Chapter 55 The Specific Immune Response: Acquired Immunity
1. Mature T cells develop from lymphoid stem cells that have migrated to the thymus.
2. T cells are a heterogeneous population of cytotoxic T cells and T-helper cells.
Interactions of Antigen-Presenting Cells and T Cells
1. Major histocompatability complex (MHC) proteins are considered the central regulators of the immune system.
2. MHC class I antigens of infected nucleated cells play a major role in activating cytotoxic T cells.
3. MHC class II antigens on antigen-presenting cells (APC) play a major role in selective activation of T-helper cells.
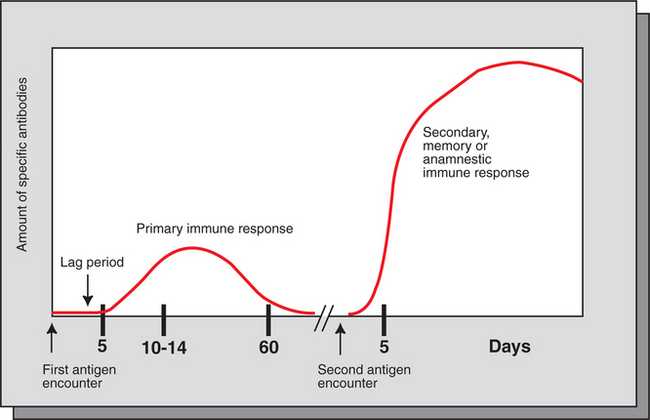
1. Initial exposure to foreign antigen induces slow onset of antibody appearance, whereas subsequent exposure induces faster, longer-lasting antibody appearance.
2. Antibodies, or immunoglobulins, are glycoprotein molecules that can be divided into five isotypes, or classes.
3. The B-cell population produces antibodies to millions of different antigens, yet the antibody-antigen interaction is specific.
4. Expansion of an antigen-specific B–memory cell population on initial antigen exposure results in a faster, more pervasive, secondary immune response.
As discussed in Chapter 54, innate immunity offers effective defense against a wide range of pathogens. Key features of innate immunity include (1) rapid response against invading pathogens, (2) nonspecificity, and (3) physical, chemical, and cellular (phagocytic cells, NK cells) barriers. The response of the innate immune system, however, is not long-lasting and does not induce immunological memory (i.e., ability to recall previous exposure to antigens and respond to these effectively and specifically). For long-lasting immunity, another arm of the immune system must be activated. This is referred to as acquired immunity, which involves activation of T and B lymphocytes. Antigen-presenting cells (APCs), a part of the innate immune system, play a central role in activating lymphocytes. Activated T lymphocytes (T cells) secrete cytokines that are essential for defense against intracellular pathogens, activation of other cells, and coordination of immune responses. B lymphocytes (B cells) have two main functions: (1) secreting antibodies that bind specifically to the antigen that induced the antibody response and (2) acting as APCs.
Before discussing how antigens are presented to specific lymphocytes, it is important to understand the different types of immune cells (Figure 55-1). All cells of the immune system are derived from multipotent stem cells that are located primarily in the marrow of long bones. These multipotent stem cells subsequently give rise to primordial stem cells, such as lymphoid stem cells or myeloid stem cells. Myeloid stem cells give rise to monocytes, which mature in tissues to become macrophages or dendritic cells. Lymphoid stem cells give rise to T, B, natural killer (NK), and lymphoid dendritic cells. Mature cells are found circulating throughout the body but concentrate in the peripheral lymphoid organs (e.g., lymph nodes, spleen) and gut-associated lymphoid tissues, where most of the complex interactions with antigens take place.
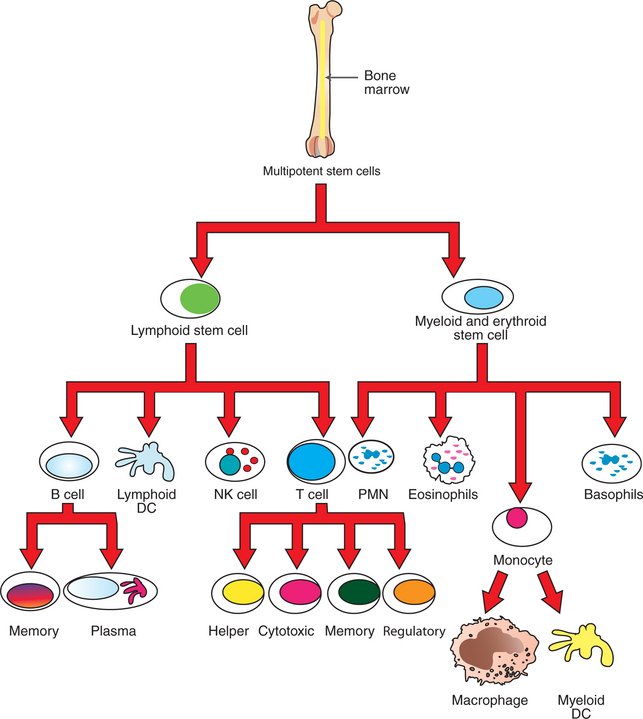
FIGURE 55-1 Lymphopoiesis: development of various types of lymphocytes. DC, Dendritic cell; NK, natural killer; PMN, polymorphonuclear neutrophil leukocytes.
Birds, unlike mammals, have a unique lymphoid organ called the bursa of Fabricius where B cells develop. This round, sac-shaped organ is located above the cloaca. Analogous to the thymus, the bursa consists of lymphocytes embedded in epithelial tissues. Mammals have no precise lymphoid organ that is equivalent to this bursa. Bone marrow and ileal Peyer’s patches are thought to be the principal mammalian organs where B cells develop.
T CELLS (T LYMPHOCYTES)
Mature T Cells Develop from Lymphoid Stem Cells That Have Migrated to the Thymus
Lymphoid stem cells that are destined to become T cells migrate to the thymus and are referred to as thymocytes. (The thymus extends approximately from the base of the trachea to the front of the heart.) The most recent immigrants from bone marrow arrive at the cortex of the thymus and lack important cell surface markers, such as T-cell receptors (TCRs), CD4, and CD8 markers, which are essential for T-cell activation. These immature thymocytes undergo a highly complex and tightly regulated development and maturation process into mature T cells. During development the cells begin to acquire both CD4 and CD8 surface markers (double positive) and TCRs. As the cells further mature, they lose either CD4 or CD8 markers. CD4+/CD8+ cells that lose the CD8 marker become CD4+/CD8− cells and are known as T-helper cells, whereas those double-positive cells that lose the CD4 marker become CD4−/CD8+ cells, or cytotoxic T cells.
The selection for survival of T cells during this developmental process is extremely stringent and discriminating. During development the thymocytes learn two important lessons: (1) T cells respond only to foreign antigens (positive selection), and (2) the cells will not respond to “self” antigens (negative selection). Learning these two critical lessons is essential for the survival of the organism. Therefore, any developing thymocytes that deviate from learning these two key lessons are terminated by apoptosis (negative selection). Consequently, greater than 90% of developing thymocytes die within the thymus. Cells that are marked for intrathymic death include those cells that are defective (i.e., cannot bind to antigens or have truncated receptors) or autoreactive (bind strongly to “self” peptides). Thus, only competent, positively selected T cells (CD4+ or CD8+) are allowed to emigrate out of the thymus as T cells.
T Cells Are a Heterogeneous Population of Cytotoxic T Cells and T-Helper Cells
All T cells express a T-cell antigen receptor (TCR), CD28 and related molecules, and either CD4 (helper cells) or CD8 (cytotoxic cells). TCR specifically binds to antigenic peptides that are presented by APCs. Based on discrete functions of T cells, these cells are subdivided into two major types: (1) helper cells and (2) cytotoxic cells. T-helper (Th) cells secrete proteins called cytokines that act on other immune cells to provide help and coordinate immune responses. Th cells express the CD4 receptor. Cytotoxic T cells kill infected or abnormal cells. These cells express the CD8 molecule (but not CD4) and have granules that are rich in serine esterase granzymes. Cytotoxic T cells also have perforins and lymphotoxins that are important in initiating cytotoxicity.
T-helper cells, based on the predominant cytokines secreted, are further divided into three major types: Th-1, Th-2, and Th-17. Th-1 cells predominantly secrete interleukin-2 (IL-2), interferon-gamma (IFN-β), and tumor necrosis factor beta (TNF-β). Th-1 immunity is critical for defense against intracellular pathogens (viral, bacterial, or protozoal) and certain types of tumors. Failure to generate Th-1 cells creates susceptibility to these infections. Abnormal activation of Th-1 cells can result in a wide variety of inflammatory conditions, including autoimmune states. Th-2 cells predominantly secrete IL-4, IL-6, IL-5, and IL-10. Formation of Th-2 cells is essential for defense against extracellular pathogens, neutralization of toxins and viruses in body fluids, and activation of other cells of the immune system. Abnormal regulation of Th-2 cells leads to allergies. Th-17 subsets predominantly secrete interleukin-17 (IL-17), a cytokine now recognized as an important mediator of inflammatory and autoimmune diseases.
Thus it is clear that the immune system must initiate the correct type of immune response to maintain homeostasis and defend the host appropriately against the invasion of different types of pathogens.
INTERACTIONS OF ANTIGEN-PRESENTING CELLS AND T CELLS
Major Histocompatibility Complex (MHC) Proteins Are Considered the Central Regulators of the Immune System
Activation of specific T cells is highly dependent on interactions with major histocompatibility complex (MHC) proteins, which have a unique ability to bind to processed antigenic peptides. Therefore, MHC proteins are considered the central regulators of the immune system. MHC proteins are encoded by a number of genes that are clustered together on a chromosome and referred to as the MHC locus. The MHC gene complex is inherited as a block of genes and is known to encode three categories of proteins or antigens: class I, class II, and class III antigens. The number of genes that encode class I antigens varies from species to species, with a large number in humans (>30) at one end of the spectrum to a limited number of genes in pigs, turkeys, and cheetahs at the other end. In general, all nucleated cells express class I antigen, which is a single α-chain peptide of approximately 45 kilodaltons (kD) linked to β2-microglobulin (a non-MHC protein thought to be essential for proper folding and stabilization of the α-chain). Class I antigens can bind to peptides (e.g., viral peptides) and serve as receptors for CD8 molecules on cytotoxic T cells. Class I antigens have a high rate of mutation, but no recombination. These mutations allow class I antigens to alter their ability to bind to endogenous, processed antigenic peptides.
MHC Class I Antigens of Infected Nucleated Cells Play a Major Role in Activating Cytotoxic T Cells
Cytotoxic killing of intracellularly infected, cancer, or autoreactive cells is an essential step in survival by containing infected cells or the spread of deleterious cells. For example, a viral infection of any cell in the body yields to viral replication within the cell, and some of these viral peptides will physically bind to intracellular MHC class I antigens (Figure 55-2). This viral peptide–MHC class I complex is carried to the surface and displayed as an altered MHC class I molecule. TCR molecules of effector CD8+ cytotoxic T cells will recognize the class I molecule–peptide complex to initiate cytotoxicity by at least four different, but complementary, mechanisms. First, contact of a cytotoxic CD8+ cell with an infected cell that is displaying a MHC class I–peptide complex will immediately result in cytoplasmic reorganization within the CD8+ cell. This includes the alignment of granules and Golgi apparatus at the site of contact. Perforins in the cytotoxic cells polymerize to form tiny injectable tubes referred to as membrane attack complexes (MACs) that “drill” holes into the target cells. Granzymes are passed from the cytotoxic cells into the target cells through these perforin tubes to initiate apoptosis.
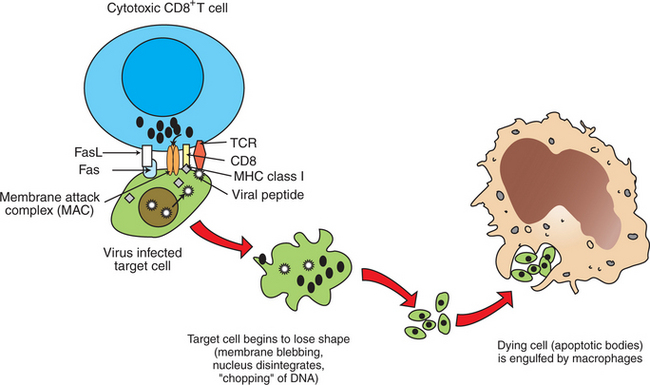
FIGURE 55-2 Mechanism of CD8-mediated cytotoxicity. FasL, Fas ligand; MHC, major histocompatability complex; TCR, T-cell receptor.
The other three mechanisms by which CD8+ cells induce apoptosis of target cells are (1) the secretion of lymphotoxin α (tumor necrosis factor alpha, TNF-α), which binds to its specific receptor on the target cells to initiate apoptosis; (2) the interactions of CD95 ligand on T cells with the CD95 “death” receptor on target cells; and (3) secretion of granulysin, an antibacterial peptide found in granules that activates lipid-degrading enzymes (sphingomyelinases). This in turn results in an increase in saponins, including ceramide, which increase apoptosis. Granulysin kills not only infected target cells, but also bacteria, thus containing the infection.
MHC Class II Antigens on Antigen-Presenting Cells Play a Major Role in Selective Activation of T-Helper Cells
The expression of cell surface MHC class II antigens is highly restricted. They are only present on select types of cells including dendritic cells, select macrophages, B cells, and keratinocytes. The presence of class II antigens on their surfaces endows these cells with a unique ability to present antigens to CD4+ Th cells (Figure 55-3). Therefore these cells are called professional APCs. Class II antigens are two-chain molecules composed of a glycoprotein, 33-kD α chain and a shorter, 27-kD β chain that form a groove onto which processed (exogenous) antigenic peptides bind. For example, when a macrophage phagocytoses an antigen and breaks it down into peptides within a vacuole, intracellular MHC class II antigens bind to these processed peptides, and this complex moves to the surface of the cell to be presented to CD4+ T cells. The processed antigenic peptide specifically binds to the TCR on T cells, and the class II MHC proteins (on APCs) specifically interact with the CD4 molecule on T cells. These interactions are the first steps in activation of Th cells. Activation of T cells is highly regulated because their inadvertent activation has profound and widespread consequences; the cytokines secreted by activated Th cells can affect a wide range of both lymphoid and nonlymphoid cells.
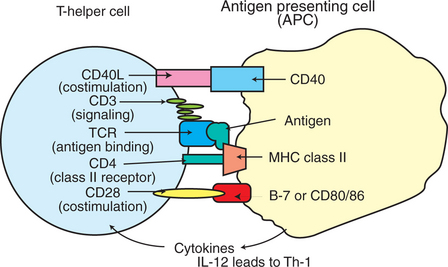
FIGURE 55-3 Interacting molecules of T-helper cells and antigen-presenting cells. IL, Interleukin; MHC, major histocompatibility complex; TCR, T-cell receptor; Th-1, T-helper cell type 1; Th-2, T-helper cell type 2.
Activation of CD4+ T cells requires at least two signals for activation. The primary activation signal determines the specificity through interactions of antigenic peptides and MHC molecules on APCs with the TCR/CD3 complex on T cells. The second signal is referred to as a co-stimulatory signal. Co-stimulatory signals include interaction of CD28 and/or CD40L, both residing on T cells, with CD80/86 and/or CD40, both residing on APCs. Cytokines that are released from APCs, such as IL-12, IL-18, and IL-27, promote the generation of a Th-1 subset of cells.
Activation of T cells is strictly controlled, with two major restrictions. First, T cells cannot recognize free antigens; rather, they recognize short peptides that are a product of processed antigens by the APCs. Second, the processed antigen must be physically associated with the MHC molecules. This results in molecular interactions of antigenic peptide bond to MHC class I or class II molecules on cells or APCs, with the TCR and CD8, or TCR and CD4 on T cells, respectively.
As mentioned, T cells will specifically interact with antigenic peptides through specific recognition by the TCR on T cells, analogous to the B-cell receptor (BCR) on B cells. The TCR on T cells belongs to the immunoglobulin superfamily and thus has variable and constant regions along with transmembrane and cytosolic domains. Similar to B cells, the variable portion of the TCR chain determines the specific binding to the antigenic peptide. Because more antigens exist in the universe than the actual number of T cells, these cells have adapted a variety of molecular mechanisms to interact specifically with an innumerable number of antigens. These mechanisms include recombination of TCR genes (similar to the BCR), unequal sister-chromatid exchange, and nucleotide insertion at various locations of the variable gene segments. An important difference between the TCR and BCR is that the TCR does not undergo somatic mutation. If the TCR could undergo somatic mutation, there would be an increased chance of inadvertently generating a TCR reactive against “self” antigens, resulting in devastating autoimmune conditions. This attribute is critical for survival because T cells, unlike B cells, can affect a large number of diverse lymphoid and nonlymphoid cells through secretion of cytokines.
ANTIBODIES
Initial Exposure to Foreign Antigen Induces Slow Onset of Antibody Appearance, Whereas Subsequent Exposure Induces Faster, Longer-Lasting Antibody Appearance
The exposure of an animal to a foreign antigen usually elicits a specific immune response. This response may involve the production of (1) specifically reactive T cells or (2) antibodies able to bind specifically with the foreign antigen. Typically, if an animal is exposed to a particular foreign antigen for the first time, no antibodies specific for that antigen will be detected in blood or secretions for several days. This “lag” period can last for up to 1 week, at which time antibodies capable of binding to the antigen appear in circulation and start increasing in quantity for the next 2 or 3 weeks. After that time, antibody quantities plateau and eventually decrease until they essentially disappear. The amount of antibodies produced and the duration of the response depend greatly on the nature of the antigen, quantity and route of exposure, and whether the antigen is given in combination with immune enhancers (adjuvants).
The type of response obtained after a first exposure to a specific antigen is called the primary immune response (Figure 55-4). If the animal is reexposed to the antigen, the lag period is very short, much higher levels of specific antibodies are obtained, and the response usually lasts for a significantly longer period. This response to a second exposure to an antigen is called a secondary immune response or anamnestic (memory) immune response.
Antibodies, or Immunoglobulins, Are Glycoprotein Molecules That Can Be Divided into Five Isotypes, or Classes
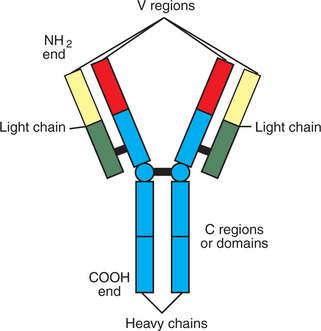
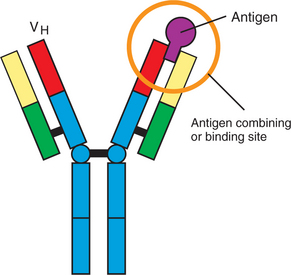
Antibodies are glycoprotein molecules that are the products of B lymphocytes. Antibodies, also called immunoglobulins, are basically made of four glycoprotein molecules. They are found on the surface of B cells, where they serve as antigen receptors (BCRs), or free in blood and secretions after being secreted by B cells. These free, or soluble, antibodies can neutralize antigens and assist in their removal. The basic structure of an antibody molecule has two identical short glycoprotein chains called light (L) chains and two identical longer chains called heavy (H) chains held together by disulfide bonds (Figure 55-5). The L chains are made of two halves, or domains; the half located at the carboxyl end of the chain is called the constant part of the L chain (CL), and the half located at the amino end is called the variable part (VL). The H chain is made of one variable domain (VH) and usually three constant domains (CH1, CH2, CH3). The amino terminal ends of the L chain (VL) and the H chain (VH) come together to form an antigen-binding or combining site (Figure 55-6). Therefore, two identical antigen-combining sites exist per basic immunoglobulin molecule. The carboxyl end of the two H chains form the Fc portion of the molecules; this end is the portion able to bind to Fc receptors on specialized cells and is the part of the molecule attached to the membrane of B cells when the immunoglobulin serves as the antigen receptor (BCR) for the cell.
Depending on molecular weight and other characteristics, immunoglobulins can be divided into classes, or isotypes. Basically, there are five isotypes: IgM, IgG, IgA, IgE, and IgD. Soluble immunoglobulin M (IgM) consists of five basic antibody molecules that bind together by disulfide bonds and an additional short protein chain to form a pentamer. Therefore, one IgM molecule has 10 identical antigen-combining sites. Its molecular weight is about 900 kD. In primary immune responses, IgM is the predominant immunoglobulin. Because of its large size, IgM is rarely found in body fluids other than blood. The BCR form of IgM is a monomer of 180 kD.
Immunoglobulin G (IgG) has the structure of the basic antibody molecule (monomer) previously described, and its molecular weight is 180 kD. IgG has two antigen-combining sites and is the predominant immunoglobulin detected in the secondary immune response. It is able to move out of the circulatory system and appears in body fluids and also in secretions.
Immunoglobulin A (IgA) is found in small amounts in circulation as a monomer and in much larger amounts in secretions, where it is found as the predominant immunoglobulin and primarily as a dimer. It is produced by plasma cells (mature B cells) located under body surfaces such as skin, mammary glands, and the intestinal, respiratory, genital, and urinary tracts. IgA is found in secretions and has an attached secretory molecule that protects IgA from intestinal proteases. Secretory IgA is the main immunoglobulin found on mucosal surfaces and has four antigen-combining sites in its dimeric structure. Its main role is to prevent antigen from attaching to these surfaces. Thus, IgA blocks penetration of antigen into the body. IgA responses are mainly elicited if antigen exposure is through contact with mucosal surfaces, such as the upper respiratory and intestinal tracts. IgM and IgG responses are elicited through parenteral contact with antigen (intradermal, subcutaneous, intramuscular, and systemic routes).
Immunoglobulin E (IgE) is a monomer, and its H chain contains four constant domains in addition to the variable domain. It is found in very low levels systemically, and most IgE is bound to basophils and mast cells (inflammatory and allergic reaction mediators) through its Fc portion. IgE is able to bind antigen while attached to these cells, thereby eliciting allergic reactions.
Immunoglobulin D (IgD) is a monomer and has only two constant domains on its H chain. IgD is primarily bound to the membrane of B cells and is secreted in negligible amounts in the serum. Negligible IgD is secreted.
The B-Cell Population Produces Antibodies to Millions of Different Antigens, Yet the Antibody-Antigen Interaction Is Specific
Antibodies bind to antigen through their antigen-binding sites. Each antigen-binding site is formed by the steric interaction of the VL and VH domains, which come in close contact because of the three-dimensional folding of the glycoprotein chains on which they reside. This steric interaction essentially forms a cleft, and any antigenic structure that fits into this cleft is recognized and binds to the antigen-binding site. Therefore, if an antigen can bind to the antigen-combining site on the BCR, the B cell is eventually (after a complex set of signal interactions) triggered to replicate (clonally expand), giving rise to many “identical” B cells, which eventually produce and secrete the soluble immunoglobulin specific for that antigen.
It is important to stress that antibody-antigen responses are specific. Antibodies produced after exposure to an antigen will only bind to that antigen or to other antigens structurally similar to the original antigen (cross-reaction). The question is, “How does the immune system manage to respond to literally several million different foreign antigens in a specific way?” Three facts are crucial to understand this situation. First, the BCR is an immunoglobulin, and the specificity of the immunoglobulin secreted by a particular B cell is the same as the specificity of its BCR. Second, an individual B cell can only have BCRs of identical specificity. Third, essentially each B cell (and there are millions) in the body has a BCR with a different antigen-combining site, because B cells undergo random genetic mutations in the genes coding for their VL and VH domains during their early development (ontogeny). Because these domains make up the antigen-combining site, a single amino acid change in either of the regions changes the steric interaction of these domains, giving rise to different “clefts” with different antigen-combining abilities.
Expansion of an Antigen-Specific B–Memory Cell Population on Initial Antigen Exposure Results in a Faster, More Pervasive, Secondary Immune Response
As the host is exposed to a foreign antigen, all existing B cells that have a BCR capable of binding the foreign antigen are able to react to such an antigen. Initially, among the millions of different B cells, the BCR of a few will bind to the antigen. This binding allows those matching B cells to multiply, creating many more B cells with the same BCR. This rather rapid expansion of antigen-specific B cells is often referred to as specific clonal expansion. The newly generated B cells start producing specific antibodies and secreting them. Specific antibodies begin appearing in circulation, and as clonal expansion increases, the quantity of specific antibodies in circulation increases. All B cells start their specific antibody production by producing IgM isotypes. Given the right milieu (e.g., appropriate sequence of signals), B cells stop producing IgM and switch production to IgG or another isotype (IgA or IgE); it is important to understand, however, that the antigenic specificity of the antibody does not change. These dynamics are consistent with those observed in a primary and secondary immune response.
As the host encounters an antigen for the first time, few of the existing B cells are able to recognize it, but once the antigen is recognized by those few B cells, they undergo clonal expansion. Therefore, antibody production starts increasing, shows up in circulation, and IgM predominates during the primary immune response. The long “lag” period represents the time needed to process significant numbers of antibody-producing B cells through clonal expansion. Some B cells start switching to IgG production, which is why some IgG appears during the later parts of a primary immune response. As antigen is neutralized by the antibodies, B-cell stimulation stops, antibody production declines until it eventually stops, and many B cells within the expanded population become long-lived memory B cells.
If the host confronts the same antigen for a second time, the antigen is recognized by the vastly expanded, antigen-specific B–memory cell population, many cells of which have switched their antibody production capability to an isotype other than IgM, mainly IgG. This expanded cell population starts antibody production quickly because of the larger population of antibody-producing cells and greater likelihood of being in an IgG-producing state. The lag period is therefore very short, and a large amount of IgG is produced, typical of a secondary immune response. Eventually, as in a primary immune response, clonal expansion stops, and antibody production decreases and eventually stops. Future confrontations with the same antigen will lead to secondary immune responses, which are characterized by short “lag periods” and high sustained production of IgG or other isotypes (IgA or IgE).
Antigen stimulation of the BCR is not a sufficient signal to initiate B-cell clonal expansion and antibody production. Many other signals must reach the B cell after its BCR has recognized antigen. These signals, often represented by interleukins, come from Th cells that have recognized the antigen through complex interaction mechanisms with APCs (see earlier discussion). Interestingly, B cells that have bound antigen through their BCR and have internalized the BCR-bound antigen are also able to interact with Th cells, helping them to recognize antigen. A B cell–T cell interaction is also necessary for B cells to switch their production from IgM to other isotypes. The B cells must interact with interleukins from Th cells.The timing and nature of the interleukins reaching the B cells play an important role in deciding to which isotype the B cell is switching.
REGULATION OF IMMUNE RESPONSES
The Actions, Secretions, and Surface Molecule Expression of Immune Cells Play an Important Role in Regulation of the Body’s Immune Response
Once the antigen is cleared by antibodies or T cells, it is imperative that the immune response return to its normal state to maintain homeostasis. Failure to downregulate the heightened immune reactivity will likely result in a number of pathological conditions, including autoimmunity, lymphoid tumors, allergies, amyloidosis, and abortion. The body has multiple mechanisms to downregulate the immune system. Immune cells themselves secrete various biomolecules that downregulate immune activity. These include prostaglandin E1 (PGE1), which increases cyclic adenosine monophosphate (cAMP) to suppress physiological activity of cells. Immune cells also secrete cytokines, such as transforming growth factor beta (TGF-β) and interleukin-10 (IL-10), which downregulate immune responses. The importance of these cytokines in downregulating immune responses is evident in the immune-mediated inflammation that results in mice with a deleted TGF-β gene. Antibodies themselves can downregulate their immune responses by binding to Fc receptors on B cells. This cross-ligation of Fc receptors results in delivery of inhibitory signals. Immune responses are antigen driven, so clearing of antigen by immune mechanisms leads to a decrease in antigenic load and thus diminishes the antigen-induced activation of lymphocytes.
As mentioned earlier, activation of T cells requires at least two signals. The second signal is altered by the increased secretion of molecules or the T cells that deliver a negative signal to dampen T-cell activation. Important in this regard is the recent discovery of T-regulatory cells (Treg), a small percentage of T cells (>5%) that are CD4+/CD25+/FoxP3+ and that have powerful downregulatory effects. The importance of these cells in downregulation of immune responses is evident because defects in these cells lead to widespread autoimmune diseases in many experimental models. Conversely, administration of Treg cells leads to prevention of inflammatory attacks. These cells hold promise in understanding the biology of immunoregulation and in therapy of various inflammatory diseases. These subsets of T cells also likely exist in domesticated animals.
CLINICAL CORRELATIONS
Unthrifty Foal
History.
You are presented with a 4-week-old Arab filly for depression, coughing, and nasal discharge. The foal was born with no apparent problems. Immunoglobulin levels were checked and were normal. However, the foal has been unhealthy and seems to be repeatedly sick with skin and respiratory infections.
Clinical Examination.
The foal has a temperature of 38.8° C (102° F) (elevated), heart rate of 60 beats/min (elevated), and a respiratory rate of 48 breaths/min (elevated). On auscultation the foal has both crackles and wheezes (abnormal lung sounds). The capillary refill time (CRT) is prolonged, and the mucous membranes are darker pink than normal. The foal also has some abrasions and cellulitis in these areas on the skin.
Comment.
With the increased temperature, abnormal lung sounds, poor perfusion (prolonged CRT, darker-than-normal mucous membranes), and increased respiratory rate, this foal probably has a respiratory infection, most likely bacterial in origin. Additionally, based on the skin abrasions, the filly also probably has a skin infection, which is uncommon in normal, healthy foals. Based on the age, breed, and recurrent infections, this foal likely has combined immunodeficiency (CID), a genetic autosomal recessive disorder. Foals with CID have a defect in a DNA-dependent protein kinase. This results in inability to produce mature B and T cells. As a result, the foal’s immune responses are limited. A normally functioning innate response is present, composed of neutrophils, macrophages, dendritic cells, and NK cells, but a normal adaptive immune response is lacking. The B cells do not make antibodies, and insufficient T cells are present to produce cytokines to help with the immune response and control infection. This foal has evidence of recurrent infections based on the history and appears to have concurrent skin and respiratory infections on presentation.
Foals with this type of history, including those with low lymphocyte counts and low immunoglobulin levels, are suspect candidates for CID. However, a definitive diagnosis is based on genetic testing and necropsy. Typically, foals with CID have rudimentary thymuses and lymph nodes because of the lack of lymphocytes and germinal centers. The spleen is usually reduced in size as well.
Treatment.
Because CID is a genetic disorder that the filly cannot overcome, the prognosis is poor. In many cases, until a definitive diagnosis is made, these foals are treated with antibiotics for the infection. Once a definitive diagnosis is made based on genetic testing, however, the foals are usually euthanized because of the poor long-term prognosis for life.
Abbas AK, Lichtman AH. Cellular and molecular immunology, ed 5. Philadelphia: Saunders, 2003.
Janeway C. Immunobiology: the immune system in health and disease, ed 6. New York: Garland Science, 2005.
Parham P. The immune system, ed 2. New York: Garland Science, 2004.
Rao CV. Immunology: a textbook. Harrow, UK: Alpha Science, 2005.
Roitt IM, Delves PJ. Essential immunology, ed 10. Malden, Mass: Blackwell, 2001.
Tizard IR. Veterinary immunology: an introduction, ed 7. Philadelphia: Saunders, 2004.
PRACTICE QUESTIONS
1. On their surface, T cells have:
2. Which of the following statements is correct with regard to CD4+ T cells?
3. Which of the following is correct concerning cytotoxic cells?
4. Cytotoxic T cells can kill their target cells through:
5. A simple antibody molecule consists of:
6. Which of the following is correct regarding primary and secondary antibody response?