CHAPTER 6 Acquired Valvular and Endocardial Disease
DEGENERATIVE ATRIOVENTRICULAR VALVE DISEASE
Chronic degenerative atrioventricular (AV) valve disease is the most common cause of heart failure in the dog. This condition is also known as endocardiosis, mucoid or myxomatous valvular degeneration, or chronic valvular fibrosis. Because clinically relevant degenerative valve disease is rare in cats, this chapter will focus on canine chronic valvular disease. The mitral valve is affected most often and to a greater degree, but degenerative lesions also involve the tricuspid valve in many dogs. However, isolated degene-rative disease of the tricuspid valve is uncommon. Thickening of the aortic and pulmonic valves sometimes is observed in older animals but rarely causes more than mild insufficiency.
Etiology and Pathophysiology
The cause of degenerative AV valve disease is unclear, but a hereditary basis is likely. Middle-aged and older small to mid-size breeds are most often affected. Disease prevalence and severity increase with age. About a third of small-breed dogs older than 10 years of age are affected. Commonly affected breeds include Toy and Miniature Poodles, Miniature Schnauzers, Chihuahuas, Pomeranians, Fox Terriers, Cocker Spaniels, Pekingese, Boston Terriers, Miniature Pinschers, Whippets, and Cavalier King Charles Spaniels. An especially high prevalence and an early onset of degenerative mitral valve disease (MVD) is reported in Cavalier King Charles Spaniels, in which inheritance is thought to be polygenic, with gender and age influencing expression. It appears that the overall prevalence of mitral regurgitation (MR) murmurs and degenerative valve disease is similar in male and female dogs, but males may have faster disease progression. Some large-breed dogs are affected also, and the prevalence may be higher in German Shepherd Dogs.
Multiple factors involving collagen degeneration, valve leaflet stress, and endothelial function are thought to be involved. Pathologic valve changes develop gradually with age. Early lesions consist of small nodules on the free margins of the valve; these become larger, coalescing plaques that thicken and distort the valve. The histologic changes have been described as myxomatous degeneration. Collagen within the affected leaflets degenerates, and acid mucopolysaccharides and other substances accumulate within the layers of the leaflets, resulting in nodular thickening, deformity, and weakening of the valve as well as its chordae tendineae. Redundant tissue between chordal attachments often bulges (prolapses) like a parachute or balloon toward the atrium. Mitral valve prolapse may be important in the pathogenesis of the disease, at least in some breeds.
Affected valves gradually begin to leak because their edges do not coapt properly. As the lesions progress, the valve insufficiency (regurgitation) becomes clinically evident. Atrial jet lesions; endocardial fibrosis; and, in patients with advanced disease, partial or even full-thickness atrial tears can form. Chronic valvular disease is also associated with intramural coronary arteriosclerosis, microscopic intramural myocardial infarctions, and focal myocardial fibrosis. The extent to which these changes cause clinical myocardial dysfunction is not clear; however, impaired myocardial contractility is observed late in the disease. Interestingly, senior dogs without valvular disease also have similar vascular lesions.
The pathophysiologic changes relate to volume overload on the affected side of the heart after the valve or valves become incompetent. Regurgitation usually develops slowly over months to years. Mean atrial pressure usually remains fairly low during this time, unless a sudden increase in regurgitant volume (e.g., ruptured chordae) occurs. With advancing valve degeneration, a progressively larger volume of blood moves ineffectually back and forth between the ven tricle and atrium, diminishing the forward flow to the aorta. Compensatory mechanisms augment blood volume to meet the circulatory needs of the body (see Chapter 3), including increased sympathetic activity, attenuated vagal tone, and renin-angiotensin-aldosterone system (RAAS) activation. Natriuretic peptide release occurs; higher atrial natriuretic peptide concentrations have been associated with marked left atrium (LA) enlargement and severe congestive heart failure (CHF). The affected ventricle and atrium dilate to accept the growing regurgitant volume and the required forward stroke volume; eccentric myocardial hypertrophy develops in an attempt to normalize the resulting increase in wall stress.
These compensatory changes in heart size and blood volume allow most dogs to remain asymptomatic for a prolonged period. Massive LA enlargement may develop before any signs of heart failure appear, and some dogs never show clinical signs of heart failure. The rate at which the regurgitation worsens, as well as the degree of atrial distensibility and ventricular contractility, influence how well the disease is tolerated. A gradual increase in atrial, pulmonary venous, and capillary hydrostatic pressures stimulates compensatory increases in pulmonary lymphatic flow. Overt pulmonary edema develops when the capacity of the pulmonary lymphatic system is exceeded. Tricuspid insufficiency may be severe enough to cause right-sided CHF. Increased pulmonary vascular pressure secondary to chronic left-sided CHF may also contribute to the development of right-sided heart failure.
Ventricular pump function is maintained fairly well until late in the disease in many dogs, even in the face of severe congestive signs. Nevertheless, chronic volume overload eventually reduces myocyte contractility. The mechanism of myocardial dysfunction may involve damage from oxygen free radicals as well as neurohormonal activation. Reduced contractility exacerbates ventricular dilation and valve regurgitation and therefore can worsen CHF. Assessment of left ventricular (LV) contractility in animals with MR is complicated by the fact that the most commonly used clinical indices (e.g., echocardiographic fractional shortening, ejection fraction) overestimate contractility because they are obtained during ejection and are therefore affected by the reduced ventricular afterload caused by MR. The echocardiographic estimation of the end-systolic volume index may be useful (see p. 41). This index suggests that myocardial function is normal to mildly depressed in most dogs with chronic mitral degeneration. A number of other echo/Doppler indices can also help assess LV systolic and diastolic function.
Complicating Factors
Although this disease usually progresses slowly, certain complicating events can precipitate acute clinical signs in dogs with previously compensated disease (Box 6-1). For example, tachyarrhythmias may be severe enough to cause decompensated CHF, syncope, or both. Frequent atrial premature contractions, paroxysmal atrial tachycardia, or atrial fibrillation can reduce ventricular filling time and cardiac output, increase myocardial oxygen needs, and worsen pulmonary congestion and edema. Ventricular tachyarrhythmias also occur but are less common.
 BOX 6-1 Potential Complications of Chronic Atrioventricular Valve Disease
BOX 6-1 Potential Complications of Chronic Atrioventricular Valve Disease
Sudden rupture of diseased chordae tendineae acutely increases regurgitant volume and can precipitate fulminant pulmonary edema within hours in previously compensated or even asymptomatic dogs. Signs of low cardiac output may also occur. Sometimes, ruptured chordae tendineae are an incidental finding (on an echocardiogram or at necropsy), especially if second- or third-order chordae are involved.
Massive LA enlargement itself can result in compression of the left mainstem bronchus and stimulate persistent coughing, even in the absence of CHF. Furthermore, massive left (or right) atrial distention can result in partial- or full-thickness tearing. Atrial wall rupture usually causes acute cardiac tamponade; there appears to be a higher prevalence of this complication in male Miniature Poodles, Cocker Spaniels, and Dachshunds. In most of these cases, severe valve disease; marked atrial enlargement; atrial jet lesions; and, often, ruptured first-order chordae tendineae are present.
Clinical Features
Degenerative AV valve disease may cause no clinical signs for years, and some dogs never develop signs of heart failure. In those that do, the signs usually relate to decreased exercise tolerance and manifestations of pulmonary congestion and edema. Diminished exercise capacity and cough or tachypnea with exertion are common initial owner complaints. As pulmonary congestion and interstitial edema worsen, the resting respiratory rate increases. Coughing tends to occur at night and early morning, as well as in association with activity. Severe edema results in obvious respiratory distress and usually a moist cough. Signs of severe pulmonary edema can develop gradually or acutely. Intermittent episodes of symptomatic pulmonary edema interspersed with periods of compensated heart failure occurring over months to years are also common. Episodes of transient weakness or acute collapse (syncope) can occur secondary to arrhythmias, coughing, or an atrial tear. Signs of tricuspid regurgitation (TR) are often overshadowed by those of MR but include ascites; respiratory distress from pleural effusion; and, rarely, subcutaneous edema. Splanchnic congestion may precipitate gastrointestinal signs. The cough caused by main bronchus compression often is described as “honking.”
A holosystolic murmur heard best in the area of the left apex (left fourth to sixth intercostal space) is typical in patients with MR. The murmur can radiate in any direction. Mild regurgitation may be inaudible or cause a murmur only in early systole (protosystolic). Exercise and excitement often increase the intensity of soft MR murmurs. Louder murmurs have been associated with more advanced disease, but in dogs with massive regurgitation and severe heart failure, the murmur can be soft or even inaudible. Occasionally, the murmur sounds like a musical tone or whoop. Some dogs with chronic mitral disease have a mid- to late-systolic click, with or without a murmur. An S3 gallop may be audible at the left apex in dogs with advanced disease. TR typically causes a holosystolic murmur best heard at the right apex. Features that aid in differentiating a TR murmur from radiation of an MR murmur to the right chest wall include jugular vein pulsations, a precordial thrill over the right apex, and a different quality to the murmur heard over the tricuspid region.
Pulmonary sounds can be normal or abnormal. Accentuated, harsh breath sounds and end-inspiratory crackles (especially in ventral lung fields) develop as pulmonary edema worsens. Fulminant pulmonary edema causes widespread inspiratory as well as expiratory crackles and wheezes. Some dogs with chronic MR have abnormal lung sounds caused by underlying pulmonary or airway disease rather than CHF. Dogs with CHF tend to have sinus tachycardia; those with chronic pulmonary disease frequently have marked sinus arrhythmia and a normal heart rate. Pleural effusion causes diminished pulmonary sounds ventrally.
Other physical examination findings may be normal or noncontributory. Peripheral capillary perfusion and arterial pulse strength are usually good, although pulse deficits may be present in dogs with tachyarrhythmias. A palpable precordial thrill accompanies loud (grade 5-6/6) murmurs. Jugular vein distention and pulsations are not expected in dogs with MR alone. In animals with TR, jugular pulses occur during ventricular systole; these are more evident after exercise or in association with excitement. Jugular venous distention results from elevated right heart filling pressures. Jugular pulsations and distention are more evident with cranial abdominal compression (positive hepatojugular reflux). Ascites or hepatomegaly may be evident in dogs with right-sided CHF.
RADIOGRAPHY
Thoracic radiographs typically show some degree of LA and LV enlargement (see p. 13), which progresses over months to years (Fig. 6-1). As LA size increases, dorsal main bronchus displacement occurs. Severe LA enlargement causes compression of the left mainstem bronchus. Fluoroscopy may demonstrate dynamic main bronchus collapse during coughing or even quiet breathing in such animals. Extreme dilation of the LA can result over time, even without clinical heart failure. Variable right heart enlargement occurs with chronic TR, but this may be masked by left heart and pulmonary changes associated with concurrent MVD.
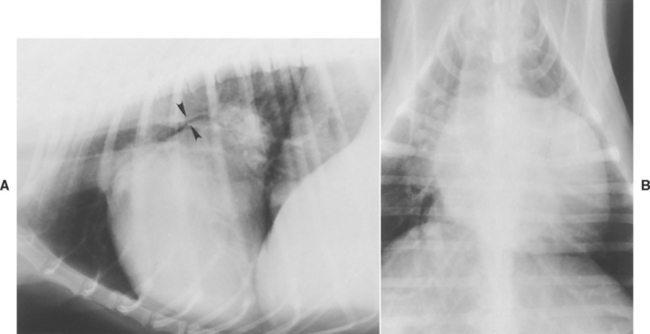
FIG 6-1 Lateral (A) and dorsoventral (B) radiographs from a Poodle with advanced mitral valve insufficiency. Note marked left ventricular and atrial enlargement and narrowing of left mainstem bronchus (arrowheads in A).
Pulmonary venous congestion and interstitial edema occur with the onset of left-sided CHF; progressive interstitial and alveolar pulmonary edema may follow. Although cardiogenic pulmonary edema in dogs typically has a hilar, dorsocaudal, and bilaterally symmetric pattern, an asymmetric distribution is seen in some dogs. The presence and severity of pulmonary edema do not necessarily correlate with the degree of cardiomegaly. Acute, severe MR (e.g., with rupture of the chordae tendineae) can cause severe edema in the presence of minimal LA enlargement. Conversely, slowly worsening MR can produce massive LA enlargement with no evidence of CHF. Early signs of right-sided heart failure include caudal vena caval distention, pleural fissure lines, and hepatomegaly. Overt pleural effusion and ascites occur with advanced failure.
ELECTROCARDOGRAPHY
The electrocardiogram (ECG) may suggest LA or biatrial enlargement and LV dilation (see p. 28), although the tracing is often normal. An RV enlargement pattern is occasionally seen in dogs with severe TR. Arrhythmias, especially sinus tachycardia, supraventricular premature complexes, paroxysmal or sustained supraventricular tachycardias, ventricular premature complexes, and atrial fibrillation are common in dogs with advanced disease. These arrhythmias may be associated with decompensated CHF, weakness, or syncope.
ECHOCARDIOGRAPHY
Echocardiography shows the atrial and ventricular chamber dilation secondary to chronic AV valve insufficiency. Depending on the degree of volume overload, this enlargement can be severe. Vigorous LV wall and septal motion are seen with MR when contractility is normal (Fig. 6-2); fractional shortening is high, and there is little to no E point–septal separation. Although ventricular diastolic dimension is increased, systolic dimension is normal until myocardial failure ensues. Calculation of end-systolic volume index may help in assessing myocardial function. Ventricular wall thickness is typically normal in dogs with chronic AV valve disease. With severe TR, paradoxical septal motion may occur along with the right ventricular (RV) and right atrial (RA) dilation. Pericardial fluid (blood) is seen after an LA tear, and evidence for cardiac tamponade may be evident. Mild pericardial effusion may also accompany signs of right-sided CHF.
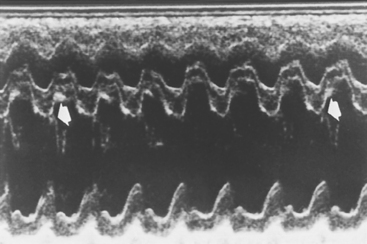
FIG 6-2 Sample M-mode echocardiogram from male Maltese with advanced mitral valve insufficiency and left-sided heart failure. Note accentuated septal and left ventricular posterior wall motion (fractional shortening = 50%) and lack of mitral valve E point–septal separation (arrows).
Affected valve cusps are thickened and may appear knobby. Smooth thickening is characteristic of degenerative disease (endocardiosis). Conversely, rough and irregular vegetative valve lesions are characteristic of bacterial endocarditis; however, clear differentiation between these by echocardiography alone may be impossible. Systolic prolapse involving one or both valve leaflets is common with degenerative AV valve disease (Fig. 6-3, A). A ruptured chorda tendinea or leaflet tip sometimes is seen flailing into the atrium during systole (Figure 6-3, B). The direction and extent of flow disturbance can be seen with color-flow Doppler (see Figure 2-35). Although the size of the disturbed flow area provides a rough estimate of regurgitation severity, there are technical limitations with this. The proximal isovelocity surface area (PISA) method is considered by some to be a more accurate way to estimate MR severity. Other Doppler techniques can be used to evaluate systolic and diastolic ventricular function. Maximal TR jet velocity indicates whether pulmonary hypertension is present and its severity.
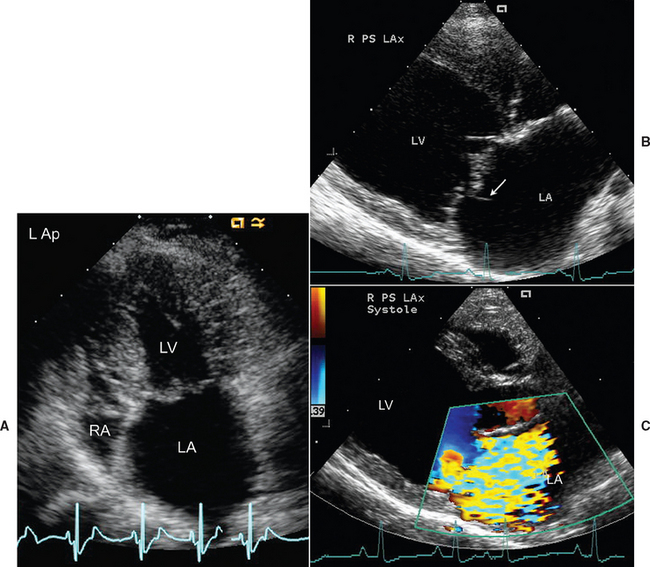
FIG 6-3 A, Thick, mildly prolapsing mitral valve and LA enlargement are seen from the left apical position in an older Dachshund with severe degenerative AV valve disease. The tricuspid valve is also thick. B, Chorda tendineae rupture is evident by the flail segment (arrow) seen in the enlarged LA of an older mixed breed dog. C, A large jet of mitral regurgitation causes a wide area of flow disturbance in another mixed breed dog on color flow echo. Note the LA and LV enlargement. LA, Left atrium; LV, left ventricle; RA, right atrium.
Clinicopathologic Findings
Clinical laboratory data may be normal or reflect changes associated with CHF or concurrent extracardiac disease. Other diseases produce signs similar to those of CHF resulting from degenerative AV valve disease, including tracheal collapse, chronic bronchitis, bronchiectasis, pulmonary fibrosis, pulmonary neoplasia, pneumonia, pharyngitis, heartworm disease, dilated cardiomyopathy, and bacterial endocarditis.
Treatment and Prognosis
Medical therapy is used to control signs of CHF as well as support cardiac function and modulate the excessive neurohormonal activation that contributes to the disease process (Box 6-2). Drugs that decrease LV size (e.g., diuretics, vasodilators, positive inotropic agents) may reduce the regurgitant volume by decreasing mitral annulus size. Drugs that promote arteriolar vasodilation enhance forward cardiac output and reduce regurgitant volume by decreasing systemic arteriolar resistance. Frequent reevaluation and medication adjustment become necessary as the disease progresses. In many dogs with advanced MR, clinical compensation can be maintained for months to years using appropriate therapy. Although congestive signs develop gradually in some dogs, severe pulmonary edema or episodes of syncope appear acutely in others. Intermittent episodes of decompensation in dogs on long-term CHF therapy often can be successfully managed. Therapy must be guided by the patient’s clinical status and the nature of complicating factors. Surgical procedures such as mitral annuloplasty, other valve repair techniques, and mitral valve replacement may be treatment options in some patients but are not widely available.
 BOX 6-2 Treatment Guidelines for Chronic Atrioventricular Valve Disease
BOX 6-2 Treatment Guidelines for Chronic Atrioventricular Valve Disease
Mild to Moderate CHF signs (Modified AHA/ACC Stage C, Chronic)*
Severe, Acute CHF Signs (Modified AHA/ACC Stage C, Acute)*
Chronic Recurrent or Refractory Heart Failure Strategies (Modified AHA/ACC Stage D)*
* See Tables 3-2, 3-3, and Box 3-1 for further details and doses.
Asymptomatic Atrioventricular Valve Regurgitation
Dogs that have shown no clinical signs of disease are generally not given drug therapy. Convincing evidence that angiotensin-converting enzyme inhibitor (ACEI) or other therapy delays time to CHF onset in asymptomatic dogs is presently lacking. Whether dogs with marked cardiomegaly might benefit from therapy to modulate pathologic remodeling is unclear.
Client education about the disease process and early signs of CHF is important. It is probably prudent to discourage high-salt foods, pursue weight reduction for obese dogs, and avoid prolonged strenuous exercise. A diet moderately reduced in salt may be helpful. Periodic reevaluation (e.g., every 6 to 12 months) of cardiac size and function as well as blood pressure is advised. Other disease conditions are managed as appropriate.
Mild to Moderate Congestive Heart Failure
When clinical signs occur in association with exercise or activity, several treatment modalities are instituted (see Box 6-2 and Tables 3-3 and Box 3-1). The severity of clinical signs and the nature of any complicating factors influence the aggressiveness of therapy. When it is unclear whether respiratory signs are caused by early CHF or a noncardiac cause, a therapeutic trial of furosemide (e.g., 1 to 2 mg/kg by mouth q8-12h) is indicated. Cardiogenic pulmonary edema usually responds rapidly.
Furosemide is used for dogs with radiographic evidence of pulmonary edema and/or more severe clinical signs. Higher and more frequent doses are used when edema is severe. After signs of failure are controlled, the dose and frequency of furosemide administration are gradually reduced to the lowest effective levels for chronic therapy. Furosemide alone (e.g., without an ACEI or other agent) is not recommended for the long-term treatment of heart failure.
An ACEI is generally recommended for dogs with early signs of failure (see Chapter 3). The ability of these agents to modulate neurohormonal responses to heart failure is thought to be their main advantage. Chronic ACEI therapy can improve exercise tolerance, cough, and respiratory effort, although the issue of enhanced survival is unclear.
Pimobendan also is being used increasingly for the management of moderate to advanced CHF (see Chapter 3). This drug has positive inotropic, vasodilator, and other actions. Its beneficial effects may exceed those of ACEIs, although they are often used together. Digoxin, with or without pimobendan, is often added to the chronic therapy of CHF resulting from advanced AV valve insufficiency. Digoxin’s sensitizing effect on baroreceptors may be more advantageous than its modest positive inotropic effect (see Chapter 3). Marked LV dilation, evidence for reduced myocardial contractility, or recurrent episodes of pulmonary edema despite furosemide and other treatment are indications for adding digoxin. Digoxin also is indicated for heart rate control in dogs with atrial fibrillation and for its antiarrhythmic effect in some cases of frequent atrial premature beats or supraventricular tachycardia. Conservative doses and measurement of serum concentrations are recommended to prevent toxicity (see p. 66).
Moderate dietary salt restriction (e.g., diets formulated for dogs with kidney disease or for senior dogs) is recommended initially. Further salt restriction may be achieved with diets formulated for patients with heart failure. Exercise restriction is important when signs of CHF exist. Mild to moderate, regular activity (not causing undue respiratory effort) may be resumed during chronic, compensated dis-ease. Strenuous exercise is not recommended. Antitussive therapy can be helpful in dogs without pulmonary edema but with persistent cough caused by mechanical mainstem bronchus compression (e.g., hydrocodone bitartrate, 0.25 mg/kg by mouth q8-12h; or butorphanol, 0.5 mg/kg by mouth q6-12h).
Severe Congestive Heart Failure
Severe pulmonary edema and shortness of breath at rest require urgent treatment (see Box 3-1). Aggressive diuresis with parenteral furosemide (e.g., 2 to 4 mg/kg q1-4h IV, initially), supplemental oxygen, and cage rest are instituted as soon as possible. Gentle handling is important because added stress may precipitate cardiopulmonary arrest. Thoracic radiographs and other diagnostic procedures are postponed until the animal’s respiratory condition is more stable.
Vasodilator therapy is also indicated. If adequate monitoring facilities are available, intravenous (IV) nitroprusside may be used for rapid arteriolar and venous dilation; however, blood pressure must be closely monitored to prevent hypotension. Another approach for acute therapy is oral hydralazine. Its direct and rapid arteriolar vasodilating effect increases forward flow and decreases regurgitation; however, oral administration can be stressful. A reduced dose is used in animals already on an ACEI. Amlodipine is an alternative arteriolar vasodilator, but it has a much slower onset of action. Topical nitroglycerin also can be used in an attempt to reduce pulmonary venous pressure by direct venodilation.
When positive inotropic therapy is indicated, pimobendan (or digoxin) may be initiated (or continued if previously prescribed) once acute dyspnea subsides. Paroxysmal atrial tachycardia or atrial fibrillation may respond to digoxin. Although several days are needed to achieve a therapeutic blood concentration with oral maintenance doses, IV digitalization is generally not recommended. Diltiazem or a β-blocker (see Table 4-2) can be used instead of or in addition to digoxin if supraventricular tachyarrhythmias require treatment (see Chapter 4). Dogs that need more intense inotropic support or that have persistent hypotension can be given an IV agent (e.g., dobutamine, dopamine, amrinone; see Box 3-1).
Ancillary therapy often includes mild sedation to reduce anxiety (e.g., butorphanol or morphine). A bronchodilator (e.g., theophylline, aminophylline) may be useful if bronchospasm is induced by severe pulmonary edema; although efficacy for this is unclear, these agents may help support respiratory muscle function.
Thoracocentesis is indicated in dogs with moderate- to large-volume pleural effusion to improve pulmonary function. Ascites that impedes respiration also should be drained. Therapy for ventricular tachyarrhythmias is warranted in some cases. Close monitoring is important for titrating therapy and identifying drug toxicities or adverse effects (e.g., azotemia, electrolyte abnormalities, hypotension, arrhythmias).
After the animal’s condition is stabilized, medications are adjusted over several days to weeks to determine optimal long-term therapy. Furosemide is titrated to the lowest dose (and longest interval) that controls signs of CHF. Institution of an ACEI is recommended for ongoing therapy if hydralazine or nitroprusside was the initial vasodilator used. As the effects of previously administered hydralazine wane, the first dose of ACEI given should be half the usual dose (i.e., 0.25 mg/kg by mouth). An ACEI can be started at the standard dose shortly after discontinuing a nitroprusside infusion.
Chronic Management of Advanced Disease
When CHF becomes refractory, therapy is intensified or modified according to individual patient needs. The following suggestions for modifying therapy are listed in approximate order of use. Recurrent pulmonary edema in some dogs responds to an increased dose of furosemide and rest for a few days. The dose can then be returned to previous or a slightly higher level, if possible. The ACEI dose should be maximized if this has not already been done (e.g., enalapril from once to twice daily).
Pimobendan and/or digoxin can be added if it is not already being used. The dose of digoxin is not titrated upward unless subtherapeutic serum concentrations are documented (see Chapter 3). Spironolactone can be added, if not already being used (see Chapter 3). This aldosterone antagonist may reduce the severity of chronic refractory pulmonary edema or effusions as well as have beneficial effects on cardiac remodeling. Conversely, another diuretic with a different mechanism of action or the spironolactone/hydrochlorothiazide combination product may be useful.
Continued monitoring, especially of renal function and serum electrolyte concentrations, is important. Dietary sodium restriction can be intensified. If the ACEI and furosemide doses are already maximal, low-dose hydralazine (e.g., 0.25 to 0.5 mg/kg by mouth q12h) or amlodipine (e.g., 0.05 to 0.2 mg/kg by mouth q24h) can be added, although blood pressure should be monitored.
Intermittent tachyarrhythmias can promote decompensated CHF as well as episodes of transient weakness or syncope. Cough-induced syncope, atrial rupture, or other causes of reduced cardiac output may also occur. Despite the periodic recurrence of signs of CHF, many dogs with chronic AV valve regurgitation can enjoy a good quality of life for several years after the signs of failure first appear.
Patient Monitoring and Reevaluation
Client education regarding the disease process, the clinical signs of failure, and the drugs used to control them is essential for long-term therapy to be successful. As the disease progresses, medication readjustment (i.e., different dosages of currently used drugs and/or additional drugs) is expected. Several common potential complications of chronic degenerative AV valve disease can cause decompensation (see Box 6-1). At-home monitoring is important to detect early signs of decompensation. Respiratory (+/− heart) rate can be monitored periodically when the dog is quietly resting or sleeping (see p. 70; a persistent increase in either can signal early decompensation.
Asymptomatic dogs should be reevaluated at least yearly in the context of a routine preventive health program. The frequency of reevaluation in dogs receiving medication for heart failure depends on the disease severity and whether any complicating factors are present. Dogs with recently diagnosed or decompensated CHF should be evaluated more frequently (within several days to a week or so) until their condition is stable. Those with chronic heart failure that appears well-controlled can be reevaluated less frequently, usually several times per year. The medication supply, administration compliance, drugs and doses being given, and diet should be reviewed with the owner at each visit.
A general physical exam with particular attention to cardiovascular parameters is important at each visit. An ECG is indicated if an arrhythmia or unexpectedly low or high heart rate is found. When an arrhythmia is suspected but not documented on routine ECG, ambulatory electrocardiography (e.g., 24-hour Holter monitoring) can be helpful. The respiratory rate and pattern are also noted; thoracic radiographs are warranted if abnormal pulmonary sounds are heard or if the owner reports coughing, other respiratory signs, or an increased resting respiratory rate. Other causes of cough should be considered if neither pulmonary edema nor venous congestion is seen radiographically and if the resting respiratory rate has not increased. Left mainstem bronchus compression by an enlarged LA can stimulate a dry cough. Cough suppressants are helpful for this, but they should be prescribed only after other causes of cough are ruled out.
Echocardiography may show evidence of chordal rupture, progressive cardiomegaly, or worsened myocardial function. Frequent monitoring of serum electrolyte concentrations and renal function is important. Other routine blood and urine tests are done periodically also. Dogs receiving digoxin should have a serum concentration measured 7 to 10 days after treatment initiation or a dosage change. Additional measurements are recommended if signs consistent with toxicity appear or if renal disease or electrolyte imbalance (hypokalemia) is suspected.
The prognosis in dogs that have shown clinical signs of degenerative valve disease is quite variable. With appropriate therapy and attentive management of complications, some dogs live well for more than 4 years after the signs of heart failure first appear. Some dogs die during an initial episode of fulminant pulmonary edema. Survival for most symptomatic dogs ranges from several months to a few years.
INFECTIVE ENDOCARDITIS
Etiology and Pathophysiology
Endocarditis is more common in dogs than in cats. Bacteremia, either persistent or transient, is necessary for endocardial infection to occur. Recurrent bacteremia may occur with infections of the skin, mouth, urinary tract, prostate, lungs, or other organs. Dentistry procedures are known to cause a transient bacteremia. Other procedures are presumed to cause transient bacteremia in some cases (e.g., endoscopy, urethral catheterization, anal surgery, and other “dirty” procedures). The likelihood of a cardiac infection becoming established is increased when organisms are highly virulent or the bacterial load is heavy.
The endocardial surface of the valve is infected directly from the blood flowing past it. Previously normal valves may be invaded by virulent bacteria, causing acute bacterial endocarditis. Subacute bacterial endocarditis is thought to result from infection of previously damaged or diseased valves after a persistent bacteremia. Such damage may result from mechanical trauma (e.g., jet lesions resulting from turbulent blood flow or endocardial injury from a vascular catheter extending into the heart). Myxomatous degeneration of the mitral valve has not been associated with a higher risk for infective endocarditis.
The lesions of endocarditis are typically located downstream from the disturbed blood flow; common sites include the ventricular side of the aortic valve in patients with subaortic stenosis, the right ventricular side of a ventricular septal defect, and the atrial surface of a regurgitant mitral valve. Bacterial clumping caused by the action of an agglutinating antibody may facilitate attachment to the valves. Alternatively, chronic stress and mechanical trauma can predispose to the development of nonbacterial thrombotic endocarditis, a sterile accumulation of platelets and fibrin on the valve surface. Nonseptic emboli may break off from such vegetations and cause infarctions elsewhere. Bacteremia can also cause a secondary infective endocarditis at these sites.
The most common organisms identified in dogs and cats with endocarditis have been Streptococcus sp., Staphylococcus sp., and Escherichia coli. Additional organisms isolated from infected valves have included Corynebacterium (Arcanobacterium) sp., Pasteurella sp., Pseudomonas aeruginosa, Erysipelothrix rhusiopathiae (E. tonsillaris), and others. Bartonella vinsonii subsp. berkhoffii and other Bartonella sp. have also been found in dogs with endocarditis. Culture-negative endocarditis may be caused by fastidious organisms or by Bartonella spp.; in a recent study of 71 dogs with infective endocarditis, Bartonella spp. was identified as the causative agent in 45% of the patients with a negative blood culture and in 20% of the overall population.
The mitral and aortic valves are most commonly affected in dogs and cats. Microbial colonization leads to ulceration of the valve endothelium. Subendothelial collagen exposure in turn stimulates platelet aggregation and activation of the coagulation cascade, leading to the formation of vegetations. Vegetations consist mainly of aggregated platelets, fibrin, blood cells, and bacteria. Newer vegetations are friable. With time, the lesions become fibrous and may calcify. As additional fibrin is deposited over bacterial colonies, they become protected from normal host defenses as well as many antibiotics. Although vegetations usually involve the valve leaflets, lesions may extend to the chordae tendineae, sinuses of Valsalva, mural endocardium, or adjacent myocardium. Vegetations cause valve deformity, including perforations or tearing of the leaflet(s), and result in valve insufficiency. Rarely, large vegetations may cause the valve to become stenotic.
Valve insufficiency and subsequent volume overload commonly lead to CHF. Because the mitral and/or aortic valve is usually affected, left-sided CHF signs of pulmonary congestion and edema are usual. Clinical heart failure develops rapidly in patients with severe valve destruction, rupture of chordae tendineae, and multiple valve involvement, or when other predisposing factors are present. Cardiac function can be compromised by myocardial injury resulting from coronary arterial embolization with myocardial infarction and abscess formation or from direct extension of the infection into the myocardium. Reduced contractility and atrial or ventricular tachyarrhythmias often result. Aortic valve endocarditis lesions may extend into the AV node and cause partial or complete AV block. Arrhythmias may cause weakness, syncope, and sudden death or contribute to the development of CHF.
Fragments of vegetative lesions often break loose. Embolization of other body sites causes infarction or metastatic infection, which results in diverse clinical signs. Larger and more mobile vegetations (based on echocardiographic appearance) are associated with higher incidence of embolic events in people; the same may occur in animals. Emboli can be septic or bland (containing no infectious organisms). Septic arthritis, diskospondylitis, urinary tract infections, and renal and splenic infarctions are common in affected animals. Local abscess formation resulting from septic thromboemboli contributes to recurrent bacteremia and fever. Hypertrophic osteopathy has also been associated with bacterial endocarditis. Circulating immune complexes as well as cell-mediated responses contribute to the disease syndrome. Sterile polyarthritis, glomerulonephritis, vasculitis, and other forms of immune-mediated organ damage are common. Rheumatoid factor and antinuclear antibody test (ANA) results may be positive.
Clinical Features
The prevalence of bacterial endocarditis is relatively low in dogs and even lower in cats. Male dogs are affected more commonly than females. An increased prevalence of endocarditis has been noted in association with age. German Shepherd Dogs and other large-breed dogs may be at greater risk. Subaortic stenosis is a known risk factor for aortic valve endocarditis. Immunocompromised animals may also be at greater risk for endocarditis, but this has not been substantiated.
The clinical signs of endocarditis are quite variable. Many affected animals have evidence of past or concurrent infections, although often a clear history of predisposing factors is absent. The presenting signs can result from left-sided CHF or arrhythmias, but cardiac signs may be overshadowed by signs of systemic infarction, infection, immune-mediated damage, or a combination of these. Nonspecific signs of lethargy, weight loss, inappetence, recurrent fever, and weakness may be the predominant abnormalities. Infective endocarditis often mimics immune-mediated disease. Dogs with endocarditis are commonly evaluated for a “fever of unknown origin.” Some of the consequences of infectious endocarditis are outlined in Box 6-3. Endocarditis has been nicknamed “the great imitator”; maintaining an index of suspicion for this disease is important.
 BOX 6-3 Potential Sequelae of Infective Endocarditis
BOX 6-3 Potential Sequelae of Infective Endocarditis
* Diseased valve most commonly associated with abnormality.
Infective valve damage may be signaled by signs of CHF in an unexpected clinical setting or in an animal with a murmur of recent onset, especially if other suggestive signs are present. But a “new” murmur can indicate noninfective acquired disease (e.g., degenerative valve disease, cardiomyopathy), previously undiagnosed congenital disease, or physiologic alterations (e.g., fever, anemia). Conversely, endocarditis may develop in an animal known to have a murmur resulting from another cardiac disease. Although a change in murmur quality or intensity over a short time frame may indicate active valve damage, physiologic causes of murmur variation are common. The onset of a diastolic murmur at the left heartbase is suspicious for aortic valve endocarditis, especially if fever or other signs are present.
Diagnosis
It may be difficult to obtain a definitive antemortem diagnosis. Presumptive diagnosis of infective endocarditis is made on the basis of positive findings in two or more blood cultures, in addition to either echocardiographic evidence of vegetations or valve destruction or the documented recent appearance of a regurgitant murmur. Endocarditis is likely even when blood culture results are negative or intermittently positive if there is echocardiographic evidence of vegetations or valve destruction along with a combination of other criteria (Box 6-4). A new diastolic murmur, hyperkinetic pulses, and fever are strongly suggestive of aortic valve endocarditis.
 BOX 6-4 Criteria for Diagnosis of Infectious Endocarditis*
BOX 6-4 Criteria for Diagnosis of Infectious Endocarditis*
Major Criteria
* Adapted from Duke criteria for endocarditis. In Durack DT et al: New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings, Am J Med 96:200, 1994.
Several samples of at least 10 ml of blood should be aseptically collected over a 24-hour period for bacterial blood culture, with more than 1 hour elapsing between collections. Ideally, different venipuncture sites should be used for each sample. Larger sample volumes (e.g., 20 to 30 ml) increase culture sensitivity. Both aerobic and anaerobic cultures have been recommended, although the value of routine anaerobic culture is questionable. Prolonged incubation (3 weeks) is recommended because some bacteria are slow-growing. Although blood culture results are positive in many dogs with this disease, negative results do not necessarily rule out infective endocarditis; in a recent study, less than 50% of the blood cultures in dogs with confirmed infective endocarditis were positive. As discussed above, Bartonella spp. is an emerging pathogen that causes blood culture-negative endocarditis in dogs; in the same study, 45% of the dogs with negative blood cultures were positive for Bartonella spp. on polymerase chain reaction (PCR). Results may be negative in the setting of chronic endocarditis, recent antibiotic therapy, intermittent bacteremia, and infection with fastidious or slow-growing organisms, as well as noninfective endocarditis. Serologic and PCR testing are also commercially available for Bartonella spp.
Echocardiography is especially supportive if oscillating vegetative lesions and abnormal valve motion can be identified (Fig. 6-4). The visualization of lesions depends on their size and location, on the image resolution, and the proficiency of the echocardiographer. Because falsenegative and false-positive findings of “lesions” may occur, cautious interpretation of images is important. Mild valve thickening and/or enhanced echogenicity may occur in patients with early valve damage. Vegetative lesions appear as irregular dense masses. As valve destruction progresses, ruptured chordae, flail leaflet tips, or other abnormal valve motion can be seen. Differentiation of mitral vegetations from degenerative thickening may be impossible, however, especially in the early stages. Nevertheless, vegetative endocarditis classically causes rough, ragged-looking valve thickening; degenerative disease is associated with smooth valvular thickening. Poor or marginal-quality images or the use of lower-frequency transducers can prevent identification of some vegetations because of suboptimal resolution. Secondary effects of valve dysfunction include chamber enlargement from volume overload and flail or otherwise abnormal valve leaflet motion. Myocardial dysfunction and arrhythmias may also be evident. Aortic insufficiency can cause fluttering of the anterior mitral valve leaflet during diastole as the regurgitant jet makes contact with this leaflet. Doppler studies illustrate flow disturbances (Fig. 6-5).
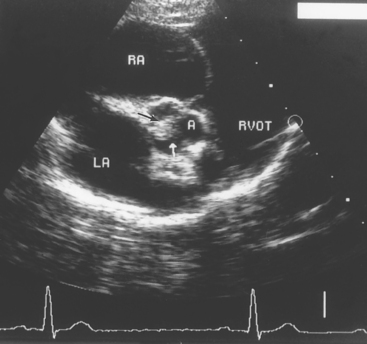
FIG 6-4 Right parasternal short-axis echocardiogram at the aortic-left atrial level in a 2-year-old male Vizsla with congenital subaortic stenosis and pulmonic stenosis. Note the aortic valve vegetation (arrows) caused by endocarditis. A, Aorta; LA, left atrium; RA, right atrium; RVOT, right ventricular outflow tract.
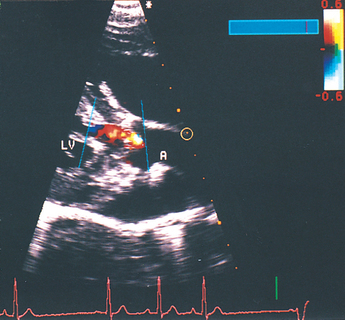
FIG 6-5 Right parasternal long axis, color flow Doppler image taken during diastole from the same dog as in Fig 6-4. The “flamelike” jet of aortic regurgitation extends from the closed aortic valve into the left ventricular outflow tract. A, Aorta; LV, left ventricle.
The ECG may be normal or document premature beats, tachycardias, conduction disturbances, or evidence of myo cardial ischemia. Radiographic findings are unremarkable in some cases; however, in others, evidence of left-sided CHF or other organ involvement (e.g., diskospondylitis) is seen. Cardiomegaly is minimal early in the disease but progresses over time as a result of valve insufficiency.
Clinicopathologic findings usually reflect an inflammatory process. Neutrophilia with a left shift is typical of acute endocarditis, whereas mature neutrophilia with or without monocytosis usually develops with chronic disease. Nonregenerative anemia has been associated with about half of canine cases. Biochemical abnormalities are variable. Azotemia, hyperglobulinemia, hematuria, pyuria, and proteinuria are common. The ANA results may be positive in dogs with subacute or chronic bacterial endocarditis; in a recent study, 75% of dogs with Bartonella vinsonii infection had positive ANA test results.
Treatment and Prognosis
Aggressive therapy with bactericidal antibiotics capable of penetrating fibrin, as well as supportive care, are indicated for infective endocarditis. Ideally, drug choice is guided by culture and in-vitro susceptibility test results. Because treatment delay while waiting for these results can be harmful, broad-spectrum combination therapy is usually begun immediately after blood culture samples are obtained. Therapy can be altered, if necessary, when culture results are available. Culture-negative cases should be continued on the broad-spectrum regimen. An initial combination of a cephalosporin, penicillin, or a synthetic penicillin derivative (e.g., ampicillin) with an aminoglycoside (gentamicin or amikacin) or a fluoroquinolone (e.g. enrofloxacin) is commonly used. This is likely to be effective against the organisms most often associated with infective endocarditis. Clindamycin or metronidazole provides added anaerobic efficacy. Azithromycin or possibly enrofloxacin or high-dose doxycycline has been suggested for Bartonella spp.
Antibiotics are administered intravenously (or at least intramuscularly) for the first week or longer to obtain higher and more predictable blood concentrations. Oral therapy is often used thereafter for practical reasons, although parenteral administration is probably better. Antimicrobial therapy is continued for at least 6 weeks; 8 weeks of therapy is often recommended. However, aminoglycosides are discontinued after 1 week or sooner if renal toxicity develops. Close monitoring of the urine sediment is indicated to detect early aminoglycoside nephrotoxicity. For documented or suspected B. vinsonii (berkhoffii) infection, repeat serologic or PCR testing is recommended between 3 and 6 months after antibiotic therapy.
Supportive care includes management for CHF (see Chapter 3) and arrhythmias (see Chapter 4) if present. Complications related to the primary source of infection, embolic events, or immune responses are addressed to the extent possible. Attention to hydration status, nutritional support, and general nursing care is also important. Corticosteroids are contraindicated. The efficacy of aspirin to inhibit platelet aggregation and vegetative lesion growth and reduce the risk of embolic events is unknown. Aspirin or oral anticoagulants appear to be of no benefit for this in people.
Long-term prognosis is generally guarded to poor. Echocardiographic evidence of vegetations and volume overload suggests a poor prognosis. Aggressive therapy may be successful if valve dysfunction is not severe and large vegetations are absent. CHF is the most common cause of death, although sepsis, systemic embolization, arrhythmias, or renal failure may be the proximate cause.
The use of prophylactic antibiotics is controversial. Experience in people indicates that most cases of infective endocarditis are not preventable. The risk of endocarditis from a specific (e.g., dental) procedure in humans is very low compared with the cumulative risk associated with normal daily activities. However, because endocarditis appears to have an increased incidence in patients with certain cardiovascular malformations, antimicrobial prophylaxis is recommended before dental or other “dirty” procedures (e.g., involving the oral cavity or intestinal or urogenital systems) in these cases. Subaortic stenosis is a well-recognized predisposing lesion; endocarditis has also been associated with ventricular septal defect, patent ductus arteriosus, and cyanotic congenital heart disease. Antimicrobial prophylaxis is recommended for animals with an implanted pacemaker or other device or with a history of endocarditis. Prophylaxis should be considered for immunocompromised animals as well. Recommendations (extrapolated from human medicine) include the administration of high-dose ampicillin or amoxicillin 1 hour before and 6 hours after an oral or upper respiratory procedure and ampicillin with an aminoglycoside (IV, 30 minutes before and 8 hours after) a gastrointestinal or urogenital procedure. Alternatively, ticarcillin or a first-generation cephalosporin intravenously 1 hour before and 6 hours after the procedure has been used.
Beardow AW, Buchanan JW. Chronic mitral valve disease in Cavalier King Charles Spaniels: 95 cases (1987–1991). J Am Vet Med Assoc. 1993;203:1023.
Borgarelli M, et al. Comparison of primary mitral valve disease in German Shepherd Dogs and in small breeds. J Vet Cardiol. 2004;6:27.
Buchanan JW. Prevalence of cardiovascular disorders. In: Fox PR, Sisson D, Moise NS, editors. Textbook of canine and feline cardiology. ed 2. Philadelphia: WB Saunders; 1999:457-470.
Buchanan JW, Sammarco CD. Circumferential suture of the mitral annulus for correction of mitral regurgitation in dogs. Vet Surg. 1998;27:182.
Corcoran BM, et al. Identification of surface morphologic changes in the mitral valve leaflets and chordae tendineae of dogs with myxomatous degeneration. Am J Vet Res. 2004;65:198.
Haggstrom J, Kvart C, Hansson K. Heart sounds and murmurs: changes related to severity of chronic valvular disease in the Cavalier King Charles spaniel. J Vet Intern Med. 1995;9:75.
Haggstrom J, et al. Effects of naturally acquired decompensated mitral valve regurgitation on the renin-angiotensin-aldosterone system and atrial natriuretic peptide concentration in dogs. Am J Vet Res. 1997;58:77.
Kitagawa H, et al. Efficacy of monotherapy with benazepril, an angiotensin converting enzyme inhibitor, in dogs with naturally acquired chronic mitral insufficiency. J Vet Med Sci. 1997;59:513.
Kittleson MD, Brown WA. Regurgitant fraction measured by using the proximal isovelocity surface area method in dogs with chronic myxomatous mitral valve disease. J Vet Intern Med. 2003;17:84.
Kvart C, et al. Efficacy of enalapril for prevention of congestive heart failure in dogs with myxomatous valve disease and asymptomatic mitral regurgitation. J Vet Intern Med. 2002;16:80.
Lombard CW, Jöns O, Bussadori CM. Clinical efficacy of pimobendan versus benazepril for the treatment of acquired atrioventricular valvular disease in dogs. J Am Anim Hosp Assoc. 2006;42:249.
Mow T, Pedersen HD. Increased endothelin-receptor density in myxomatous canine mitral leaflets. J Cardiovac Pharmacol. 1999;34:254.
Muzzi RAL, deAraujo RB, Muzzi LAL, et al. Regurgitant jet area by Doppler color flow mapping: quantitative assessment of mitral regurgitation severity in dogs. J Vet Cardiol. 2003;5:33.
Olsen LH, Fredholm M, Pedersen HD. Epidemiology and inheritance of mitral valve prolapse in Dachshunds. J Vet Intern Med. 1999;13:448.
Orton EC, et al. Technique and outcome of mitral valve replacement in dogs. J Am Vet Med Assoc. 2005;226:1508.
Pedersen HD, et al. Auscultation in mild mitral regurgitation in dogs: observer variation, effects of physical maneuvers, and agreement with color Doppler echocardiography and phonocardiography. J Vet Intern Med. 1999;13:56.
Pedersen HD, Lorentzen KA, Kristensen BO. Echocardiographic mitral prolapse in Cavalier King Charles spaniels: epidemiology and prognostic significance for regurgitation. Vet Rec. 1999;144:315.
Serres F, Chetboul V, Tissier R, et al. Chordae tendineae rupture in dogs with degenerative mitral valve disease: prevalence, survival, and prognostic factors (114 cases, 2001-2006). J Vet Intern Med. 2007;21:258-264.
Smith PJ, et al. Efficacy and safety of pimobendan in canine heart failure caused by myxomatous mitral valve disease. J Small Anim Pract. 2005;46:121.
Straeter-Knowlen IM, et al. ACE inhibitors in heart failure restore canine pulmonary endothelial function and ANG II vasoconstriction. Am J Physiol. 1999;277:H1924.
Breitschwerdt EB. Bartonella species as emerging vector-transmitted pathogens. Comp Cont Educ Pract Vet. 2003;25(Suppl):12.
Chan KL, et al. A randominzed trial of aspirin on the risk of embolic events in patients with infective endocarditis. J Am Coll Cardiol. 2003;42:775.
DiSalvo G, et al. Echocardiography predicts embolic events in infective endocarditis. J Am Coll Cardiol. 2001;37:1069.
Durack DT, et al. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200.
Elwood CM, Cobb MA, Stepien RL. Clinical and echocardiographic findings in 10 dogs with vegetative bacterial endocarditis. J Small Anim Pract. 1993;34:420.
MacDonald KA, et al. A prospective study of canine infective endocarditis in Northern California (1999-2001): emergence of Bartonella as a prevalent etiologic agent. J Vet Intern Med. 2004;18:56.
Miller MW, Fox PR, Saunders AB. Pathologic and clinical features of infectious endocarditis. J Vet Cardiol. 2004;6:35.
Peddle G, Sleeper MM. Canine bacterial endocarditis: a review. J Am Anim Hosp Assoc. 2007;43:258-263.
Smith BE, Tompkins MB, Breitschwerdt EB. Antinuclear antibodies can be detected in dog sera reactive to Bartonella vinsonii subsp. berkhoffii, Ehrlichia canis, or Leishmania infantum antigens. J Vet Intern Med. 2004;18:47.
Sykes JE, et al. Evaluation of the relationship between causative organisms and clinical characteristics of infective endocarditis in dogs: 71 cases (1992-2005). J Am Vet Med Assoc. 2006;228:1723.
Tou SP, Adin DB, Castleman WL. Mitral valve endocarditis after dental prophylaxis in a dog. J Vet Intern Med. 2005;19:268.
Wall M, Calvert CA, Greene CE. Infective endocarditis in dogs. Compend Contin Educ Pract Vet. 2002;24:614.