CHAPTER 9 Pericardial Disease and Cardiac Tumors
GENERAL CONSIDERATIONS
Several diseases of the pericardium and intrapericardial space can disrupt cardiac function. Normally, the pericardium anchors the heart in place and provides a barrier to infection or inflammation from adjacent tissues. The pericardium is a closed serosal sac that envelops the heart and is attached to the great vessels at the heartbase. Directly adhered to the heart is the visceral pericardium, or epicardium, which is composed of a thin layer of mesothelial cells. This layer reflects back over itself at the base of the heart to line the outer fibrous parietal layer. A small amount (∼0.25 ml/kg body weight) of clear, serous fluid normally serves as a lubricant between these layers. The pericardium helps balance the output of the right and left ventricles and limits acute distention of the heart, although there are few overt clinical consequences associated with its removal.
Excess or abnormal fluid accumulation in the pericardial sac is the most common pericardial disorder, and it occurs most often in dogs. Other acquired and congenital pericardial diseases are seen infrequently. Acquired pericardial disease causing clinical signs is uncommon in cats.
CONGENITAL PERICARDIAL DISORDERS
PERITONEOPERICARDIAL DIAPHRAGMATIC HERNIA
Peritoneopericardial diaphragmatic hernia (PPDH) is the most common pericardial malformation in dogs and cats. It occurs when abnormal embryonic development (probably of the septum transversum) allows persistent communication between the pericardial and peritoneal cavities at the ventral midline. The pleural space is not involved. Other congenital defects, such as umbilical hernia, sternal malformations, and cardiac anomalies may co-exist with PPDH. Abdominal contents herniate into the pericardial space to a variable degree and cause associated clinical signs. Although the peritoneal-pericardial communication is not trauma induced, trauma can facilitate movement of abdominal contents through a preexisting defect.
Clinical Features
The initial onset of clinical signs associated with PPDH can occur at any age (ages between 4 weeks and 15 years have been reported). The majority of cases are diagnosed during the first 4 years of life, usually within the first year. In some animals clinical signs never develop. Males appear to be affected more frequently than females, andWeimaraners may be predisposed. The malformation is common in cats as well.
Clinical signs usually relate to the gastrointestinal (GI) or respiratory system. Vomiting, diarrhea, anorexia, weight loss, abdominal pain, cough, dyspnea, and wheezing are most often reported; shock and collapse may also occur. Possible physical examination findings include muffled heart sounds on one or both sides of the chest; displacement or attenuation of the apical precordial impulse; an “empty” feel on abdominal palpation (with herniation of many organs); and, rarely, signs of cardiac tamponade (discussed in more detail later).
Diagnosis
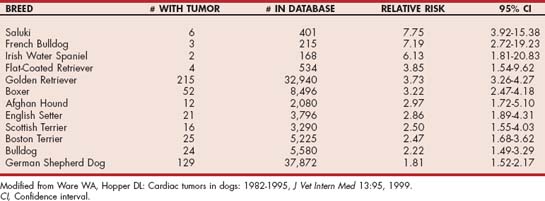
Thoracic radiographs are often diagnostic or highly suggestive of PPDH. Enlargement of the cardiac silhouette, dorsal tracheal displacement, overlap of the diaphragmatic and caudal heart borders, and abnormal fat and/or gas densities within the cardiac silhouette are characteristic findings (Fig. 9-1, A and B). A pleural fold, extending between the caudal heart shadow and the diaphragm ventral to the caudal vena cava on lateral view, is usually evident. Gas-filled loops of bowel crossing the diaphragm into the pericardial sac, a small liver, and few organs within the abdominal cavity may also be seen. Echocardiography helps confirm the diagnosis when radiographic findings are equivocal (Fig. 9-2). A GI barium series is diagnostic if the stomach and/or intestines are in the pericardial cavity (Fig. 9-1, C). Fluoroscopy, nonselective angiography (especially if only falciform fat or liver has herniated), celiography, or pneumopericardiography also can aid in diagnosis. ECG changes are inconsistent; decreased amplitude complexes and axis deviations caused by cardiac position changes sometimes occur.
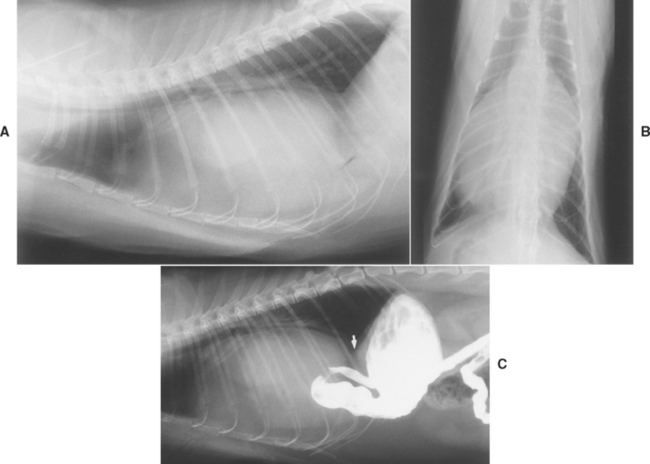
FIG 9-1 Lateral (A) and dorsoventral (B) radiographs from a 5-year-old male Persian cat with a congenital peritoneopericardial diaphragmatic hernia (PPDH). Note the greatly enlarged cardiac silhouette containing fat, soft tissue, and gas densities as well as tracheal elevation. There is overlap between the cardiac and diaphragmatic borders on both views. Presence of a portion of the stomach and duodenum within the pericardium is evident after barium administration (C); omental fat and liver are also present within the pericardial sac. In C, the dorsal pleural fold between pericardium and diaphragm is best appreciated (arrow).
Treatment
Therapy involves surgical closure of the peritonealpericardial defect after viable organs are returned to their normal location. The presence of other congenital abnormalities and the animal’s clinical signs influence the decision to operate. The prognosis in uncomplicated cases is excellent. Older animals without clinical signs may do well without surgery, especially because organs chronically adhered to the heart or pericardium may be traumatized during attempted repositioning.
OTHER PERICARDIAL ANOMALIES
Pericardial cysts are rare anomalies. They may originate from abnormal fetal mesenchymal tissue or from incarcerated omental or falciform fat associated with a small PPDH. The pathophysiologic signs and clinical presentation can mimic those seen with pericardial effusion. Radiographically, the cardiac silhouette may appear enlarged and deformed. Echocardiography or pneumopericardiography can reveal the diagnosis. Surgical cyst removal, combined with partial pericardiectomy, usually resolves the clinical signs.
Congenital defects of the pericardium itself are extremely rare in dogs and cats; most are incidental postmortem findings. Sporadic cases of partial (usually left-sided) or complete absence of the pericardium are reported. A possible complication of partial absence of the pericardium is herniation of a portion of the heart; this could cause syncope, embolic disease, or sudden death. Echocardiography or angiocardiography may allow antemortem diagnosis.
PERICARDIAL EFFUSION
HEMORRHAGE
Hemorrhagic effusions are common in dogs. The fluid usually appears dark red, with a packed cell volume (PCV) >7%, a specific gravity >1.015, and a protein concentration >3 g/dl. Cytologic analysis shows mainly red blood cells, but reactive mesothelial, neoplastic, or other cells may be seen. The fluid does not clot unless hemorrhage was very recent. Neoplastic hemorrhagic effusions are more likely in dogs older than 7 years. Middle-aged, large-breed dogs are most likely to have idiopathic “benign” hemorrhagic effusion.
Hemangiosarcoma (HSA) is by far the most common neoplasm causing hemorrhagic pericardial effusion in dogs; it is uncommon in cats. Hemorrhagic pericardial effusion also occurs in association with various heartbase tumors; pericardial mesotheliomas; malignant histiocytosis (MH); and, rarely, metastatic carcinoma. HSAs (see p. 167) usually arise within the right heart, especially in the right auricular appendage. Chemodectoma is the most common heartbase tumor; it arises from chemoreceptor cells at the base of the aorta. Thyroid, parathyroid, lymphoid, and connective tissue neoplasms also occur at the heartbase. Pericardial mesothelioma develops in some dogs and cats and may mimic idiopathic disease. Lymphoma involving various parts of the heart is seen more often in cats than in dogs. Dogs with MH and pericardial effusion usually have pleural effusion and ascites despite the fact that they do not have cardiac tamponade.
Idiopathic (benign) pericardial effusion is reported most frequently in medium- to large-breed dogs. Golden Retrievers, Labrador Retrievers, and Saint Bernards may be predisposed. Although dogs of any age can be affected, the median age is 6 to 7 years. More cases have been reported in males than females. Mild pericardial inflammation, with diffuse or perivascular fibrosis and focal hemorrhage, is common on histologic exam. Layers of fibrosis suggest a recurrent process in some cases. Constrictive pericardial disease is a potential complication.
Other, less common causes of intrapericardial hemorrhage include left atrial rupture secondary to severe mitral insufficiency (see p. 116), coagulopathy, penetrating trauma (including iatrogenic laceration of a coronary artery during pericardiocentesis), and possibly uremic pericarditis.
TRANSUDATES
Pure transudates are clear, with a low cell count (usually <1000 cells/μl), specific gravity (<1.012), and protein content (<2.5 g/dl). Modified transudates may appear slightly cloudy or pink tinged. Their cellularity (∼1000 to 8000 cells/μl) is still low, but total protein concentration (∼2.5-5.0 g/dl) and specific gravity (1.015-1.030) are higher than those of a pure transudate. In some dogs and cats, transudative effusions occur with congestive heart failure (CHF), hypoalbuminemia, PPDH and pericardial cysts, and toxemias that increase vascular permeability (including uremia). These conditions usually are associated with relatively small-volume pericardial effusion; cardiac tamponade is rare.
EXUDATES
Exudative effusions are cloudy to opaque or serofibrinous to serosanguineous. They typically have a high nucleated cell count (usually much higher than 3000 cells/μl), protein content (often much above 3 g/dl), and specific gravity (>1.015). Cytologic findings are related to the etiology. Exudative pericardial effusions are found only rarely in small animals, except in cats with feline infectious peritonitis (FIP).
Infectious pericarditis is usually related to plant awn migration, bite wounds, or extension of a pleural or mediastinal infection. Various bacteria (aerobic and anaerobic), actinomycosis, coccidioidomycosis, disseminated tuberculosis, and, rarely, systemic protozoal infections have been identified. Sterile exudative effusions are reported with leptospirosis, canine distemper, and idiopathic pericardial effusion in dogs and with FIP and toxoplasmosis in cats. FIP is the most important cause of symptomatic pericardial effusion in cats. Chronic uremia occasionally causes a sterile, serofibrinous or hemorrhagic effusion.
Pathophysiology
Fluid accumulation within the pericardial space causes clinical signs when it raises intrapericardial pressure to or above normal cardiac filling pressure. This accumulation impedes venous return and cardiac filling. As long as intrapericardial pressure remains low, cardiac filling and output remain relatively normal. If fluid accumulates slowly, the pericardium may distend enough to accommodate the increased effusate volume at relatively low pressure. However, pericardial tissue is relatively noncompliant. Rapid fluid accumulation or a very large effusion causes a steep rise in intrapericardial pressure, leading to cardiac tamponade. Pericardial fibrosis and thickening further limit the compliance of this tissue.
Pericardial effusion of very large volume may cause clinical signs by virtue of its size, even without overt cardiac tamponade. Lung and/or tracheal compression can compromise ventilation and stimulate cough; esophageal compression can cause dysphagia or regurgitation.
CARDIAC TAMPONADE
Cardiac tamponade develops when pericardial fluid accumulation raises intrapericardial pressure to or above the normal cardiac diastolic pressure. This external compression of the heart progressively limits filling, initially of the right heart, then the left. Cardiac output subsequently falls while systemic venous pressure rises. Pressure in all cardiac chambers and the great veins eventually becomes equilibrated during diastole. Neurohormonal compensatory mechanisms are activated as tamponade develops. Gradual pericardial fluid accumulation results in signs of CHF because of compensatory volume retention and the direct effects of impaired cardiac filling. Manifestations of systemic venous congestion and right-sided CHF (ascites and pleural effusion) usually predominate because of the right heart’s thinner wall and low pressures. Pericardial effusion does not typically affect cardiac contractility directly, but reduced coronary perfusion during tamponade can impair both systolic and diastolic function. Low cardiac output, arterial hypotension, and poor organ perfusion can ultimately lead to cardiogenic shock and death.
The rate of pericardial fluid accumulation and the distensibility of the pericardial sac determine whether and how quickly cardiac tamponade develops. Rapid accumulation of even a relatively small volume can raise intrapericardial pressure sharply. A gradual process is implied when the pericardial fluid volume is large. Cardiac tamponade is relatively common in dogs but rare in cats.
Pulsus paradoxus is the term used to describe the exaggerated variation in arterial blood pressure that occurs during the respiratory cycle as a result of cardiac tamponade. During inspiration intrapericardial and right atrial (RA) pressures fall, which facilitates right heart filling and pulmonary blood flow. At the same time, left heart filling is reduced as more blood is held in the lungs and the interventricular septum bulges leftward from the inspiratory increase in right ventricular (RV) filling; consequently, left heart output and systemic arterial pressure decrease during inspiration. The variation in systolic arterial pressure between inspiration and expiration is usually >10 mm Hg in patients with cardiac tamponade and pulsus paradoxus. Pulsus paradoxus is not always discernible using palpation of the femoral pulse.
Clinical Features
Clinical findings in patients with cardiac tamponade usually reflect right-sided CHF as well as poor cardiac output. Before obvious ascites develops, possible nonspecific signs include lethargy, weakness, poor exercise tolerance, and inappetence. The history typically includes complaints of weakness, exercise intolerance, abdominal enlargement, tachypnea, syncope, and cough. A history of collapse is more common in dogs with neoplastic disease; dogs without a mass lesion are more likely to have obvious ascites. Marked loss of lean body mass occurs in some chronic cases (Fig. 9-3).
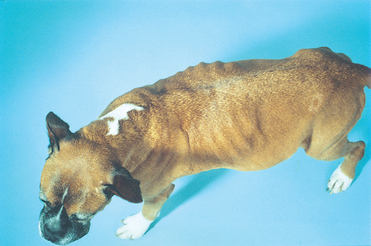
FIG 9-3 Older male Boxer with chronic cardiac tamponade and right-sided congestive heart failure secondary to chemodectoma. The abdomen is greatly distended with ascites; chronic loss of lean body mass is evident along the spine, pelvis, and rib cage.
Jugular vein distention and/or positive hepatojugular reflux, hepatomegaly, ascites, labored respirations, and weak femoral pulses are common physical examination findings. Pleural effusion and ascites are also common in cats, as well as dogs, with cardiac tamponade. A palpable decrease in arterial pulse strength during inspiration (pulsus paradoxus) might be discernible in some dogs with tamponade. Sinus tachycardia, pale mucous membranes, and prolonged capillary refill time are manifestations of high sympathetic tone. The precordial impulse is weak when the pericardial fluid volume is large. Heart sounds are muffled in patients with moderate to large pericardial effusions. Lung sounds are muffled over the ventral thorax in those with pleural effusion. Although pericardial effusion does not cause a murmur, concurrent cardiac disease may do so. If fluid has rapidly accumulated, acute tamponade can lead to shock and death without obvious signs of pleural effusion, ascites, or radiographic evidence of cardiomegaly. In such cases, jugular venous distention, hypotension, and pulmonary edema may be evident. Infectious pericarditis may be accompanied by fever; rarely, a pericardial friction rub might be heard.
Diagnosis
A central venous pressure (CVP) above 10 to 12 cm H2O is common; normally, CVP is <8 cm H2O. CVP measurement is helpful when the jugular veins are difficult to assess or it is unclear whether right heart filling pressure is elevated. Moderate- to large-volume pleural effusion should be drained before CVP measurement, not only to stabilize the patient but also to minimize artifactual CVP elevation.
RADIOGRAPHY
Pericardial effusion enlarges the cardiac silhouette (Fig. 9-4). A massive amount of pericardial fluid causes the classic globoid-shaped heart shadow on both radiographic views. Smaller fluid volumes allow various cardiac contours to be identified, especially dorsally. Other findings associated with tamponade include pleural effusion, a distended caudal vena cava, hepatomegaly, and ascites. Pulmonary infiltrates of edema and distended pulmonary veins are seen ocasionally. Some heartbase tumors cause tracheal deviation or a soft-tissue mass effect. Metastatic lung lesions are common in dogs with hemangiosarcoma.
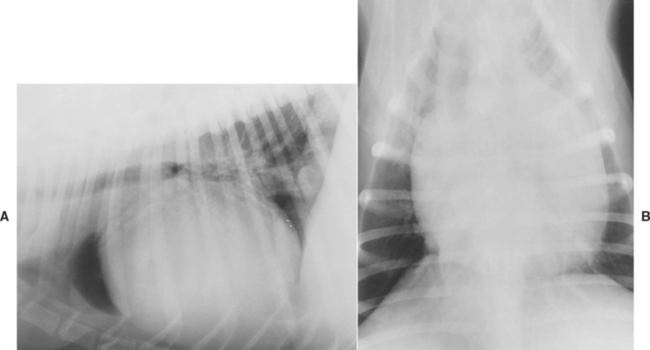
FIG 9-4 Lateral (A) and dorsoventral (B) radiographs from a mixed-breed dog with large pericardial effusion. Note globoid shape of cardiac silhouette and distended caudal vena cava (A).
When used, fluoroscopy demonstrates diminished to absent motion of the cardiac shadow because of the fluid surrounding the heart. Angiocardiography is used only rarely now to evaluate patients with pericardial effusion and cardiac tumors; it typically reveals increased endocardialto-pericardial distance. Cardiac neoplasms can cause displacement of normal structures, filling defects, and vascular “blushing” (opacification of excessive, abnormal tumor-associated vessels). Pneumopericardiography has also been replaced by echocardiography. Pneumopericardiography uses carbon dioxide or air injected into the drained pericardial sac to outline the heart, but it is rarely used these days. Radiographs are taken from different orientations, but the left lateral and dorsoventral views are most helpful. These views allow the injected gas to outline the right atrial and heartbase areas, respectively, where tumors are most common.
ELECTROCARDIOGRAPHY
Although there are no pathognomonic electrocardiographic (ECG) findings, the following abnormalities are suggestive of pericardial effusion: small amplitude QRS complexes (<1 mV in dogs), electrical alternans, and ST segment elevation (epicardial injury current). Electrical alternans is a recurring alteration in the size of the QRS complex (or sometimes the T wave) with every other beat (Fig. 9-5). It results from the back-and-forth swinging motion of the heart within the pericardium. Electrical alternans is most likely to be seen in patients with large-volume pericardial effusion; it may be most evident at heart rates between 90 and 140/min and/or in the standing position. Sinus tachycardia is common with cardiac tamponade. Atrial or ventricular tachyarrhythmias may also occur.
ECHOCARDIOGRAPHY
Echocardiography is highly sensitive for detecting pericardial fluid, and it is the preferred diagnostic modality if radiographic changes are equivocal. Because fluid is sonolucent, pericardial effusion appears as an echo-free space between the bright parietal pericardium and the epicardium (Fig. 9-6). Abnormal cardiac wall motion and chamber shape and intrapericardial or intracardiac mass lesions can also be imaged. With large-volume pericardial effusion, the heart may appear to swing back and forth within the pericardial sac. Cardiac tamponade is manifested by diastolic compression/collapse of the right atrium and sometimes the right ventricle (Fig. 9-7). It must be remembered that the volume of effusion is not the main determinant of hemodynamic compromise but rather the intrapericardial pressure. The RV and RA walls are often well visualized and may appear hyperechoic because of the surrounding fluid. Better visualization of the heartbase and mass lesions is generally obtained before pericardiocentesis is performed. Careful evaluation of all portions of the right atrium and auricle, right ventricle, ascending aorta, and pericardium itself is important to screen for neoplasia. The left cranial parasternal (and transesophageal) transducer positions are especially useful. Some mass lesions are difficult to visualize. Mesothelioma may not cause discrete mass lesions and therefore may be indistinguishable from idiopathic pericardial effusion.
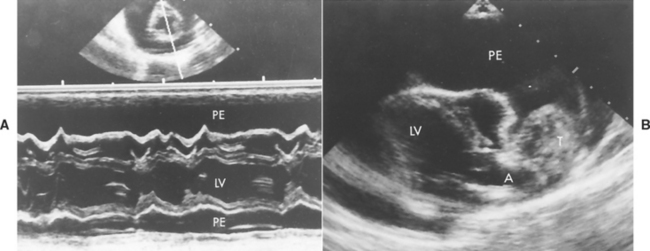
FIG 9-6 Echocardiographic examples of pericardial effusion. A, Short-axis M-mode view at mitral valve and chordal levels. Large echo-free (fluid) spaces are seen on either side of the heart; the right ventricular wall is clearly visualized. The small two-dimensional image above the M-mode shows the heart (transected by the M-mode cursor line) surrounded by pericardial fluid (which appears black on the image). B, Long-axis two-dimensional view from left parasternal position depicting a large heartbase tumor and pericardial effusion in a Schnauzer. PE, Pericardial effusion; T, tumor; LV, left ventricle; A, aorta.
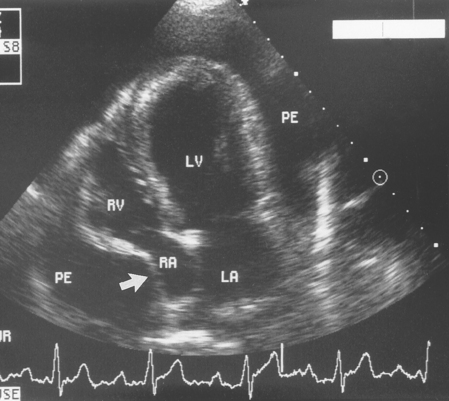
FIG 9-7 Diastolic compression of the right atrial wall (arrow) is evident in this left caudal four-chamber echocardiogram from a 3-year-old female Saint Bernard with cardiac tamponade. LA, Left atrium; LV, left ventricle; PE, pericardial effusion; RA, right atrium; RV, right ventricle.
Sometimes pleural effusion, a markedly enlarged left atrium, a dilated coronary sinus, or persistent left cranial vena cava can be confused with pericardial effusion. Careful scanning from several positions helps in differentiating these conditions. Identification of the parietal pericardium in relation to the echo-free fluid helps differentiate pleural from pericardial effusion. Because the pericardium is a relatively strong ultrasound reflector, by progressively dampening the returning echo signals, pericardial echos are seen to be the last to disappear. Most pericardial fluid accumulates near the cardiac apex because the pericardium adheres more tightly to the heartbase; there is usually little fluid behind the left atrium. Furthermore, evidence of collapsed lung lobes or pleural folds can often be seen within pleural effusion.
CLINICOPATHOLOGIC FINDINGS
Hematologic and biochemical test results are generally nonspecific. The complete blood count (CBC) may suggest inflammation or infection. Cardiac HSA may be associated with a regenerative anemia, increased numbers of nucleated red blood cells and schistocytes, and thrombocytopenia. Mild hypoproteinemia is seen in some cases of pericardial effusion. Cardiac troponin concentration or enzyme activities may be increased as a result of ischemia or myocardial invasion; mild increases in liver enzyme activities and prerenal azotemia may occur secondary to heart failure. Pleural and peritoneal fluids in dogs and cats with cardiac tamponade are usually modified transudates.
Pericardiocentesis (discussed in the next section) usually yields a hemorrhagic effusion; occasionally the fluid is suppurative. Samples are submitted for cytologic analysis and saved for possible bacterial (or fungal) culture. Nevertheless, differentiation of neoplastic effusions from benign hemorrhagic pericarditis is usually impossible on the basis of cytology alone. Reactive mesothelial cells within the effusion may closely resemble neoplastic cells; furthermore, chemodectomas and HSAs may not shed cells into the effusion. Therefore identifying a mass lesion with echocardiography is helpful for diagnosis. The effusions in patients with lymphoma or MH typically are consistent with a modified transudate. Neoplastic cells usually are easily identified in dogs and cats with lymphoma and in dogs with MH. Many neoplastic (and other non-inflammatory) effusions have a pH of 7.0 or greater, whereas inflammatory effusions generally have lower pH. However, there appears to be too much overlap for pericardial pH to be used as a reliable discriminator. Pericardial fluid culture is performed if cytology and pH suggest an infectious or inflammatory cause. In some patients fungal titers (e.g., coccidioidomycosis) or other serologic tests are helpful. It is currently unclear whether analysis of pericardial fluid for cardiac troponins or other substances will allow better differentiation of the underlying etiology.
Treatment and Prognosis
It is important to differentiate cardiac tamponade from other causes of right-sided CHF because the treatment is very different. Positive inotropic drugs do not ameliorate the signs of tamponade; diuretics and vasodilators can further reduce cardiac output and exacerbate hypotension and shock. Pericardiocentesis (discussed in the next section) is the immediate treatment of choice, and it also provides diagnostic information. Most signs of CHF resolve after pericardial fluid is removed. A dose or two of a diuretic may be useful after pericardiocentesis in some animals. Pericardial effusions secondary to other diseases that cause CHF, congenital malformations, or hypoalbuminemia do not usually cause tamponade and often resolve with management of the underlying condition.
Dogs with idiopathic pericardial effusion are initially treated conservatively by pericardiocentesis. After an infectious cause is ruled out by pericardial fluid culture or cytologic analysis, a glucocorticoid is often used (e.g., oral prednisone, 1 mg/kg/day, tapered over 2-4 weeks); however, its efficacy in preventing recurrent idiopathic pericardial effusion is unknown. Sometimes a 1- to 2-week course of a broad-spectrum antibiotic is used concurrently. Periodic reevaluation of these dogs by radiography or echocardiography is advised to detect recurrence. Apparent recovery occurs after one or two pericardial taps in about half of affected dogs. Cardiac tamponade recurs after a variable time span (days to years) in other cases. Some cases of recurrent effusion are caused by mesothelioma, MH, or other neoplasia, which may become evident on repeated echocardiographic exam.
Recurrent effusion that does not respond to repeated pericardiocenteses and antiinflammatory therapy is usually treated by subtotal pericardiectomy. Removal of the pericardium ventral to the phrenic nerves allows pericardial fluid drainage to the larger absorptive surface of the pleural space. The less invasive technique of thoracoscopic partial pericardiectomy has also been used successfully to treat idiopathic and some cases of neoplastic pericardial effusion; biopsy samples of the mass or masses (if identified) can be obtained through thoracoscopy. Lateral and subxiphoid approaches have been described. Percutaneous balloon pericardiotomy also appears to be an effective and even less invasive option for some cases. This procedure is performed under general anesthesia with fluoroscopic guidance. It involves placing a percutaneous sheath introducer through the chest wall into the pericardial space, then inserting a large balloon dilation catheter. The sheath is adjusted so that the balloon can be positioned across the pericardial membrane; as the balloon is inflated, it stretches the hole in the parietal pericardium. There is some concern that adhesions developing around a small pericardiotomy opening may result in fluid reaccumulation or increased risk of constrictive pericarditis.
Neoplastic pericardial effusions are also initially drained to relieve cardiac tamponade. Therapy may involve attempted surgical resection (depending on tumor size and location) or surgical biopsy, a trial of chemotherapy (based on biopsy or clinicopathologic findings), or conservative therapy until episodes of cardiac tamponade become unmanageable. Surgical resection of HSA is often not possible because of the size and extent of the tumor. Small tumors involving only the tip of the right auricle have been successfully removed; use of a pericardial patch graft may allow resection of larger masses involving the lateral RA wall. However, auriculectomy alone rarely results in prolonged long-term survival. Partial pericardiectomy may prevent the recurrence of tamponade. The increased potential for tumor dissemination throughout the thoracic cavity does not appear to affect survival time, compared with pericardiocentesis alone, in dogs with HSA or mesothelioma. The prognosis in dogs with RA HSA treated with surgery alone or in those in which treatment is declined by the owners is poor (median survival of 2-3 weeks); most dogs with atrial HSA have objective responses to multiagent chemotherapy (VAC protocol) and survival times of 4 to 8 months. Survival time in dogs with mesothelioma may be slightly longer than in those with HSA, but the overall prognosis is poor.
Heartbase tumors (e.g., chemodectoma) tend to be slow growing and locally invasive and have a low metastatic potential. Partial pericardiectomy may prolong survival for years. Percutaneous balloon pericardiotomy may also be an effective palliative procedure. Because of local invasion, complete surgical resection is rarely possible; attempts at aggressive resection often result in severe bleeding and death. However, small, well-defined masses may be completely resectable. Surgical biopsy is indicated if chemotherapy is contemplated. Effusion secondary to myocardial lymphoma, usually easily diagnosed cytologically, often responds to pericardiocentesis and chemotherapy.
Infectious pericarditis should be treated aggressively with appropriate antimicrobial drugs, as determined by microbial culture and sensitivity testing. Surgical therapy is likely to be more effective than continuous drainage with an indwelling pericardial catheter, and it also allows removal of penetrating foreign bodies. The prognosis is guarded. Even with successful elimination of infection, epicardial and pericardial fibrin deposition may lead to constrictive pericardial disease.
Pure hemorrhage into the pericardial space, whether the result of trauma, rupture of the left atrium, or a systemic coagulopathy, should be removed if signs of cardiac tamponade exist. Only enough blood to control signs of tamponade should be removed because continued drainage may predispose to further bleeding. The remaining blood is usually resorbed through the pericardium (autotransfusion). Surgery may be needed to stop continued bleeding or remove large clots. Dogs that survive an initial episode of intrapericardial bleeding from rupture of the left atrium still have a guarded to poor prognosis because of recurrent tearing of the left atrium. Animals with intrapericardial hemorrhage of unclear cause should be evaluated for a coagulation disorder. When trauma-induced intrapericardial hemorrhage persists in an animal with normal hemostasis, surgical exploration is indicated.
Complications
Complications of diseases causing pericardial effusion relate to (1) sequelae of the fluid accumulation itself (e.g., cardiac tamponade and compression of surrounding structures [lung, esophagus, trachea]), (2) immediate effects of associated inflammatory processes (e.g., arrhythmias, local and systemic effects of infectious agents, further fluid formation), (3) pericardial fibrosis and subsequent constrictive pericarditis, (4) sequelae of neoplastic processes (e.g., further bleeding, metastases, local invasion and obstruction, seeding of the pleura, loss of function), and (5) complications of pericardiocentesis (discussed in the next section). Overly aggressive surgical attempts to remove cardiac tumors or the entire pericardial sac can be fatal, and partial pericardiectomy may enhance intrathoracic dissemination of certain tumors such as mesothelioma and carcinoma.
PERICARDIOCENTESIS
Pericardiocentesis should be done immediately in animals with cardiac tamponade. Administration of diuretics or vasodilators without pericardiocentesis may cause further hypotension and cardiogenic shock. Pericardiocentesis is a relatively safe procedure when performed carefully. Removal of even a small volume of pericardial fluid can markedly decrease intrapericardial pressure in animals with tamponade.
Pericardiocentesis is usually done from the right side to minimize the risk of trauma to the lung (via the cardiac notch) and major coronary vessels (located mostly on the left). The need for sedation depends on the clinical status and temperament of the animal. The animal is usually placed in left lateral or sternal recumbency for more secure restraint, especially if the animal is weak or excitable. Sometimes needle pericardiocentesis can be successfully performed on the standing animal, but the risk of injury increases if the patient suddenly moves. An elevated echocardiography table with a large cutout can also be used with good success; the animal is placed in right lateral recumbency, and the tap is performed from underneath. An advantage to this method is that fluid moves to the right side with gravity; however, if adequate space is not available for wide sterile skin preparation or needle/catheter manipulation, this approach is not advised. Echo guidance can be used but is not necessary unless the effusion is of very small volume or appears compartmentalized.
A variety of equipment can be used for pericardiocentesis. A butterfly needle/catheter (19- to 21-gauge) or appropriately long hypodermic or spinal needle attached to extension tubing is adequate in emergency situations. An over-the-needle catheter system is a safer alternative because it reduces the risk of cardiopulmonary laceration during fluid aspiration. The catheter is chosen according to patient size (e.g., 12- to 16-gauge, 4- to 6-inch long catheter for large dogs, down to 18- to 20-gauge, 11/2- to 2-inch long catheter for small dogs or cats). A few extra small side holes may be smoothly cut (with sterile scissors) near the tip of larger catheters to increase fluid removal rate. During initial catheter placement the extension tubing is attached to the needle stylet. After the catheter is advanced into the pericardial space, the extension tubing is reattached directly to the catheter. With all methods, a three-way stopcock is placed between the tubing and a collection syringe.
An ECG monitor should be in place during pericardiocentesis because needle/catheter contact with the heart commonly induces ventricular arrhythmias. The skin is shaved over a wide area of the right precordium (from about the third to seventh intercostal spaces and from sternum to costochondral junction) and surgically prepared. Sterile gloves and aseptic technique are used for the procedure. The puncture site is located by palpating to identify the point at which the cardiac impulse feels strongest (usually between the fourth and sixth ribs just lateral to the sternum). Local anesthesia is necessary when using large catheters and recommended for needle pericardiocentesis. Lidocaine (2%) is infiltrated with sterile technique at the skin puncture site, into underlying intercostal muscles, and into the pleura. A small stab incision is made in the skin to allow catheter entry.
Intercostal vessels are located just caudal to each rib and must be avoided when entering the chest. Once the needle has penetrated the skin, the operator’s assistant should apply gentle negative pressure to the attached syringe as the operator slowly advances the needle toward the heart. It is sometimes helpful to aim the tip of the needle toward the animal’s opposite shoulder. The tubing is observed so that fluid will be seen as soon as it is aspirated. Pleural fluid (usually straw colored) may enter the tubing first and is drained as much as possible. The pericardium creates increased resistance to needle advancement and may produce a subtle scratching sensation. Gentle pressure is used to advance the needle through the pericardium. A loss of resistance may be noted with needle penetration, and fluid aspirated into the tubing usually appears dark red. If the needle comes into contact with the heart, a marked scratching or tapping sensation is usually felt, the needle may move with the heartbeat, and ventricular premature complexes are often provoked. The needle should be retracted slightly if cardiac contact occurs. It is important to avoid excessive needle motion within the chest. When a catheter system is used, after the needle/stylet is well within the pericardial space, the catheter is advanced, the stylet removed, and the extension tubing attached to the catheter. Initial fluid samples are saved for cytologic exam and possible culture, and then as much fluid as possible is aspirated.
Pericardial effusion usually appears quite hemorrhagic. It can be distressing to see dark, bloody fluid being aspirated from near the heart, but pericardial fluid can be differentiated from intracardiac blood in several ways. Unless the fluid is caused by very recent pericardial hemorrhage, it will not clot. (A few drops can be placed on the table or into a serum tube to check.) The PCV of pericardial fluid is usually much lower than that of peripheral blood (except in some dogs with HSA); also, the supernatant is xanthochromic (yellow tinged) when spun in a hematocrit tube. As the pericardial fluid is drained, the animal’s ECG complexes increase in amplitude, tachycardia diminishes, and the patient often takes a deep breath and appears more comfortable.
Complications
Complications of pericardiocentesis include (1) cardiac injury or puncture causing arrhythmias (the most common complication, although usually self-limiting when the needle is withdrawn), (2) lung laceration causing pneumothorax and/or hemorrhage, (3) coronary artery laceration with myocardial infarction or further bleeding into the pericardial space, and (4) dissemination of infection or neoplastic cells into the pleural space.
CONSTRICTIVE PERICARDIAL DISEASE
Etiology and Pathophysiology
Constrictive pericardial disease is diagnosed occasionally in dogs but only rarely in cats. This condition occurs when thickening and scarring of the visceral and/or parietal pericardium restrict ventricular diastolic expansion and prevent normal cardiac filling. Both ventricles are affected. Usually the entire pericardium is involved symmetrically. Fusion of parietal and visceral pericardial layers obliterates the pericardial space in some cases. In others the visceral layer (epicardium) alone is involved. A small amount of pericardial effusion (constrictive-effusive pericarditis) may be present.
Increased fibrous connective tissue and variable amounts of inflammatory and reactive pericardial infiltrates are seen on histopathologic exam. Although the etiology of constrictive pericardial disease is often unknown, acute inflammation with fibrin deposition and possibly varying degrees of pericardial effusion are thought to precede its development. Some cases in dogs are attributable to recurrent idiopathic hemorrhagic effusion, infectious pericarditis (resulting from actinomycosis, mycobacteriosis, coccidioidomycosis), a metallic foreign body in the pericardium, tumors, and idiopathic osseous metaplasia and/or fibrosis of the pericardium.
In advanced constrictive pericardial disease, ventricular filling is limited essentially to early diastole, before ventricular expansion is abruptly curtailed. Any further ventricular filling is accomplished only at high venous pressures. Compromised filling reduces cardiac output, and compensatory mechanisms of heart failure cause fluid retention, tachycardia, and vasoconstriction.
Clinical Features
Middle-aged, large- to medium-breed dogs are most often affected. Males and German Shepherd Dogs may be at higher risk. Some dogs have a history of pericardial effusion. Clinical signs of right-sided CHF predominate. Abdominal distention (ascites), tachypnea or labored breathing, tiring, syncope, weakness, and weight loss are common complaints. These signs may develop over weeks to months. Ascites and jugular venous distention are the most consistent clinical findings, as in dogs with cardiac tamponade. Weakened femoral pulses and muffled heart sounds are also typical. A diastolic pericardial knock sound, resulting from abrupt deceleration of ventricular filling in early diastole, has been described but is not often identified in dogs. A systolic murmur or click, probably caused by valvular disease rather than the pericardial pathology, or a diastolic gallop sound may be heard.
Diagnosis
The diagnosis of constrictive pericardial disease may be difficult. Typical radiographic findings include mild to moderate cardiomegaly, pleural effusion, and caudal vena cava distention. Reduced cardiac motion may be evident on fluo roscopy. Echocardiographic changes in dogs with constrictive pericardial disease may be subtle; suggestive findings include diastolic flattening of the left ventricular freewall and abnormal septal motion. The pericardium may appear thickened and intensely echogenic, but differentiating this from normal pericardial echogenicity may be impossible. Possible ECG abnormalities include sinus tachycardia, P-wave prolongation, and small QRS complexes.
A CVP >15 mm Hg is common. Intracardiac hemodynamic measurements are most useful diagnostically. In addition to high mean atrial and diastolic ventricular pressures, the atrial pressure waveform shows a prominent y descent (during ventricular relaxation). This is in contrast to cardiac tamponade, wherein the y descent is diminished. During tamponade, ventricular diastolic expansion immediately raises intrapericardial pressure and impairs caval flow into the right atrium, thus preventing the normal early diastolic decrease in CVP (y descent), although flow into the right atrium (and x descent on atrial waveform) continues during ventricular contraction. With constrictive pericardial disease, filling pressure is low only in early diastole (during the time of y descent). Another classic finding with constrictive pericardial disease is an early diastolic dip in ventricular pressure, followed by a mid-diastolic plateau, but this is not consistently seen in dogs. Results of angiocardiography may be normal, or they may show atrial and vena caval enlargement with increased endocardial-pericardial distance.
Treatment and Prognosis
Therapy for constrictive pericardial disease consists of surgical pericardiectomy. This is more successful when only the parietal pericardium is involved. Constrictive pericardial disease involving the visceral layer requires epicardial stripping. This procedure increases the surgical difficulty and associated complications. Pulmonary thrombosis is reportedly a common postoperative complication and can be life-threatening. Tachyarrhythmias are another complication of surgery. In the postoperative period, a diuretic and possibly an angiotensin-converting enzyme inhibitor (ACEI) may be helpful. Positive inotropic and vasodilating drugs are not usually indicated. Constrictive pericardial disease is progressive and, without successful surgical intervention, ultimately fatal.
CARDIAC TUMORS
Etiology and Pathophysiology
Echocardiography has made the antemortem diagnosis of cardiac tumors more common, although the overall prevalence of such neoplasms is low. Some cardiac tumors cause severe clinical signs, whereas others are diagnosed fortuitously. Dogs with cardiac tumors tend to be middle-aged and older. More than 85% of affected dogs are between 7 and 15 years of age; however, very old dogs (>15 years) have a surprisingly low prevalence. Reproductive status influences the relative risk for cardiac tumors in dogs, despite a similar frequency of occurrence in males and females overall. Neutered dogs have a greater relative risk, especially spayed females, which have a risk that is four to five times greater compared with that of intact females. Intact and neutered males also have greater risk than intact females. Certain breeds of dog have a higher prevalence of cardiac tumor compared with the general population (Table 9-1). The age distribution of cats with cardiac tumors is different from that of dogs; about 28% are 7 years old or younger. It is unknown whether reproductive status affects relative risk for cardiac tumors in cats.
The most common cardiac tumor in dogs is HSA. Most are located in the right atrium and/or right auricle; some also infiltrate the ventricular wall. HSAs usually are associated with hemorrhagic pericardial effusion and cardiac tampon ade (see p. 158). Metastases are common by the time of diagnosis. Golden Retrievers, German Shepherd Dogs, Afghan Hounds, Cocker Spaniels, English Setters, and Labrador Retrievers, among others, are at higher risk for this tumor.
Masses at the heartbase are the second most frequently reported cardiac tumor in dogs. They are usually neoplasms of the chemoreceptor aortic bodies (chemodectoma, aortic body tumors); ectopic thyroid or parathyroid, or mixed-cell–type tumors also occur here. Heartbase tumors tend to be locally invasive around the root of the aorta and surrounding structures; metastases to other organs occur rarely. Chemodectomas are reported more frequently in brachycephalic dogs (specifically Boxers, Boston Terriers, and Bulldogs) but affect individuals of other breeds as well. Clinical signs associated with heartbase tumors are usually related to pericardial effusion and cardiac tamponade.
Mesothelioma occurs sporadically; there may be geographical variation in its prevalence. Other primary tumors involving the heart are rare in dogs but include myxoma, various types of sarcoma, and other neoplasms. Most cases involve right-heart structures. Metastatic tumors, including lymphoma, other sarcomas, and various carcinomas, may involve the heart as well. MH may involve the heart or pericardium; most affected dogs are either Golden Retrievers, Labrador Retrievers, Rottweilers, or Greyhounds. Mild pericardial effusion, without overt signs of cardiac tamponade, co-exists with pleural and abdominal effusion.
Lymphoma is the most common cardiac tumor in cats. Various (mostly metastatic) carcinomas are the next most common cardiac neoplasms in cats. HSA is uncommon; other tumors (such as aortic body tumor, fibrosarcoma, rhabdomyosarcoma) are reported only rarely in cats.
Cardiac tumors cause several pathophysiologic abnormalities, depending on their location and size. Ultimately, the patient’s clinical signs can be referred to one or a combination of these. Many tumors impede cardiac filling by causing pericardial effusion and cardiac tamponade (discussed earlier). An intrapericardial mass can itself externally compress the heart as well as cause pericardial effusion. Alternatively, a tumor that grows in an intracardiac location can physically obstruct cardiac inflow or outflow. Myocardial tumor infiltration or secondary ischemia can disrupt the cardiac rhythm and impair contractility. If the tumor is small or has not yet markedly impaired cardiac function, clinical signs may be absent.
Clinical Features
Signs of right-sided CHF result from blood flow obstruction within the right atrium or ventricle or from cardiac tamponade. Syncope, weakness associated with exertion, and other low output signs also result from cardiac tamponade, blood flow obstruction, arrhythmias, or impaired myocardial function secondary to cardiac tumors. Tachyarrhythmias of any type may also occur; intracardiac conduction disturbances sometimes result from tumor infiltration. Lethargy or collapse may relate to bleeding tumors (e.g., HSA) present in extracardiac locations as well.
Auscultation findings vary. Arrhythmias or muffled heart sounds (if large pericardial effusion is present) are common. Occasionally a murmur is caused by neoplastic obstruction of intracardiac blood flow, but murmurs associated with unrelated disease (e.g., degenerative mitral regurgitation) are more common. Auscultation findings may be normal.
Diagnosis
Radiographic findings are also quite variable. The cardiac silhouette may be normal or show an unusual bulge, a mass effect adjacent to the heart, or a globoid cardiac silhouette compatible with pericardial effusion. Intrapericardial masses are obscured by pericardial effusion. Other radiographic findings that occur secondary to impaired cardiac filling include pleural effusion, evidence of pulmonary edema, widening of the caudal vena cava (and/or pulmonary veins), hepatomegaly, and ascites. Dorsal deviation of the trachea and increased perihilar opacity are seen in some dogs with heartbase tumors. Evidence of pulmonary metastases is found with some primary or secondary (metastatic) cardiac neoplasms.
ECG findings sometimes show abnormalities suggesting the location and sequelae of the underlying disease, such as chamber enlargement, pericardial effusion, and various arrhythmias. Echocardiography can depict cardiac masses and determine the presence or absence of pericardial effusion as well as secondary changes in cardiac chamber size, shape, and ventricular function. Doppler techniques allow assessment of associated blood flow abnormalities. Heartbase tumors that extend into the pericardial space are easier to see when there is pericardial effusion, just as intracardiac masses are accentuated by the echolucent intracardiac blood surrounding them (Fig. 9-8). The left cranial parasternal transducer position may be especially useful in evaluating the ascending aorta, right auricle, and surrounding structures. Echocardiographic assessment of the tumor’s location, size, attachment (pedunculated or broad based), and extent (superficial or deeply invading adjacent myocardium) may help in determining whether surgical resection or biopsy is possible. Visualizing a suspected mass lesion in more than one echocardiographic plane helps verify it and prevent the misinterpretation of artifacts.
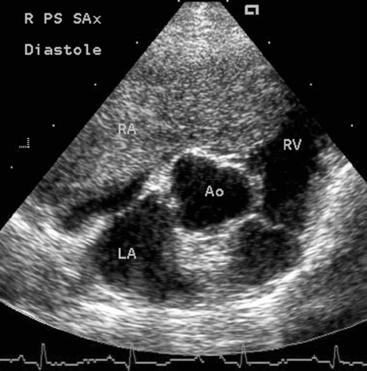
FIG 9-8 Right parasternal short-axis echocardiogram from a 16-year-old Cocker Spaniel and Poodle mix with ascites and weakness. A large right atrial tumor extends across the tricuspid orifice into the ventricle in this diastolic frame. Pericardial effusion was not present in this dog. Ao, Aorta; LA, left atrium; RA, right atrium; RV, right ventricle.
Pericardial fluid analysis is recommended, although definitive diagnosis of neoplasia cannot usually be made on the basis of cytologic findings alone (see p. 163). Cardiac lymphoma or MH is more likely to be diagnosed on pericardial fluid cytology. Nevertheless, visualization of a cardiac mass using echocardiography, computed tomography, pneumopericardiography, angiography, or another modality is usually necessary for diagnosis. Hematologic and serum biochemical tests are generally nonspecific in dogs and cats with cardiac tumors. Cardiac enzyme activities or circulation troponin concentrations may be high because of ischemia or myocardial invasion; mild increases in serum alanine aminotransferase activity and azotemia may occur with CHF. HSA is often associated with a regenerative anemia, increased number of nucleated red blood cells and schistocytes, leuko cytosis, and thrombocytopenia. If present, pleural and peritoneal fluids are usually modified transudates.
Treatment and Prognosis
Unfortunately, there are few good long-term options in most patients with a heart tumor. Cardiac tamponade is managed when it occurs (see p. 163). Conservative therapy (pericardiocentesis as needed, possibly with glucocorticoid administration to decrease inflammation) is used in some animals. Partial pericardiectomy or pericardiotomy may be helpful in animals with recurrent tamponade.
Surgical tumor resection may be possible depending on the location, size, and invasiveness of the mass. Tumors involving only the tip of the right auricular appendage or a pedunculated mass in a surgically accessible location are more likely to be resectable. Intracardiac masses within the right side of the heart might be reached using venous inflow occlusion techniques and rapid cardiotomy; however, surgical access to lesions on the left side of the heart and large or medially attached masses in the right heart generally requires cardiopulmonary bypass.
Surgical biopsy of a nonresectable mass may be helpful if chemotherapy is being contemplated. Although many cardiac tumors appear to be fairly unresponsive to chemotherapy, some are treated with short-term success. Some cardiac hemangiosarcomas respond to vincristine, doxorubicin, and cyclophosphamide combination chemotherapy for 4 to 8 months. Lymphoma and MH should be treated using standard protocols.
Aronsohn MG, Carpenter JL. Surgical treatment of idiopathic pericardial effusion in the dog: 25 cases (1978–1993). J Am Anim Hosp Assoc. 1999;35:521.
Aronson LR, Gregory CR. Infectious pericardial effusion in five dogs. Vet Surg. 1995;24:402.
Brisson BA, Holmberg DL. Use of pericardial patch graft reconstruction of the right atrium for treatment of hemangiosarcoma in a dog. J Am Vet Med Assoc. 2001;218:723.
Closa JM, Font A, Mascort J. Pericardial mesothelioma in a dog: long term survival after pericardiectomy in combination with chemotherapy. J Small Anim Pract. 1999;40:383.
Cobb MA, et al. Percutaneous balloon pericardiotomy for the management of malignant pericardial effusion in two dogs. J Small Anim Pract. 1996;37:549.
Davidson BJ, Paling AC, Lahmers SL, et al. Disease association and clinical assessment of feline pericardial effusion. J Am Anim Hosp Assoc. 2008;44:5-9.
Day MJ, Martin MWS. Immunohistochemical characterization of the lesions of canine idiopathic pericarditis. J Small Anim Pract. 2002;43:382.
Dunning D, et al. Analysis of prognostic indicators for dogs with pericardial effusion: 46 cases (1985–1996). J Am Vet Med Assoc. 1998;212:1276.
Ehrhart N, et al. Survival of dogs with aortic body tumors. Vet Surg. 2002;31:44.
Fine DM, Tobias AH, Jacob KA. Use of pericardial fluid pH to distinguish between idiopathic and neoplastic effusions. J Vet Intern Med. 2003;17:525.
Jackson J, Richter KP, Launer DP. Thorascopic partial pericardiectomy in 13 dogs. J Vet Intern Med. 1999;13:529.
Miller MW, Sisson DD. Pericardial disorders. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine. ed 5. Philadelphia: WB Saunders; 2000:923-936.
Reimer SB, et al. Long-term outcome of cats treated conservatively or surgically for peritoneopericardial diaphragmatic hernia: 66 cases (1987-2002). J Am Vet Med Assoc. 2004;224:728.
Rush JE, Keene BW, Fox PR. Pericardial disease in the cat: a retrospective evaluation of 66 cases. J Am Anim Hosp Assoc. 1990;26:39.
Sidley JA, et al. Percutaneous balloon pericardiotomy as a treatment for recurrent pericardial effusion in 6 dogs. J Vet Intern Med. 2002;16:541.
Stepien RL, Whitley NT, Dubielzig RR. Idiopathic or mesothelioma-related pericardial effusion: clinical findings and survival in 17 dogs studied retrospectively. J Small Anim Pract. 2000;41:342.
Stafford Johnson M, et al. A retrospective study of clinical findings, treatment and outcome in 143 dogs with pericardial effusion. J Small Anim Pract. 2004;45:546.
Thomas WP, et al. Constrictive pericardial disease in the dog. J Am Vet Med Assoc. 1984;184:546.
Vicari ED, et al. Survival times of and prognostic indicators for dogs with heart base masses: 25 cases (1986–1999). J Am Vet Med Assoc. 2001;219:485.
Ware WA. Cardiac neoplasia. In: Bonagura JD, editor. Kirk’s current veterinary therapy XII. Philadelphia: WB Saunders; 1995:873-876.
Ware WA, Hopper DL. Cardiac tumors in dogs: 1982-1995. J Vet Intern Med. 1999;13:95.
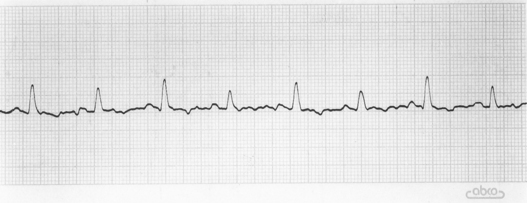
 TABLE 9-1
TABLE 9-1