CHAPTER 45 Urinary Tract Infections
URINARY TRACT INFECTIONS
Bacterial infections of the urinary tract occur more frequently in dogs than in cats. Although inflammatory disease of the lower urinary tract is common in cats, bacterial infections are rare. Fewer than 2% of the cases of lower urinary tract disease (LUTD) in cats are caused by a primary urinary tract infection (UTI). Most of the UTIs in dogs involve bacterial inflammation of the lower urinary tract (bladder, urethra); however, the ascension of bacteria into the ureters and kidneys is a potential sequela of lower UTIs. Compared with the prevalence of bacterial UTIs, mycoplasmal, chlamydial, viral, and fungal UTIs are rare in dogs. Most bacterial infections of the lower urinary tract respond quickly to appropriate antibiotic treatment; however, UTIs associated with defects in the host immune system (complicated UTIs) often fail to respond to antibiotic therapy, or the infection relapses shortly after antibiotic withdrawal.
Etiology and Pathogenesis
The most common bacterial pathogens associated with UTIs in the dog include Escherichia coli, Staphylococcus, Streptococcus, Enterococcus, Enterobacter, Proteus, Klebsiella, and Pseudomonas organisms. E. coli is the most common isolate from canine and feline urine (Table 45-1). Although UTIs usually involve a single organism, as many as 20% to 30% may be mixed bacterial infections (i.e., two or more species). Most bacterial UTIs are thought to be caused by intestinal or cutaneous flora that ascend through the urethra to the bladder. Although many enteric organisms are anaerobes, the oxygen tension in urine probably inhibits the growth of strict anaerobic bacteria; therefore anaerobes rarely cause UTIs.
 TABLE 45-1 Approximate Percentages of Bacterial Isolates in Dogs with Urinary Tract Infections
TABLE 45-1 Approximate Percentages of Bacterial Isolates in Dogs with Urinary Tract Infections
| ISOLATES | PERCENTAGE OF TOTAL |
|---|---|
| E. coli | 45 |
| Staphylococcus spp. | 13 |
| Proteus spp. | 10 |
| Enterococcus | 8 |
| Klebsiella spp. | 7 |
| Streptococcus spp. | 6 |
| Enterobacter spp. | 3 |
| Pseudomonas spp. | 3 |
| Other organisms | 5 |
Bacterial virulence of invading organisms is a major factor that determines whether a UTI becomes established (Box 45-1). The ability of bacteria to adhere to the epithelial surface of the urinary tract prevents bacterial washout during voiding and allows bacteria to proliferate between urine voidings. Infection of the urinary tract usually involves bacterial colonization of the genitalia, migration of the bacteria along the urethra, and adherence of the organisms to the uroepithelium. Uroepithelial adherence is facilitated by fimbriae, which are rigid, filamentous, proteinaceous appendages found on many gram-negative bacteria. Other factors that increase bacterial virulence include capsular K antigens, which interfere with opsonization and phagocytosis, and O antigens in endotoxin, which decrease smooth muscle contractility. The latter may stop ureteral peristalsis and facilitate the ascension of bacteria from the bladder to the kidney. E. coli isolates from dogs have a greater ability to produce colicins (resulting in increased vascular permeability), hemolysins (increasing their invasiveness through tissue damage), and β-lactamase (causing resistance to β-lactam antibiotics) and to ferment dulcitol (which is associated with resistance to phagocytosis), but they have a decreased ability to agglutinate red blood cells (RBCs; associated with uroepithelial adherence) compared with human E. coli isolates. Finally, cell wall–deficient bacterial variants may thrive in hypertonic environments such as the renal medulla and urine, where white blood cell (WBC) migration and phagocytosis may be compromised.
Bacterial resistance to antimicrobial drugs may result from inherent resistance, from mutation and selection, or from the transfer of resistance factors (R factors) between organisms through DNA transfer. An entire bacterial population can acquire resistance by genetic transfer after only one dose of an antibiotic. The R factor phenomenon has been identified in gram-negative bacteria, including E. coli, Enterobacter, Klebsiella, and Proteus. R factor resistance to multiple drugs is common, and R factors are known to confer resistance to penicillins, cephalosporins, aminoglycosides, tetracyclines, chloramphenicol, sulfonamides, and trimethoprim.
Mycoplasmal organisms have also been associated with UTIs in dogs, but this type of infection is uncommon. Clinical signs of mycoplasmal cystitis may include hematuria, pollakiuria, stranguria, incontinence, polydipsia-polyuria, and fever; however, some dogs with positive urine culture results are asymptomatic. Whether mycoplasmas are primary urinary tract pathogens remains unclear.
HOST DEFENSE MECHANISMS
The status of the host defense mechanisms appears to be the most important factor influencing the pathogenesis of UTI (Table 45-2). Normal voiding is an efficient natural defense mechanism against UTI. The mechanical washout that occurs as a result of complete voiding is responsible for removing more than 95% of nonadherent bacteria that gain entrance into the urinary bladder. Washout is enhanced by increased urine production and frequency of voiding. Disorders that decrease the frequency of voiding or the volume of voided urine or that result in an increased urine residual volume may predispose animals to the development of UTIs. The normal urine residual volume for dogs and cats is less than 0.2 to 0.4 ml/kg.
 TABLE 45-2 Host Defense Mechanisms and Abnormalities that May Lead to Complicated Urinary Tract Infections
TABLE 45-2 Host Defense Mechanisms and Abnormalities that May Lead to Complicated Urinary Tract Infections
| HOST DEFENSES | ABNORMALITIES |
|---|---|
| Normal Micturition | |
| Normal urine volume | Urinary incontinence |
| Normal voiding frequency | Urine outflow tract obstruction |
| Incomplete bladder emptying | |
| Small residual urine volume | |
| Anatomic Structures | |
| Urethral high-pressure zone | |
| Urethral contraction and peristalsis | |
| Urethral length | Vesicoureteral reflux |
| Vesicoureteral valvelike junction | |
| Ureteral contractions and peristalsis | |
| Mucosal Defense Barriers | |
| Antimicrobial Properties of Urine | |
| Hyperosmolality | Decreased urine concentration |
| High urea concentration | Glucosuria |
| Acidic pH | |
| Systemic Immunocompetence | |
| Cell-mediated immunity? | Immunosuppressive drug therapy |
| Humoral immunity | |
Bacteria are normally present in increasing numbers from the midurethra to the distal urethra, but these organisms seldom cause UTIs in normal dogs. The high-pressure zone in the midurethra and the spontaneous urethral contractions help prevent the ascension of bacteria. Differences in epithelial morphology (decreased epithelial receptor sites) also help decrease the number of bacteria that can colonize the proximal and middle sections of the urethra. The length of the urethra and zinc-containing bacteriostatic/bactericidal prostatic secretions contribute to a lower incidence of UTIs in male dogs than in female dogs. In both genders the valvelike nature of the vesicoureteral junction confers protection against the ascension of bacteria to the kidneys.
The colonization of vulval and preputial luminal mucous membranes by nonpathogenic flora also serves to decrease colonization by uropathogens. Normal flora occupy most of the epithelial receptor sites, produce bacteriocins that interfere with uropathogen metabolism, and have a high affinity but low requirement for the essential nutrients needed by uropathogens. In addition, mucosal secretions help prevent the adherence of uropathogens to the epithelium; specifically, secretory immunoglobulins do so by coating pathogenic bacteria, and glycosaminoglycans by forming a protective barrier over the epithelial surface.
The antibacterial properties of urine constitute an important host defense mechanism against UTIs. Urine is frequently bacteriostatic and sometimes can be bactericidal, depending on its composition. The combination of a low pH and high concentrations of urea and weak organic acids in concentrated urine inhibits bacterial growth. The increased urine-concentrating ability of cats compared with dogs is thought to be one of the reasons that normal cats have so few bacterial UTIs. Dilute urine formed in animals with polydipsic-polyuric disorders has less antibacterial activity than hypersthenuric urine does. For example, the prevalence of bacterial UTI is higher in both dogs and cats with chronic kidney disease (CKD). Animals with CKD also often have decreased concentrations of antibiotic in their urine during treatment associated with decreased renal excretion of the drug.
COMPLICATED VERSUS UNCOMPLICATED URINARY TRACT INFECTIONS
Uncomplicated UTIs occur in the absence of underlying structural or functional abnormalities in the host defense mechanisms. They are easier to treat than complicated UTIs and are usually cleared soon after appropriate antibiotic treatment is initiated. Complicated UTIs are associated with defects in the host defense mechanisms (i.e., interference with normal micturition, anatomic defects, damage to mucosal barriers, alterations in urine volume or composition, or systemic immunocompromise). It is usually not possible to eliminate the clinical and clinicopathologic signs of complicated UTIs with antibiotic treatment alone; signs either persist during antibiotic treatment or recur shortly after antibiotic withdrawal. Because of the relatively low prevalence of UTIs in male dogs compared with female dogs, any UTI in a male dog should be considered a complicated infection.
Disorders of micturition are often complicated by UTI. Urine retention or incomplete voiding allows more time for bacteria to multiply within the urinary tract. Urine retention may also cause bladder wall distention that can compress intramural vessels and thereby decrease the number of WBCs and other antimicrobial factors that enter the bladder lumen. Conversely, urinary incontinence associated with decreased urethral sphincter tone may predispose the patient to an ascending UTI. Damage to mucosal barriers (e.g., transitional cell carcinoma [TCC]) may also result in the development of a complicated UTI depending on the extent of the lesion and whether uropathogens are concurrently introduced. Interestingly, bacterial inoculation of the urinary bladder in experimental animals usually fails to establish a UTI that lasts beyond 2 to 3 days, unless the uroepithelium is first damaged by a chemical or mechanical insult.
Whenever the urinary bladder is catheterized, bacteria are carried up the urethra to the bladder. If the catheter is inserted too far and damages the bladder mucosa, the chance of infection increases greatly. Anatomic defects may also allow the ascending migration of bacteria (e.g., indwelling urinary catheter, ectopic ureter) or may damage mucosal barriers (e.g., urolithiasis, neoplasia, urachal remnant, thickened bladder wall caused by chronic inflammation). In one study of 137 dogs cared for in an intensive care unit, indwelling urethral catheters were associated with UTI in 26 cases (19%); another similar study of 39 dogs demonstrated a UTI rate of 10%. Decreased urine volume may also be associated with a heightened risk for UTI because of decreased washout (although concentrated urine has greater antibacterial properties), and altered urine composition (glucosuria or the excretion of irritating substances such as cyclophosphamide metabolites that result in hematuria) can make the environment more receptive to bacterial growth. In addition to these local factors, systemic disorders, such as renal failure, hyperadrenocorticism, prolonged corticosteroid administration, neoplasia, and diabetes mellitus, can result in a complicated UTI. Potential mechanisms suggested to increase the risk of UTI in dogs with hyperadrenocorticism and/or diabetes mellitus include enhanced bacterial growth in urine caused by glucosuria or decreased urine concentration, decreased neutrophil chemotaxis associated with glucosuria, and decreased inflammatory response and/or urine retention (detrusor muscle weakness) associated with hypercortisolemia. UTI is also common in dogs with thoracolumbar (T-L) disk disease. In a recent study of 92 dogs that underwent surgery for T-L disk disease, 25 (27%) had UTI. Risk factors for UTI in this study included female gender, the inability to ambulate or voluntarily void, lack of perioperative cefazolin administration, and decreased body temperature (<35° C) during the anesthetic period.
RELAPSES VERSUS REINFECTIONS
Recurrences of clinical and clinicopathologic signs of UTI can be classified into two categories: relapses and reinfec tions. Relapses are infections caused by the same species of bacteria; the clinical signs recur relatively shortly after antibiotic withdrawal. In these cases the previous antibacterial treatment has failed to eliminate the organism. Relapses may result from the use of an improper antibiotic or dosage, the emergence of drug-resistant pathogens, or failure to eliminate factors that alter normal host defense mechanisms and allow the bacteria to persist (e.g., bacteria inside a urolith). Relapsing UTIs are frequently associated with a greater antimicrobial resistance than that observed in the original infection. Relapses in male dogs may result from chronic prostatic infections. Because of the blood-prostate barrier, antibiotics must be lipid soluble and have an alkaline or neutral pKa (e.g., fluoroquinolones, trimethoprim-sulfa, chloramphenicol, carbenicillin) in order to gain access to the prostate.
Recurrent UTIs may also result from reinfection. In this case the previous antibacterial treatment cleared the first infection, but the urinary tract subsequently became infected with another bacterium. In most cases the interval between reinfections is longer than the interval between relapses (>2-4 weeks). The occurrence of reinfections often indicates that the factors that alter normal host defense mechanisms have not been eliminated. Alternatively, reinfections may be iatrogenic and occur as a result of follow-up catheterization. Reinfections with less invasive bacteria (Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter cloacae) generally suggest that the host’s immune system is compromised. Similarly, Corynebacterium urealyticum UTI in dogs and cats has been associated with preexisting urinary tract disorders (e.g., incontinence and urine retention).
Clinical Features
Inflammation of the lower urinary tract often results in pollakiuria, stranguria or dysuria, and gross or microscopic hematuria. Urinalysis findings compatible with a lower UTI include bacteriuria, hematuria, pyuria, and increased numbers of transitional epithelial cells in the urine sediment. In addition, an increased urine protein concentration and alkaline urine may be observed. However, bacteria as well as other urine sediment abnormalities are not always observed during urine sediment examination in animals with a bacterial UTI, especially if the urine is hyposthenuric or isosthenuric. Ideally, urine bacterial cultures should be performed to confirm the presence and type of bacteria. Research has shown that the testing of canine urine with commercially available dipstick leukocyte esterase assays is not reliable, and the false-negative rate can exceed 10% in the absence of a urine sediment examination. Some urine dipsticks also have a nitrate pad to detect nitrate-reducing bacteria, but this test has also been shown to be inaccurate in dogs and cats.
Cystocentesis constitutes the best way to collect urine for urinalysis and bacterial culture because it prevents urine from being contaminated by bacteria inhabiting the distal urethra, prepuce, or vulva. If urine collected by catheterization, voiding, or bladder expression is cultured, it is important to quantify the number of organisms per milliliter to differentiate a true infection from contamination (see Table 41-1). Bacterial antibiotic sensitivity testing should be performed to guide the selection of antibiotic treatment and, in cases of recurrent UTI, help differentiate relapses from reinfections. It may be difficult to differentiate a lower UTI from upper urinary tract involvement (as well as prostatitis), but this should be attempted to prevent renal damage in dogs and cats with pyelonephritis, which requires long-term antibiotic treatment and close monitoring (Box 45-2). Animals with acute bacterial pyelonephritis or prostatitis may manifest nonspecific systemic signs of lethargy, depression, anorexia, fever, and leukocytosis, which rarely occur in the setting lower UTIs. However, these systemic signs are frequently absent in animals with chronic pyelonephritis or prostatitis. Bilateral pyelonephritis may result in renal failure and subsequent azotemia and the loss of urineconcentrating ability. Cylindruria, especially WBC cellular casts, indicates the presence of renal disease and, if coupled with a significant bacteriuria, is highly suggestive of bacterial pyelonephritis. Several tests have been developed to differentiate upper and lower UTIs in people (see Box 45-2); however, these tests are difficult to perform and have not always proved reliable in veterinary medicine.
 BOX 45-2 Clinicopathologic Findings that Can Be Associated with Bacterial Pyelonephritis in Dogs and Cats
BOX 45-2 Clinicopathologic Findings that Can Be Associated with Bacterial Pyelonephritis in Dogs and Cats
Treatment
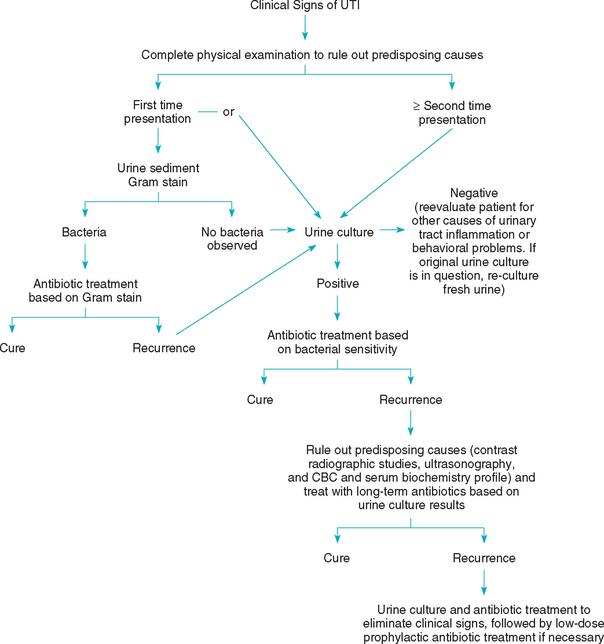
It is important to try to identify those animals with potentially treatable immune system defects or disorders (e.g., diabetes mellitus, hyperadrenocorticism, chronic renal failure, urolithiasis, urachal remnants, excessive perivulvar skin folds or pyoderma, incontinence) that predispose to the development of UTIs. Therefore a complete physical examination should be performed in all animals with signs of a UTI. Similarly, urinalysis and culture should be performed in all dogs and cats with suspected immune system defects. Although antibiotic treatment is the cornerstone of management, the status of host defense mechanisms is thought to be the single most important determinant of the outcome of treatment for a UTI. Antibiotic treatment should control the pathogenic bacterial growth for enough time to allow host defense mechanisms to prevent colonization of the urinary tract without the need for further antibiotic administration. Although it is advisable to evaluate the bacterial sensitivity to antimicrobial drugs, the treatment of acute, uncomplicated UTIs is often dictated by economic and time considerations. If bacterial sensitivity results are not available, the antibiotic should be chosen on the basis of bacterial identification or the Gram’s staining characteristics of the bacteria (Fig. 45-1). Clinical experience at several veterinary teaching hospitals has shown that intelligent guesses can be made regarding bacterial susceptibility to antibiotics. In the absence of bacterial sensitivity testing, the following are the drugs of choice for the treatment of infection with the bacteria listed: E. coli, trimethoprim-sulfa or enrofloxacin; Proteus, amoxicillin; Staphylococcus, amoxicillin; Streptococcus spp., amoxicillin; Enterobacter spp., trimethoprim-sulfa or enrofloxacin; Klebsiella spp., first-generation cephalosporins or enrofloxacin; and Pseudomonas spp., tetracycline (Table 45-3). It should be noted, however, that it is often difficult to predict the sensitivity of gram-negative enteric bacteria. If the identity of the bacteria is unknown, treatment should be determined on the basis of the Gram’s staining characteristics (i.e., ampicillin, amoxicillin, or amoxicillin-clavulanic acid for gram-positive bacteria and trimethoprim-sulfa or enrofloxacin for gram-negative bacteria).
 TABLE 45-3 Antimicrobial Agents to Which More than 90% of Urinary Isolates Are Susceptible In Vitro at Concentrations Less than One Fourth of the Expected Urinary Concentration
TABLE 45-3 Antimicrobial Agents to Which More than 90% of Urinary Isolates Are Susceptible In Vitro at Concentrations Less than One Fourth of the Expected Urinary Concentration
| ORGANISM | ANTIMICROBIAL AGENTS |
|---|---|
| E. coli* | |
| Coagulase-positive | Amoxicillin |
| Staphylococcus spp. | |
| Proteus mirabilis | |
| Klebsiella pneumoniae* | |
| Streptococcus spp. | |
| Pseudomonas aeruginosa | |
| Enterobacter spp.* | |
| Enterococcus spp. |
* These bacteria are capable of major changes in their susceptibility to antibiotics and are therefore less predictable.
The steps to follow in the management of a UTI are given in Box 45-3, and a flow diagram is shown in Fig. 45-1. The duration of therapy for a lower UTI must be individualized and should be based on the cessation of clinical signs and elimination of the abnormal urine sediment as well as negative urine culture results. In general, uncomplicated lower UTIs should be treated for 2 weeks, whereas complicated UTIs should be treated for a minimum of 4 weeks. Proper selection of antibiotic therapy can be verified after 3 to 5 days of therapy by determining whether the urine is sterile. The urine sediment, however, may still be abnormal at this time.
 BOX 45-3 Ideal Steps to Follow in the Management of Urinary Tract Infections in Dogs and Cats
BOX 45-3 Ideal Steps to Follow in the Management of Urinary Tract Infections in Dogs and Cats
Diagnosis should be determined on the basis of history; urine sediment; and, ideally, urine culture and sensitivity findings.
Select an antimicrobial agent.
Reculture urine in 3 to 5 days to ascertain effectiveness of selected antimicrobial agent.
Examine urine sediment 3 to 4 days before discontinuing antibiotic treatment.
Repeat urinalysis and culture 10 to 14 days after cessation of antibiotic therapy.
Patients with recurrent urinary tract infections should undergo contrast-enhanced radiography and/or ultrasonography, a complete blood count, and serum biochemistry profile to determine whether they have underlying predisposing factors.
It may be necessary to treat frequent reinfections with prophylactic doses of antibiotics after the initial inflammation has been cleared up in response to standard-dose antibiotic treatment.
Reasons for a poor therapeutic response are listed in Box 45-4. Urine culture and sensitivity testing should always be done in animals with recurrent UTIs. In addition, attempts should be intensified to identify defects in the host’s immune system. Double contrast–enhanced cystography and ultrasonography may be used to identify anatomic abnormalities, mucosal lesions of the bladder, or urolithiasis. In intact male dogs semen and prostatic wash cytologic and culture studies as well as ultrasonography should be done to rule out or identify bacterial prostatitis. Excretory urographic, ultrasonographic, and renal biopsy findings may confirm the presence of pyelonephritis; however, results of these studies may be normal in dogs and cats with chronic pyelonephritis. In patients with moderate to marked pyeloectasia, ultrasound-guided pyelocentesis can be used to obtain samples for cytology and culture. Finally, the possibility of otherwise asymptomatic hyperadrenocorticism causing the recurrent UTIs should be considered, especially in animals with infections associated with low numbers of WBCs and RBCs in the urine sediment. Long-term (4 to 6 weeks) antibiotic treatment is required for patients with complicated UTIs, and careful follow-up examinations should be performed in such animals (see Box 45-3). When antibiotic treatment is used for this period of time, the adverse effects of long-term antibiotic therapy should also be considered. Keratoconjunctivitis sicca and folate deficiency anemia may occur in association with long-term use of trimethoprim-sulfa (although they are rare), and nephrotoxicity is always a concern in animals receiving aminoglycosides, even for a short time.
 BOX 45-4 Reasons for Poor Therapeutic Response in Dogs and Cats with Urinary Tract Infections
BOX 45-4 Reasons for Poor Therapeutic Response in Dogs and Cats with Urinary Tract Infections
Use of ineffective drugs or ineffective duration of therapy
Failure of owner to administer prescribed dose at proper intervals
Gastrointestinal tract disease or concurrent oral intake of food and drug, resulting in decreased drug absorption
Impaired action of drugs, either because bacteria are not multiplying or because they are sequestered in an inaccessible site (e.g., prostate or uroliths)
Failure to recognize and eliminate predisposing causes
Presence of mixed bacterial infections in which only one of the pathogens is eradicated by antimicrobial therapy
The prognosis for an animal with a complicated UTI, as opposed to an uncomplicated UTI, is always guarded. The single most important treatment for a complicated UTI is correction of the underlying defect in the host defense mechanisms. If predisposing factors cannot be identified or eliminated, relapses and reinfections are common. Low-dose (one third to one half of the conventional daily dose) antimicrobial treatment administered at bedtime (after the last evening void) may be recommended for animals with frequent infections associated with host defense mechanism problems that cannot be cured. This allows the drug to be present in the bladder overnight, supplementing the animal’s defense mechanisms. Penicillins are recommended for the treatment of recurrences caused by gram-positive bacteria, whereas trimethoprim-sulfa or enrofloxacin is recommended for the treatment of recurrences caused by gram-negative bacteria. It should be noted, however, that low-dose, long-term antibiotic treatment can predispose the animal to the development of a very resistant UTI.
Urinary acidification (ammonium chloride) has been advocated as adjunctive therapy for lower UTIs because acidic urine provides a less favorable environment for bacterial growth. However, the antimicrobial activity of acidic urine is inferior to that of antibiotics and should not be expected to eradicate infection; ammonium chloride should be used only in conjunction with other modes of therapy. Urinary acidification may also be an effective adjunctive therapy to adjust the urine pH and thereby optimize the efficacy of certain antibiotics (penicillin, ampicillin, carbenicillin, tetracycline, nitrofurantoin). Ammonium chloride (60 to 100 mg/kg) should be given orally twice daily to maintain a urine pH of less than 6.5. The use of ammonium chloride is not without risk, however, especially in male dogs, because oxalate, silicate, urate, and cystine are all less soluble in acidic urine and urolithiasis may result from excessive acidification. In addition, urinary acidification would be contraindicated in dogs with liver or kidney disease. Urinary antiseptics have also been advocated as adjunctive therapy in the control or prophylaxis of lower UTIs. Although they are less effective than specific antimicrobial therapy in eradicating infections, they are probably more effective than urinary acidifiers. Methenamine mandelate is a cyclic hydrocarbon and is the most commonly used urinary tract antiseptic. The dose for dogs is 10 mg/kg, administered orally every 6 hours. In an acidic environment (pH < 6), methenamine hydrolyzes to form formaldehyde. It should be used in conjunction with ammonium chloride to enhance its effectiveness. Methylene blue (tetramethylthionine chloride) is a weak urinary antiseptic agent that used to be common in combination products designed to treat lower urinary tract inflammation in people. These products should not be used in cats, however, because methylene blue has the potential to cause Heinz bodies and hemolytic anemia. Similarly, phenazopyridine, a urinary tract analgesic, should not be used in cats.
Cranberry juice extracts, glycosaminoglycans, and vaccines directed against bacterial fimbria are additional adjunctive treatments that can decrease bacterial adherence to uroepithelium in other species but require further evaluation in the dog before clinical recommendations can be made.
Adams LG, Syme HM. Canine lower urinary tract diseases. In Ettinger SJ, Feldman EC, editors: Textbook of veterinary internal medicine, ed 6, St Louis: Elsevier/Saunders, 2005.
Bartges JW. Urinary tract infections. In Ettinger SJ, Feldman EC, editors: Textbook of veterinary internal medicine, ed 6, St Louis: Elsevier, 2005.
Cohn LA, et al. Trends in fluoroquinolone resistance of bacteria isolated from canine urinary tracts. J Vet Diag Invest. 2003;15:338.
Crawford JT, et al. Influence of vestibulovaginal stenosis, pelvic bladder, and recessed vulva on response to treatment for clinical signs of lower urinary tract disease in dogs: 38 cases (1990–1999). J Am Vet Med Assoc. 2002;221:995.
Forrester SD, et al. Retrospective evaluation of urinary tract infection in 42 dogs with hyperadrenocorticism or diabetes mellitus or both. J Vet Intern Med. 1999;13:557.
Hess RS, et al. Concurrent disorders in dogs with diabetes mellitus: 221 cases (1993–1998). J Am Vet Med Assoc. 2000;217:1166.
Ling GV. Bacterial infections of the urinary tract. In: Ettinger SJ, et al, editors. Textbook of veterinary internal medicine. Philadelphia: WB Saunders, 2000.
Ling GV, et al. Interrelations of organism prevalence, specimen collection method, and host age, sex, and breed among 8,354 canine urinary tract infections (1969–1995). J Vet Intern Med. 2001;15:341.
Norris CR, et al. Recurrent and persistent urinary tract infections in dogs: 383 cases (1969–1995). J Am Anim Hosp Assoc. 2000;36:484.
Ogeer-Gyles J, et al. Evaluation of catheter-associated urinary tract infections and multi-drug-resistant Escherichia coli isolates from the urine of dogs with indwelling urinary catheters. J Am Vet Med Assoc. 2006;229:1584.
Oluch AO, et al. Nonenteric Escherichia coli isolates from dogs: 674 cases (1990–1998). J Am Vet Med Assoc. 2001;218:381.
Seguin MA, et al. Persistent urinary tract infections and reinfections in 100 dogs (1989–1999). J Vet Intern Med. 2003;17:622.
Senior DF. Management of urinary tract infections. In Elliott JA, Grauer GF, editors: BSAVA manual of canine and feline nephrology and urology, ed 2, Gloucester, England: British Small Animal Veterinary Association, 2007.
Smarick SD, et al. Incidence of catheter-associated urinary tract infection among dogs in a small animal intensive care unit. J Am Vet Med Assoc. 2004;224:1936.
Stiffler KS, et al. Prevalence and characterization of urinary tract infection in dogs with surgically treated type 1 thoracolumbar intervertebral disc extrusion. Vet Surg. 2006;35:330.
Swenson CL, et al. Evaluation of modified Wright-staining of urine sediment as a method for accurate detection of bacteriuria in dogs. J Am Vet Med Assoc. 2004;224:1282.