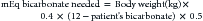Chapter 21 Drug Therapy for Endocrinopathies
Hormones fall into two broad categories. The majority are peptides and amino acid derivatives, including complex polypeptides (e.g., thyroid-stimulating hormone [TSH]), intermediate-size peptides (e.g., insulin), small peptides (e.g., thyrotropin-releasing hormone [TRH]), dipeptides (e.g., T4 and T3), and derivatives of single amino acids (e.g., catecholamines). The remainder consists of steroid derivatives of cholesterol, of which there are two types: those with an intact steroid nucleus (e.g., adrenal and gonadal steroids) and those in which the B ring of the steroid has been cleaved (e.g., vitamin D). In general, the cellular effects of hormones are achieved either through interactions with cell membrane receptors, thus stimulating a cascade of intracellular reactions often involving secondary messenger systems, or through passive diffusion to the cellular nucleus, stimulation of protein synthesis, and subsequent formation of the effector protein(s). Protein hormones typically interact with cell membrane receptors, an exception being thyroid hormones, whereas steroidal hormones passively diffuse through the cell membrane to the nucleus.
Drug therapy for the endocrine system is implemented to either replace a deficient hormone or prevent or reduce the formation or effects of an overabundant hormone. Hormones may also be administered to provocatively test for the presence of an endocrine disease. Understanding the proper uses of the drugs depends on an appreciation of the normal physiology of each endocrine system, including mechanisms of control and behavior of target tissues (Table 21-1).
Diseases of the Thyroid Gland
Synthesis of Thyroid Hormones
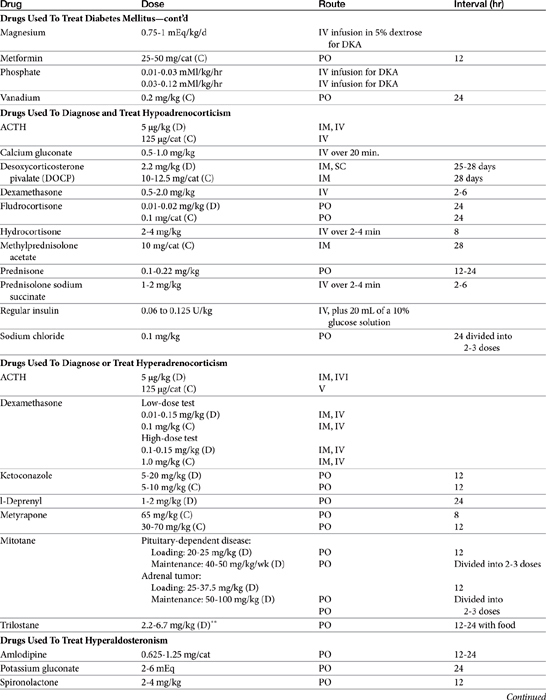
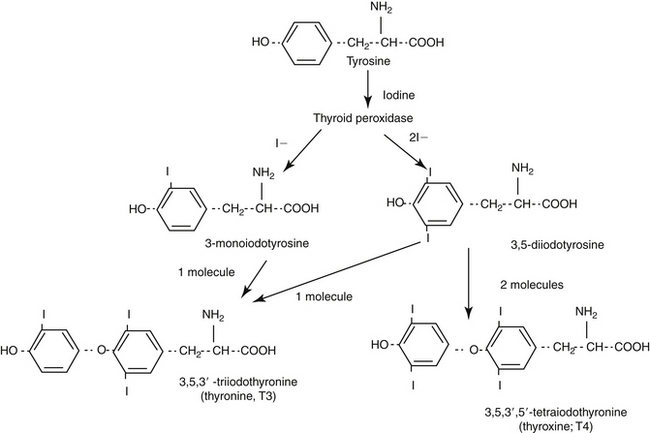
The protein thyroglobulin is synthesized by the endoplasmic reticulum and Golgi apparatus of thyroidal follicular cells and is released in vesicles into the colloid within follicular lumens. Iodine is accumulated by an active transport process in thyroidal follicular cells (Figure 21-1). Once in the follicular lumen, iodine is rapidly oxidized and combined with a tyrosine residue within thyroglobulin to form monoiodotyrosine. Monoiodinated tyrosines are then joined to form diiodinated tyrosines; further combinations yield triiodinated tyrosine (i.e., triiodothyronine [T3] and thyroxine [T4]), which contains four iodine molecules (Figure 21-2). The enzyme thyroid peroxidase mediates oxidation of iodine and formation of monoiodinated and diiodinated tyrosines as well T3 and T4. Four or more sites exist within the thyroglobulin molecule for the generation of thyroid hormones; generally, each molecule contains three or four molecules of T4 and zero to one of T3 (humans). The preformed hormones are released from the follicular colloid upon stimulation with TSH, with much greater quantities of T4 being released than T3. The hormones are transported bound to one of several transport proteins in the bloodstream and are delivered to target cells. At a target cell, both hormones are taken up. Intracellularly, T4 is deiodinated on its outer ring to produce T3, which is actually the active hormone that causes physiologic effects. In contrast, deiodination of the inner ring produces physiologically inactive reverse T3 (rT3). Thyroid hormones stimulate many metabolic processes, including activity of many enzymes; metabolism of vitamins and minerals; regulation of other hormones; and stimulation of calorigenesis, protein and enzyme synthesis, and carbohydrate and lipid metabolism. They also have marked cardiac inotropic and chronotropic effects, stimulate erythropoiesis, and affect virtually every body tissue.
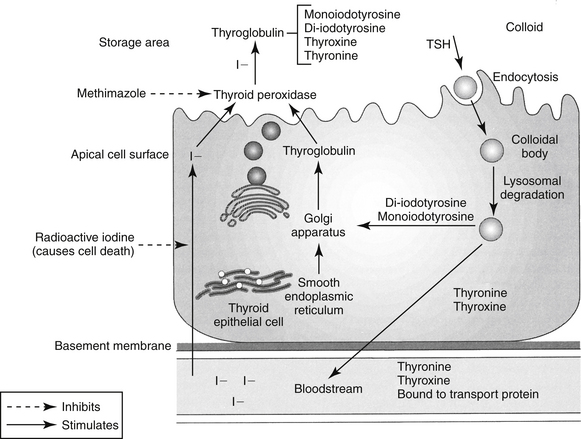
Figure 21-1 Synthesis of thyroid hormones. Iodine is concentrated in the apical cell colloid. At the same time, thyroglobulin is synthesized by the smooth endoplasmic reticulum and Golgi apparatus. At the apical cell surface, thyroglobulin and then tyrosine are iodinated, iodotyrosyl precursors are coupled to form thyronine and thyroxine, and all are stored in colloid. Thyroxine peroxidase mediates iodinization of the thyroglobulin–iodotyrosyl complexes. When signaled by thyroid-stimulating hormone (TSH), thyroglobin (as a colloid droplet) is engulfed by pinocytosis into the apical cell. Lysosomal degradation releases thyroxine and thyronine, which enter the blood stream, and the iodotyrosyl precursors, from which iodine is released and recirculated. The thyroid hormones reach target tissues bound to a circulating protein. Once inside the cell, thyroxine is converted to thyronine, the physiologically active thyroid hormone. Targets of thyroid hormone inhibition include administration of radioactive iodine, which is accumulated in active cells, ultimately leading to their destruction; methimazole, an inhibitor of thyroid peroxidase (controls but does not cure hyperthyroidism); and control of peripheral tissue response to excessive thyroid hormone release. I, Iodine.
Hypothyroidism
Pathophysiology
Spontaneous hypothyroidism is a common endocrinopathy in dogs and exceedingly rare in cats. In primary hypothyroidism, which accounts for 99% of cases of spontaneous canine disease, the thyroid gland itself is affected. Cells are lost as a result of either lymphocytic thyroiditis or idiopathic atrophy; neoplastic destruction is rare. Secondary hypothyroidism results from a deficiency of pituitary TSH. Congenital secondary hypothyroidism can result from pituitary malformation or isolated thyrotroph (i.e., TSH-secreting cell) abnormalities. Acquired secondary hypothyroidism is rarely seen due to pituitary neoplasia. Tertiary hypothyroidism results from a lack of hypothalamic TRH and has not been reported in dogs. Congenital absence of the thyroid gland and iodine deficiency are rarer causes of hypothyroidism. Cats suffering from clinically evident hypothyroidism generally develop the disorder in response to iatrogenic removal or destruction of thyroidal tissue as treatment for hyperthyroidism. Congenital primary hypothyroidism occurs uncommonly in kittens, and iodine deficiency is even rarer.
The clinical signs of hypothyroidism refer to multiple body systems. Dermatologic signs include bilateral, nonpruritic, localized, or diffuse alopecia. The hair coat is dry, coarse, and slow to grow. Hyperkeratosis, scaling, or seborrhea may be present. Immunosuppression may lead to secondary pyoderma. Cardiac abnormalities may develop with severe hypothyroidism, including bradycardia and a weak apex beat. Various mild electrocardiographic and echocardiographic changes may be noted, such as increased left ventricular end-systolic diameter, decreased systolic left ventricular posterior wall thickness, and decreased fractional shortening. However, no substantiated reports of congestive heart failure secondary to canine hypothyroidism exist. Possible reproductive disorders include abnormal estrous cycles and lack of libido. Neuromuscular dysfunctions include weakness or stiffness, muscle wasting, neuropathies, and facial muscle weakness. Gastrointestinal signs are uncommon, but constipation is most likely to occur, if anything. Diarrhea may occur in hypothyroid dogs, but a cause-and-effect relationship has not been established. In cats lethargy and obesity are the most common clinical manifestations. Clinical laboratory abnormalities of hypothyroidism in dogs and cats include normocytic, normochromic, nonregenerative anemia and hypercholesterolemia with hypertriglyceridemia or hyperlipidemia.
Myxedema coma is a rare endocrine emergency resulting from decompensation of severe chronic hypothyroidism. Myxedema refers to dermal accumulation of glycosaminoglycans, which bind water and cause an increased skin thickness and nonpitting edema, mostly in the face, jowls, and distal extremities.1 Myxedema coma is characterized by profound weakness, bradycardia, hypotension, hypoventilation, hypothermia without shivering, and altered mentation ranging from dullness and depression to stupor or coma. The actual mortality rate is not known but is thought to be high, primarily because myxedema coma is not widely recognized. The diagnosis should be made clinically, and therapy should be initiated without waiting for results of thyroid hormone concentrations.1 In addition to the biochemical abnormalities typical of hypothyroidism, additional findings may include hypoxia, hypercarbia, hyponatremia, and hypoglycemia. Serum thyroid hormone concentrations are typically very low or undetectable.
In humans the presence of concurrent disease and altered mentation are essential features for supporting a diagnosis of myxedema coma. In one study of seven hypothyroid dogs for which intravenous levothyroxine therapy was deemed necessary, five dogs had altered mentation. Of those five, all had concurrent disease and four had myxedema. Because of the low incidence of myxedema coma, it is not known if there is a breed predilection, but in a study by Pullen and Hess,2 three were Rottweilers, two were mixed breeds, one was a Cocker Spaniel, and one was a Shetland Sheepdog. Rottweilers were overrepresented. Treatment is with intravenous levothyroxine (discussed later).
Baseline and Provocative Testing of Thyroid Status
Diagnosis of hypothyroidism can be challenging. Hormones typically measured include T4, free T4, and TSH. If serum total T4 concentration is normal, it is highly unlikely that the dog is hypothyroid.3-6 Because nonthyroidal factors such as drugs, illness, and age affect T4, if the T4 concentration is below normal, the dog may or may not be hypothyroid,3-57 and further testing is required. Breed may also affect reference ranges.8,9,10
Thyroid hormones are bound to plasma proteins. Approximately only 0.1%, of circulating hormone is unbound and thus available to cells and physiologically active. Most laboratories measure total (bound and unbound) T3 and T4 concentrations. A number of techniques purportedly quantify the fraction of unbound thyroxine (i.e., free T4 [fT4]), including equilibrium dialysis, the gold standard for comparison with other methods, and several immunoassays that indirectly detect fT4. Currently, methods that depend on equilibrium dialysis are the most accurate measurements of fT4; measurement of fT4 by analog radioimmunoassay techniques offer no diagnostic advantage over measurement of total T4. Regardless of the test, normal thyroid hormone concentrations will vary with the laboratory and the kit used; any test must be validated for the target species (i.e., dog or cat).
Because the pituitary–thyroid axis functions to maintain free, not total, T4 within a certain range, fT4 is less affected by nonthyroidal factors; therefore measurement of serum fT4 concentration more accurately reflects thyroidal axis status. Accordingly, fT4 is a more sensitive and more specific test for diagnosis of hypothyroidism, but it is also not as good a stand-alone test as once believed (discussed later). Measurement of serum fT4 concentration can be the initial test for diagnosis of hypothyroidism or can be used in dogs found to have low total T4 concentrations.
Serum TSH concentration can also be measured to help establish a diagnosis of canine hypothyroidism. In primary hypothyroidism TSH concentration should be elevated owing to lack of negative feedback of serum T4 on the pituitary (i.e., in normal dogs T4 feeds back and suppresses TSH secretion). Although 99% of canine hypothyroidism cases are believed to be due to primary thyroidal failure, only approximately 63% to 82% of hypothyroid dogs have elevated serum TSH concentrations.11-14 The reason for the discrepancy is unknown but is likely due to inadequacy of currently available assays. Of the hormones measured to diagnose hypothyroidism, however, TSH measurement is the most specific—that is, it has the smallest chance of a false-positive result.
The effect of nonthyroidal illness on testing can be quite significant. Dogs that are ill because of a disease but that do not have hypothyroidism can have low T4 and fT4 concentrations; if the disease is severe (e.g., a dog is in intensive care), a 44% chance exists that the fT4 concentration will be below normal even though the dog is not hypothyroid. Serum TSH concentration, however, is less affected by nonthyroidal factors. Therefore in sick dogs the first choice for diagnosis of hypothyroidism would be a combination of TSH, fT4, and T4; second choice a combination of TSH and fT4; and third choice a combination of TSH and T4. If the results are ambiguous (i.e., some parameters suggest hypothyroidism, and others do not), the ideal would be to resolve the nonthyroidal illness, if possible, and retest the dog at that time or possibly to perform a TSH stimulation test, if available.
Bovine TSH previously was used as the provocative agent for TSH stimulation testing but is no longer available. Thyrogen, recombinant human TSH (rhTSH), can be used instead. The optimal protocol for distinguishing normal and hypothyroid dogs, however, remains to be determined, although results of studies evaluating rhTSH in dogs are promising.15-17 Doses of 50 to 100 μg/dog given intravenously with samples taken before and at 4 or 6 hours after injection are likely appropriate. Euthyroid animals should respond to TSH stimulation with increased T4 concentration, whereas hypothyroid animals should not. Reconstituted rhTSH can be stored at 4°C for 4 weeks and at −20°C for 8 weeks without loss of biological activity.18
Measurement of serum T3 concentration is not helpful for diagnosing hypothyroidism. Most T3 is derived from intracellular metabolism or conversion by peripheral tissues of T4 to T3 and reverse T3 (rT3). Thus most body T3 is located within cells, and serum T3 concentration does not reflect total body levels. A previous argument for measuring serum T3 concentration was the belief that some dogs were “poor converters” and could produce T4 but not T3. The theory was apparently substantiated by finding normal serum T4 but nondetectable T3 concentrations in a small percentage of dogs. Such discrepant results are now known, however, to be an artifact caused by the presence of T3 autoantibodies. Insofar as poor conversion has never been proved and currently is not believed to exist, measurement of serum T3 concentration is not recommended.
Autoantibodies made against T4 or T3 can be measured and can be a marker of immune-mediated destruction of the thyroid gland. Autoantibodies typically cause spurious elevations in serum T3 or T4 concentrations, so their presence should be suspected if either hormone is measured in a patient suspected to be hypothyroid and the concentration of either or both hormones is reported to be greater than the reference range. However, the clinical and prognostic significance of autoantibodies is unknown. The presence of autoantibodies does not mean a patient is hypothyroid. If autoantibodies are suspected, serum fT4 concentration should be measured by equilibrium dialysis for the best assessment of function.19 If the serum fT4 concentration is within the reference range, thyroidal function is normal at that time but the patient should be reevaluated periodically (e.g., every 3 months) for development of hypothyroidism. If serum fT4 concentration is low, the dog is likely hypothyroid. One study followed 234 dogs with normal T4 and TSH levels and elevated antithyroglobulin antibodies (TGAA) for 1 year. Only 19% developed clinical signs of hypothyroidism or consistent laboratory values (or both). Another 57% remained TGAA positive without signs or laboratory evidence of hypothyroidism, 8% went from positive to borderline results, and 15% became TGAA negative.20
Calculation of the percentage of uptake of radioactive pertechnetate (99mTcO4-) has proved to be a useful tool aiding in the diagnosis of thyroidal illness in humans and cats and may also be valuable for dogs with primary hypothyroidism. Normal lobes of canine thyroid glands are uniformly intense, symmetric ovals, slightly smaller than the parotid salivary glands, and have smooth and regular margins. The parotid glands also concentrate 99mTcO4-, and the normal uptake ratio between these the thyroid and parotid glands is 1:1. In 14 dogs with histologically confirmed primary hypothyroidism, the percentage of uptake of 99mTcO4- distinguished hypothyroid dogs from dogs with nonthyroidal illness.21 The dogs with nonthyroidal illness had 99mTcO4- uptake ranging from 0.39% to 1.86%, similar to what has been described in healthy Beagles. The hypothyroid dogs had 99mTcO4- uptake ranging from 0.03 to 0.26%. In fact, of the tests examined in the study (T4, fT4, TSH, and TSH and TRH stimulation tests), scintigraphy was the only one that did not have overlap between the two groups of dogs. Limitations of the test include requirement for specialized equipment and the fact that reference ranges have not been defined in a large number of dogs from a variety of breeds. Additionally, increased 99mTcO4- uptake can occur with a diet high in iodine and has been reported in a hypothyroid dog with thyroiditis,22 so false-negative results are possible. The final complicating factor is that in humans with thyroiditis, the 99mTcO4- uptake pattern depends on disease stage. The role scintigraphy holds in aiding in the diagnosis of canine hypothyroidism remains to be defined.
Another area that has received recent attention as an ancillary test in the diagnosis of canine hypothyroidism is ultrasonography. In healthy dogs and dogs with nonthyroidal illness, the thyroid gland lobes are fusiform in the longitudinal plane and triangular in the transverse plane, have a smooth thyroid capsule, and homogenous echotexture, usually hyperechoic or isoechoic to the sternothyroid muscle.23,24 The ultrasonographic description of the thyroid gland lobes of hypothyroid dogs is more variable. However, they tend to be either round or ovoid on the transverse view. Echotexture may depend on whether the animal has thyroglobin autoantibodies. Thyroid lobes from antibody-positive hypothyroid dogs were homogenously hypoechoic to the sternothyroid muscle, whereas the lobes of those that were antibody negative were heterogeneous in echotexture.23 However, another study did not find a difference based on thyroglobin autoantibody status; thyroid lobes were reported to be hypoechoic to the sternothyroid muscle.24 Additionally the volume of the glands is smaller in hypothyroid dogs. A thyroid volume of less than 0.05 mL/kg was reported to be 81% sensitive, 96% specific, and 91% accurate for the diagnosis of primary hypothyroidism.23 Care should be taken with the use of this modality for diagnosing canine hypothyroidism because ultrasonography is highly user dependent and substantial interobserver variability exists when taking measurements.
Therapy of Hypothyroidism
Disposition of thyroid hormones
Thyroidal hormone disposition varies between preparations and species. Although thyroid hormones are bioavailable after oral administration, T4 absorption can be decreased by a number of factors such as the type of preparation and intraluminal contents of the ileum or colon, where most absorption occurs. In humans bioavailability ranges from 40% to 80%. Because of differences among formulations, some authors recommend initiating therapy with a brand-name product.25 However, the pharmacokinetics of T4 is idiosyncratic; some dogs achieve higher postpill concentrations when treated with a generic brand. No evidence exists that a single brand is consistently better than another. Therapy can probably be started with any brand, but if a dog does not respond, another brand should be administered to see whether better results and higher postpill levels can be achieved; if the first brand tried is generic, the second should not be. In contrast to T4, T3 is well absorbed (95% in humans) from the gastrointestinal tract.
In dogs the half-life of T3 and T4 is 5 to 6 hours and 12 to 15 hours, respectively. However, the half-life of T4 may be dose dependent.26 The short half-life makes avoidance of fluctuations in serum concentrations during a 12- to 24-hour dosing interval difficult. However, the status of intracellular T3 concentration is not well known; the impact of fluctuating T4 may be minimized by physiologic conversion. Steady-state concentrations will occur at five drug half-lives (i.e., 75 hours for T4 and 30 hours for T3 in dogs). On the other hand, clinical response varies depending on the complication of hypothyroidism present. The first evaluation after starting supplementation should occur after about 4 weeks of therapy in order to judge blood levels as well as therapeutic response. Certain abnormalities (e.g., dermatological) can take up to 3 months to resolve once therapeutic blood levels have been achieved.
Preparations
Thyroid hormones can be supplemented as crude extracts of animal origin or as synthetic preparations. The biological activities of animal-origin products, such as desiccated thyroid and thyroglobulin, vary, however, and therapeutic failure with these products is not uncommon. Thus synthetic products are recommended.
Several synthetic thyroid hormone products are available as T4 or T3 individually or in combination. Sodium levothyroxine (T4) is the drug of choice for most patients. If T4 therapy has failed to achieve a response in a dog with confirmed hypothyroidism, T3 can be administered, but failure of T4 therapy is very rare. If no apparent response to T4 is seen or inadequate blood levels are achieved with appropriate dosing, another brand of T4 should be used first before prescribing T3. The diagnosis of hypothyroidism should also be reevaluated if resolution of clinical signs does not occur with documentation of adequate blood concentrations and a sufficient duration of therapy. Combination products generally contain T4 and T3 at a ratio of 4:1, the proportion of thyroid hormones secreted in normal humans. Use of combination products is not recommended. The administration frequency of T4 and T3 should differ, orally absorbed T4 is converted as needed to T3 by target cells so therapy with T4 alone usually achieves adequate T3 concentrations, and use of combinations may result in serum T3 concentrations that produce thyrotoxicosis.1
Plasma half-life of T4 varies among dogs, and a marked variability in the dose necessary to achieve therapeutic concentrations exists. Beginning doses of T4 should be 20 μg/kg every 12 hours for dogs and 50 to 100 μg once daily in cats. After resolution of clinical signs, approximately 25% of dogs maintain adequate blood levels and a good therapeutic response with once-daily dosing. Initial doses as low as 5 μg/kg may be indicated in dogs with concurrent illness, especially cardiac, to allow the body to slowly adapt to the increase in oxygen demand that may accompany thyroid hormone replacement. The dosage can then be increased over the following 3 to 4 weeks.
Pharmacokinetics of oral liquid levothyroxine suggest that once-daily dosing should be adequate,27 and administration of liquid levothyroxine (Leventa Intervet/Schering-Plough) once daily in 35 hypothyroid dogs was recently evaluated.28 Dogs were started at a dose of 20 μg/kg once daily and monitored every 4 weeks (adjustment phase). At the reevaluation visit, serum T4 and TSH concentrations were obtained 4 to 6 hours after the pill was administered, and dose adjustments were made if the T4 value did not fall within the target therapeutic range. Once clinical signs resolved and T4 concentrations were within the target range, the adjustment phase ended and the maintenance phase began. To assess long-term efficacy, dogs were then reevaluated at 9 and 22 weeks, with the same parameters measured at each visit and dosages adjusted as needed.
The starting dose of 20 μg/kg was also the maintenance dose in 79% of the dogs. Maintenance doses for the remaining dogs were 10 μg/kg (3%), 15 μg/kg (3%), and 30 μg/kg (15%). In the maintenance phase, dose adjustments were required for 16% of dogs. The median dose was 24 μg/kg once daily. Clinical signs improved within 4 weeks in 90% of dogs and within 4 weeks in 100%. Body condition scores initially assessed as overweight or obese had normalized in 52% of dogs by week 22, and dermatologic abnormalities had resolved in 68%. Peak serum T4 concentrations were within the target therapeutic range in 65% of dogs at the first follow-up visit after beginning the maintenance phase of the study and in 79% of dogs at the 22-week examination. The remaining dogs had clinical control of the disease, but transiently low T4 concentrations. Reported adverse events included sudden death in one dog after 47 days of treatment, vomiting after administration of the solution in two dogs, and reddish discoloration of hair coat in one dog.
Intravenous levothyroxine is used in the treatment of dogs with myxedema coma. In 7 dogs given intravenous levothyroxine, the median dose was 7 μg/kg. Three of the seven dogs were given intravenous levothyroxine once. To resolve the neurologic issues, the remaining dogs received a total of 5, 6, 10, or 13 doses each. The injections were given every 12 hours in three of four dogs and every 8 hours in the remaining dog. The dogs were started on oral levothyroxine within 24 hours of the last intravenous dose.2
Sodium liothyronine (T3) should be reserved for patients not responding to T4 therapy. Dosing should start at 4 to 6 μg/kg every 8 hours for dogs and 4.4 μg/kg every 8 to 12 hours for cats. Clinical improvement may take 4 to 6 weeks; twice-daily administration can begin at that time.
Response to Therapy
Therapeutic Drug Monitoring
Variability in blood concentrations after oral administration of T4 can be very large because of differences in disposition, including bioavailability, so monitoring is an important tool with which to guide therapy. Blood concentrations should not be monitored until steady-state concentrations of the hormone have been reached and sufficient time for a physiologic response to the new concentration has passed. Thus concentrations should be monitored 1 month after therapy has begun.
For monitoring a sample should be drawn 4 to 6 hours after the pill is administered. At that time, serum T4 concentration should be in or slightly above the upper half of the reference range. TSH concentration can also be measured in the same sample and can be helpful. Increased values are associated with inadequate therapy, but TSH concentrations within the reference range are not interpretable; dogs with both adequate and inadequate control can have TSH concentrations within the reference range.29 Measurement of fT4 probably is unnecessary and does not add any more information, except in patients who have T4 autoantibodies; in such patients T4 concentration as measured by radioimmunoassay is falsely elevated. Whether measurement of prepill hormone concentrations is helpful remains controversial. If T3 is the sole supplement, peak concentrations can be collected 3 hours after administration of the pill if an 8-hour dosing interval is being used. With subsequent retesting, samples should be collected at the same time as previously, so comparisons between tests across time in the same patient are more valid.
Interpretation of thyroid hormone concentrations must be made in the context of clinical signs. Animals should be supplemented for 1 to 3 months before clinical efficacy can be judged. Although no clinical signs of thyrotoxicosis may be present, whether increased serum T4 concentrations without clinical signs of hyperthyroidism are detrimental has never been studied. In humans elevated postpill T4 concentrations are strictly avoided. Low serum T4 concentrations should also be interpreted with response to therapy. The effects of concurrent drug therapy or other diseases that might influence the metabolism of thyroid hormones must be considered. Drug dose can be changed to achieve the therapeutic range. However, the pharmacokinetics of T4 are not linear—that is, to achieve a 15% change in serum T4 level, a 25% change in dose, not a 15% change, may be required.30
Effects of other drugs on thyroid hormones
Numerous drugs can affect thyroid hormone concentrations. The reader is referred elsewhere for complete information.31,32
Glucocorticoids, phenobarbital, sulfa antibiotics, tricyclic antidepressants, and nonsteroidal antiinflammatory agents, among others, have been shown to decrease T4, T3, fT4, and TSH.
Hyperthyroidism
Pathophysiology
Neoplasia of the thyroid gland, whether benign or malignant, is the most common cause of hyperthyroidism. In cats a benign tumor is the etiology in approximately 99% of cases, whereas in dogs a thyroid tumor leading to hyperthyroidism is almost always malignant. (Note, however, that most canine thyroid tumors do not cause hyperthyroidism; at least 90% of canine thyroid tumors are nonfunctional, and they can cause hypothyroidism as a result of the destruction of normal thyroidal tissue.) Occasionally, administration or accidental ingestion of thyroid hormones can cause thyrotoxicosis.
The clinical signs of hyperthyroidism reflect abnormalities in several body systems as a result of excessive concentrations of T4 and T3. Increased energy expenditure results in weight loss and polyphagia. As skin protein synthesis and blood flow increase, the hair coat changes. Increased renal blood flow, glomerular filtration rate (GFR), and renal tubular activity account for polydipsia and polyuria; loss of medullary interstitial tonicity can also contribute. Vomiting may reflect a direct effect on the chemoreceptor trigger zone, overeating, or gastrointestinal hypermotility. Malabsorption and hypermotility can cause diarrhea. Thyroid hormones may directly stimulate the central nervous system, causing behavioral changes that typify hyperthyroid cats (i.e., nervousness, hyperkinesis, agitation). Occasionally, hypokalemia develops, which may explain the weakness that affects some cats.
Heat and stress intolerance, panting, and respiratory distress may be related to decreased pulmonary vital capacity, decreased pulmonary compliance, slightly elevated body temperature, and cardiac stimulation (associated with catecholamine release). Cardiac disturbances include tachycardia, premature cardiac contractions, and gallop rhythms. Secondary cardiac hypertrophy can occur, which is distinct at a microscopic level from that seen with primary idiopathic hypertrophic cardiomyopathy. Thus cats with hypertrophy secondary to hyperthyroidism have thyrotoxic cardiac disease, not hypertrophic cardiomyopathy. Long-standing untreated thyrotoxic cardiac disease can progress to congestive heart failure. Thyroid hormones directly affect the cardiac muscle, as well as increase the needs of peripheral tissues, causing a high cardiac output state and an increase in myocardial oxygen demand. Peripheral resistance, on the other hand, is generally decreased. Catecholamines may contribute to the positive inotropic and chronotropic effects of thyroid hormones on the heart. Thyrotoxicosis probably increases the myocardial responsiveness to catecholamines by increasing the number of catecholamine receptors.
Baseline and Provocative Testing
Baseline serum T4 concentration can be measured to diagnose hyperthyroidism and is sufficient in the majority of cases as it is elevated in approximately 91% of hyperthyroid cats.33 (It is important to remember, however, that rare normal cats may have an elevated T4.) The other 9% of cats represent those with early or mild hyperthyroidism where serum T4 concentration can fluctuate in and out of the normal range34 or cats with concomitant nonthyroidal illness. Nonthyroidal illness can suppress serum T4 concentration,35 so the serum T4 concentration in hyperthyroid cats can be within the normal range.36,37 As a result, resting serum T4 concentrations obtained from cats must be critically evaluated and the possibility of further testing considered.
If the serum T4 concentration of a hyperthyroid cat is within the reference range, it will typically be in the upper half of the range. If a serum T4 concentration is in the lower half of the reference range, it is highly unlikely (but not impossible) that the cat is hyperthyroid, and another diagnosis should be considered. In sick, older cats with normal thyroidal function, serum T4 concentration is usually low,35 so the finding of a T4 even in the upper half of the reference range in such a cat may indicate hyperthyroidism. If the T4 concentration is in the upper half of the reference range but there hyperthyroidism is still suspected, further diagnostics should be pursued. Options for additional tests include the following: (1) measurement of serum fT4 concentration by equilibrium dialysis; (2) repeat measurement of total T4 concentration (Because serum T4 concentration may fluctuate in and out of the normal range, on a second test the sample may be drawn by chance while the T4 concentration is above normal and diagnostic. Unfortunately, there is no way to predict when this will occur.); (3) performance of a T3 suppression test or TRH stimulation test. These latter two tests are valid, good tests, although they are more labor intensive and often not necessary.
As in dogs, serum fT4 concentration in cats is less affected by nonthyroidal factors than is total T4 concentration and is a more accurate reflection of thyroid function. For example, fT4 concentration is elevated in 94% of mildly hyperthyroid cats, whereas total T4 concentration is elevated in only 61%.33 However, fT4 concentration may also be elevated in 6% to 12% of sick, euthyroid cats.33,38 To help discriminate between hyperthyroid and sick euthyroid cats, a total T4 should be measured along with fT4 concentration. Sick euthyroid cats with an elevated fT4 typically have T4 concentrations that are within the lower half of or below the reference range. Thus if the T4 concentration is in the upper half of the normal range or above and the fT4 concentration is elevated, this is consistent with a diagnosis of hyperthyroidism. If the T4 concentration is in the lower half of the normal range or below and the fT4 concentration is elevated, the cat is very unlikely to be hyperthyroid, and another diagnosis should be sought.
Measurement of serum T3 is not very helpful for diagnosis and is not recommended. Overall, only 67% of hyperthyroid cats have an elevated T3 concentration.33 In cats with mild hyperthyroidism, only 21% actually have an elevated serum T3 concentration.33
Performance of pertechnetate scans to diagnose hyperthyroidism is quite useful overall. Uptake of radioactive pertechnetate can confirm hyperthyroidism as well as delineate functional thyroid tissue, establish the extent of thyroid involvement, and possibly detect metastasis (some metastases, but not all, will concentrate radioiodine). For cats in which biochemical evidence of hyperthyroidism is clear, scans will clearly identify functioning tissue. However, false-positive results may occur;39 in other words, nonhyperthyroid cats can have a scan suggesting the presence of hyperthyroidism, and timing of a scan after methimazole therapy may affect results.40
Relationship of Treatment for Feline Hyperthyroidism to Renal Disease
Treatment of hyperthyroidism can lead to decreases in GFR and unmask chronic renal disease,41-45 and no therapy appears to be safer than another. However, how best to assess cats before definitive therapy (i.e., radioactive iodine [131I therapy] or surgery) is unknown. Although one study determined that a GFR value of 2.25 mL/kg/min might represent a cutoff for deciding whether renal failure is a possibility,43 measurement of GFR is not easily obtained and GFR measurements vary depending on the technique used,46 with each laboratory needing to determine its own guidelines. Furthermore, in two studies, hyperthyroid cats with GFR greater than 2.25 mg/kg/min were azotemic 30 days after treatment;42,44 whether this simply reflects a difference in methodology is unclear. Unfortunately, no readily available clinical indicators, including urine specific gravity, exist.47,48 Cats with pretreatment urine specific gravity greater than 1.035 may develop azotemia with treatment.48
Therefore the best option is probably to treat cats transiently with methimazole until serum T4 concentration is adequately controlled and then maintain euthyroidism for 30 days. When the serum T4 concentration is maintained within the normal range, renal function and the effect of definitive therapy can be assessed. Many cats exhibit an increase in serum blood urea nitrogen (BUN) or creatinine concentration (or both) with therapy, but the clinical result must be assessed as the most important parameter. Most cats improve clinically despite the increased renal parameters when their hyperthyroidism is treated. If they improve and the renal failure is not clinically apparent, the hyperthyroidism can be definitively treated.
Whether all cats that are to undergo 131I treatment or thyroidectomy need to have their kidney function evaluated with a methimazole trial remains to be determined. We prefer performing a trial on all cats before definitive therapy is undertaken. Certainly, if there is any question about the adequacy of renal function, trial therapy with methimazole is warranted. If renal failure becomes clinically apparent during the trial, methimazole administration should be stopped and therapy for renal failure instituted. Once the cat is stable again, the hyperthyroidism should be controlled as best as possible for life with methimazole and therapy for renal failure continued. Alternatively, if a trial is not performed and renal failure becomes overt because of definitive correction of hyperthyroidism, exogenous thyroid hormone can be supplemented in an attempt to support the kidneys, but the efficacy of such therapy is unknown and may be questionable.49 A balance must then be struck between creating iatrogenic hyperthyroidism and maintaining renal function.
Drugs Used To Control Hyperthyroidism
Hyperthyroidism may be medically controlled with methimazole and ipodate. Both can be used as either the sole drug to manage hyperthyroidism or in preparation for surgery or radioiodine administration. Propylthiouracil administration is not recommended because of associated possible severe adverse effects. In general, methimazole blocks synthesis of thyroid hormones and, specifically, thyroid peroxidase activity necessary for coupling of tyrosine residues by acting as a preferential substrate for the enzyme. As a result, T3 and T4 are not secreted. Carbimazole is a methimazole prodrug currently used in Europe but not available in the United States. It appears to be equal in efficacy but safer than methimazole. Controlled-release carbimazole tablets are now available in Europe. Based on pharmacokinetics in normal cats the controlled-release formulation may be appropriate for once-daily dosing;50 however, dosing has not been evaluated in hyperthyroid cats. Ipodate is a cholecystographic agent that acts primarily by inhibiting conversion of T3 to T4 but also has some direct inhibitory effects on thyroid hormone secretion.
Overall, methimazole is highly effective at reversing thyrotoxicosis and maintaining euthyroidism. In 262 spontaneously hyperthyroid cats, methimazole treatment lowered the serum T4 in more than 99%.51 A very small percentage of cats may be truly methimazole resistant. In the 262 cats, clinical side effects occurred, unrelated to the dose of methimazole used, in 18%, including anorexia (11%), vomiting (11%), lethargy (9%), excoriation of the face and neck (2%), bleeding (2%), and icterus (2%). Anorexia, vomiting, and lethargy typically happened during the first month of therapy and resolved despite continued drug administration. However, in eight cats, gastrointestinal side effects persisted and required cessation of therapy. Treatment with methimazole was also permanently stopped in cats that developed liver failure (e.g., vomiting, anorexia, and icterus), excoriated faces or necks, or a bleeding tendency.51 Myasthenia gravis has been reported after treatment with methimazole in four cats. In two cats, prednisone was used to control the myasthenia.52 Lymphadenomegaly, which resolved with discontinuation of methimazole administration, was reported in a single cat.53
On hematologic screening, eosinophilia, lymphocytosis, leukopenia, thrombocytopenia, and agranulocytosis may be noted. The milder adverse effects—eosinophilia, lymphocytosis, and leukopenia—are usually noted within 1 to 2 months of initiation of treatment and are transient despite continued therapy. The more serious complications (e.g., thrombocytopenia, agranulocytosis) occur in a minority of cats (≤3%) within the first 3 months of therapy and necessitate permanent discontinuation of methimazole administration.51 The mechanism of hematologic disorders induced by methimazole is not understood. Interestingly, bleeding occurred in one cat without a decrease in platelet number, so thrombocytopenia is not the only mechanism that can cause a bleeding tendency. In human patients receiving propylthiouracil, vitamin K therapy reduced bleeding caused by hypoprothrombinemia; however, the benefits of vitamin K therapy have not been studied in cats receiving thyroid peroxidase inhibitors.54 Immunologic effects, including induction of positive antinuclear antibodies (ANAs), can occur. The risk of developing a positive ANA result appears to increase with length of therapy and dose. However, clinical signs of a lupuslike syndrome (e.g., dermatitis, polyarthritis, glomerulonephritis, thrombocytopenia, fever) or hemolysis do not occur.51
A starting dose of 10 to 15 mg/day divided into two or three daily doses depending on the severity of the hyperthyroidism has been recommended. The goal for cats on methimazole is to have a serum T4 concentration in the lower half of the reference range. Postpill timing does not matter. Although some cats require methimazole only once daily for adequate control, methimazole generally is more effective twice daily.55 For the first 3 months, the period during which most adverse effects develop, cats receiving methimazole should be evaluated every 2 to 3 weeks with a complete physical examination, determination of serum T4 concentration, complete blood count (CBC), and measurement of liver enzymes and bilirubin. Renal parameters should also be monitored to assess kidney function. Although cats with a subnormal serum T4 concentration typically are not clinically hypothyroid, development of a positive ANA titer may be related to dose. Thus the minimal dose necessary to maintain serum T4 concentration in the lower half of the reference range, and not below, should be used. If serum T4 concentration remains high and poor compliance or difficulty in giving the medication has been ruled out as the cause of persistent hyperthyroidism, the methimazole dose should be increased in 2.5- to 5-mg increments to a maximum of 20 mg/day. If hepatopathy, facial excoriation, a bleeding tendency, or serious hematologic consequences occur, the medication should be halted permanently and alternative therapy used. After the first 3 months, serum T4 concentration should be determined every 3 to 6 months to evaluate adequacy of therapy. Because blood dyscrasias are unlikely but not impossible after 3 months of therapy, a CBC need be performed only if clinical signs suggest agranulocytosis, hemolysis, or thrombocytopenia.51
To prevent development of adverse effects, other authors have recommended an initial dose of 2.5 mg twice daily for 2 weeks.56 If after this period an owner observes no untoward side effects, the physical examination reveals no new problems, and a CBC (including platelets) is within normal limits, the dosage should be increased to 2.5 mg thrice daily for an additional 2 weeks. A similar recheck should then be completed, including measurement of a serum T4 concentration. If serum T4 concentration is within or near the normal reference range, the dose may be maintained for 2 to 6 weeks to determine the need for any further dosage adjustments. The dosage should be increased by 2.5 mg/day increments to a maximum of 20 mg/day (assuming correct methimazole administration) or until the hyperthyroidism is controlled.56 Monitoring for adverse effects should be done as previously described.
Because of the relationship between hyperthyroidism and renal disease (as previously discussed), a third protocol has been advocated if abnormal renal parameters are present. Methimazole should be administered at a dose of 2.5 mg twice daily for 2 weeks, then 2.5 mg thrice daily for 2 weeks, then 5 mg twice daily for 2 weeks, and finally 5 mg thrice daily as needed. The serum T4 concentration, BUN, creatinine, phosphate, and a CBC should be evaluated at the end of each 2-week period. The dose escalation should stop once serum T4 concentration has normalized. If the serum T4 can be decreased to within the reference range and the renal parameters remain stable or improve, antithyroid medications may be continued or a permanent therapy may be considered. If clinical signs of renal disease worsen with therapy, treatment of the hyperthyroidism should be reevaluated. Some cats may be healthier without treatment.56 Alternatively, the dose of methimazole can be titrated to achieve the best control possible of the hyperthyroidism while maintaining adequate renal function.
Methimazole can be given transdermally. Although methimazole in pleuronic lecithin organogel (PLO) is absorbed poorly in healthy cats after a single dose,57 it is likely that chronic dosing leads to improved absorption and resolution of hyperthyroidism as transdermal methimazole can be used to treat hyperthyroidism.58-60 The transdermal route may take longer, however, to bring about remission. In a randomized, prospective study of hyperthyroid cats, owners dosed their cats with methimazole orally (tablets) or transdermally (in PLO; 50 mg/mL) at 2.5 mg every 12 hours.59 Of cats treated transdermally 56% were euthyroid at 2 weeks, which was significantly fewer cats than the control rate in response to oral administration (88%); by 4 weeks the difference was no longer statistically significant (67% control with transdermal methimazole versus 82% for oral), but the lack of difference may have been due to a small number of cats remaining in the study at 4 weeks. Whether transdermal administration for a longer period of time would have controlled the hyperthyroidism in more cats was not evaluated. An advantage of transdermal methimazole is a significantly decreased rate of gastrointestinal adverse effects. However, the incidence of hepatopathy, facial excoriation, and blood dyscrasias is similar for both the transdermal and oral routes. Some cats develop erythema at the transdermal dosing site, but it is typically not severe enough to require drug discontinuation.59
Methimazole administration does not affect tumor size. Clinical signs will recur with discontinuation of the drug. Methimazole can be used before surgery to decrease serum T4 concentrations to within the reference range to stabilize the patient. Discontinuation of methimazole 2 weeks before radionuclide scanning or therapy has been recommended. Because radionuclide uptake is increased in normal tissue for 9 days after discontinuation of methimazole therapy,40 treatment within that window may increase the risk of iatrogenic hypothyroidism with radioiodine administration.
In humans, use of cholecystographic agents for treatment of hyperthyroidism has been studied, and the radiopaque organic iodine agent ipodate has shown some success. Experience with ipodate in veterinary medicine has been limited. A single study of 12 spontaneously hyperthyroid cats was performed using calcium ipodate granules reformulated into 50-mg capsules.61 Initial dosage was 50 mg/cat, administered orally twice daily. The dosage was increased to 150 mg (100 mg in the morning, 50 mg at night) and then 200 mg (100 mg orally twice daily) at 2-week intervals if serum T3 did not normalize or if other abnormalities attributable to hyperthyroidism failed to resolve satisfactorily. (Because the main mechanism of action of ipodate is to inhibit conversion of T4 to T3, monitoring of serum T3 concentrations is necessary to judge efficacy.) Only eight cats responded during the 14-week study period; seven of these were treated at a dosage of 100 mg/day, and one required 150 mg/day. Interestingly, serum T3 concentration decreased in the responders and declined into the normal range in two nonresponders as well. No adverse clinical signs or hematologic abnormalities attributable to ipodate treatment were noted.61 The reason for poor response in four cats was not apparent. Lack of efficacy of ipodate has been noted in human patients, especially those with Graves’ disease and severe hyperthyroidism. The effect of ipodate may be transient in some cats, given that two cats clearly had relapsed at the end of the 14 weeks.
Thus cholecystographic agents may be a feasible alternative to methimazole for medical treatment of feline hyperthyroidism and may be a good option for cats that require medical treatment but cannot tolerate methimazole. The need for special formulation of capsules may limit availability. Unfortunately, efficacy of treatment cannot be predicted, although cats with severe hyperthyroidism are less likely to respond. If treatment is efficacious, serum T3 concentrations will normalize and clinical signs will abate. Owners should also be warned that a positive response might be transient. Unfortunately, ipodate is no longer available. Iopanoic acid has been recommended for use at the same dosage, but no studies exist proving its efficacy.
Drugs Used To Cure Hyperthyroidism
Compared with medical therapy that simply controls hyperthyroidism, 131I therapy provides a cure. The goal of 131I therapy is to restore euthyroidism with a single dose of radiation without producing hypothyroidism. Surgery can also cure the disease, but 131I is the definitive treatment of choice in cats with ectopic thyroid tumors that are not surgically accessible (e.g., intrathoracic). In addition, 131I may be the best way to treat metastatic carcinoma. Radioactive iodine, like stable iodine, is actively taken up by and stored in thyroidal tissue. The emitted β particles cannot travel far, thus limiting damage to adjacent normal tissues. Because normal tissue has atrophied and is quiescent, 131I will be concentrated within a thyroid tumor(s), and normal tissue is relatively spared from destruction by the radioactive particles.
The 131I dose can be administered orally, subcutaneously, or intravenously but is usually given subcutaneously to avoid the stress of intravenous catheterization or injection and the possibility of vomition of radioactive material. The major disadvantages of radioactive iodine therapy include accessibility (a limited number of facilities are licensed to use radiopharmaceuticals, but this is changing), cost, and the possible extent of hospital stay while radioactivity decreases in the patient (potentially up to 2 weeks, depending on local radiation safety regulations). After discharge, cats will continue to excrete a small amount of radiation for 2 to 4 weeks, and close contact with the cat should be minimized during this time.
TSH increases 131I uptake of the thyroid gland in humans, and rhTSH is routinely administered before 131I treatment to lessen the 131I dose and minimize irradiation of nonthyroidal tissues. Whether rhTSH would increase the uptake of 131I in thyroid glands of hyperthyroid cats was recently examined.62 Five hyperthyroid cats were given 25 μg rhTSH intravenously 1 hour before 123I administration; 123I can be used to visualize hyperfunctional tissue and will be handled by the body like 131I but will not cure hyperthyroidism. The same cats were given another 131I injection 8 days later without first receiving rhTSH. The rhTSH increased radioactive iodine uptake by the thyroid gland by 7.33%, which was statistically significant. Thus hyperthyroid cats undergoing 131I therapy may require a lower radioactive iodine dose if given rhTSH before treatment, but further studies are needed to optimize the rhTSH dose and the interval between its administration and the 131I injection.
Administration of 131I by any route appears to be relatively safe. Pain or discomfort in the area of the thyroid gland presumably reflects radioactive thyroiditis and should resolve within several days of therapy. A transient voice change has been noted in one cat63 and transient dysphagia in eight.64 Although many cats develop a subnormal serum T4 concentration following 131I administration, a low serum T4 concentration in itself does not mean hypothyroidism is present. Because nonthyroidal diseases can suppress serum T4 concentration, nonthyroidal disease should always be excluded before a diagnosis of hypothyroidism is made. Supplementation is required only if clinical signs of hypothyroidism develop. Although 11% of cats have a low serum T4 concentration after 131I therapy, approximately only 2% require l-thyroxine supplementation.64
Clinical signs of euthyroidism generally occur within 1 to 3 weeks of 131I administration; the first sign generally is normalization of appetite and weight gain. Therapeutic failure rate is approximately 2%. If T4 is still elevated 3 to 6 months after 131I administration, the hyperthyroid state is unlikely to resolve without further treatment. Most of these cats will respond to a second dose of 131I.63-67 In one study 2.5% of cats had a relapse of hyperthyroidism 1.1 to 6.5 years after initial radioiodine treatment.64
Percutaneous ultrasound-guided intrathyroidal injection of ethanol or percutaneous radiofrequency ablation of thyroidal tissue has been tried as a means of targeting and destroying thyroid tumors. After treatment of unilateral disease with percutaneous ethanol injection (PEI), resolution was obtained lasting at least 12 months.68 However, only a small number of cats were studied. Bilateral PEI for bilateral disease led to mortality in one cat, likely because of laryngeal paralysis. Treating bilateral disease with staged injections has not shown long-term success, with the longest remission obtained being 27 weeks.69 Adverse effects include mild gagging, voice change, Horner’s syndrome, and laryngeal paralysis; are usually transient; and resolve in 8 weeks or less.68,69
Radiofrequency ablation was used to treat four cats with unilateral thyroid disease and five cats with bilateral disease.70 The nine cats were administered 14 treatments. Of the cats with unilateral disease, three became clinically and biochemically euthyroid for 1, 6, and 18 months; the fourth cat became clinically euthyroid for 6 weeks, although the serum T4 concentration remained slightly above the reference range. Interestingly, all cats with bilateral disease had treatments on one side and remission was obtained after 1 to 3 treatments for 1 to 6 months. Repeat therapy was used in some cats to induce a second or third remission when clinical signs returned. Transient Horner’s disease was the sole clinical complication and was seen after 3 of the 14 treatments. Clinically inapparent laryngeal paralysis was noted in one cat during laryngeal examination.70
Neither ethanol injection nor radiofrequency ablation is currently widely available for treatment of feline hyperthyroidism, and both require considerable experience. Radiofrequency ablation also requires specialized equipment.
Drugs Used To Control Clinical Signs of Hyperthyroidism
Thyroid hormones may increase the number or sensitivity of β-receptors in the myocardium.56 Tachycardia, myocardial hypertrophy, heart failure, and cardiac arrhythmias have been associated with thyrotoxicosis in hyperthyroid cats. Beta-adrenergic blockers (e.g., propranolol) have no effect on thyroid hormone concentration but decrease the neuromuscular and cardiovascular effects of hyperthyroidism, such as hyperexcitability, hypertension, and cardiac hypertrophy. These agents can be used in combination with an antithyroid drug such as methimazole or alone if a patient cannot tolerate antithyroid medications, and they may be helpful in preparing a patient for thyroidectomy or radioactive iodine by making the cat a better candidate for surgery or hospitalization. Nonselective β blockade by propranolol can reduce the hyperdynamic effects of thyroid hormones on the myocardium. In addition, propranolol inhibits conversion of T4 to T3 by peripheral tissues in hyperthyroid humans. Because propranolol does not directly affect the thyroid gland, however, patients are not returned to a euthyroid state. Propanolol dosing in cats suffering from cardiac disorders associated with hyperthyroidism should begin at 2.5 mg orally every 12 hours and be increased to 7.5 mg every 8 hours as necessary to control heart rate. If propranolol is being used to prepare a patient for surgery, therapy should continue for 14 days preoperatively. Care should be taken in patients with congestive heart failure because the negative chronotropic effects of propranolol may decrease myocardial reserve. In addition, as a nonselective β blocker, propranolol can cause bronchospasms, which may be lethal in cats with respiratory distress or may exacerbate feline asthma. Atenolol, a selective β1blocker, might be used instead of propranolol (2 mg/kg or 6.25 mg/cat once daily).
Administration of large doses of iodide (e.g., sodium or potassium iodide) for a short time (1 to 2 weeks) will cause transient hypothyroidism in normal animals. Organification of thyroid hormones is prevented and hormone secretion is reduced. The clinical effects of high-dose iodine therapy will occur in 7 to 14 days in humans; however, refractoriness to these effects will develop in several weeks to months. In hyperthyroid cats, iodine (50-100 mg orally, once daily) has been used to prevent an acute thyroid crisis (i.e., a thyroid storm) in patients undergoing thyroidectomy. One to two drops of a saturated solution of potassium iodide can be administered in gelatin capsules beginning 10 days before surgery.71
Diseases of the Parathyroid Glands
Normal Calcium Homeostasis
Calcium balance is maintained by the integrated influences of parathyroid hormone (PTH) on calcium and phosphorus reabsorption in bone and distal renal tubular cells and by the intestinal absorption of calcium as mediated by vitamin D (Figure 21-3). Of the total calcium present in serum, approximately 40% is protein bound, 10% is bound to other factors such as citrate or phosphate, and 50% is ionized. Serum ionized calcium concentration, which is the biologically active portion, normally fluctuates less than 0.1 mg/dL. Secretion of PTH is exquisitely sensitive to changes in ionized calcium concentration. Decreases in serum calcium concentration stimulate PTH secretion, which in turn causes increased calcium resorption from urine (distal renal tubule), increased mobilization of calcium and phosphorus from bone, and increased vitamin D synthesis. Parathyroid hormone mediates the activation of vitamin D (see Figure 21-3).
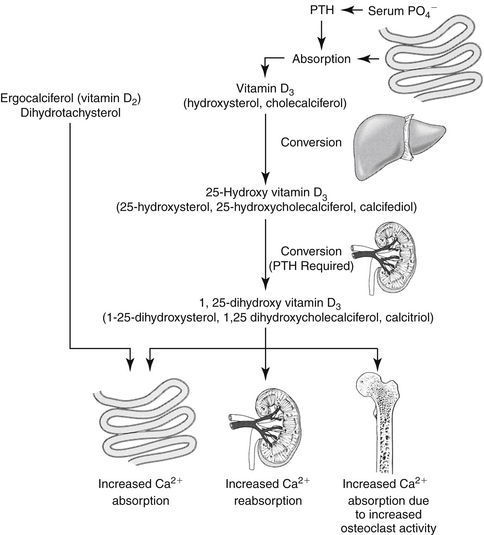
Figure 21-3 Calcium is very tightly regulated through the combined effects of parathyroid hormone (PTH), calcitonin, and vitamin D. The figure shows the activation of vitamin D and the way vitamin D and PTH act to increase serum calcium concentrations. Vitamin D must undergo several sequential activation steps before it is fully functional; calcitriol, the end product, acts to increase serum calcium concentrations and is essential for synthesis of PTH. Calcitonin, on the other hand, decreases serum calcium concentrations.
The main function of vitamin D is to increase gastrointestinal calcium and phosphorus absorption. In humans vitamin D (cholecalciferol) can be ingested or made by irradiation of cutaneous 7-dehydrocholesterol; the serum half-life of vitamin D is 19 to 25 hours, although the vitamin is stored in fat depots. In dogs vitamin D must be ingested; what occurs in cats is unknown. Cholecalciferol is converted to 25-hydroxycholecalciferol (25-OHD, calcidiol) in the liver by hepatic microsomal enzymes (see Figure 21-3). Once in circulation, 25-OHD is bound to a binding globulin. In humans 25-OHD has an elimination half-life of about 19 days. Calcidiol is further hydroxylated to the most potent form, 1,25-dihydroxycholecalciferol (calcitriol), in the renal proximal tubules. The enzyme responsible for hydroxylation of 25-OHD is inhibited by calcitriol in a negative feedback manner and by hyperphosphatemia. On the other hand, conversion of 25-OHD to calcitriol requires PTH; thus without PTH, little to no calcitriol is made and there is practically no functioning vitamin D in the body. Renal and, to some degree, bone activities mediate the acute response to calcium homeostasis. Intestinal calcium reabsorption may take several days to occur, in part because of the time necessary for vitamin D synthesis or activation. Calcitriol is further hydroxylated to 1,24,25-(OH) 3-D3 and subsequent metabolites that have variable activity.
Calcitonin, a polypeptide hormone secreted from thyroidal C (parafollicular) cells in response to hypercalcemia, is the least important regulator of calcium metabolism. It has a mild blood calcium–lowering effect by decreasing both the absorptive activity of osteoclasts and the formation of new osteoclasts. The main role of calcitonin may be to limit postprandial hypercalcemia. In normal animals the role of calcitonin is more important in juveniles. Calcitonin has minor effects on calcium handling by the gastrointestinal tract and at high doses promotes urinary calcium excretion.
Because ionized Ca is available to cells and is thus the biologically active form, it is critically important in the diagnosis of calcium disturbances. If ionized calcium is normal, even if total calcium is not, no further diagnostics are warranted. The correction formulas previously advocated and used to correct calcium concentration for serum albumin or protein concentration are no longer recommended.72 To know a patient’s calcium status, the clinician must measure ionized calcium concentration.
Hypoparathyroidism
Pathophysiology and Diagnosis of Primary Hypoparathyroidism
Primary hypoparathyroidism in dogs can reflect destruction of the parathyroid glands by disease (e.g., lymphocytic parathyroiditis) or trauma, including surgical removal. The most common cause in cats is injury or removal of the parathyroid glands during thyroidectomy. Hypomagnesemia can be a cause or effect of hypoparathyroidism in dogs and cats. Cessation of PTH secretion results in the loss of calcium mobilization from bone, of calcium retention by the kidneys, and of calcium absorption from the intestines. The primary clinical manifestation of hypoparathyroidism reflects decreased serum calcium concentration.
Hypoparathyroid patients may be hyperphosphatemic as a result of decreased renal phosphorus excretion. Measurement of serum PTH concentration is required to establish a diagnosis of hypoparathyroidism. Serum PTH concentrations below the reference range in hypocalcemic dogs and cats confirm the diagnosis, assuming the assay used is reliable and validated. Serum PTH concentrations in the low end of the reference range are not appropriate in hypocalcemic individuals, and a diagnosis of hypoparathyroidism can also be made.73
Hypocalcemia
Hypocalcemia and, in particular, decreased serum ionized calcium concentration leads to neuromuscular hyperexcitability as the stabilizing influence of calcium on neuronal sodium permeability is lost. Although both central and peripheral nerves are affected, clinical signs are usually peripheral, ranging from latent tetany (e.g., muscle cramping, lameness, irritability) to muscle fasciculations and stiff gait to tetanic seizures. The concentration of ionized calcium below which tetany develops depends on the rate at which hypocalcemia develops. Furthermore, cerebrospinal fluid concentrations may be more critical and may remain stable in the face of fluctuations in serum concentrations. Despite the importance of calcium in cardiac contractility, clinical evidence of cardiac dysfunction generally does not develop. Differential diagnoses for hypocalcemia include primary hypoparathyroidism, acute and chronic renal failure, acute pancreatitis, puerperal tetany (eclampsia), intestinal malabsorption syndromes, nutritional secondary hyperparathyroidism, ethylene glycol toxicity, administration of phosphate-containing enemas, congenital vitamin D receptor defects, and hypomagnesemia.
Treatment of Hypocalcemia and Hypoparathyroidism
No treatment fully compensates for the absence of PTH.74 The need for calcium therapy is based on serum calcium concentrations and clinical signs. Total serum calcium concentrations of 6.5 mg/dL might be considered critical; below 6 mg/dL generally results in clinical tetany, and below 4 mg/dL may be fatal.
Hypocalcemic tetany is a life-threatening condition, requiring immediate intravenous replacement. Because calcium can be lethal, it must be administered slowly to effect over 20 to 30 minutes. The amount of calcium in both oral and intravenous preparations varies with the salt. Because it is much less caustic if administered perivascularly, the calcium gluconate salt (10% solution: 9.3 mg calcium /mL) is preferred to the chloride (10% solution: 27.2 mg calcium/mL) and glucoheptonate (22% solution: 18 mg calcium/mL) salts. The dose of calcium for intravenous administration is 5 to 15 mg/kg (i.e., for the 10% gluconate salt solution [0.5 to 1.5 mL/kg] given slowly and intravenously. An electrocardiogram should be monitored during infusion; if bradycardia, premature ventricular contractions, or shortening of the Q-T interval is seen, the infusion should be slowed or briefly discontinued. Fluids containing bicarbonate, lactate, acetate, or phosphate cannot be used to administer calcium because the calcium will precipitate out of solution. Although seizures will stop once eucalcemia is established, other signs, such as nervousness, panting, and behavioral changes, may persist for 30 to 60 minutes after normalization of serum calcium concentration.73 Correction of tetany should help resolve hyperthermia that developed as a result of excess muscle activity. Life-threatening hyperthermia should be treated as an emergency.
Duration of response to a single dose of calcium varies from 1 to 12 hours. The type of future therapy required depends on the cause of hypocalcemia. If primary hypoparathyroidism is diagnosed, long-term treatment with oral vitamin D is required insofar as no suitable commercial PTH preparations exist. Oral calcium supplementation will also be needed in the long-term, but parenteral calcium administration is required in the short-term. Oral maintenance therapy with calcium should begin as soon as possible, but oral calcium is not absorbed from the gastrointestinal tract until vitamin D therapy begins to take effect, which is usually in 1 to 5 days. In the meantime, continuous calcium infusion is recommended (60-90 mg/kg/day elemental calcium) until oral medications provide control. If initial hypocalcemia was severe, the dose administered should be in the upper end of the range. The dose can be decreased as the oral medications begin to work and based on serum calcium concentration.73 Intermittent intravenous infusions are not recommended because of the wide fluctuations in serum calcium concentrations that will occur.74
In theory, the gluconate salt can be given subcutaneously, diluted 1 part calcium to 2 to 4 parts saline, but this is not recommended. Although not fully conclusive that calcium gluconate was the cause, two case reports suggest that severe calcinosis cutis and subsequent sloughing resulted from subcutaneous administration of calcium gluconate.75,76 Calcium carbonate solutions can never be given subcutaneously.
The daily oral calcium dose is 25 to 50 mg/kg, divided in several doses. Parenteral calcium administration can be slowly discontinued beginning 1 to 2 days after initiation of oral treatment; the rate will depend on serum calcium concentrations. Calcium salts available for oral administration include lactate (13% elemental calcium available), gluconate (10% elemental calcium available), carbonate (40% elemental calcium available [tablet form]), chloride (27% elemental calcium available) and citrate (21% elemental calcium available). The carbonate form has the highest percentage of calcium; does not cause gastric irritation, as do other forms; and is a phosphate binder, lowering serum phosphate, which has positive effects on endogenous calcitriol synthesis. Thus it is the preferred oral form. Other salts containing smaller amounts of calcium may require many tablets to be administered as a single dose. Over-the-counter preparations (e.g., Tums, which contains calcium carbonate) can also be used for oral maintenance therapy. As vitamin D therapy becomes effective, calcium supplementation should no longer be necessary (discussed later).
Vitamin D therapy (Figure 21-4) is necessary for both absorption of orally administered calcium and normalization of calcium homeostasis. Patients should be hospitalized during induction of vitamin D therapy, until serum calcium concentrations remain between 8 and 10 mg/dL without parenteral support. Vitamin D preparations vary in potency, time to onset (time to steady-state concentrations), and cost. Vitamin D can be administered as ergocalciferol (vitamin D2) and calcitriol (active vitamin D3). Vitamin D3 is likely, however, to be better absorbed orally than is ergocalciferol. Bile is essential for adequate oral absorption; the majority of absorbed vitamin D occurs in chylomicrons in the lymph. Dihydrotachysterol (DHT), a synthetic form of vitamin D previously used to treat hypoparathyroidism, is no longer available.
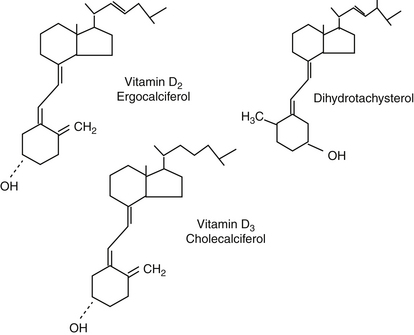
Figure 21-4 Structures of ergocalciferol, vitamin D2, present in plants, and cholecalciferol, vitamin D3, present in animals. Dihydrotachysterol is a congener of vitamin D2, currently not available commercially.
Ergocalciferol, or vitamin D2 (4000 to 6000 U/kg per day), is inexpensive and widely available. Large doses are necessary, however, to compensate for decreased potency in hypoparathyroid patients—it is an inactive form, and conversion to the active form will be impaired by low PTH concentrations in the patient. Large doses are also required to saturate fat depots (vitamin D is a fat-soluble vitamin). The effect of the medication is usually obvious 5 to 14 days after beginning therapy, and parenteral calcium can usually be discontinued 1 to 5 days after starting oral treatment.73 Once the serum calcium concentration is in the target range of 8 to 10 mg/dL, ergocalciferol can be administered every other day. Serum calcium concentration should be monitored weekly, with the ergocalciferol dose adjusted to maintain calcium in the target range.73 The required maintenance dose is 1000 to 2000 U/kg once daily to once weekly.73
In patients in whom development of hypocalcemia can be anticipated (e.g., after parathyroid gland tumor removal), it may be necessary to start vitamin D supplementation preemptively. Guidelines exist, although they are unproven. If the serum calcium is below 14 mg/dL, vitamin D therapy is not recommended preoperatively. If calcium is above 15 mg/dL or a dog has more than one parathyroid mass, calcitriol therapy should be started the morning of surgery or immediately thereafter.74
Even after a pet appears stable, rechecks are recommended monthly for the first 6 months and then every 2 to 3 months for life.73 Hypercalcemia must be avoided because it can cause renal and other organ damage and numerous other problems. A disadvantage of ergocalciferol therapy is that because of high lipid solubility and body fat–store saturation, serum calcium concentrations can take up to 4 weeks to decline once ergocalciferol administration has been discontinued. Hypercalcemia should be treated aggressively if it occurs. Because of the risk of hypercalcemia and the difficulty of treatment, ergocalciferol is not recommended for treatment of hypoparathyroidism.
Calcitriol is the vitamin D of choice for treatment of hypoparathyroidism. Although calcitriol is significantly more expensive than ergocalciferol, it is much safer. As calcitriol is already active, small doses may be used and the dose may be adjusted frequently because of its rapid onset of action (1 to 4 days versus 5 to 21 days for ergocalciferol) and brief biological effect. If hypercalcemia occurs, the effects of this drug abate quickly after stopping therapy or with dose reduction.73 A loading dose of 20 to 30 ng/kg daily can be administered for 3 to 4 days and then decreased to a maintenance dose of 5 to 15 ng/kg daily.74 Doses should be divided and given twice daily. Reformulation may be required for veterinary patients; alternatively, a liquid form can be used to facilitate dose adjustments.
When calcitriol is used, dose adjustments should be made in 10% to 20% increments and only after enough time has elapsed for a previous alteration to take effect. Total calcium measurement is recommended daily in the initial phase, weekly during maintenance until a satisfactory serum calcium concentration is obtained, and then quarterly thereafter. The target for total serum calcium concentration, regardless of the form of calcium or vitamin D used, is just below the reference range.
If hypomagnesemia is documented, magnesium sulfate (1 to 2 mEq/kg/day) should be administered. Correction of serum magnesium concentrations can decrease needed doses of vitamin D or calcium (or both).74
If hypoparathyroidism is due to surgical intervention and expected to be temporary (e.g., at least one parathyroid gland was left after the thyroidectomy in cats or a parathyroid tumor was removed in dogs), calcitriol can be discontinued over time. In cats parathyroid function usually recovers in 2 weeks but can take up to 3 months; after removal of a parathyroid tumor in dogs, return of function of the remaining gland (or glands) can take up to 12 weeks.74 Tapering of the calcitriol dose can begin after 4 weeks. If hypocalcemia returns, the calcitriol dose should be increased to the previous level and attempts at tapering made again later. If tapering after 3 months still results in hypoparathyroidism, permanent therapy with calcitriol will likely be necessary.74
Oral calcium should be continued until serum calcium is maintained between 8 and 10 mg/dL, usually 1 to 2 days to several weeks after vitamin D therapy is started (depending on the vitamin D product used). Calcium can gradually be tapered over weeks as normal serum concentrations are maintained.
Because of potential nephrotoxic and other effects, hypercalcemia should be avoided. If hypercalcemia (>12 mg/dL) occurs at any time regardless of the product used, vitamin D administration should be discontinued until serum calcium concentration returns to normal. In addition, oral calcium supplements, if being given, also need to be ceased and the patient should be placed on a calcium-restricted diet.
Primary Hyperparathyroidism
Hypercalcemia
Multiple rule-outs for hypercalcemia exist. They can be remembered by the mnemonic GOSHDARNIT: Granulomatous disease, Osteolytic disease, Spurious (e.g., sample lipemia or hemolysis), Hyperparathyroidism, D toxicosis (i.e., vitamin D toxicosis), Addison’s disease, Renal failure, Neoplasia, Idiopathic (only recognized in cats), and Temperature (hypothermia has been noted as a cause). The most common cause of hypercalcemia in dogs is malignancy.77 Lymphosarcoma is most likely, but multiple myeloma and anal sac apocrine cell adenocarcinoma and others are possible. In cats idiopathic hypercalcemia is most likely, but neoplasia or renal failure are also possibilities. Squamous cell carcinoma and lymphosarcoma are the most common hypercalcemia-causing neoplasias in cats.78
Assessing the phosphorus levels can aid in ranking differential diagnoses. The net effect of PTH is to increase serum calcium but decrease phosphorus. In primary hyperparathyroidism (PHPTH) the normal feedback mechanism of calcium on the parathyroid glands is lost. With elevated PTH hypercalcemia occurs with a low normal or low serum phosphorus concentration. The most common cause of PHPTH is neoplasia (functioning adenoma) of the chief cells of the parathyroid glands.79 Hyperplasia of the parathyroid gland(s), multiple endocrine neoplasia, and hereditary hyperparathyroidism are less common causes. Secondary hyperparathyroidism generally occurs as calcium and phosphorus homeostasis becomes unbalanced (e.g., renal or nutritional secondary hyperparathyroidism).
Most neoplasias cause hypercalcemia by secreting the hormone parathyroid hormone related–peptide (PTHrP), which binds to PTH receptors affecting serum calcium and phosphorus just as PTH does, mimicking PHPTH. Hematologic malignancies growing in the bone marrow or metastases of solid tumors to bone can also cause hypercalcemia.
Vitamin D toxicosis leads to elevations in both calcium and phosphorus and can result from ingestion of cholecalciferol-containing rodenticides or human antipsoriasis creams containing calcipotriene, a synthetic vitamin D3 (e.g., Dovonex)80 or from oversupplementation. Toxicity may not be evident until the drug has accumulated, which may take several weeks to months depending on the product. Granulomatous disease is an uncommon cause of hypercalcemia and may be due to the ability of macrophages to activate vitamin D independent of feedback mechanisms.
Unfortunately, the presence of renal failure can make interpretation of results difficult. Hypercalcemia can lead to renal failure and vice versa. If azotemia is present, phosphorus is likely to be elevated and cannot be used to help in ranking differential diagnoses. In addition, it can be hard to determine if the hypercalcemia or the renal failure came first. The clinician should be sure always to assess ionized calcium concentration. In the presence of azotemia, ionized calcium is often normal (and therefore not a cause for worry) when total serum calcium concentration is high. If ionized calcium is high, the diagnosis is likely PHPTH, whereas if the ionized calcium is normal to low, it is likely primary renal failure.
Clinical signs of hypercalcemia can be severe but are usually insidious and unnoticed. Polyuria and polydipsia are the most common in dogs. Hypercalcemia can cause secondary nephrogenic diabetes insipidus, initially increased and then decreased GFR, nephrocalcinosis, and soft tissue mineralization. Muscle weakness and atrophy, depression, anorexia, vomiting, shivering, bone pain, constipation, a stiff gait, and cardiac arrhythmias can also be seen. In cats presenting signs include vomiting, weight loss, dysuria, anorexia, inappropriate urination, lethargy, diarrhea, hematuria, pollakiuria, and stranguria. Signs of lower urinary tract disease may be seen in up to one third of hypercalcemic dogs and cats, which are prone to forming calcium-containing uroliths. Fibrous osteodystrophy of all bones, but particularly the skull, can occur with hyperparathyroidism.
Pathophysiology and Diagnosis of Primary Hyperparathyroidism
PHPTH results in hypercalcemia from autonomous PTH secretion from solitary or multiple parathyroid gland adenomas or hyperplasia or parathyroid gland carcinoma. Although no epidemiologic studies have been done, PHPTH is uncommon in dogs and rare in cats, primarily affecting older animals. It can occur in any breed, but it is an autosomal dominant, genetically transmitted disease in the Keeshond.81 Because clinical signs tend to be absent to mild, hypercalcemia can be diagnosed on a serum biochemistry profile obtained for unrelated concerns. Given the numerous diseases that cause hypercalcemia, a minimum database of a hemogram, serum biochemistry profile, urinalysis, and urine culture should be obtained and a full, careful physical examination performed in all hypercalcemic animals.
If clinical signs are present, the most common ones involve the renal, gastrointestinal, or neuromuscular systems. Of the clinical signs reported in dogs with PHPTH, 81% had polyuria or polydipsia; 73% had either urinary tract calculi, a urinary tract infection, or both; 53% had decreased activity; and 37% had a decreased appetite; azotemia was documented in only 5%. The most commonly reported clinical signs in cats are anorexia, lethargy, and vomiting. In contrast to dogs, in which a parathyroid mass is rarely palpable, 11 of the 19 reported cats with PHPTH had a palpable mass.79
In uncomplicated PHPTH, the biochemistry profile usually demonstrates persistently elevated total and ionized calcium, decreased to low-normal phosphorus, and normal BUN and creatinine levels. Assays for PTH and PTHrP are relatively sensitive, readily available diagnostic tools to help identify the cause of hypercalcemia. In general, in animals with PHPTH, serum PTH levels are within the normal reference range or elevated, and PTHrP is usually undetectable. In the face of hypercalcemia, a PTH concentration within the upper half of the reference range is wholly inappropriate and consistent with a diagnosis of PHPTH. In the face of hypercalcemia, a PTH concentration in the lower half of the normal range should raise suspicion for PHPTH, although it does not confirm it diagnostically.
Although previous reports state that 90% of dogs and cats with PHTPH have a solitary adenoma,79 in one recent study, 42% of dogs had multiglandular disease.82 It is therefore important to identify all abnormal glands before or at the time of definitive treatment. Selective sampling and determination of serum PTH concentrations in both jugular veins have proved inconsistent in predicting the affected side, and this procedure is not recommended.79,82 Cervical ultrasonography can be a useful diagnostic aid in locating and identifying abnormal parathyroid glands, with reported sensitivity rates ranging from 42% to 100% when performed by highly experienced ultrasonographers.82,83 However, finding parathyroid glands with ultrasound requires a great deal of practice and skill.
Treatment of Primary Hyperparathyroidism
For PHPTH the only effective long-term therapy is ablation of the abnormal parathyroid tissue. Several methods of definitive treatment for PHPTH exist, but surgical excision is the most common, with cure rates up to 95% if all autonomously functioning tissue is removed.83 In humans undergoing parathyroidectomy, PTH concentrations are measured preoperatively and intraoperatively 10 minutes after removal of the abnormal gland. A greater than 50% decrease in PTH concentration between the preexcision and postexcision samples is associated with successful removal of autonomously functioning tissue in humans. A minimum period of 6 months of normocalcemia postoperatively is considered curative in human medicine. With this in mind, a rapid intact PTH assay was recently validated in dogs. In 12 dogs the assay accurately detected increased serum PTH concentrations in all dogs with PHPTH preoperatively. A greater than 50% decrease in serum PTH levels between the preexcision and postexcision samples corresponded with a return to normocalcemia for at least 6 months in 92% of the dogs in the study.82
Ethanol injection84 or application of radiofrequency85 has been reported for treatment of PHPTH. In a retrospective study, ethanol injection had only a 72% efficacy rate, whereas radiofrequency ablation and surgery had success rates of 90% and 94%, respectively, for medians of approximately 560 days.83 Thus chemical ablation is not recommended. Unfortunately, radiofrequency ablation is not widely available and requires considerable expertise. With bilateral disease, staged treatments are needed.
Treatment of Hypercalcemia
Because hypercalcemia is best treated by addressing the underlying disorder, the cause should be delineated as quickly as possible and appropriate therapy instituted. Hypercalcemia can be damaging, especially to the kidneys, with severity appearing to depend on the phosphorus concentration. If the product of multiplying the serum calcium concentration by the serum phosphorus concentration is above 60 to 80, nephrotoxicity is likely.79 If the etiology cannot be identified rapidly and continuing hypercalcemia is judged to be deleterious, clinical signs are present, or the hypercalcemia is idiopathic, the hypercalcemia should be addressed directly.
If treatment is deemed necessary, intravenous fluid therapy should be initiated. Dehydration may be present and should be corrected because it decreases GFR and can perpetuate hypercalcemia. Saline (0.9%) is the fluid of choice. Fluid deficits should be replaced and then fluid rates maintained at 120 to 180 mL/kg daily to promote calciuresis. Potassium supplementation is usually required to prevent hypokalemia.79
Furosemide may be given to increase calcium excretion, but patients should be well hydrated first. Constant-rate infusion is typically recommended (5 mg/kg intravenously followed by a 5 mg/kg/hr infusion).79 Lower doses (2-4 mg/kg 2 to 3 time daily, intravenously, subcutaneously, or orally) may produce a milder reduction in hypercalcemia. Thiazide diuretics are discouraged because they may increase renal calcium reabsorption.
Glucocorticoids have many beneficial effects; they decrease intestinal calcium absorption, reduce bone resorption, and increase renal calcium excretion. Prednisone (1-2.2 mg/kg twice daily, orally, subcutaneously, or intravenously) or dexamethasone (0.1-0.22 mg/kg twice daily, orally, subcutaneously, or intravenously) can be used. Steroid-sensitive hypercalcemias include lymphoma/leukemia, multiple myeloma, vitamin D toxicity, granulomatous disease, hypoadrenocorticism, and feline idiopathic hypercalcemia. Glucocorticoids should not be administered unless a definitive diagnosis is known. Even one dose can cause remission in a patient with lymphosarcoma. Although this could be beneficial in the short-term, it will make diagnosis impossible until remission ends. Furthermore, if standard combination chemotherapy is started after remission from prednisone alone is lost, it will be less effective, and, accordingly, overall prognosis will be worse.
Calcitonin (salmon calcitonin, 4-6 IU/kg subcutaneously, 2 to 3 times daily) can be used if other treatments fail. It can be an effective treatment for vitamin D toxicosis or other diseases in which bone resorption is a major cause of the hypercalcemia. In dogs the response may be short-lived, and anorexia, vomiting, or allergic reactions may occur. Information in cats is lacking.
Bisphosphonates, “osteoclast poisons,” are believed to act by interfering with hydroxyapatite crystal dissolution or by direct action on osteoclasts. They may also be used if bone resorption is a major source of the hypercalcemia. Pamidronate disodium has been used to treat vitamin D toxicosis secondary to calcipotriene ingestion at a dose of 1.3 to 2 mg/kg,86,87 as well as hypercalcemia associated with nocardiosis (one cat), idiopathic hypercalcemia and renal failure (one cat), lymphoma (two dogs), thyroid carcinoma (one dog), and multiple endocrine neoplasia (one dog).87 Given its potential nephroxicity, pamidronate should be diluted in saline and infused intravenously over a 4-hour period with a minimum of 4-hour diuresis both before and after the treatment.87 Calcium and phosphorus normalize in 24 to 48 hours.86,87 The decrease in total and ionized calcium concentration after treatment is proportional to the degree of hypercalcemia.87 Treatment can be repeated if necessary, but may not be needed depending on the cause of the hypercalcemia. In one cat with idiopathic hypercalcemia, although a first treatment worked, a second and third did not. For the second and third treatments, previously frozen pamidronate was thawed and administered.87 Whether lack of response was due to progression of the disease state or to instability of the pamidronate with freezing, storage, and thawing is unknown. Etidronate is an oral drug that has had limited use in dogs but may be helpful (5-15 mg/kg once to twice daily). Clodronate, which is not available in the United States, has been used successfully to treat experimentally induced vitamin D toxicity if given within 24 hours after vitamin D ingestion.88 Clodronate toxicity and efficacy for treating accidental vitamin D overdose remain to be determined.
Sodium bicarbonate (1 to 4 mEq/kg intravenously by slow bolus) can be given during a hypercalcemic crisis. This does not always lower total serum calcium concentration but decreases the ionized portion, so adverse effects of the hypercalcemia are less common.
It is unclear whether therapy for treatment of feline idiopathic hypercalcemia is required. One guideline is to make the decision using the previously described guidelines for emergency treatment: Treat if calcium continues to rise, the calcium–phosphorus product is greater than 60 mg/dL, the ionized calcium concentration is greater than 1 mg/dL above the reference range, or clinical signs or azotemia are present.89 The aggressiveness of therapy depends on the clinical status and parameters present. If treatment is not pursued at first, calcium concentrations should be monitored every 1 to 3 months and the need for therapy reevaluated. With any treatment, ionized calcium may not normalize even though total calcium does,90 so measurement of the ionized form is required for accurate assessment of therapeutic success.
Dietary therapy with a high-fiber diet was reported to be successful in five cats based on measurement of total serum calcium and/or resolution of clinical signs.91 Although dietary management was not effective in other cats,90 the recommended first line of treatment for idiopathic hypercalcemia is to place an affected cat on a diet that is not designed to acidify the urine; urine-acidifying diets have been theorized to contribute to development of hypercalcemia. High-fiber, oxalate-prevention, or renal diets can be implemented. If ionized calcium has not normalized after 3 months, prednisone therapy should be added89 and may be successful.90 The recommended starting dose is 5 to 10 mg per cat per day, but it can be increased to 10 mg twice daily if needed. Prednisolone may be preferred to prednisone.89 Bisphosphonates may be useful, and intravenous pamidronate has been used in one cat.87 Although no peer-reviewed publications exist, use of alendronate (10 mg/cat/once weekly) has been successful for up to 1 year.89 Because erosive esophagitis has been reported in women taking alendronate, the weekly pill should be followed with 6 mL of water given by a dosing syringe and then a small dab of butter on the cat’s lips to increase licking and salivation.89 Therapy for renal failure should be instituted as needed. Subtotal parathyroidectomy has not been successful.90
Diseases of the Pancreas: Diabetes Mellitus
Normal Physiology
Glucagon stimulates glucose production from both hepatic conversion of glycogen and metabolism of noncarbohydrates, such as amino acids and lipids, into glucose or glucose precursors (e.g., lipids to fatty acids and glycerol). Low blood glucose concentrations increase glucagon secretion from pancreatic α cells. Should glucose concentrations become too high, insulin secretion is stimulated from pancreatic β cells. Insulin lowers blood glucose by stimulating formation of glycogen from glucose in the liver, inhibiting peripheral formation of glucose from amino acids and lipids, and facilitating diffusion of glucose into cells in insulin-dependent tissues such as muscle and adipose tissue. As a result, amino acids are synthesized in muscle, and adipocytes produce and store fat. Insulin secretion is regulated by a negative feedback mechanism sensitive to blood glucose concentrations. As the blood glucose concentration decreases, pancreatic β cells secrete less insulin and blood glucose does not decline further. With lower serum insulin concentrations, insulin-dependent cells can no longer utilize blood glucose, whereas insulin-independent cells such as neurons, which obtain glucose by simple diffusion, continue to use any glucose that remains in the blood.
Diabetes mellitus (DM) is a progressive disease resulting from an absolute or relative insulin deficiency caused by insufficient insulin secretion from pancreatic β cells and is characterized by four stages in human patients. Prediabetic patients are normal but subsequently develop the disease; subclinical DM can be diagnosed only by sophisticated provocative tests. In latent DM, patients are clinically normal but respond abnormally to a glucose load, and in overt DM, patients have persistent fasting hyperglycemia. The duration of progression from stage I to stage IV can be weeks to years, depending on the type of DM.
How best to classify DM in veterinary medicine is controversial, with the current classification scheme adapted from human medicine. Type 1 DM is characterized by β-cell destruction with progressive, eventually complete, insulin insufficiency. Genetic susceptibility plays a role, and β-cell destruction is usually due to immunologic processes. Causes of type 1 DM in animals may include hereditary factors and pancreatic destruction by pancreatitis, viruses, or autoimmune disease. Insulin resistance, dysfunctional β cells, and increased hepatic gluconeogenesis characterize type 2 DM. A classification of “other specific types of diabetes” also exists, formerly referred to as “secondary” DM. On the basis of pancreatic islet histology, lack of β-cell antibodies, risk factors, and clinical behavior of the disease, type II DM appears to be the most common form in cats.92 Approximately 10% to 20% of diabetic cats have “other specific types of DM”, which includes diseases that lead to β-cell loss, such as pancreatic neoplasia, and conditions such as hyperadrenocorticism or acromegaly that cause marked insulin resistance.93 The remaining cats are believed to be type I diabetics. DM in dogs is believed to be mainly type 1.94
DM can also be classified as insulin-dependent DM (IDDM) or non-insulin-dependent DM (NIDDM), depending on the need for insulin therapy to control glycemia, prevent ketoacidosis, and survive. Individuals with IDDM must receive insulin to prevent ketoacidosis, whereas control of glycemia and avoidance of ketoacidosis can be accomplished through diet, exercise, and oral hypoglycemic drugs in patients with NIDDM.95 At the time of diagnosis, all dogs are likely to be insulin dependent, as would be expected with type 1 diabetics, and at least 75% of cats with type 2 DM are insulin dependent.93
Management of persistent hyperglycemia is important to the progression of the syndrome, especially in cats. Cats may alternate between being insulin dependent and not, and the DM can go into remission for weeks to years with appropriate therapy. Persistent hyperglycemia can profoundly reduce insulin secretion and induce apoptosis in β cells, ultimately causing permanent DM in cats that at one point had a normal β cell mass,96 a situation referred to as glucose toxicity. The effects begin within 2 days of onset of persistent hyperglycemia, and the impact on β cells increases with the magnitude of hyperglycemia.97 Pancreatic βcell death by apoptosis occurs within 10 days of persistent hyperglycemia.97 The impact of glucose toxicity is more severe in the presence of reduced β cell mass, underscoring the importance of effective control and βcell preservation. Control of blood glucose, ideally with insulin but potentially with oral hypoglycemic therapy, may allow β cells to regain insulin secretory capacity. As a result, the diabetic state is transient in 15% to 68% of cats96,98-100 and can require as much as 20 to 52 weeks of insulin therapy before remission is achieved.101,102
Diagnosis
Presence of appropriate clinical signs (e.g., polyuria or polydipsia, polyphagia, and weight loss) and documentation of persistent fasting hyperglycemia (>200 mg/dL) and glycosuria are used to diagnose overt DM. Occasionally, a definitive diagnosis of DM may be difficult to make. Mild hyperglycemia, such as that induced by stress or certain diseases (e.g., hyperadrenocorticism), may uncommonly result in glycosuria. In nondiabetic cats, blood glucose concentrations may reach 400 mg/dL. Thus diagnosis of DM must be based on blood glucose and the presence of the classic signs. Unfortunately, the clinical signs are not unique to DM, and other differential diagnoses must be considered. On the other hand, some latent diabetic animals become overtly diabetic because of the presence of concurrent diseases; clinical signs of the precipitating disease may obscure the classic signs of DM. Hospitalization of a patient and reevaluation of blood glucose after the patient has adjusted to the new environment may help identify nondiabetic hyperglycemic states. Alternatively, urine can be monitored for the persistent presence of glucose at home. The presence of ketonuria strongly supports a diagnosis of DM. Identifying current drug therapy can also be important; progesterone or prolonged glucocorticoid therapy, which can precipitate DM, might support its diagnosis.
Measurement of glycosylated proteins can be used to corroborate a diagnosis of DM. Non-enzymatic, irreversible binding of glucose to hemoglobin results in the formation of glycosylated hemoglobin (GHb). Fructosamine refers to glycosylated serum proteins, mainly albumin, but glycated albumin (GA) also can specifically be measured in dogs and cats.103,104 Glycosylated proteins form at a rate proportional to the average blood glucose concentration, so the higher the mean blood glucose concentration over time, the greater the glycosylated protein concentrations should be. Glycosylated protein levels are also affected by the half-life of the native protein; the shorter the half-life, the quicker the concentration of glycosylated protein falls after correction of hyperglycemia. Albumin has a short half-life compared with that of red blood cells. Thus in dogs and cats, GHb reflects glycemic control over the previous 2 to 3 months. In comparison, in dogs fructosamine reflects the previous 2 to 3 weeks and GA the previous 1 to 3 weeks.104 In cats plasma proteins may be more rapidly metabolized, and fructosamine concentrations reflect the glucose concentrations in the previous 7 days.105
One study suggested that fructosamine might be more sensitive than GHb for monitoring control,106 but measurement of either can be helpful. Both parameters are typically not affected by stress.107-112 However, measurement of either is neither perfect nor absolute. Although in general, the higher the blood glucose concentration over time, the higher serum glycosylated protein concentrations should be, normal animals or well-controlled diabetics can have elevated concentrations of these substances, and, conversely, uncontrolled diabetic animals can have normal levels of either.110,111,113 Thus the glycosylated protein concentration must be interpreted in conjunction with all other data.
Dietary Therapy
Dietary therapy is very important for treatment of DM. Through unknown mechanisms, dietary fiber can delay gastrointestinal glucose absorption, reducing postprandial fluctuations in blood glucose and enhancing glycemic control. High-fiber diets have been traditionally recommended for diabetics, but this is now being questioned. Insoluble fiber is beneficial in diabetic dogs.114,115 However, the response of diabetic dogs to fiber varies between individuals. A recent study showed that high-fiber, moderate-starch diets were not advantageous for dogs with stabilized DM compared with a moderate-fiber, low-starch diet.116 Insoluble fiber, the type present in commercial feline high-fiber diets, can also improve glycemic control in diabetic cats.117 However, recent theories suggest that high-carbohydrate diets may lead to DM in cats, and high-protein diets may be more beneficial. The DM of cats on a high-protein, low-carbohydrate diet either resolved or was treatable with a reduced insulin dose.118,119
Although veterinary low-carbohydrate, high-protein prescription diets such as Purina DM or Hill’s m/d are the first-choice dietary recommendation for most cats with DM, a carefully selected over-the-counter high-protein, low-carbohydrate diet can provide the same degree of effective glycemic control as prescription diets120 when financial constraints dictate the less-expensive option or when a cat will not readily eat a veterinary diet. Many canned over-the-counter diets are relatively low in carbohydrate content (<5.0g/100kcal), but information must be obtained from the manufacturer on specific brands and flavors to ensure that the target nutrient composition is being met. Most dry over-the-counter diets are higher in carbohydrate content. Thus, if a prescription dry veterinary low-carbohydrate, high-protein diet is not an option, it may, unfortunately, be more difficult to identify a good-quality dry food with low carbohydrate content. Caution should be used in feeding diabetic cats with concurrent renal disease a high-protein diet; a high-fiber diet may be a better choice.
Drug Therapy
Insulin is the mainstay of therapy in diabetic dogs and cats. Its administration must be integrated with meals, exercise, owner’s needs, and other characteristics of the patient or pet owner’s lifestyle; these factors have been described elsewhere.95,121-123 Although the ideal goal of insulin therapy is to maintain blood glucose concentrations as close to physiological as possible, this is difficult to do because exogenous insulin is administered as one or two large daily doses rather than in response to glucose concentrations. The realistic goal for insulin therapy, at least, should be elimination of the clinical signs of DM. Because secondary complications that dramatically alter the quality of health of human diabetics such as retinopathy or nephropathy appear to be rare in animals, near normalization of glucose concentrations may not be as important for diabetic dogs and cats as it is for human diabetic patients. Glucose concentrations should, however, be sufficiently controlled to prevent development of ketoacidosis and detrimental effects of hyperglycemia. Hyperglycemia is associated with an increased risk of bacterial infections; hepatic lipidosis; pancreatitis; and renal, hepatic, and pulmonary disease. Complicating problems that may alter response to insulin therapy must be identified in diabetics, as well as underlying diseases that led to the development of clinical signs in a covert diabetic.
Insulin Preparations
Insulin preparations are defined by modifications that alter time of onset and duration of action. They also vary in the source species and therefore in potential antigenicity. Chemical extracts of cattle and swine pancreases traditionally were the primary source. More recently, bacterially produced human recombinant products have been developed, but these are more expensive. More problematic, development of human products has led to discontinuation of several animal-source products that had been used successfully for controlling DM in dogs and cats.
The kinetics of individual insulin products vary markedly among species. Insulin products are generally classified as short-acting (e.g., regular insulin), intermediate-acting (e.g., neutral protamine Hagedorn [NPH], lente), or long-acting (e.g., PZI, Ultralente). The duration of action of regular insulin is the same in dogs and cats, but the duration of action of other insulins is typically shorter in cats than dogs. Intermediate- and long-acting insulins tend to be less bioavailable and thus less potent than short-acting insulins when given subcutaneously.
Regular insulin (zinc insulin crystals) is unmodified and acts the same whether crystalline or noncrystalline. Given intravenously, it begins working immediately, with maximal effects occurring at 0.5 to 2 hours and duration of effect being 1 to 4 hours. When insulin is given intramuscularly, time to effect is 10 to 30 minutes, time to peak effect is 1 to 4 hours, and duration of effect is 3 to 8 hours. When it is given subcutaneously, onset of effects occurs in 10 to 30 minutes, maximal effects occur at 1 to 5 hours, and duration is 4 to 10 hours. Inhaled insulin has recently been shown to be systemically bioavailable and physiologically active in dogs and cats, with an onset of action between 5 and 15 minutes124 and a duration of action of 3 hours.125 Neutral regular insulin will maintain its potency for as long as 18 months when stored at 5° to 25° C and will maintain 95% of its potency when stored at 37° C for 12 months.
Regular insulin is generally reserved for treatment of ketoacidosis. Use in daily maintenance therapy might include administration in combination with longer-acting products to provide a rapid onset with long duration of action; however, in general, such combinations are not needed or used. Insulin is available commercially in stable preparations of 70% NPH/30% regular or 50% NPH/50% regular (e.g., 70/30 or 50/50 insulin, respectively), but these are often quite potent, causing a rapid decrease in blood glucose concentration, and duration of effect is usually short.95
Protamine zinc insulin (PZI) was developed to prolong the effects of regular insulin. The preparation is formed by mixing insulin, zinc, and protamine, a fish protein, in a buffered solution such that it precipitates as a poorly soluble form. Poor solubility prolongs the absorption time after subcutaneous administration and provides a slower onset of activity and a longer duration of action. For PZI insulin the onset of action occurs at 1 to 4 hours, and maximum effect occurs at 3 to 12 hours. Duration is 12 to 24 hours in cats, and it may be necessary to administer the drug twice daily (in approximately 25% of cats). Use of PZI Vet (Idexx Pharmaceuticals) is limited to the existing supply; in April 2008 the manufacturer announced that after the sale of its existing inventory, it would no longer manufacture or sell animal-based insulin. Although many pharmacists can compound bovine PZI insulin, the authors do not recommend its use because the potency of compounded insulins varies between bottles.
To replace PZI Vet, a similar formulation based on recombinant human insulin (Prozinc) has recently been developed by Idexx. Limited data exist evaluating its efficacy and safety in diabetic cats, and no published pharmacodynamic studies of Prozinc in cats are available. One study compared glycemic control achieved with Prozinc with that of PZI Vet in diabetic cats. Fifty cats with stable glycemic control on PZI Vet were switched to Prozinc on day 0. Serum fructosamine concentrations, body weight, and insulin dose were measured on days 0, 15, and 30. On day 30, the cats were switched back to PZI Vet. Owners’ subjective assessments of their cats’ glycemic control were obtained at all time points. There were 47 cats completing the study; three were removed because of diabetic remission (n = 1), hypoglycemia (n = 1), and fractious behavior (n = 1). In the cats that completed the study, no difference in glycemic control between the insulin types was detected. The only adverse event reported was hypoglycemia in one cat.126 A second study evaluated Prozinc in 126 newly diagnosed and 13 poorly controlled diabetic cats.127 Control was assessed on days 7, 14, 30, and 45 by evaluating clinical response, body weight, serum fructosamine, and serial blood glucose concentrations over a 9-hour blood glucose curve. Based on the measured parameters, 84% of cats were judged to have good glycemic control by day 45. Thus Prozinc appeared safe and efficacious in diabetic cats. Prozinc has gained FDA approval for use in diabetic cats.
Isophane (NPH) insulin, developed as a compromise between short-acting regular insulin and slow-acting PZI, is made by manipulating the ratio between insulin and protamine. Isophane insulin is more potent and faster-acting than PZI but shorter in duration. For NPH administered subcutaneously, onset of action occurs in 0.5 to 3 hours and maximum effects occur at 2 to 8 hours. Duration of action is as little as 6 to as long as 12 hours in cats and 18 hours in dogs. Twice-daily administration is usually necessary for adequate diabetic control.
Lente insulin does not incorporate protamine but contains insulin and high concentrations of zinc (10 times greater than that in regular insulin) in an acetate rather than a phosphate buffer. Adjustment of pH causes insoluble precipitates to form that are longer-acting (i.e., ultralente) compared with soluble, shorter-acting (i.e., semilente) preparations. Lente insulin is a mixture of approximately 70% ultralente and 30% semilente, and its effects begin immediately. In dogs, time to maximum effect is 2 to 10 hours and duration is 8 to 20 hours. In diabetic cats, time to maximum effect for lente is 2 to 5 hours and duration of effects is 8 to 12 hours.128 Human recombinant forms of lente and ultralente insulins are no longer available. The only veterinary preparation of lente insulin is Vetsulin (Intervet), which is porcine in origin. Vetsulin is currently approved in the United States by the Food and Drug Administration for use in both dogs and cats; it has been available for many years in other countries under the name Caninsulin. It is the only insulin approved for use in dogs. Currently, ultralente is not formulated as a veterinary preparation.
Lastly, “designer” recombinant insulins are made in which the amino acid structure of the protein has been altered slightly in order to change the pharmacokinetic profile. One of these products, glargine (Lantus), is used in veterinary medicine. Glargine differs from human insulin in that glycine is substituted for asparagine at position 21 in the α chain of insulin and by addition of two arginine residues to the βchain. Glargine is a clear aqueous solution with a pH of 4. The interaction of the acidic insulin and the relatively neutral pH of subcutaneous tissues causes microprecipitates to form and thus gives a relatively constant systemic absorption profile. As formation of microprecipitates depend on the solution’s acidity, glargine cannot be mixed or diluted.129 Although marketed as a “peakless” insulin in humans, it does have peaks and nadirs in healthy cats. In nondiabetic cats given 0.5 U/kg glargine insulin once daily, the mean time to the glucose nadir was 14 hours.130 It is long-acting, with blood glucose concentrations suppressed below baseline at 24 hours in approximately half the cats studied, whether given once daily (0.5 U/kg) or twice daily (0.25 U/kg).130,131 Another long-acting insulin analog, detemir, has lower within-subject variability compared with NPH and glargine in human type 1 or type 2 diabetics.132,133 In one study of detemir using healthy cats, the findings suggest that glargine may have a more rapid onset then detemir, but the peak effect of detemir may be more predictable. It also appears that detemir may have a longer duration of action than glargine.134 There are no published studies evaluating detemir insulin in diabetic cats or dogs at this time.
The species of origin of insulin products potentially plays a role in therapeutic efficacy. Canine and porcine insulin have identical amino acid sequences, so porcine insulin is less immunogenic in dogs.135 Reduced antigenicity may, however, be undesirable (discussed later). Porcine insulin has a shorter duration of action than does beef insulin in dogs; human insulin appears more potent than beef–pork insulin in cats, thus requiring lower (25%) dosing.
Commercial insulins are available in concentrations of 40 (U-40), 100 (U-100), and 500 (U-500) U/mL. One unit of insulin is equal to 36 μg. Syringes are available for the corresponding insulin concentration. Accurate dosing requires that the appropriate syringe be used. For U-100 insulin, low-dose syringes are highly recommended for patients requiring low doses.
Insulin should be mixed before administration by gently rolling the bottle between the palms of one’s hands; aggressive shaking may denature the product. Although refrigeration is not necessary, extreme sunlight and heat can destroy insulin. Refrigeration can protect the insulin.
Insulin Therapy
Individual response to insulin varies greatly. In cats, differences in required doses probably reflect, at least in part, availability of endogenous insulin. Both dose and frequency of administration should be designed for an individual patient; it may be necessary to base frequency on a compromise between meeting client needs and minimizing fluctuations in blood glucose. Although long-acting preparations can initially be tried every 24 hours, a 12-hour interval usually proves necessary; the authors prefer starting with twice-daily dosing to achieve control sooner. In one study 94% of dogs required twice-daily dosing regardless of insulin type used for adequate control.136
Regular insulin can be given by intravenous, intramuscular, or subcutaneous routes, but all other preparations should be given subcutaneously. Injection should occur in the lateral, not dorsal, thoracic or lumbar areas. The dorsum can be poorly vascularized, especially between the shoulder blades, and drug absorption can be erratic. Although some authors recommend a starting dose of 0.25 U/kg twice daily,95 others recommend using 0.5 U/kg if the blood glucose is greater than 360 mg/dL and 0.25 U/kg if it is less than 360 mg/dL.137 Dogs should be fed 50% of their daily food immediately before each injection to ensure appropriate caloric intake before administration of a full dose of insulin. Semimoist foods are discouraged.
The type of insulin to use is a matter of personal opinion and experience. For dogs, therapy should begin with an intermediate-acting insulin (e.g., NPH, lente). In one study, 53 dogs with uncomplicated diabetes were treated with Vetsulin for 60 days after a variable initial dose determination period. Therapy started once daily and was changed to twice daily as needed. The starting dose was 1 U/kg, with a supplemental dose depending on body weight (dogs below 10 kg received 1 U supplement, 10-11 kg 2 U, 12-20 kg 3 U, and above 20 kg 4 U), as formerly recommended in the package insert. (Note that the dosing scheme was based on the package insert at the time of the study, but recommendations are now different.) Efficacy and safety were evaluated at the end of the dose determination period (time 0) and 30 (time 1) and 60 days (time 2) later. At times 0, 1, and 2, 100%, 66%, and 75% of the dogs, respectively, were judged to be adequately controlled based on blood glucose concentrations and clinical signs. By day 60 66% were receiving twice-daily injections. The median number of days required to achieve adequate glycemic control was 35 (range 5-151). No unexpected side effects were observed, but 22 dogs had signs at some time that could have been caused by hypoglycemia and two died of presumed hypoglycemia. The owners of seven dogs reported swelling or pain (or both) at the injection site, but neither symptom was noted by the investigators.138
In cats, good options are glargine, PZI, and lente insulins. Ultralente is no longer available. Prozinc currently is an acceptable insulin choice for cats; detemir (discussed previously) may be a good option for the treatment of diabetic cats in the future, but because of the lack of availability and limited data, it cannot be recommended at this time.
One study evaluated use of PZI in 67 diabetic cats. Initial PZI dosage ranged from 0.2 to 0.6 U/kg twice daily. After 45 days, the mean dose was 0.9 U/kg (range 0.2 to 1.8). Mean blood glucose nadir occurred approximately 5 to 7 hours after insulin injection but ranged from 1 to 9 hours.139 Overall, 90% of owners believed their cat improved. Clinical hypoglycemia occurred in five cats, and hypoglycemia without clinical signs occurred in another 21 (31%). Ten cats were not controlled by day 45. Whether longer treatment and more dosage adjustments would have achieved control is unknown. In general, cats with newly diagnosed DM had a better response than those with previously treated DM; perhaps the cats that failed previous treatment had an underlying cause of insulin resistance. Most diabetic cats will require PZI twice daily for adequate control, but once-daily injections may suffice in up to 25%. Initial PZI dosage should be low (e.g., 1 U/injection) to prevent hypoglycemia.139 The initial reports on Prozinc also support initial low doses with this insulin. In one study one cat was removed because of documented hypoglycemia, and in another study 160 of 690 serial blood glucose curve values documented hypoglycemia in 87 of 139 diabetic cats. The reports do not state whether any episodes of hypoglycemia were clinically apparent.126,127
In diabetic cats, the use of glargine appears extremely promising. Glargine has a long duration of action and predictable blood glucose–lowering effects. In eight newly diagnosed cats treated with a high-protein, low-carbohydrate diet, the DM resolved in all cats within 4 months.140 Glargine appears to be appropriate for use in any cat, providing control of blood glucose concentrations throughout most of the day. Long-term diabetic cats have been switched to and treated with glargine insulin as well with excellent results;141 remission is less likely after long-term treatment with another insulin, but it may still occur.120 In a study evaluating remission rates in diabetic cats initially treated with insulin, 55 diabetic cats were evaluated whose owners followed a highly intensive monitoring and blood glucose regulation (blood glucose concentrations maintained between 50-100mg/dL) protocol using insulin glargine and fed a low-carbohydrate diet. A total of 35 cats (64%) achieved remission. Cats that received glucocorticoid treatment within 6 months before diagnosis of DM, that required a lower maximum insulin dose, or that were intensively managed using glargine within 6 months of diagnosis were more likely to achieve remission, whereas cats with a peripheral neuropathy present at diagnosis (e.g., difficulty climbing stairs or a plantigrade stance) were less likely to do so. Other factors examined that were not predictors of entering remission were age at diagnosis, gender, obesity, evidence of diabetic ketoacidosis at diagnosis, development of azotemia during therapy, concurrent hyperthyroidism, and frequency of asymptotic hypoglycemia.142 In the authors’ opinion, glargine insulin is the current preferred insulin choice for diabetic cats, be they newly diagnosed or long-term diabetics with poor glycemic control on their current insulin regimen. It is also the only commercially available long-acting insulin that has been extensively studied in diabetic cats.
Cats should be started at a glargine dose of 0.5 U/kg if the blood glucose concentration is above 360 mg/dL or 0.25 U/kg if the blood glucose concentration is below 360 mg/dL. In either case, twice-daily administration is recommended. Compared with other types of insulin, with which dose adjustments are typically made based on the blood glucose nadir, for glargine dose adjustments should be made based on the fasting blood glucose value. Because the doses are typically small, 0.3 mL, low-dose syringes should be used for accurate dosing.
Lente insulin can be used in cats, but because it has a shorter duration of action than glargine or PZI, it almost always, if not always, must be administered twice daily.128 A recommended starting dose is 0.25 U/kg twice daily if the blood glucose concentration is 216 to 360 mg/dL or 0.5 U/kg twice daily if the blood glucose concentration is above 360 mg/dL.143 Alternatively, a dose of 1 U/cat twice daily for cats weighing less than 4 kg and 1.5 to 2 U/cat twice daily for cats weighing more than 4 kg can be used to initiate therapy.144
A multicenter study recently assessed the efficacy and safety of porcine lente insulin in 46 diabetic cats.102 Therapy was initiated twice daily at a dose of 0.25 U/kg if the blood glucose concentration was 270 to 360 mg/dL and 0.5 U/kg if the blood glucose concentration was above 360 mg/dL. All cats were followed for a stabilization period of approximately 16 weeks, and 23 cats were followed for an additional variable period of up to 49 weeks. Diabetic remission was achieved in 15%; all were newly diagnosed diabetics, and time to remission ranged from 2 to 56 weeks. The mean insulin dose both at the end of the stabilization period (week 16) and the end of the study (week 49) was 2 U per cat twice daily. The authors concluded that lente insulin was safe and effective in diabetic cats; achieving diabetic stability with lente may take 3 to 4 months; cats were more likely to go into remission if they were newly diagnosed, compared with previously diagnosed, diabetics; and it could take as long as 56 weeks to achieve remission.
Long-term response to insulin was assessed in 54 diabetic cats that were evaluated numerous times over a minimum of 3 months.145 Insulins used included beef/pork PZI (n = 14), beef/pork ultralente (n = 26), and beef/pork lente (n = 14). Response was based on resolution of clinical signs and mean blood glucose, with good, mediocre, and poor response considered to be a blood glucose concentration less than 200 mg/dL, between 200 and 300 mg/dL, and greater than 300 mg/dL, respectively. No difference was found among the types of insulin regarding therapeutic success, although this may reflect the small number of animals studied rather than variability of the outcomes.145
Interestingly, the percentage of cats that were judged to have responded was different depending on which criteria were used: clinical signs versus mean blood glucose concentration. For PZI and ultralente, 14% and 15% of cats achieved good control based on mean blood glucose, whereas no animals were considered well controlled for lente. If using clinical signs as the means to assess control, the percentage of good responders was greater for all treatment groups: 50% for PZI and ultralente and 79% for lente. Improvements in mean blood glucose were considered mediocre in 64% of the PZI group and 50% each for ultralente and lente, whereas improvements in clinical signs were considered mediocre in 50%, 42%, and 21% of the PZI, ultralente, and lente groups, respectively. Glycemic control was significantly better in the cats without concurrent disease compared with cats that did have it. Survival was evaluated in a total of 104 cats. Mean and median survival times in cats with good glycemic control (on the basis of mean blood glucose) for all treatment groups were 24 and 16 months compared with 17 and 20 months for cats with mediocre control, respectively.145
Monitoring of Therapy and Alteration of Insulin Dose
Marked variation in insulin kinetics—particularly in cats—makes monitoring diabetic control crucial. Options include performance of serial glucose curves either in a hospital or at home, measurement of serum glycosylated protein concentrations, monitoring of presence and degree of glycosuria, and assessment of the presence or absence of clinical signs of DM.
Performance of in-hospital blood glucose curves has long been the gold standard for assessing diabetic control. Glucose curves should establish the insulin effectiveness, time to peak effect, duration of effect, glucose nadir, and degree of fluctuation in blood glucose concentration and identify the Somogyi phenomenon (discussed later), if present. To construct a curve, blood glucose concentration is measured in general every 2 hours for one interval between injections (i.e., for 12 hours if insulin is administered twice daily or for 24 hours if insulin is given once daily). When blood glucose concentration is below 125 mg/dL, the concentration should be measured hourly. A normal insulin and feeding schedule must be maintained as much as possible. If a patient does not eat the normal amount of the normal food at the usual time, the serial glucose curve should probably not be performed. The patient should be fed its standard diet at the usual time and the insulin given by the owner at home or, if possible, in the hospital so a veterinarian or veterinary technician can assess the owner’s injection technique. Obtaining a fasting blood sample for measurement of blood glucose concentration before insulin injection can aid in appraisal of glycemic control, but this may not be possible for the morning value if normal feeding time occurs before the hospital opens. Clearly, cooperation between client and veterinarian is necessary to maximize the information obtained with minimal disturbance to routine.
A curve should be performed the first day insulin therapy is initiated or changed. Glucose concentrations may be lower than expected after the first 24 to 48 hours of insulin therapy, especially in cats as stress hyperglycemia resolves.146 The first curve is done solely to ensure that hypoglycemia does not occur. If hypoglycemia is found, the insulin dose should be decreased 25% and another curve done the following day to check for hypoglycemia. The insulin dose should not be increased based on the first day’s curve. A patient requires 5 to 7 days on a dose of insulin to equilibrate and reach maximal effect. Another blood glucose curve should be performed 7 to 10 days after discharge. Based on assessment of the curve, the insulin dose can be increased or decreased as necessary.
The pattern of insulin effect should be used to determine dose, interval, and feeding schedule. Ideally, glucose concentrations should reach a nadir (i.e., the lowest point) of 80 to 150 mg/dL. The highest glucose concentration should be close to 200 to 250 g/dL in dogs or 300 mg/dL in cats. The actual nadir and peak concentrations in a patient will probably be lower or higher, respectively, than measured because the exact timing of nadir and peak effects of insulin are not known and can change day to day; therefore blood glucose concentration is not often measured exactly at those times. Changes in insulin dose can usually be made without affecting true (as compared to apparent) duration of effect. The glucose differential is the difference between the nadir and the blood glucose concentration before the next dose and can be a measure of insulin effectiveness.147 If the curve is relatively flat (e.g., differential of 50 to 100 mg/dL), the insulin may not be having a desired effect. However, the glucose differential should be interpreted in conjunction with the absolute blood glucose concentrations obtained from the curve. If all blood glucose concentrations are less than 200 mg/dL, then the insulin is very effective. However, if all blood glucose concentrations are between 350 and 400 mg/dL, then the insulin is ineffective or stress hyperglycemia is present.
When assessing a glucose curve, whether it is the first curve performed on a patient or the last of many, the clinician should ask three basic questions. First, has the insulin succeeded in lowering the blood glucose? Second, if the insulin has lowered the blood glucose, what was the lowest blood glucose value? Third, how long has the insulin lasted? By answering these questions, logical changes in dosing regimen can be made if necessary. Results of a serial glucose curve should always be interpreted in light of clinical signs. Curves vary from day to day in dogs148 and cats.149 Stress hyperglycemia can falsely elevate results, and a patient’s refusal to eat while hospitalized can falsely decrease results.150 If a patient is not polyphagic or polydipsic and polyuric and body weight is stable or increasing, diabetic control is likely good.
The first aim in regulating a diabetic is to achieve an acceptable nadir, with blood glucose concentrations ideally falling between 80 and 150 mg/dL. In general, if an acceptable nadir is not achieved, the insulin dosage should be adjusted depending on the size of the animal and the degree of hypoglycemia or hyperglycemia. Usually, changes of approximately 10% are appropriate. Hypoglycemia should always be avoided. No matter what other blood glucose concentrations are during the day, if the nadir blood glucose concentration is below 80 mg/dL, decrease the dose by 25%. Blood glucose concentrations should be reevaluated 7 to 10 days after an insulin dose adjustment is made if hyperglycemia was the issue. If a dose is lowered because of the presence of hypoglycemia, a curve should be performed the next day to ensure that hypoglycemia does not recur.
Once an acceptable nadir exists, duration of action can be determined by a blood glucose curve. Duration of action is the time from the insulin injection through the lowest glucose concentration until the blood glucose concentration exceeds 200 to 250 mg/dL. If the insulin dose is inadequate and the target glucose nadir has not yet been achieved, the dose must be increased until the nadir is acceptable before duration of effect of the insulin can be determined.
If insulin with too short a duration of activity is used, obtainment of an acceptable glucose nadir may not be possible. In these patients, blood glucose concentration is typically quite high in the morning because control has been inadequate for most of the previous day. A blood glucose curve in this situation shows a noticeable but brief decrease in serum glucose concentration after the insulin injection. Increasing dosing frequency from once to twice a day or changing to a longer-lasting insulin type is indicated.
Based on duration of action, the following general recommendations can be made. If the duration is 22 to 24 hours, once-daily therapy is adequate. If the duration is 16 to 20 hours, a shorter-acting insulin should be used twice daily. If the duration is 13 to 16 hours, twice-daily insulin therapy can be tried, but the evening dose should be lower than the morning dose. If the duration is 10 to 12 hours, the patient should receive insulin twice daily. If the duration appears to be less than 8 hours, a blood glucose nadir higher than 80 mg/dL must be ensured. If blood glucose drops below 60 mg/dL at any time, the Somogyi phenomenon can occur. The Somogyi phenomenon or overswing, also called hypoglycemia-induced hyperglycemia, refers to a period of hypoglycemia followed by marked hyperglycemia. The phenomenon results from a normal physiologic response when blood glucose concentrations decline to less than 60 mg/dL in response to an insulin dose that is too high or when blood glucose concentration decreases rapidly regardless of the nadir.95 In either case a number of reflexes are triggered that act to increase blood glucose. Counterregulatory hormones such as epinephrine, cortisol, glucagon, and growth hormone (GH) are secreted. The net effect of these hormones is to increase hepatic glycogenolysis and gluconeogenesis and to decrease peripheral tissue glucose utilization. Hyperglycemia usually occurs rapidly, thus preventing a hypoglycemic seizure. Insulin secretion does not occur in response to the rise in glucose, however, as would occur in normal dogs and cats, and diabetics become extremely hyperglycemic (400 to 800 mg/dL). If the Somogyi phenomenon is observed, the insulin dosage should be decreased so the nadir is above 80 mg/dL; counterregulatory hormones will no longer interfere with the action of the exogenous insulin, and the true duration of effect will become apparent. If the duration of insulin action is truly less than 8 hours, adequate therapy with that type of insulin requires injections more frequently than twice daily, which is impractical for most owners. A switch between different types of intermediate-acting insulin can also be beneficial. For example, a dog or cat may metabolize NPH insulin quickly, resulting in too short an effect, but lente insulin may have a longer duration.
Once control has been achieved, blood glucose curves should be performed every 3 to 6 months to assess adequacy of glycemic control or earlier if clinical signs suggest that control has been lost. The more precarious the control, the more frequently rechecks should be done. As during the initial curves, if the nadir is unacceptable, the insulin dose must be lowered or raised accordingly. If duration of action appears to have changed, then the same modifications as previously discussed can be made.
If using glargine insulin in cats, interpretation of blood glucose curves and dose adjustment is different than for other insulin types. For the first 3 days, 12-hour blood glucose curves should be performed (i.e., the curve should be performed for the interval between the morning and evening dose). The purpose of the blood glucose curve is to detect hypoglycemia, if present, and lower the glargine dose as needed. Many cats require dose reduction within the first 3 days. It is important to note that the insulin dose should not be increased regardless of the appearance of the curves. After the first 3 days, the cat should be sent home and then return for a curve 7 days later. Subsequent blood glucose curves should be performed at 1, 2, and 4 weeks and then as required.
Recommendations for dose adjustments are based on the pre-insulin blood glucose concentration, compared with other insulins for which dose is altered on the basis of the nadir. If at recheck the pre-insulin blood glucose concentration is higher than 290 mg/dL, the glargine dose should be increased by 1 U per cat. The dose should not be changed if the pre-insulin blood glucose concentration is 220 to 290 mg/dL. In either of these first two scenarios, a curve should be done to ensure that hypoglycemia does not occur. The dose should be decreased 0.5 to 1 U/cat if the pre-insulin blood glucose concentration is 80 to 180 mg/dL. If biochemical hypoglycemia is present (i.e., blood glucose concentration is below 80 mg/dL but no clinical signs of hypoglycemia are present), the dose should be decreased by 1 U per cat. If clinical signs of hypoglycemia are present, the glargine dose should be decreased 50%. Administration of glargine should not be discontinued within 2 weeks of starting treatment even if euglycemia is present; the clinician should decrease the dose if needed but should not stop the insulin.151
To determine if a cat is in remission, insulin administration should be continued until the cat is receiving 1 U twice daily. Then, if the pre-insulin blood glucose concentration is below 180 mg/dL, once-daily administration is appropriate. If the next day the pre-insulin blood glucose concentration is still below 180 mg/dL, the clinician should not administer insulin and should do a complete curve. If the pre-insulin blood glucose concentration is above 180 mg/dL when the cat is receiving once-daily insulin, the clinician can go back to twice-daily administration. An attempt to wean the cat can again be made in a couple weeks. Diabetic cats in remission should stay on a low-carbohydrate diet.
If performance of a curve is impossible because of the cat’s temperament or the owner’s financial constraints, the clinician may start glargine at 0.25 U/kg (with a maximum dose of 2 U/cat) subcutaneously twice daily and have the owner monitor urine glucose concentration or water intake. A cat well regulated on glargine should have trace urine glucose at most, and urine glucose should be negative most of the time. If after 2 weeks of receiving glargine, urine glucose is greater than trace, the dose should be increased 1 U per cat weekly until urine glucose is negative or water intake is below 20 mL/kg in a 24-hour period if the cat is eating canned food and below 60 mL/kg in a 24-hour period if the cat is eating dry food. At this point, the cat should be kept on the same dose for 2 weeks, and then the clinician can start decreasing the dose by 1 U per cat weekly until urine glucose is positive or the insulin has been discontinued.152
Performance of blood glucose curves has become controversial, insofar as they are certainly not perfect. Blood glucose curves can be affected by the stress of hospitalization, especially in cats, and deviation from normal routine, and they vary day to day. In one study, glucose curves were performed in diabetic dogs on 2 consecutive days, with all conditions being identical on both days (e.g., type and dose of insulin, amount and type of diet). Parameters such as minimum, maximum, and mean blood glucose concentration; fasting blood glucose concentration before the morning or evening injection (all dogs were treated twice daily); and time from insulin injection to nadir were significantly different between the two curves. In some dogs, the curve showed better control on day 1, whereas others showed better control on day 2. To examine the clinical implications of day-to-day variability of the curves, a theoretical recommendation for adjustment of the dog’s insulin dose was based on the results of each curve. The researchers assessed 30 sets of paired 12-hour curves. Opposite theoretical recommendations for adjustment of a dog’s insulin dose were made on day 2 compared with day 1 in 27% of occasions. For 17% of the curves, a different but not opposite recommendation resulted. The same dosage adjustment recommendation was made on both days in 57%. Given this variation, predicting the timing of a diabetic’s nadir based on previous serial blood glucose curves and obtaining a single sample at that time is unlikely to give a reliable result—in other words, spot-checking does not provide helpful information.148
To avoid some of the problems associated with in-hospital curves, performance of glucose curves at home has taken on new importance. For glucose curves it is not necessary for venous blood to be collected. Capillary blood is suitable,153 with the ear being the best site for blood collection. Two types of lancing device are available. If conventional automatic devices designed for pricking human fingertips are used, a device with a variable needle depth should be chosen and the appropriate depth for each patient used.154 Warming of the ear with a hair dryer or a warm, wet washcloth inside a plastic bag may be necessary but not well tolerated, and it may take up to 2 minutes to obtain an adequate sample depending on the size of the drop required.154 A device that creates a vacuum after the skin is lanced (e.g., Microlet Vaculance, Bayer) does not require warming of the ear and generates an adequate drop of blood within approximately 30 seconds,154 but mastery may be a bit difficult and require repeated instruction.155 Glucometers that require minimal amounts of blood as well as those that “sip” the blood into the strip are desirable. Studies support that owners are both willing and able to generate home serial glucose curves, although some owners may require additional demonstrations.156-158
Unfortunately, home-generated curves have not eliminated all the problems associated with serial glucose curves, because variability remains a problem. Interestingly, a study of diabetic cats compared four serial blood glucose curves generated at home and in the clinic.158 Owners obtained a 12-hour glucose curve once monthly for 4 months. Within a week of obtaining the at-home curve, the cats were brought to the clinic, where the curve was repeated. The mean glucose concentrations tended to be lower in the clinic than at home. The mean glucose concentrations for each of the four clinic curves compared with the home-generated curves were 336 mg/dL versus 363 mg/dL, 316 mg/dL versus 381 mg/dL, 235 mg/dL versus 307 mg/dL, and 235 mg/dL versus 345 mg/dL. One explanation put forth for these findings was that the cats commonly refused to eat while in clinic. Theoretical treatment recommendations based on nadir glucose concentrations of the home-generated and in-clinic curves were compared, and decisions differed in 38% (14 of 37). In three of these instances, treatment decisions would have been completely contrary.158 Additionally, day-to-day variability in curves persists, even if home-generated. In seven diabetic cats, paired glucose curves were generated on 2 consecutive days at home or in the clinic. Considerable day-to-day variability existed in both, with no greater agreement between home curves and clinic curves.156 These findings augment the recommendation that changes in insulin dose be made on the basis of biochemical indicators of glycemic control (e.g., glucose concentration, fructosamine) as well as clinical support of glycemic control (e.g., weight gain, absence of polyuria or polydipsia).
Continuous monitoring of glucose concentrations has also received attention of late.149,159,160 The CGMS (Continuous Glucose Monitoring System, MiniMed) is a device that can be strapped onto a patient and a small needle inserted into the subcutaneous tissue. Interstitial glucose concentrations are sampled every 5 minutes for up to 72 hours. Using such a device gives many more data points for evaluation and avoids the stress of multiple venipunctures or catheterization. Furthermore, a patient could wear the device at home if necessary.
The device has been assessed in normal and diabetic dogs and cats. Interstitial and blood glucose concentrations were highly correlated overall.149,159,160 However, postprandial increases in blood glucose concentration may not be detected in the interstitial fluid.160 Accuracy varied between patients, and the difference between blood and interstitial glucose concentrations was more marked in some patients than others. The greatest discrepancies occurred at higher glucose concentrations.160 The working range of the CGMS is approximately 40 to 400 mg/dL—that is, blood glucose concentrations outside the range cannot be measured. In fact, if the device repeatedly detects values outside the range, it is interpreted as a machine error and the device shuts off. No irritation resulted from sensor placement.149,159,160 Preliminary results suggest that the CGMS is useful for clinical management of insulin therapy.
Measurement of urine glucose concentration at home can aid in monitoring. First, urine glucose levels can be determined as needed to aid in assessment of glycemic control, especially when other data are conflicting. Consistently negative readings on urine glucose may indicate that insulin dosages are either adequate or excessive. A serial glucose curve will differentiate between adequate insulin therapy and use of excessive doses that could result in hypoglycemia. Uniformly high urine glucose readings coupled with unresolved clinical signs indicate that the insulin dose may be inappropriate. Negative urine glucose concentrations in the afternoon, followed by high urine glucose readings the following morning, may be indicative of the Somogyi phenomenon; however, documentation of the Somogyi phenomenon requires performance of a glucose curve. Second, urine glucose concentrations can be determined regularly (at least weekly) to help in the assessment of ongoing control. Changes in urine glucose levels may alert the owner and clinician to loss of glycemic control and the need for reevaluation. If a cat’s urine cannot be collected, use of Glucotest (Purina), a product that is sprinkled in a litter box and changes color to reflect the urinary glucose concentration, may help.161
Monitoring of GHb or fructosamine may be helpful. Measurement of glycated proteins alone is probably not adequate for assessment of overall control, but looking at trends can be illuminating. Concentration of either glycated protein should be measured when a diabetic is first diagnosed, and then reassessed with every recheck examination. In general, if the concentration is increasing, control is deteriorating, and if the concentration is decreasing, control is improving. A study of healthy cats infused with intravenous glucose to maintain moderate or marked hyperglycemia (mean glucose 306 mg/dL and 522 mg/dL, respectively) found considerable overlap in fructosamine concentrations between the two groups.105 Although elevated in the cats with marked hyperglycemia, fructosamine concentrations only intermittently exceeded the reference range in cats with moderate hyperglycemia.105 Finally, to attribute a change in fructosamine concentration over time to altered glycemic control in the patient and not just inherent variability in the test, a critical difference of more than 33 μmol/L was identified.105 Thus trends of sufficient magnitude in fructosamine concentrations of individual patients may be the best use of this tool to monitor glycemic control in diabetic patients.
Lastly, home monitoring of clinical signs has been advocated as a useful adjuvant tool in assessing glycemic control. (It should be emphasized, however, that home monitoring of clinical signs alone is not an appropriate means of monitoring a diabetic animal but should be one of several monitoring modalities employed. This is discussed later in this chapter.) One study evaluated the usefulness of a variety of different clinical and biochemical measurements in 23 cats treated with lente insulin.162 The investigators compared subjective clinical control and water intake to biochemical markers of glycemic control (i.e., serial blood glucose, fructosamine, beta-hydroxybutyrate, cholesterol, triglycerides, glycerol, and urine glucose concentrations). No single measurement best correlated with the level of clinical control identified. The amount of water drunk over 24 hours, maximum blood glucose concentration, mean blood glucose concentration, and urine glucose were the most useful practical indicators of clinical control. Owners were often happy with the level of control in their cats despite not having laboratory evidence of tight glycemic control, emphasizing the importance that the long-term aim of treatment in diabetic cats is to control the clinical signs associated with hyperglycemia.
In one study of 53 dogs, control was judged to be good or poor based on clinical signs, physical examination findings, and body weight. Then, clinical determination of good or poor control was compared with fasting blood glucose, serial glucose curve, and serum fructosamine and GHb concentrations. Although all parameters of glucose control were significantly lower in dogs with good control, considerable overlap existed between the two groups for all. All blood glucose measurements and fructosamine and GHb concentrations were consistent with good glycemic control in 60% of dogs judged to have good clinical control or with poor control in only 39% of dogs judged to have poor clinical control. The initial fasting blood glucose was 100 to 300 mg/dL in 80% of dogs with good clinical control and in 21% of dogs with poor clinical control.163
Although the importance of home monitoring of clinical signs cannot be overemphasized, these authors believe that glucose curves should be performed periodically in all diabetic patients (for aggressive animals or those who experience stress hyperglycemia in the hospital, the curves are most appropriately performed at home, if possible). If diabetic control appears inadequate based on clinical signs, the only way to know how to adjust the dose is by performing a curve. Furthermore, hypoglycemia can be clinically nondetectable until a crisis is reached (e.g., the patient begins to have seizures). Performance of curves can demonstrate occurrence of hypoglycemia, and the insulin dose can be changed before clinical signs occur.
Complications of Insulin Therapy
Periods of hyperglycemia in otherwise well-controlled diabetics are difficult to avoid. Evaluation is indicated if hyperglycemia is present most of the time or clinical signs exist. Owner compliance regarding storage, mixing, and administration of insulin should be re-examined first in such a scenario, as well as patient management (e.g., diet, exercise). After these causes of poor control have been eliminated, a glucose curve should be performed to identify causes of poor control such as the Somogyi phenomenon (discussed previously), short duration of insulin action, or insulin resistance.
Interestingly, not every hypoglycemic event triggers the Somogyi phenomenon. The precise reason is not known, but it may have to do with how quickly the blood glucose concentration drops; the Somogyi phenomenon may be more likely to occur with a more rapid decline. Cats, in general, may be less likely to have a Somogyi phenomenon than dogs. In a recent study that evaluated intensive blood glucose control using glargine in 55 diabetic cats,164 asymptomatic hypoglycemia (blood glucose concentration <50 mg/dL) was measured in most (93%) cats at least once. However, symptomatic hypoglycemia was rare, with only a single event in one cat that had mild signs of restlessness.164 Despite the high number of hypoglycemic periods, only four single documented Somogyi events occurred in four cats.165
The Somogyi phenomenon can occur in dogs or cats receiving any insulin dose, but it should be particularly considered as a possibility in patients receiving more than 2.2 U/kg of insulin per dose in the face of persistent glycosuria (>1 g/dL) and clinical signs indicative of poor control (i.e., polyuria, polydipsia, and polyphagia). In addition, it may occur particularly when insulin dose adjustments are made based on early-morning urine glucose concentrations. Diagnosis of the phenomenon is based on documenting nadir and peak glucose concentrations of below 65 mg/dL and above 300 mg/dL, respectively, within a single dosing interval or a rapid decrease in blood glucose concentration followed by a rapid rise and a marked hyperglycemia, regardless of whether hypoglycemia occurred. Because the timing of the blood glucose nadir varies day to day,148 the presence of the Somogyi phenomenon cannot be documented by measuring blood glucose concentration at a single time point.
Documenting the presence of the Somogyi phenomenon can be challenging. The hormones secreted (e.g., GH, cortisol) may induce insulin resistance that lasts for as long as 72 hours after a hypoglycemic episode. Depending on when a glucose curve is performed, the overswing may be seen; alternatively, if a curve is done on the day after the overswing, only consistently high blood glucose concentrations may be measured. If the presence of glucosuria is monitored, it may be absent or quite elevated, depending on when a sample is collected.
If a Somogyi overswing is present, the daily insulin dose should be decreased by at least 25% and by 50% if clinical signs of hypoglycemia are noted. A glucose curve should be performed immediately after the reduced insulin dose is administered to ensure that hypoglycemia is no longer occurring. Once hypoglycemia has been eliminated, another curve should be performed in approximately 5 to 7 days and the dose adjusted further if necessary. If the cause of the overswing is rapid lowering of blood glucose concentration and not hypoglycemia, a change in insulin type is required.
Rapid metabolism of insulin can result in a clinical situation that looks similar to the Somogyi overswing. With rapid insulin metabolism, the patient may become markedly hyperglycemic before the next insulin dose, but the hyperglycemia is not preceded by hypoglycemia or a rapid decline in blood glucose concentration, as with the Somogyi phenomenon. Morning urine glucose concentrations will be high, and clinical signs of DM generally persist. Increasing the insulin dose will only cause a lower nadir, not prolong duration of action, and hypoglycemia may result. The syndrome can be diagnosed only through construction of a curve. Treatment involves changing to a longer-acting insulin or increasing injection frequency. The vast majority of diabetic dogs require twice-daily insulin therapy for adequate control.136 Evaluation of glycemic control should be done approximately 5 to 7 days after initiating a new insulin dose, and further dose adjustments made as needed.
Hypoglycemia
Hypoglycemia is the most common complication of insulin therapy in dogs and cats. Insulin overdose, failure to eat, presence of vomiting, and increased exercise raise the risk of hypoglycemia. Humans can suffer from hypoglycemic unawareness, a condition associated with poorly controlled DM, in which mild hypoglycemia is not sensed and clinical signs of hypoglycemia do not develop until the blood glucose concentration is quite low. Although not documented, the same may occur in dogs and cats.
Clinical signs of hypoglycemia are neurologic, and tissues with the highest metabolic activity are impaired first. Cortical signs include disorientation, weakness, and hunger followed by lethargy and ataxia. Seizures and coma ensue if hypoglycemia persists. Blindness may be a permanent sequela. Death can occur as a result of central respiratory and cardiac depression.
In a retrospective study, the most common clinical signs of hypoglycemia in diabetic dogs were seizures, ataxia, and weakness, occurring at a median of 3 months after initiation of insulin therapy; in cats seizures, recumbency, anorexia, shaking, vomiting, ataxia, and dullness occurred after a median duration of 8 months of therapy. Other clinical signs noted in dogs were anorexia, diarrhea, restlessness, pacing, blindness, and coma; in cats amaurosis, vocalization, circling, lethargy, weakness, diarrhea, urination, stupor, and coma were noted. Obese cats were considered at greater risk for overdosage.166
The development of hypoglycemia in a previously well-controlled animal suggests that a change has occurred either exogenously (e.g., use of an incorrect syringe, overly concentrated insulin because of improper mixing) or endogenously (e.g., DM was transient, patient is vomiting or anorectic, patient has developed maldigestion or malabsorption). Overlap of action of injections should also be considered (i.e., one injection is given when blood glucose concentration is still low from the previous injection).
Treatment of hypoglycemia varies according to the severity of signs. Mild hypoglycemia can be treated by feeding a normal meal; moderate signs may require treatment with sugar or syrup (e.g., Karo syrup) being fed to the animal or rubbed on the buccal membranes. Convulsions require intravenous administration of 50% dextrose. A slow intravenous bolus of 50% dextrose (0.5 g/kg diluted 1:4) should be administered, followed by a continuous-rate infusion of 5% dextrose until the patient can be fed. A single dose of dextrose may not be adequate. Dextrose doses from 0.25 to 19.2 g/kg and 0.2 to 6.3 g/kg were required in dogs and cats, respectively, to attain euglycemia after an insulin overdose, and continuaton of a dextrose drip may be necessary for up to 4 days to maintain normal blood glucose concentrations.166 Insulin therapy should be discontinued until hyperglycemia is documented. Once the animal is sufficiently conscious, food can be offered.
Insulin resistance
Insulin resistance should be suspected in any pet in which marked hyperglycemia persists throughout the day despite insulin doses of more than 1.5 U/kg per injection or when large doses of insulin (i.e. >2.2 U/kg per injection) are needed to maintain adequate glycemic control. However, use of these doses does not mean that insulin resistance is present. The problem could lie with owner technique of insulin administration, patient management (e.g., exercise, diet), or insulin choice. Lack of response to high doses of one insulin type does not mean all insulins will be ineffective; for example, 20% of cats did not respond to high doses of ultralente insulin but could be effectively managed by twice-daily lente.167 In addition, longer-acting insulin (PZI) will be more slowly absorbed and less bioavailable than shorter-acting insulin; thus slightly more than 2.2 U/kg of long-acting insulin may be required.
Before a thorough and costly workup for insulin resistance is initiated, factors that mimic insulin resistance should be ruled out. The owner’s technique and insulin handling should always be evaluated first. Possible causes for an unsatisfactory response to insulin include inadequate mixing of insulin before withdrawal into the syringe; use of the incorrect syringe (e.g., using a U100 syringe with U40 insulin); misunderstanding of how to read the insulin syringe; problems with insulin injection technique; inactivation of insulin as a result of improper handling; and, if diluted insulin is being used, improper dilution. A bottle of insulin should be discarded after 2 to 3 months of use because activity may begin to decrease. If owner issues are suspected, a glucose curve should be performed after the owner administers insulin using a new, undiluted bottle and while being observed. Second, the owner should be questioned to ensure consistent and appropriate diet and exercise. If hyperglycemia is believed to be due to a postprandial surge from feeding a meal when the insulin’s effects are waning, timing of meals should be adjusted. Alternatively, addition of an oral hypoglycemic agent such as acarbose can be considered. Third, if no response is seen to one type of insulin, then another should be tried to see if it might be effective. Fourth, absorption of insulin can vary among subcutaneous sites, so another injection site should be used; the lateral thorax or abdomen is recommended. Lastly, a glucose curve should be performed to eliminate other possible mimics of insulin resistance, such as the Somogyi phenomenon and inadequate duration of insulin action.
Once true insulin resistance has been documented, the following differential diagnoses should be considered.
Insulin antibodies are a commonly discussed cause of insulin resistance. The clinical significance of anti-insulin antibodies (AIAs) remains unclear at this time. Although antibodies may form against exogenous insulin, associated clinical insulin resistance appears rare. AIAs were measured in diabetic subjects after starting therapy with porcine lente insulin (n=100), bovine lente insulin (n=100), or bovine protamine zinc insulin (n=20); 12%, 56%, and 90%, respectively, developed AIA. No association was detected between AIA concentration and insulin dose or fructosamine concentrations.135 If AIAs are believed to be a cause of resistance, a commercial assay for measurement of AIAs now exists. Alternatively, in pursuing this diagnosis for insulin resistance, use of a radioimmunoassay (RIA) for insulin may be helpful.168 Circulating AIAs can interfere with measurement of serum insulin by RIA, causing spuriously high insulin concentrations, and the artifact can provide evidence of antibody presence. Typically, 24 hours after the last insulin injection in a diabetic pet, serum insulin concentration should be less than 50 μU/mL. In comparison, apparent serum insulin concentration is above 400 μU/mL if AIAs are present.168 If AIAs are believed to be causing insulin resistance, the insulin source should be switched to a different one. Glycemic control should improve within 2 weeks of changing the species of insulin if insulin-binding antibodies are causing resistance.168
Infection, ketoacidosis, and concurrent illness can cause insulin resistance. The urinary tract and oral cavities are common sites of infection; a urinalysis and urine culture, regardless of urinalysis finding,169 and complete oral examination should always be performed. Renal disease, hepatic insufficiency, cardiac insufficiency, pancreatitis, and starvation should be considered as possible causes of insulin resistance. Malnutrition can lead to insulin resistance and diminished insulin secretion. Obesity has been linked to glucose intolerance and abnormal insulin secretion in cats and dogs, but its role in creating insulin resistance is unclear insofar as obese diabetic pets generally remain insulin responsive. Hyperthyroidism, hypothyroidism, and hyperadrenocorticism can cause insulin resistance through diverse mechanisms.
Based on data from several recent studies, acromegaly may be a more common cause of insulin resistance in cats than previously recognized, and several reliable methods exist by which a diagnosis may be obtained, especially when the tests are used together. Computed tomography (CT) has proved useful in demonstrating the presence of a mass lesion in the region of the pituitary gland in insulin-resistant diabetic cats suspected of having acromegaly or hyperadrenocorticism, with a mass visualized on CT in all 16 such cats in one study.170 In another study designed to screen diabetic cats for the presence of acromegaly, serum IGF-1 concentrations were measured in 184 variably controlled diabetic cats. Of the 184 cats screened, 59 had increased serum insulin-like growth factor I (IGF-I) concentrations—that is, greater than 1000ng/mL (reference range 208-443 ng/mL). Eighteen of the 59 cats underwent further examination, including intracranial CT, contrast-enhanced CT, or magnetic resonance imaging (MRI), and acromegaly was confirmed in 17. Contrast-enhanced CT allowed detection of most, but not all, pituitary masses, and one cat with a postmortem examination diagnosis of acromegaly did not have a visible mass on CT or MRI.171 In another recent study, diabetic cats were screened for concurrent acromegaly by measuring plasma GH and IGF-1 concentrations and examining the pituitary fossa using MRI.172 Of the 16 cats in the study, six required an insulin dosage at or above 1.5 U/kg per injection and had an enlarged pituitary gland on MRI; one cat receiving less than 1.5 U/kg of insulin per injection had an enlarged pituitary gland. Five of the six cats were diagnosed with acromegaly based on the enlarged pituitary gland on MRI and elevated plasma GH concentrations. Finally, a study evaluating the usefulness of IGF-I concentrations as a screening test for the diagnosis of acromegaly in diabetic cats found that 25.6% (19 of 74) of all diabetic cats and 47.5% (19 of 40) of poorly controlled diabetics had acromegaly.173 Currently, no one test appears to be completely effective in diagnosing feline acromegaly, but a combination of serum IGF-1 concentrations, feline GH concentrations where available, and contrast-enhanced CT or MRI are options (see also the section on GH later in this chapter).
Certain drugs can cause insulin resistance, most notably progestogens and glucocorticoids. Although cats are resistant to development of many of the common adverse effects of glucocorticoids, such as polyuria and polydipsia, they may develop glucocorticoid-associated glucose intolerance readily. If possible, use of these medications should be slowly discontinued in diabetic patients. Otherwise, the patients may need to be treated as insulin-resistant. Neoplasia has been associated with insulin resistance in 5% to 10% of diabetic cats and dogs.95 Hyperlipidemia should be considered as a possible cause of insulin resistance.
When a cause for insulin resistance is sought, the easiest causes to rule out and the most likely should be eliminated first, proceeding through to the least likely. The following order, in general, has been recommended in cats: concurrent drugs, obesity, concurrent disease (including infection and ketoacidosis), hyperthyroidism, acromegaly, hyperadrenocorticism, and insulin antibodies. The order to use in dogs, in general, is as follows: concurrent drugs, diestrus/acromegaly, obesity, concurrent disease (including infection and ketoacidosis), hyperadrenocorticism, hypothyroidism, hyperlipidemia, and insulin antibodies.174 This order is not absolute. If strong evidence exists for a differential diagnosis lower in the order, that possibility should be ruled out first.
Management of insulin resistance requires correcting the underlying disorder, if possible. For causes such as a simple bacterial infection or concurrent administration of diabetogenic medications, eliminating the underlying problem can be relatively easy; other problems, such as acromegaly, may be more difficult to correct. If insulin antibodies are suspected, the insulin can be switched to a less antigenic form.
If the cause cannot be determined or eliminated, the following guidelines are suggested:174 (1) Administer insulin at least twice daily. (2) Avoid long-acting insulins, unless regular insulin is added. Intermediate-acting insulins are more effective in overcoming insulin resistance and lowering blood glucose concentrations. (3) Consider using mixtures of short-acting and longer-acting insulins. (4) Administer insulin shortly before or at the time of feeding to help control postprandial hyperglycemia. Large insulin doses may be required, but it will be necessary to determine the actual dosage using serial blood glucose curves, as for any diabetic.
Oral Antidiabetic Agents
Oral antidiabetic agents, including the sulfonylureas (e.g., glipizide), biguanides (e.g., metformin), α-glucosidase inhibitors (e.g., acarbose), and the thiazolidinediones (e.g., darglitazone), are commonly used to treat human patients with NIDDM. Mechanisms of action vary and, with the exception of acarbose, are directed toward the underlying abnormalities of type 2 diabetics (Figure 21-5).
Figure 21-5 Diagram of main metabolic abnormalities underlying type 2 diabetes mellitus and the way they are targeted by the oral hypoglycemic agents. The abnormalities are shown in red, along with their accompanying minor abnormalities and consequences. The oral hypoglycemics are noted in blue, and the blue arrows show which of the main metabolic abnormalities they target.
Sulfonylureas stimulate pancreatic beta cells to secrete insulin and have been among the most popular oral antidiabetic agents in human medicine. Extrapancreatic effects include decreased hepatic gluconeogenesis and increased tissue sensitivity to insulin. Hypoglycemia is the main adverse effect of sulfonylureas in humans, although it may be least likely to occur with glipizide.175
Mechanisms of action of metformin, a biguanide, include decreased hepatic gluconeogenesis (perhaps the primary mechanism) and increased insulin sensitivity by hepatic and peripheral tissues.175 Metformin appears more effective than glipizide in humans in resolving disorders associated with insulin resistance. Side effects occur in up to 20% of human patients and include diarrhea, abdominal discomfort, nausea, and anorexia. Lactic acidosis is a potentially severe adverse effect of biguanide antidiabetic agents in general but occurs rarely with metformin.175 Among the oral hypoglycemic drugs, metformin is recommended as initial therapy in obese human diabetic patients because it may ameliorate insulin resistance and is less likely to be associated with weight gain.175 As with glipizide, the initial dose of metformin in humans is low and is progressively increased on the basis of glucose monitoring. Both glipizide and metformin are given with meals in human diabetics.
The alpha-glucosidase inhibitors such as acarbose are complex oligosaccharides of bacterial origin that competitively inhibit small intestinal enzymes responsible for degradation of complex carbohydrates into absorbable monosaccharides.175 Some of the antihyperglycemic effects of acarbose may be mediated through its effect on thyroid hormones.176 When acarbose is ingested, the postprandial glucose concentration surge is delayed and diminished. The drug is not absorbed, so systemic effects are uncommon. Flatulence, soft stools, and diarrhea, however, occur because of the osmotic effect and bacterial fermentation of nondigested carbohydrates. The adverse effects appear to be transient, however, and are minimized in humans by starting therapy at a low dose. Acarbose is used in humans primarily to reduce postprandial glucose fluctuations and to improve glycemic stability when response to traditional oral antidiabetic agents is insufficient.177
The thiazolidinediones (e.g., rosiglitazone, darglitazone) bind to a novel receptor called the peroxisome proliferator-activated receptor-γ (PPAR-γ) and enhance insulin action and promote tissue glucose utilization. They are referred to as insulin sensitizers because they stimulate nuclear receptors that enhance the expression of proteins involved in glucose and lipid metabolism. Like metformin, troglitazone may target disorders associated with insulin resistance.
Clinical Use
Therapy of any disease is ideally aimed toward the underlying abnormality. In type 1 DM, insulin is lacking, so treatment provides an exogenous source. Type 1 diabetic patients do not have the metabolic abnormalities present in type 2 DM that are addressed by oral hypoglycemic agents (i.e., insulin resistance, dysfunctional β cells, and increased hepatic gluconeogenesis). Consequently, administration of these agents to type 1 diabetics is inappropriate, with the exception of acarbose. For type 2 DM, oral hypoglycemic drugs can be used initially, but as type 2 DM progresses, exogenous insulin injections will be required.
Because knowing the type of DM is helpful when choosing therapy, distinguishing between types 1 and 2 in cats and dogs would be extremely helpful. As dogs are mainly type 1 diabetics, oral hypoglycemics have not been used widely in this species. Cats are believed to be mainly type 2, at least initially. However, patients with advanced type 2 DM and glucose toxicity, a population likely to represent the majority of diabetic cats, will have totally lost insulin secretory ability. Accordingly, some diabetic cats will respond to oral hypoglycemic agents, but most will require insulin therapy.
The most studied and used oral hypoglycemic agent in cats is the sulfonylurea glipizide. Because of the mechanism of action, the ability of sulfonylureas to further the progression of DM remains controversial. Beta cells normally co-secrete the hormone amylin with insulin. Amyloid deposits can be found in the pancreatic islets of more than 90% of human patients with type 2 DM and in 65% of diabetic cats, and in diabetic cats the deposits are associated with significant loss of islet beta cells.178 Amylin is the precursor of the islet amyloid. Although the mechanisms underlying the transformation of amylin into amyloid fibrils are largely unknown, findings in one recent study support the hypothesis that islet amyloidosis results from prolonged beta cell stimulation. In this study, DM was induced in eight healthy domestic cats by a partial pancreatectomy, followed by induction of insulin resistance with corticosteroid and GH treatment. The cats were then treated with either glipizide (n = 4) or insulin (n = 4) for 18 months. All cats were negative for the presence of islet amyloid at the time of pancreatectomy. At the end of the study, all of the glipizide-treated cats and one of the four insulin-treated cats had pancreatic islet amyloid deposits. The glipizide-treated cats had threefold higher basal and fivefold higher glucose-stimulated plasma amylin concentrations than insulin-treated cats, suggesting an association between elevated amylin secretion and islet amyloidosis.179 Whether this is a positive association between glipizide treatment and development of islet amyloidosis or a negative association between insulin treatment and the development of islet amyloidosis or whether similar events would occur in naturally occurring feline DM remains uncertain.
The long-term success rate with glipizide is estimated to be approximately 35%,100,145 but which cats will respond cannot be predicted. The ideal patient for treatment with glipizide is a stable, nonketotic diabetic cat of optimal to obese body weight that has mild clinical signs with no complicating diseases. Patients that are emaciated, dehydrated, or debilitated or that have recently lost 10% or more of their body weight or have concomitant disease are not good candidates. Additionally, glipizide can be tried in any cat whose owners refuse to give injections.
Adverse effects are minimal. Vomiting is most common (approximately 15%).99,100 Increased liver enzymes and icterus develop within 4 weeks of initiating therapy in approximately 10%. Hypoglycemia occurs in approximately 12% to 15% of responder cats; usually these cats are transient diabetics. Most cats that respond without continued adverse effects can be treated with glipizide for life, but glipizide loses effectiveness in at least 5% to 10%. The period from initiation of therapy until failure is unpredictable, ranging from weeks to more than 3 years.121
Glipizide therapy should be instituted at a dosage of 2.5 mg/cat orally twice daily with food, and the cat should be examined after 1 and 2 weeks. A history, complete physical examination, body weight, blood glucose concentration, and urine glucose and ketones test should be evaluated. If no problems occurred during the first 2 weeks, the dosage should be increased to 5 mg/cat twice daily. If ketonuria is found, the medication should be discontinued and insulin therapy initiated. If vomiting or icterus is present, the drug should be discontinued until the problem resolves. Most cats will tolerate the medication if started at a lower dosage and gradually increased. If hepatic enzyme elevation or icterus occurred, liver enzymes and serum bilirubin concentration should be checked periodically after reinitiation. If problems recur, drug administration should be stopped and the cat placed on insulin.
Once a dosage of 5 mg twice daily has been given for 2 weeks, the previously mentioned parameters and a 10- to 12-hour glucose curve should be checked every 4 weeks.121 Response to therapy is demonstrated by resolution of clinical signs, blood glucose concentrations during the curve at or below 200 to 300 mg/dL, and lack of glycosuria. Time until response varies, so therapy at the full dosage should continue for 12 weeks unless a contraindication develops.121
If a cat becomes hypoglycemic while on glipizide therapy, discontinue drug administration and reevaluate the cat, potentially with a glucose curve, to assess if continued glipizide therapy is needed. If the cat becomes hyperglycemic after discontinuation of glipizide treatment, restart therapy with a 50% dose reduction and continue to monitor as described above.
If no response is seen after 12 weeks, glipizide administration should be stopped and insulin therapy instituted. If clinical signs and glycosuria resolve and blood glucose concentrations are at or below 200 mg/dL, glipizide therapy should be stopped and the blood glucose concentration reevaluated in 1 week. If hyperglycemia is present then, glipizide should be reinitiated. If euglycemia is present, no medication is warranted. Glipizide can be used again, however, at any time if hyperglycemia recurs. The patient should be rechecked every 3 months to ensure ongoing control.
Cats that have resolution of clinical signs according to the owner, stable body weight, and normal physical examinations but serial blood glucoses at or above 300 mg/dL present a clinical dilemma. Either the clinical signs have not truly resolved or the hyperglycemia is due to stress. Such cats should ideally be monitored by serum glycated protein concentrations and urine glucose concentrations to determine overall glycemic control. If glycosuria is absent and glycated protein concentrations are at a level or downward trend, glipizide therapy can proceed. If glycosuria is present or glycated protein levels are trending upward, insulin should be used instead.
Biguanides
Metformin doses of 25 to 50 mg/cat twice daily should attain plasma concentrations used for treating human DM,180,181 but the use of the drug in diabetic cats is not promising.180 In the single published study evaluating metformin use in diabetic cats, five newly diagnosed cats received metformin at a gradually increasing dosage, and only one achieved glycemic control after 8 weeks of therapy. In one nonresponder, serum alanine aminotransferase activity was within the reference range at the start of the study but had increased to 1440 U/L at week 7; 3 weeks after discontinuation of the medication, serum alanine aminotransferase activity was 95 U/L. One cat was found dead after 2 weeks, and no response was seen after 7 to 8 weeks in four cats. The responding cat was treated with 10 mg metformin daily for 1 week, then 10 mg twice daily for 2 weeks, followed by 25 mg twice daily for 5 weeks. Subsequently, the dose was increased to 50 mg twice daily, and 3 weeks later the owner reported resolution of clinical signs of DM. Metformin was used successfully for 4 months until pancreatitis developed, at which time therapy was changed to insulin. The serum insulin concentration was within or greater than the reference range in the responder diabetic cat and was undetectable or at the low end of the reference range in the nonresponders, suggesting that metformin may be beneficial only in diabetic cats with detectable serum insulin concentration at the time treatment is initiated. Adverse effects are common and include inappetence, weight loss, and vomiting. The size of pills commercially available is not amenable to cats, and use of this drug for felines requires compounding.180
Alpha-glucosidase inhibitors
In five dogs, a combination of acarbose and insulin provided better glycemic control over insulin alone. However, the final conclusion was that because of the expense and adverse effects, acarbose is primarily indicated for poorly controlled diabetic dogs in which the cause for the poor control cannot be identified.182 Acarbose, in conjunction with a low-carbohydrate diet, was an effective means of decreasing exogenous insulin dependence and improving glycemic control in one group of diabetic cats,118 but how much of the dose reduction in insulin was due to the diet and how much to acarbose is unclear. Acarbose may be helpful in cats with renal disease that cannot be placed on a low-carbohydrate diet because of the high protein content of these diets. Acarbose may be administered at a dosage of 25 to 200 mg/dog or 12.5 to 25 mg/cat twice daily with meals. Side effects include flatulence, semiformed stools, or diarrhea.
Thiazolidinediones
Although recent work suggests that darglitazone has beneficial effects in obese nondiabetic cats to decrease insulin secretion and glucose concentrations in a glucose tolerance test,183 no work has been done in diabetic cats.
Transition metals
Transition metals are insulin mimetic. In healthy nonobese cats, dietary chromium supplementation causes a small but statistically significant, dose-dependent improvement in glucose tolerance.184 The most commonly reported side effects are mild GI upset. Unfortunately, however, large clinical studies on the effect of vanadium or chromium in diabetic cats are lacking. In one study, chromium had no effect in concert with insulin treatment in diabetic dogs.185
Acute Metabolic Complications
Diabetic Ketoacidosis
Diabetic ketoacidosis (DKA) is a complex catabolic disorder caused by either relative or absolute insulin deficiency. Diabetogenic hormones (e.g., cortisol, progesterone, GH), probably contribute to its development. Thus any disease that increases the secretion of these stress hormones can predispose diabetic patients to the development of ketoacidosis, and insulin therapy may become ineffective.
Glycogen, protein, and fat, rather than glucose, are used by the body for energy in the absence of insulin. Glycogen is broken down into glucose. As a physiologic survival mechanism during starvation (insulin deficiency is perceived by the body as a state of starvation because glucose is not available for use by most cells), fatty acids formed from fat breakdown are transported into mitochondria and metabolized to ketone bodies to be used as a fuel source. The ketone bodies are acetone, acetoacetate, and β-hydroxybutyrate. The last is formed from acetoacetate and hydrogen ions. A lack of insulin decreases tissue utilization of ketone bodies. As ketones accumulate, the body’s buffering systems become overwhelmed, and metabolic acidosis develops. Glucose formation continues unchecked and even accelerates. Glycosuria that accompanies hyperglycemia causes an osmotic diuresis with subsequent depletion of sodium, chloride, and potassium. If an animal is unable to ingest sufficient water to keep up with ongoing losses, dehydration and prerenal azotemia develop. Severe hyperosmolarity may result. Ketoacidosis can cause vomiting and diarrhea, further complicating acid–base and electrolyte disorders. Production of glucose counterregulatory hormones increases in response to the stress of illness, but the hormones antagonize insulin and worsen the hyperglycemia and ketonemia. Patients with DKA usually are ill when they are brought to the veterinarian, and 6% to 12% are dehydrated. Blood glucose concentration is generally greater than 300 mg/dL, and the patient may be severely acidotic (arterial bicarbonate <11 mEq/L).
Hypokalemia, hypophosphatemia, and hypomagnesemia may accompany DKA. Hypokalemia may develop because of decreased food intake or increased renal loss associated with osmotic diuresis. However, acidosis may cause intracellular potassium ions to shift extracellularly, thus masking total body potassium depletion. Rehydration, correction of acidosis, and insulin therapy may further decrease serum potassium concentrations. The risk of hypophosphatemia is similarly increased by diminished intake, urinary losses, fluid therapy, and translocation after insulin therapy. Energy (adenosine triphosphate [ATP]) depletion becomes evident in high-energy use cells such as skeletal muscle, brain, and red blood cells. Hemolytic anemia is the most common and serious sequela to hypophosphatemia but does not usually occur until serum phosphorus concentration is at or below 1 mg/dL.186 Neuromuscular signs include weakness, ataxia, and seizures as well as anorexia and vomiting secondary to intestinal ileus. Lastly, osmotic diuresis may cause hypomagnesemia, and because of acid–base imbalances, magnesium may shift intracellularly. Clinical signs of hypomagnesemia (e.g., lethargy, anorexia, muscle weakness [including dysphagia and dyspnea], muscle fasciculations, seizures, ataxia, and coma) do not usually occur until serum total magnesium concentration is below 1 mg/dL.186
Diagnosis of DKA includes documentation of the presence of DM, ketone bodies, and acidosis. The existence of ketonuria establishes a diagnosis of ketosis; acidosis must be documented to differentiate ketosis from ketoacidosis. Urine test strips and nitroprusside reagents (e.g., Ketostix) do not detect β-hydroxybutyrate, which can be the predominant ketone present in the acidotic state. If ketoacidosis is suspected but a urine dipstick is negative, other means for detecting ketone bodies, such as Acetest tablets (Ames Division, Miles Laboratories, Elkhart, Ind.) should be used, if available. A commercially available hand-held ketone meter (PrecisionXtra, Abbott, Alameda, Calif.) will detect plasma β-hydroxybutyrate and can be used to aid in the diagnosis of DKA in dogs and cats if urine strips are negative for ketone bodies or urine cannot be obtained.187
Treatment of ill ketoacidotic patients should be aggressive, with the treatment goals being to restore water and electrolyte imbalances, provide sufficient insulin to begin normalization of metabolism, correct acidosis, identify precipitating factors for the DKA, and provide a carbohydrate substrate when required by the insulin treatment.186 Care should be taken not to return blood glucose concentrations to normal too rapidly, because biochemical and osmotic problems could be created by overly aggressive therapy, as described in human “fragile” DKA patients that experience rapid changes in serum tonicity and develop cerebral edema. Cerebral cells of diabetic patients may accumulate sorbitol, an osmotically active polyol formed in response to excessive blood glucose concentrations. Thus as a hypertonic patient achieves normal tonicity and euglycemia with treatment, water may move into neurons that have become hypertonic relative to extracellular fluid.
The common use of isotonic, sodium-containing fluids may minimize large osmotic shifts by maintaining serum sodium concentrations as glucose concentrations decrease,188 so development of cerebral edema may be uncommon, at least in diabetic cats. A recent retrospective study evaluated 13 diabetic ketotic cats treated in an intensive care unit and similarly managed with intravenous constant-rate infusions of regular insulin and 0.9% saline. The median change in glucose concentration was 122% (range 1% to 1230%). In cats with abnormal sodium concentrations, the median change in sodium (irrespective of direction) was 5.4% (range 1.3% to 11.6%). The median percentage change in serum tonicity (irrespective of direction) was only 2.9% (range 1.4% to 5.7%). At all time points examined, sodium was the major determinant of serum tonicity, with only minimal contributions from glucose. If sodium levels had not increased during treatment, the total decrease in serum tonicity owing to glucose alone would have been 13%. The minimal fluctuations in serum tonicity likely explain the low incidence of osmotic-mediated neurologic complications seen during treatment of diabetic cats.188 Caution should still be exercised in treatment, especially of hyperglycemic, hyperosmolar, nonketotic diabetic patients. Blood glucose concentrations in hyperglycemic, hyperosmolar diabetics patients may be elevated to the point that they are a major determinant of serum tonicity; alternatively, a diabetic patient not managed with isotonic sodium-containing fluids may be lacking the sodium concentrations needed to buffer the changes in serum tonicity once measures are initiated to reduce the hyperglycemia. In either case, complications from too rapid a decrease in blood glucose concentrations could occur.
The first step in treating DKA should always be to initiate replacement and maintenance fluid therapy to enhance renal blood flow, promote urinary glucose excretion, and decrease the effects of diabetogenic hormones. Sodium chloride (0.9% saline) containing appropriate potassium supplementation is the fluid of choice. Because of the possible risk of cerebral edema formation, rapid fluid administration is indicated only in life-threatening situations. Fluid administration should be directed at gradually replacing hydration deficits over 24 hours while supplying maintenance fluid needs and matching ongoing losses. Once out of the critical phase, fluid replacement should be decreased in an effort to correct fluid imbalances in a slow but steady manner.186 When serum sodium concentration is 140 to 155 mEq/L, Ringer’s solution should be used; if serum sodium concentration increases to more than 155 mEq/L, 0.45% saline should be administered.
Ideally, potassium supplementation should be based on actual measurement of serum potassium concentration. If measurement is not available, 40 mEq potassium should be added to each liter of intravenous fluids.186 Potassium supplementation should be adjusted every 6 to 8 hours, again ideally based on actual measurements, until the patient is stable and serum electrolytes are within the reference range. Total hourly potassium administration should not exceed 0.5 mEq/kg body weight. If the serum potassium concentration is greater than 5, 4 to 5.5, 3.5 to 4., 3 to 3.5, 2.5 to 3, 2 to 2.5, or less than 2 mEq/L, then no potassium, 20 to 30, 30 to 40, 40 to 50, 50 to 60, 60 to 80, and 80 mEq of potassium should be added to each liter of fluids administered, respectively.186
Phosphate therapy is indicated if clinical signs of hypophosphatemia or hemolysis are identified or if the serum phosphorus concentration is below 1.5 mg/dL. An intravenous constant-rate infusion of potassium phosphate (the solution contains 4.4 mEq/mL of potassium and 3 mM/mL of phosphate) should be a given at a rate of 0.03 to 0.12 mM phosphate/kg/hour. Administration of potassium must be taken into account when giving potassium phosphate for correction of hypophosphatemia.189 Serum phosphate concentration must be measured to assess response and adjust the dose. Because adverse effects of phosphate administration include hypocalcemia, serum calcium concentration, preferably ionized calcium concentration, should also be monitored.
Whether magnesium supplementation is required is controversial. Hypomagnesemia is usually not treated unless persistent lethargy, anorexia, weakness, or refractory hypokalemia or hypocalcemia are encountered after 24 to 48 hours of therapy and another cause for the problem cannot be identified.186 The initial intravenous dose for the first day is 0.75 to 1 mEq/kg administered by constant-rate infusion in 5% dextrose in water. The supplementation can be continued at half the initial dose for an additional 3 to 5 days if necessary. The dose should be decreased 50% to 75% in azotemic animals. Parenteral magnesium therapy can cause hypocalcemia, cardiac arrhythmias, hypotension, and respiratory depression.186
Bicarbonate therapy in the management of patients with DKA is contentious. Proponents point to potential deleterious effects of acidosis on cardiac hemodynamics, whereas opponents are concerned by possible creation of paradoxical central nervous system acidosis or a shift in the oxygen-hemoglobin dissociation curve resulting in tissue hypoxia. A prospective trial of human DKA patients with moderate to severe acidosis (pH 6.9 to 7.1) found no difference in outcome in patients treated with bicarbonate and those who were not.190 Clinical presentation in conjunction with acid–base status should be used to determine the need. If plasma bicarbonate concentration is above 12 mEq/L, bicarbonate therapy is not indicated, especially if the patient is alert;186 correction of dehydration and insulin administration usually eliminates acidosis. When plasma bicarbonate concentration is below 11 Eq/L (total venous CO2 <12 mEq/L), specific therapy should be initiated. The amount of bicarbonate needed to correct acidosis to 12 mEq/L over 6 hours is calculated as
The factor 0.4 corrects for the extracellular fluid space in which bicarbonate is distributed (i.e., 40% of body weight). Using the factor 0.5 means that 50% of the full bicarbonate dose is infused, so a conservative dose is given. The bicarbonate should be infused intravenously over 6 hours (never as a bolus), then the serum bicarbonate concentration reassessed and a new dosage calculated and administered, if needed.186
Recommendations regarding insulin doses and routes vary for ketoacidotic patients, but authors agree that therapy should begin with low doses. Insulin therapy should be delayed at least 1 to 2 hours after the start of intravenous fluid therapy. At that time serum electrolytes should be measured; if hypokalemia is present, insulin therapy can be delayed an additional 1 to 2 hours to allow fluid therapy to replenish potassium.
The goal of insulin therapy is to lower the blood glucose concentration to 200 to 250 mg/dL over 6 to 10 hours. Regular insulin is used because it is short-acting and more easily regulated compared with longer-acting insulins. Blood glucose concentrations should be monitored hourly. The major risks associated with insulin therapy are hypoglycemia, hypokalemia, and hypophosphatemia.
The three techniques used are hourly intramuscular,191 continuous low-dose intravenous infusion,192 and intermittent intramuscular/subcutaneous.193 An intermittent intravenous regimen is discouraged because the biologic effects of regular insulin administered intravenously last only 20 minutes. For the hourly intramuscular technique, an initial loading dose of 0.2 U/kg of regular insulin is given, followed by 0.1 U/kg every 1 to 2 hours. Lower doses can be used at first if hypokalemia is a concern. The rear legs are recommended sites for administration. If the blood glucose concentration declines at a rate greater than 75 mg/dL/hr, the dose should be decreased to 0.05 U/kg/hr. If the decrease is less than 50 mg/dL/hr, the dose should be increased to 0.2 U/kg/hr. When the blood glucose concentration is below 300 mg/dL, regular insulin can be given subcutaneously (0.1 to 0.3 U/kg, every 6 to 8 hours) and the dose altered as required. In addition, when blood glucose concentration is 250 to 300 mg/dL, 100 mL of 50% dextrose should be added to each liter of fluid to achieve a 5% dextrose solution. Blood glucose concentration should be maintained between 150 and 300 mg/dL until the patient is stable and eating. When the patient is no longer receiving fluids, is eating and drinking, has stopped vomiting, and is no longer ketoacidotic, maintenance insulin therapy with longer-acting insulin can be instituted.
For the constant-rate low-dose infusion technique, regular crystalline insulin (2.2 U/kg for dogs and 1.1 U/kg for cats) is added to 250 mL of 0.9% saline and initially administered at 10 mL/hr in a line separate from that used for fluid administration.192 The first 50 mL of an insulin–electrolyte mixture run through intravenous tubing should be discarded because of adsorption of insulin to glassware and plastic. An intravenous infusion pump should be used to ensure a constant administration rate. The infusion rate can be slowed for 2 to 3 hours if hypokalemia is a concern and should be altered as needed so that blood glucose concentrations decline by approximately 50 mg/dL/hr. Once the blood glucose concentration reaches 250 mg/dL, the fluid should be changed to 0.45% saline with 2.5% dextrose. The insulin infusion can then be discontinued and regular insulin given every 6 to 8 hours, as previously described. Alternatively, the infusion rate can be slowed to maintain blood glucose concentrations between 150 and 300 mg/dL until the patient is stable enough to institute maintenance insulin therapy. If infusion is used, insulin should be added to the new fluid at the same concentration.
For the intermittent, intramuscular/subcutaneous insulin technique, the initial regular insulin dose is 0.25 U/kg intramuscularly every 4 hours. Once the patient is rehydrated, subcutaneous administration is substituted. Dose is adjusted based on serum glucose concentrations. This technique is not recommended, however. Blood glucose concentrations decrease rapidly, and the risk for hypoglycemia is great.
Hyperglycemic, Hyperosmolar, Nonketotic Syndrome
The true incidence of hyperglycemic, hyperosmolar nonketotic syndrome (HHNS) in the canine and feline diabetic pet population is unknown, but the condition is relatively uncommon. In one retrospective study of diabetic cats, HHNS constituted 6.4% of emergency room visits of diabetic cats.194 However, it is associated with a high mortality rate in both humans with type 2 DM and feline diabetics.194,195
The pathogenesis of HHNS is not fully understood but may be a result of decreased insulin activity rather than a complete absence. Insulin deficiency causes excess glucagon secretion and decreased glucose use by peripheral tissues. As with DKA, decreased glucose utilization causes muscle and fat breakdown, thus supplying precursors needed for hepatic gluconeogenesis. Insulin deficiency in combination with excess glucagon promotes hepatic gluconeogenesis, and profound hyperglycemia develops. The insulin activity present prevents ketone bodies from forming.
Why some animals develop DKA and others HHNS is unclear. In both disorders patients commonly have concurrent diseases, so insulin resistance is likely to be important in both.194 Interestingly, certain types of diseases may be more common in HHNS compared with DKA. In one study of cats with HHNS or DKA, overall presence of concurrent disease did not differ between the two groups but presence of specific diseases did. Chronic renal disease occurred in 58.8% of cats with HHNS but only 12.5% of those with DKA, and congestive heart failure was present in 29.4% of the HHNS cats but only 3% of cats with DKA.194 Thus the presence of certain diseases may predispose one syndrome to develop over another, but this remains to be proven.
The diagnostic criteria for HHNS in veterinary medicine are the presence of severe hyperglycemia (>600 mg/dL) and serum hyperosmolarity (>350 mOsm/L). Severe dehydration causes hyperosmolarity, which leads to the central nervous system abnormalities such as dullness, circling, pacing, or unresponsiveness common with this disorder. Ketones are usually not present, but lactic acidosis often is.194 The therapeutic goal is to correct the extreme volume depletion and hyperosmolarity. Despite the hyperosmolarity, the initial fluid of choice is isotonic (0.9%) saline.186 Half the estimated dehydration deficit plus maintenance requirements should be replaced in the first 12 hours and the remainder in the following 24 hours. Potassium and phosphorus supplementation should occur as for patients with DKA. Insulin therapy should be delayed 4 to 6 hours until the positive benefits of fluid therapy are documented (e.g., correction of dehydration, stabilization of blood pressure, and improvement in urine production).186 Insulin can be administered as for DKA, but the dosages should be decreased by 50% to dampen the decrease in blood glucose concentration and prevent a rapid decrease in extracellular fluid osmolarity.
Prognosis
To some degree, prognosis for a diabetic dog or cat depends on its owner’s desire to treat, the presence and severity of underlying disorders, and how well the animal responds to therapy. One must also bear in mind that DM is most commonly diagnosed in the geriatric pet population. The mean survival time for diabetic dogs has been stated to be 3 years after diagnosis, with the highest mortality within the first 6 months.95 A recent study assessing the incidence, survival, and breed distribution of insured dogs in the United Kingdom diagnosed with DM supports this estimation. Median survival time for 686 dogs was 57 days after the first insurance claim; if 223 dogs that survived less than 1 day were excluded from the analysis, median survival was 2 years. For dogs surviving at least 30 days (n=347), the median survival time was not reached by the end of the study.196 Median survival times in newly diagnosed diabetic cats is reported to be 17 months,145 and the life expectancy of diabetic cats that survive the first 6 months after diagnosis has been estimated to be 5 years.121