever, bilateral mucopurulent discharge, and normal lung sounds and respiratory rate, this disease is likely an upper respiratory infection. The vaccination history and clinical signs indicate S. equi is the most likely pathogen.
Horses are exposed to S. equi through inhalation, ingestion, or exposure to conjunctival surfaces; association with other infected horses; or a contaminated environment. The bacteria enter the cells of the tonsillar crypts and ventral surface of the soft palate and migrate throughout the upper respiratory tract, infecting the lymph nodes as well and replicating there extracellularly. Most cases resolve over a prolonged period. In other horses, however, the guttural pouches can become infected, or the animal can become systemically infected and develop abscesses in other parts of the body (e.g., lymph nodes). In the latter situation the syndrome is known as “bastard strangles.”
The immune response associated with infection begins with the mucosal innate response. Horses will have increased levels of neutrophils in their blood (neutrophilia). The number of lymphocytes in the blood may vary, but there is a marked reaction in the local lymph nodes to the infection. These horses have severe lymphadenopathy of the regional nodes from increased numbers of neutrophils and lymphocytes. A combination of both the innate and the acquired immune response is capable of controlling the infection, and eventually the horse eliminates the pathogen. Again, however, in a small percentage of the infected horses, S. equi migrates to the systemic lymph nodes to cause bastard strangles at some later time point in life.
A diagnosis can be made based on history, clinical signs, and combined diagnostic testing. Traditionally, these horses have neutrophilia in their peripheral blood. Definitive diagnosis is based on culturing the agent, often from the regional lymph nodes using an aspirate. In many cases, there is concern about an outbreak within a particular barn because the agent is highly infectious, and infected horses and barns must be quarantined to prevent further spread of the disease. Another test for a definitive diagnosis is polymerase chain reaction (PCR).
Treatment.
The treatment of S. equi infection varies based on clinical signs and the population at risk. In the early stages of infection, with clinical signs of fever, depression, anorexia, and bilateral mucopurulent nasal discharge, but no or limited lymphadenopathy, these horses can often be treated systemically with antibiotics, and the disease will usually resolve. However, once the lymph nodes begin to become enlarged or abscessed, antibiotic treatment will often prevent further spread of infection but will not aid in clearance. When antibiotics are discontinued, the disease continues to progress. Some horses become greatly depressed, with marked lymphadenopathy that can affect breathing. In these animals the lymph nodes are often drained. Sometimes a tracheostomy is needed, in which case horses are treated with systemic antibiotics to prevent secondary infections. Disinfecting the environment is critical to the control of strangles within populations.
Abbas AK, Lichtman AH. Cellular and molecular immunology, ed 5. Philadelphia: Saunders, 2003.
Janeway C. Immunobiology: the immune system in health and disease, ed 6. New York: Garland Science, 2005.
Parham P. The immune system, ed 2. New York: Garland Science, 2004.
Rao CV. Immunology: a textbook. Harrow, UK: Alpha Science, 2005.
Roitt IM, Delves PJ. Essential immunology, ed 10. Malden, Mass: Blackwell, 2001.
Tizzard IR. Veterinary immunology: an introduction, ed 7. Philadelphia: Saunders, 2004.
Acknowledgment
The authors would like to thank Deena Khan and Rebecca Phillips for their contributions in the editing of the chapters in this section.
PRACTICE QUESTIONS
1. Which of the following statements is correct in regard to macrophages?
2. Which of the following is correct about neutrophils?
3. Antigen-presenting cells (APCs) include which of the following?
4. Which of the following is correct with regard to antigen?