CHAPTER 41 Clinical Manifestations of Urinary Disorders
GENERAL CONSIDERATIONS
This chapter begins with a discussion of urinary tract problems that are likely to be identified by pet owners (e.g., pollakiuria and dysuria-stranguria, hematuria, urinary incontinence, and polydipsia and polyuria). Problems that are usually identified on the basis of a physical examination, a minimum database, or with imaging techniques, including proteinuria, azotemia, and renomegaly, are discussed subsequently.
POLLAKIURIA AND DYSURIA-STRANGURIA
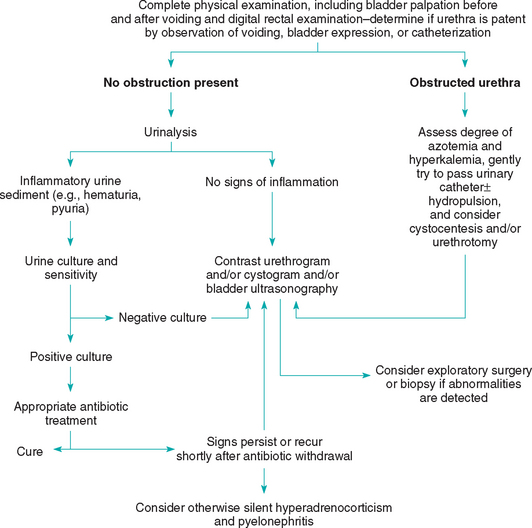
Lower urinary tract inflammation (LUTI) usually results in increased frequency of urination (pollakiuria) and difficult urination (dysuria) associated with straining (stranguria; Fig. 41-1). LUTI in dogs is often caused by bacterial infection; in contrast, primary bacterial infection of the urinary tract is relatively rare in cats. Sterile inflammation (e.g., some cases of calcium oxalate urolithiasis and idiopathic cystitis) or space-occupying masses of the lower urinary tract (e.g., neoplasia, ureterocele) can result in pollakiuria and dysuria-stranguria in both dogs and cats. When an animal has clinical signs suggestive of LUTI or obstruction, transabdominal palpation of the bladder may confirm the presence of a distended bladder, a thickened bladder wall, a bladder mass, or urolithiasis. If possible, urinary bladder palpation should be performed before and after the patient voids because a full bladder may obscure the presence of intraluminal masses or uroliths. Digital rectal examination in smaller male and female dogs and in cats often allows the clinician to evaluate the trigone of the bladder and the pelvic urethra in a search for masses or uroliths. Urinalysis, urine bacterial culture, ultrasonography of the bladder, and/or plain or contrast-enhanced radiography of the bladder and urethra often demonstrate the cause of the pollakiuria and dysuriastranguria; occasionally, advanced imaging modalities (e.g., computed tomography (CT) scan) may be necessary to evaluate the lower urinary tract. Currently, cystoscopy is widely used in specialty practices and academic hospitals for evaluation of patients with lower urinary tract diseases. If systemic signs (e.g., depression, lethargy, anorexia, vomiting) are present in animals with LUTI, a complete blood count (CBC) and serum biochemistry profile should also be obtained, and the kidneys, prostate, and uterus/uterine stump should be evaluated as a possible source of the signs.
URETHRAL OBSTRUCTION
Urethral obstruction, either functional (e.g., reflex dyssynergia, urethral spasm) or anatomic (e.g., urolithiasis, granulomatous urethritis, neoplasia), usually causes pollakiuria, dysuria-stranguria, or both, with an attenuated or absent urine stream. A urethral catheter will pass relatively easily in patients with a functional obstruction, whereas an anatomic obstruction will result in “grating,” difficult passage or the inability to pass the catheter. If there is any question, a positive contrast retrograde urethrogram will confirm the presence of an anatomic lesion or obstruction. If a complete urethral obstruction exists, the degree of postrenal azotemia and hyperkalemia should be assessed immediately. Hyperkalemia can cause life-threatening cardiac arrhythmias and should be treated promptly (see Fig. 41-1).
URINARY TRACT INFECTION
Urine for urinalysis and bacterial culture may be obtained by antepubic cystocentesis, urinary bladder catheterization, or a midstream catch during voiding. However, the number of organisms isolated in a normal dog or cat varies according to the technique used (Table 41-1). Ideally, urine should be obtained by cystocentesis, and urine specimens should be plated within 30 minutes of collection. If this is not possible, the urine sample should be refrigerated in a closed container because bacteria may double their numbers in urine every 45 minutes at room temperature, resulting in false-positive culture findings. On the other hand, false-negative urine culture results may be obtained if the urine has been frozen or refrigerated for 12 to 24 hours or more.
 TABLE 41-1 Numbers of Bacteria per Milliliter Considered Significant According to Method of Urine Collection in Dogs and Cats
TABLE 41-1 Numbers of Bacteria per Milliliter Considered Significant According to Method of Urine Collection in Dogs and Cats

Animals with recurrent or refractory urinary tract infections (UTIs) should undergo ultrasonography or contrast- enhanced radiography in a search for underlying anatomic disorders. Bladder tumors or polyps, uroliths, pyelonephritis, prostatitis, ureteroceles, and urachal remnants are common causes of recurrent or unresponsive UTIs. In some cases, systemic disorders such as hyperadrenocorticism, chronic kidney disease, and diabetes mellitus may be associated with recurrent UTIs, as can long-term corticosteroid treatment. UTIs are discussed in greater depth in Chapter 45.
TRANSITIONAL CELL CARCINOMA
Transitional cell carcinoma (TCC) is the most common malignant bladder tumor in dogs and should be suspected in older dogs with hematuria, pollakiuria, and dysuriastranguria. TCCs are rare in cats, where they are usually detected as a diffuse thickening of the bladder wall during palpation or imaging. TCCs most frequently arise in the bladder trigone region; therefore rectal palpation can often detect their presence. Urinary bladder ultrasonography or double contrast-enhanced cystography will confirm that a bladder mass exists. In some cases, unilateral or bilateral hydroureter-hydronephrosis is observed as a result of obstruction of one or both ureters at the vesicoureteral junction. Tumor biopsy and histopathologic evaluation should be done to confirm the tumor type and stage and to direct the nature of specific treatment. The bladder tumor antigen test (V-BTA test: www.polymedco.com) is usually not recommended as a diagnostic aid because it does not reliably differentiate dogs with bladder cancer from dogs with LUTI resulting from other causes. In some specialty practices, cystoscopy provides a simple method to obtain a diagnostic sample for histopathology and assess the extent of bladder involvement in dogs and cats with infiltrative bladder diseases.
UROLITHIASIS
Urinary bladder and urethral uroliths can often be palpated during abdominal or rectal examination; however, a full bladder or a thickened, inflamed bladder wall may obscure small uroliths. In male dogs with dysuria, the urethra should be palpated subcutaneously from the ischial arch to the os penis in a search for urethral uroliths. Ultrasonography or plain or contrast-enhanced radiography of the urinary tract may be necessary to confirm the presence of uroliths. Calcium oxalate and struvite uroliths are the most radiodense, whereas urate uroliths are relatively radiolucent, and contrast-enhanced radiographs may be required for their diagnosis. Silicate and cystine uroliths have an intermediate radiodensity, and unless the stones are small (<5 mm in diameter), they can usually be observed on plain film radiographs.
Urinalysis findings in dogs and cats with urolithiasis often indicate the presence of urinary tract inflammation (e.g., hematuria, pyuria, increased numbers of epithelial cells, and proteinuria). The urine pH varies depending on the stone type, on the presence or absence of a concurrent bacterial infection, and on the animal’s diet. In general, struvite uroliths are associated with an alkaline urine (especially if urease-producing bacteria are present); cystine uroliths with an acidic urine; and oxalate, urate, and silicate uroliths with a neutral-to-acidic urine. Crystalluria may be observed depending on the urine concentration, pH, and temperature. Although crystalluria may exist in the absence of uroliths, and uroliths may be present in the absence of crystalluria, if the two coexist, the identity of the crystals is usually the same as that of the urolith (Figs. 41-2 to 41-6). Exceptions do occur, however; for example, a urease-producing bacterial infection could generate struvite crystals in the presence of silicate or calcium oxalate uroliths. Bacterial urine culture and sensitivity testing should be performed in all animals with urolithiasis to identify and properly treat any concurrent UTI. If a cystotomy is performed to remove stones, a small piece of the bladder mucosa or urolith should be submitted for bacterial culture. This is because urine may be sterile in dogs and cats that have previously been treated with antibiotics, whereas the stone or bladder mucosa may still harbor bacteria.
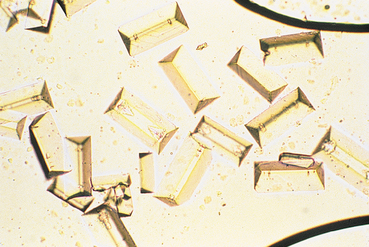
FIG 41-2 Struvite crystals in urine sediment. These crystals are normally colorless.
(From Grauer GF: Canine urolithiasis. In Allen DG, editor: Small animal medicine, Philadelphia, 1991, JB Lippincott.)
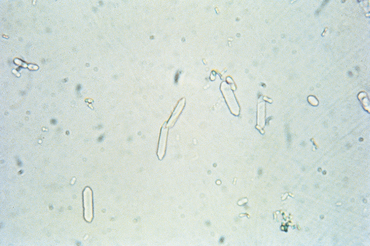
FIG 41-3 Monohydrate calcium oxalate crystals in urine sediment. These crystals are normally colorless.
(From Grauer GF: Canine urolithiasis. In Allen DG, editor: Small animal medicine, Philadelphia, 1991, JB Lippincott.)
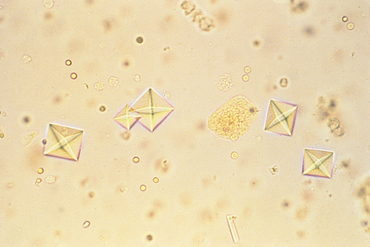
FIG 41-4 Dihydrate calcium oxalate crystals in urine sediment. These crystals are normally colorless.
(From Grauer GF: Canine urolithiasis. In Allen DG, editor: Small animal medicine, Philadelphia, 1991, JB Lippincott.)
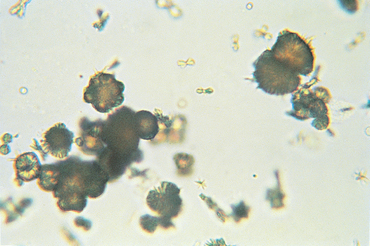
FIG 41-5 Ammonium biurate crystals in urine sediment. These crystals are normally dark yellow.
(From Grauer GF: Canine urolithiasis. In Allen DG, editor: Small animal medicine, Philadelphia, 1991, JB Lippincott.)
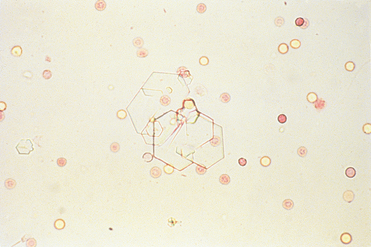
FIG 41-6 Cystine crystals in urine sediment. These crystals are normally clear to light yellow.
(From Grauer GF: Canine urolithiasis. In Allen DG, editor: Small animal medicine, Philadelphia, 1991, JB Lippincott.)
The animal’s signalment, as well as the clinicopathologic and radiographic findings, are often helpful in determining the type of urolith (Box 41-1); however, a quantitative urolith analysis should be performed if uroliths are passed or removed surgically. Identification of the urolith type facilitates the use of specific measures to dissolve them or prevent their recurrence. Qualitative commercial kit analysis of uroliths is not recommended because these kits do not detect silicic acid salts, frequently fail to detect calcium-containing uroliths, and yield false-positive results for uric acid more than half of the time in animals with cystine uroliths. Quantitative urolith analysis, available at most teaching hospitals and reference laboratories, is recommended instead.
 BOX 41-1 Factors That May Aid in the Identification of Uroliths in Dogs
BOX 41-1 Factors That May Aid in the Identification of Uroliths in Dogs
Struvite
Calcium Oxalate
Urolithiasis is discussed in greater detail in Chapters 46 and 47.
FELINE LOWER URINARY TRACT DISEASE (LUTD)
Cats with LUTD (often referred to as feline urologic syndrome, feline lower urinary tract inflammation, or feline interstitial cystitis; see Chapter 47) usually are presented because of pollakiuria, dysuria-stranguria, microscopic or gross hematuria, or inappropriate voiding. In male cats with a urinary tract obstruction, the presenting signs depend on how long the obstruction has been present. Within the first 6 to 24 hours, most obstructed cats will make frequent attempts to urinate, pace, vocalize, hide under beds or behind couches, lick their genitalia, and display anxiety. If the obstruction is not relieved within 36 to 48 hours, characteristic clinical signs of postrenal azotemia and hyperkalemia, including anorexia, vomiting, dehydration, depression, weakness, collapse, stupor, hypothermia, acidosis with hyperventilation, or bradycardia, may be observed. Sudden death may also occur.
On physical examination an unobstructed cat is apparently healthy, except for a small, easily expressible bladder. The bladder wall may be thickened, and palpation may cause the animal to void. Abdominal palpation may be painful to the unobstructed cat; however, the obstructed cat will always resent manipulation of the caudal abdomen unless it is severely depressed or comatose. The most significant physical examination finding in an obstructed cat is a turgid, distended bladder that is difficult or impossible to express. Care should be exercised in manipulating the distended bladder, however, because the wall has been injured by the increased intravesical pressure and is susceptible to rupture. In a male cat with a urethral obstruction, the penis may be congested and it may protrude from the prepuce. Occasionally, a urethral plug is seen extending from the urethral orifice, and in some cases the cat may lick its penis until it becomes excoriated and bleeds.
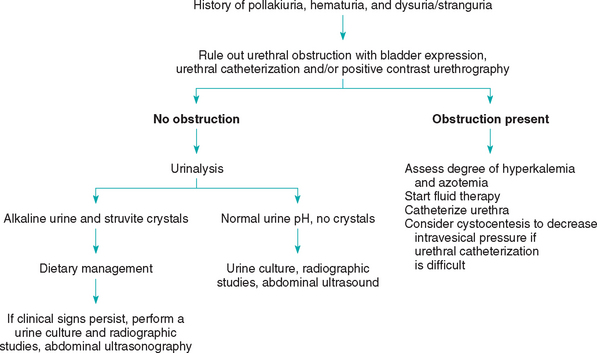
A history of acute onset of pollakiuria, dysuriastranguria, and hematuria in an otherwise healthy cat indicates LUTD. Physical examination should include digital rectal palpation of the caudal bladder and urethra in an attempt to determine whether there are masses or calculi, as well as abdominal palpation of the bladder before and after voiding to determine the residual urine volume and whether there are intraluminal masses or uroliths. The minimal diagnostic workup in cats with pollakiuria and dysuria-stranguria should always include a complete urinalysis. The urine should preferably be obtained by cystocentesis; however, if manipulation of the bladder during abdominal palpation results in voiding, a sample obtained from a clean tabletop may be used to assess urine pH and sediment.
An extensive diagnostic evaluation of the unobstructed cat is usually not warranted. In most of these cases the urine is bacteriologically sterile, and clinical signs respond to canned food dietary therapy. However, if clinical signs persist beyond 5 to 7 days of instituting dietary therapy, a second urinalysis with a urine culture and sensitivity, radiography of the abdomen, ultrasonography, and/or contrast-enhanced cystography-urethrography should be performed (Fig. 41-7).
HEMATURIA
Hematuria, the presence of red blood cells in the urine, is frequently encountered in clinical veterinary medicine. Because the urine strip reagents detect hemoglobin and myoglobin, a positive “blood” test in a urine dipstick does not necessarily mean that the patient has hematuria, and the sediment should be evaluated microscopically (discussed in more detail later). Hematuria occurring in conjunction with pollakiuria and dysuria-stranguria is usually associated with LUTI. Conversely, hematuria that occurs in the absence of other clinical signs often originates from the upper urinary tract. Hematuria may be gross (macroscopic hematuria) or occult (microscopic hematuria). Occult hematuria (more than five red blood cells per high-power field) is often present in dogs and cats with pollakiuria and dysuria-stranguria. The diagnostic workup in dogs and cats with hematuria is directed toward identifying the origin of the hemorrhage as well as the underlying disease.
In most cases hematuria is caused by inflammation, trauma, or neoplasia of the urogenital tract; however, hematuria may also be caused by systemic bleeding disorders, strenuous exercise, heat stroke, or renal infarcts. The renal telangiectasia that occurs in Cardigan Welsh Corgis may also cause hematuria, as can the renal hematuria in Weimaraners. The timing of gross hematuria during voiding may provide clues as to the source of the hemorrhage. Hematuria that occurs at the beginning of voiding (initial hematuria) is suggestive of hemorrhage originating from the lower urinary tract (bladder neck, urethra, vagina, vulva, penis, or prepuce). Extraurinary causes such as proestrus, metritis, pyometra, prostatic disease, or neoplasia of the genital tract may also cause initial hematuria (Table 41-2). Hematuria that occurs at the end of voiding (terminal hematuria) usually results from hemorrhage originating from the upper urinary tract (bladder, ureters, or kidneys). In this case the hemorrhage may be intermittent, which allows the red blood cells to settle in the bladder and be expelled with the last of the bladder contents. If hematuria occurs throughout voiding (total hematuria), the hemorrhage usually originates in the bladder, ureters, or kidneys. Pseudohematuria may be caused by myoglobin or hemoglobin, drugs, and natural or artificial food dyes in the urine. In cases of pseudohematuria, the urine supernate remains discolored after centrifugation.
 TABLE 41-2 Potential Causes of Hematuria
TABLE 41-2 Potential Causes of Hematuria
| URINARY CAUSES | EXTRAURINARY CAUSES |
|---|---|
| Initial hematuria | |
| Total or terminal hematuria | |
| Pseudohematuria | |
In dogs and cats with hematuria caused by inflammation, trauma, or neoplasia of the lower urinary tract, concurrent clinical signs usually include pollakiuria and dysuriastranguria. Hematuria associated with upper urinary tract disease may be associated with systemic signs, including depression, lethargy, anorexia, vomiting, diarrhea, weight loss, and abdominal pain, or it may be asymptomatic. In some cases upper urinary tract hemorrhage can result in the formation of blood clots in the bladder, leading to subsequent dysuria-stranguria. If hemorrhage from the genital tract is causing hematuria, spontaneous bleeding not associated with voiding may also be observed. Additional signs indicating that the genital tract is the source of hemorrhage include a purulent vaginal or urethral discharge independent of voiding, behavioral changes (e.g., proestrus), or straining to defecate in association with a stilted gait (e.g., prostatic disease).
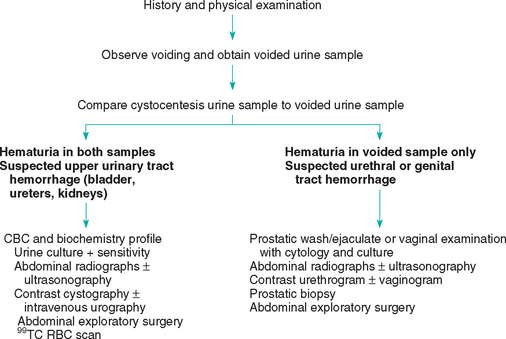
A complete physical examination often helps localize the source of the hematuria. If possible, the kidneys should be palpated and assessed in terms of their size, shape, consistency, and symmetry and for the presence of pain. The urinary bladder should be palpated before and after voiding, because, as already noted, a full bladder may obscure intraluminal masses, uroliths, or wall thickening. Observation of voiding should also be part of the physical examination and provides the opportunity to obtain a voided urine sample (Fig. 41-8). In addition, the timing of the hematuria can be confirmed and the character of the urine stream, as well as the presence or absence of dysuria, can be noted. Rectal palpation allows evaluation of the prostate in male dogs and of the pelvic urethra in dogs and cats of both sexes. The trigone region of the bladder can also be palpated rectally in small dogs and cats; this is facilitated by concurrent abdominal palpation, with the examiner pushing the bladder toward the pelvic inlet. In larger female dogs digital vaginal palpation and the use of a vaginal speculum or scope allow for the urethral orifice to be evaluated; vaginal masses, strictures, and lacerations can be ruled in or out in this way. In male dogs the perineal urethra should be palpated subcutaneously from the ischial arch to the os penis, and the penis should be extruded from the prepuce and examined to determine whether there are masses, signs of trauma, or urethral prolapse. Finally, catheterization of the urethra in dysuric animals allows assessment of urethral patency; when indicated, positive contrast retrograde urethrography or ultrasonography can be employed to outline urethral anatomic abnormalities.
Comparison of urine obtained by cystocentesis with voided urine may help differentiate lower urinary tract or genital tract disease from upper urinary tract disease. Cystocentesis prevents the urine from being contaminated with bacteria, cells, and debris from the urethra, vagina, vulva, prepuce, or uterus; however, prostatic disease may alter the characteristics of urine obtained by cystocentesis (as a result of the reflux of fluid into the bladder). Abnormal urinalysis findings in urine collected by cystocentesis indicate involvement of the bladder, ureters, kidneys, or prostate. It should be remembered, however, that catheterization or bladder expression, and to a greater extent cystocentesis, may result in traumatic microscopic hematuria.
Urinalysis should be performed as soon as possible after urine collection. In addition to evaluating the urine sediment for red blood cells, the clinician should look for white blood cells, epithelial cells, tumor cells, casts, crystals, parasite ova, and bacteria. If urine remains at room temperature for more than 30 minutes, urease-producing bacteria can proliferate, resulting in an increase in the urine pH, which may cause red and white blood cells and casts to fragment and lyse and may alter the crystal composition. In addition, hyposthenuria can result in the lysis of red and white blood cells, and lysed red blood cells in urine may create confusion between hemoglobinuria and hematuria. Refrigeration is the easiest way to preserve the stability of a urine sample. Although overnight refrigeration in a closed sterile container is acceptable for urine to be used for bacterial culture samples, it is not recommended for urine intended for chemical and cellular analysis.
Reagent strips used to detect blood in urine do so by detecting the peroxidase-like activity of hemoglobin from lysed cells. The test can detect approximately 0.05 to 0.3 mg of hemoglobin per deciliter of urine (equivalent to 10,000 lysed red blood cells per milliliter of urine, or approximately three lysed red blood cells per high-power field). These reagent test strips also show a positive reaction for blood in the presence of myoglobinuria.
A CBC and serum biochemistry profile should be evaluated in dogs and cats with hematuria and concurrent systemic signs. An inflammatory leukogram is compatible with metritis-pyometra, acute bacterial pyelonephritis, or prostatitis. Azotemia occurring in association with hematuria usually indicates the presence of renal parenchymal disease or a rent in the urinary excretory pathway; however, prerenal causes of azotemia should also be ruled out. If the blood loss caused by hematuria is severe or if signs of generalized bleeding exist, a hemostasis profile, platelet count, and bleeding time should be evaluated (see Chapter 87).
Plain and contrast-enhanced radiography, ultrasonography, and/or cystoscopy will often help show the location and cause of hematuria. In some cases, abdominal exploratory surgery and biopsy may be necessary to arrive at a diagnosis. Biopsy specimens may be obtained from the kidneys, bladder, and prostate gland; if indicated, individual ureteral catheterization through a cystotomy or visualization through a cystoscope may be performed to determine if renal hematuria is unilateral or bilateral. Nuclear medicine (technetium-labeled red blood cells) can also be used to localize renal hematuria to one individual kidney.
DISORDERS OF MICTURITION
Disorders of micturition include both urine retention and urine leakage (incontinence). Incontinence, the inappropriate passage of urine, may be caused by congenital abnormalities or acquired disorders. In evaluating an animal with incontinence, the clinician may find it helpful to determine whether the urinary bladder is distended, small, or normal in size (Table 41-3). Distended bladders are associated with urine retention and are usually caused by either detrusor hypocontractility or increased outflow resistance. Increased outflow resistance may be anatomic (e.g., urethral urolith) or functional (e.g., reflex dyssynergia). Urinary incontinence will occur with a primary urine retention disorder when intravesicular pressure overcomes outflow resistance pressure. This type of incontinence is termed paradoxic. More commonly, however, incontinence is associated with a small or normal-sized bladder that is typically caused by either decreased outflow resistance or increased detrusor contractility.
 TABLE 41-3 Causes of Urinary Incontinence and Associated Clinical Signs
TABLE 41-3 Causes of Urinary Incontinence and Associated Clinical Signs
| DISORDERS | CLINICAL SIGNS |
|---|---|
| Large bladder | |
| Lower motor neuron lesions | Dribbling of urine; distended bladder that is easily expressed; history of trauma or surgery in pelvic region |
| Upper motor neuron lesions | Distended bladder that is difficult to express; possible presence of paresis or paralysis |
| Reflex dyssynergia | Often, large-breed male dog; distended bladder that is difficult to express but easy to catheterize; urine stream initiated and then interrupted |
| Outflow tract obstruction | Usually male animals; dysuria-stranguria, dribbling of urine; distended bladder that is |
| Small bladder | |
| Urethral sphincter mechanism incompetence | Middle-aged or older neutered or spayed dogs; dribbling of urine usually occurring when animal is relaxed or asleep, normal voiding otherwise |
| Detrusor hyperreflexia/instability | Pollakiuria, dysuria-stranguria, hematuria, bacteriuria |
| Congenital abnormalities | Young animal; constant dribbling of urine possible, voiding possibly normal otherwise |
Initial Evaluation
The age of onset, reproductive status, age at neutering, current medications, and history of trauma or previous urinary tract disorders are important anamnestic points to cover when obtaining the history in an animal with disorders of micturition. The physical examination should include an evaluation of the perineum for evidence of urine scalding or staining. Thorough palpation of the bladder to assess its size and wall thickness and a rectal examination to assess anal tone, the prostate gland, the pelvic urethra, and the trigone region of the bladder should be performed in all cases. A digital vaginal examination is indicated, and vaginoscopy may be used to help identify congenital defects (e.g., vaginal strictures, ectopic ureters) in female dogs.
A neurologic examination should include evaluation of the perineal and bulbospongiosus reflexes. The perineal reflex causes the anal sphincter to contract and the tail to ventroflex in response to pinching of the perineal skin. The bulbospongiosus reflex causes the anal sphincter to contract in response to gentle compression of the bulb of the penis or the vulva. Both of these reflexes are dependent on an intact pudendal nerve (sensory and motor) and intact sacral spinal cord segments S1-S3. If both reflexes are normal, the pudendal reflex arc is intact. Because the pelvic nerve (sensory and motor parasympathetic innervation to the detrusor muscle) arises from the same sacral cord segments, damage to the pudendal nerve may also affect the pelvic nerve.
Dogs should be walked outside so that the voiding posture and urine stream size and character can be observed. Immediately after the animal has attempted to void, the bladder should be palpated to estimate the residual volume (normal residual volume is approximately 0.2 to 0.4 ml/kg). Catheterization to quantify the residual volume is indicated if a large bladder is palpable after voiding (in male dogs behavioral urine marking can make it difficult to assess the true residual urine volume).
A urinalysis should be performed in all animals with urinary incontinence. If a bacterial urine culture is indicated, as noted earlier, cystocentesis is the preferred method of collection; however, dogs and cats with a distended bladder should ideally be catheterized to empty the bladder and prevent the possibility of urine from leaking from the cystocentesis site.
Pharmacologic Testing and Treatment
Frequently, the diagnosis of disorders of micturition (see Chapter 48) is based to some degree on the animal’s response to pharmacologic testing and therapy. For example, detrusor hypocontractility should improve in response to a parasympathomimetic drug such as bethanechol, and urethral hypotonicity should respond to α-adrenergic agents such as phenylpropanolamine or hormone replacement therapy. Urethral hypertonicity is treated with α-sympatholytics (e.g., phenoxybenzamine) and striated muscle relaxants (e.g., diazepam). Detrusor hypercontractility often responds to treatment of the underlying inflammatory process (e.g., bacterial cystitis or urolithiasis); however, smooth muscle antispasmodics (e.g., oxybutynin) and parasympatholytics (e.g., propantheline) may be useful in cases of severe inflammation.
DISTENDED BLADDER
Causes of incontinence that are typically associated with a distended bladder include neurogenic disorders (lower and upper motor neuron lesions and reflex dyssynergia) and urine outflow tract obstructive disorders (paradoxic incontinence; see Table 41-3). If neurologic lesions or deficits are detected during a neurologic examination, the status of the bladder helps localize the lesion and classify the injury as an upper motor neuron (UMN) lesion (located above the fifth lumbar vertebral body) or a lower motor neuron (LMN) lesion (located at or below the fifth lumbar vertebral body). The most characteristic sign of an LMN lesion to the bladder is a distended bladder that is easily expressed. An LMN injury affecting innervation to the bladder creates both sphincter and detrusor hyporeflexia; if the lesion involves the S1-S3 spinal cord segments, both perineal and bulbospongiosus reflexes are absent.
Animals with UMN lesions to the bladder characteristically have a large, distended bladder that is difficult to express; the UMN lesion may also cause paresis or paralysis. Animals with a UMN lesion have no voluntary control of micturition, and the urethral sphincter shows reflex hyperexcitability because there is a lack of inhibition to the somatic efferents in the pudendal nerve, making expression of the bladder difficult. With time, UMN bladders may develop reflex contraction and partial emptying in response to detrusor stretching. This “automatic” emptying occurs without control or sensation.
Reflex dyssynergia or detrusor-urethral dyssynergia is a condition observed primarily in large-breed male dogs. The cause is usually difficult to determine but may include any of several neurologic lesions of the spinal cord or autonomic ganglia. Reflex dyssynergia results from active contraction of the detrusor without relaxation of the internal or external urethral sphincters. Characteristic signs of reflex dyssynergia include a normal or near-normal initiation of voiding, followed by a narrowed urine stream. Urine may be delivered in spurts, or flow may be completely disrupted and the animal will often strain to produce urine. After a while, the dog lowers his leg and then often begins dribbling urine as he walks away. Although it is difficult to express urine from the bladder of a dog with reflex dyssynergia, urethral catheterization is usually easy.
Incontinence in an animal with urinary outflow tract obstruction is called paradoxic incontinence. It occurs because intravesical pressure exceeds the pressure within the urethra, allowing urine to leak past the obstruction before a urethral or bladder rupture occurs. Clinical signs associated with an anatomic urethral obstruction include dribbling of urine, straining to urinate without producing urine, restlessness, and abdominal pain. The most common causes of urethral obstruction are calculi and neoplasia in dogs and urethral plugs in cats; however, urethral strictures and granulomatous urethritis can also create obstructions to urine flow. Prostatic disease in dogs may cause an outflow tract obstruction. Older male dogs with benign prostatic hyperplasia may be evaluated because of stranguria and tenesmus; however, prostatic neoplasia and prostatic abscess formation are more likely causes of urinary outflow tract obstruction in such animals.
SMALL OR NORMAL-SIZED BLADDER
Causes of urinary incontinence in animals with a small or normal-size bladder include urethral sphincter mechanism incompetence (USMI), detrusor hyperreflexia or instability, and congenital abnormalities. Estrogen and testosterone are believed to contribute to the integrity of urethral muscle tone by increasing its responsiveness to α-adrenergic innervation. Thus middle-aged to older, spayed female dogs are prone to the development of incontinence associated with decreased estrogen concentrations. This incontinence is most pronounced when the animal is asleep or relaxed and often responds to estrogen replacement therapy. Less frequently, incontinence develops in male dogs after castration; the condition seems to occur most commonly in dogs castrated at an older age and often responds to intramuscular testosterone administration. Diagnosis of both processes is based on the history, physical examination, and urinalysis findings (no evidence of LUTI) and on the response to therapy. Frequently, α-adrenergic treatment (e.g., phenylpropanolamine) is effective in both male and female dogs with USMI incontinence, and in severe cases may be combined with hormone replacement treatment. Testosterone treatment is contraindicated in dogs that were neutered because of behavioral, prostatic, or perineal problems. In these cases α-adrenergic treatment should be used; α-adrenergic treatment should be used with caution (or not at all) in patients wih hypertension.
Detrusor hyperreflexia or instability is the inability to control voiding because of a strong urge to urinate. Inflammation of the bladder or urethra may create a sensation of bladder fullness, which triggers the voiding reflex. Clinical signs of this type of incontinence include pollakiuria, dysuria-stranguria, and frequently hematuria. Bacterial UTI is the most common cause in the dog, and sterile LUTD is the most common cause in cats. A urinalysis that reveals evidence of UTI or inflammation (e.g., bacteriuria, pyuria, or hematuria) initially supports a tentative diagnosis of urge or inflammatory incontinence. If clinical signs persist after appropriate treatment for the urinary tract inflammation has been initiated, further diagnostic testing, including ultrasonography, contrast-enhanced radiography, and/or cystoscopy, are indicated because infiltrative disease of the bladder (e.g., neoplasia, chronic cystitis), polyps, uroliths, or urachal remnants can also result in pollakiuria and stranguria. It should also be noted that detrusor hyperreflexia/instability may also be a primary or idiopathic disorder that is not associated with bladder or urethral inflammation.
Urinary incontinence in a young animal may be associated with a variety of congenital defects of the urinary and genital systems. The most common defects are ectopic ureters and vaginal strictures, but a patent urachus, urethrorectal and urethrovaginal fistulas, and female pseudohermaphroditism have also been associated with urinary incontinence. Ectopic ureters are most commonly observed in female dogs. Breeds at high risk for ectopic ureters include Siberian Huskies, Miniature and Toy Poodles, Labrador Retrievers, Smooth Fox Terriers, West Highland White Terriers, Collies, and Cardigan Welsh Corgis. Ectopic ureters are rarely seen in cats, but the gender predisposition is reversed; the prevalence is higher in males than in females.
The most common clinical sign in an animal with ectopic ureters is a constant dribbling of urine, although dogs and cats with a unilateral ectopic ureter may void normally. Because 70% of ectopic ureters in dogs terminate in the vagina, vaginoscopy may allow visualization of the opening of the ectopic ureter; however, the opening can be difficult to see, even if the vagina is fully distended with air. Intravenous urography, retrograde vaginourethrography, and cystoscopy are additional diagnostic tests for characterizing the defect. In contrast to the incontinence associated with ectopic ureters, that associated with a vaginal stricture is often intermittent, occurring with changes in body position. Vaginal strictures can be diagnosed using digital vaginal examination, vaginoscopy, or contrast-enhanced vaginography.
Incontinence may also be caused by cognitive dysfunction, decreased bladder capacity, or decreased mobility in senior animals. Polyuric-polydipsic disorders such as chronic kidney disease and diabetes mellitus in senior animals also often exacerbate incontinence. Likewise, diuretic and corticosteroid therapy should be avoided in incontinent animals because of their negative effects on urine concentration.
POLYDIPSIA AND POLYURIA
Increased thirst and urine production are frequent presenting complaints in small animals. Polydipsia (PD) and polyuria (PU) in the dog and cat have been defined as a water consumption greater than 80 to 100 ml/kg/day and a urine production greater than 40 to 50 ml/kg/day, respectively. However, it is possible for thirst and urine production to be within the normal range and yet be abnormal in individual animals. Polydipsia and polyuria usually co-exist, and determining the primary component of the syndrome is one of the initial diagnostic considerations in an animal showing increased water consumption and urine production.
Thirst is stimulated primarily by osmotic factors. Hyperosmolality of the extracellular fluid usually occurs secondary to water loss, or it may result from the ingestion or intravenous infusion of hypertonic solutions. This hyperosmolality results in the dehydration of osmoreceptors, which stimulate thirst. Nonosmotic factors, including decreased arterial blood pressure, increased body temperature, pain, and certain drugs, can also stimulate thirst. Thirst is inhibited by expansion of the extracellular fluid volume, increased arterial blood pressure, drinking, and fullness of the stomach. Thirst is abnormally stimulated in animals with primary polydipsia, resulting in water consumption that exceeds physiologic need. Renal function in these animals is usually normal, and secondary polyuria occurs to rid the body of the excess water.
The kidneys maintain body fluid composition and volume by resorbing water and solutes from the glomerular filtrate. The resorption of solute in excess of water results in the formation of dilute urine. Conversely, the resorption of water in excess of solute results in the formation of concentrated urine. For concentrated urine to form, antidiuretic hormone (ADH) must be produced and released, and the renal tubules must be responsive to the ADH. For the latter to occur, the renal medullary interstitium must be hypertonic and at least one third of the total nephron population must be functional. ADH is synthesized in the supraoptic and paraventricular nuclei of the hypothalamus and is stored in the posterior pituitary gland. Its release is stimulated by the same factors that stimulate thirst. In the presence of ADH, the distal portion of the distal convoluted tubule and the collecting duct become permeable to water, and water is resorbed from the tubular lumen. The hypertonicity of the renal medullary interstitium produces the osmotic pressure that drives the water resorption. A primary polyuria associated with a relative or absolute lack of ADH is termed central or pituitary diabetes insipidus (CDI), whereas a polyuria caused by nonresponsiveness to ADH is termed nephrogenic diabetes insipidus (NDI; Box 41-2).
Even though PU and PD usually occur together, the owner may not be aware of one or both components, depending on their severity and how closely the animal is observed. Conversely, owners frequently confuse pollakiuria with polyuria. Polyuria is often manifested by nocturia, pollakiuria and incontinence, whereas polydipsia is often manifested by a constantly empty water bowl and drinking from unusual sources, including toilets and puddles, and eating snow. It is relatively easy for most pet owners to measure 24-hour water consumption in a single-pet household, and this is a good way to confirm the presence of polydipsia; measuring water consumption in a multipet household is relatively difficult, unless the patient can be isolated.
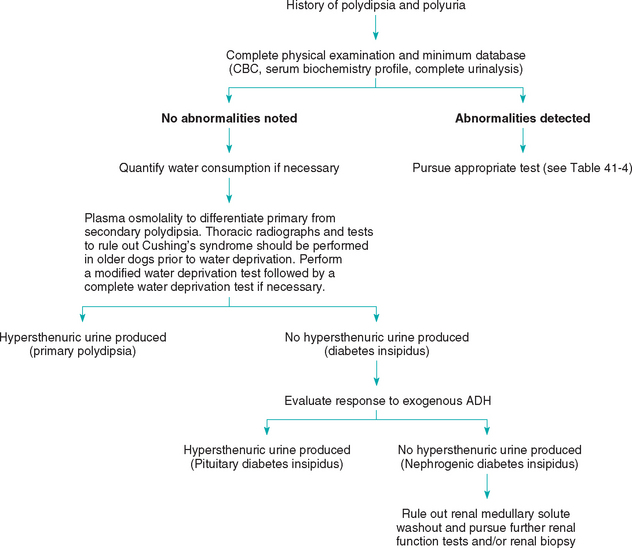
A complete history and physical examination may suggest the underlying cause in animals with polydipsia and polyuria (Fig. 41-9); these include lymphadenopathy in dogs (lymphoma with hypercalcemia); perineal mass (anal sac adenocarcinoma with hypercalcemia); cataracts (diabetes mellitus); symmetric truncal alopecia (hyperadrenocorticism); vaginal discharge (pyometra); and small, irregular kidneys (chronic kidney disease). A minimum workup consisting of a CBC, serum biochemistry profile, urinalysis, thoracic radiography, and abdominal radiography or ultrasonography may confirm or suggest a diagnosis in many animals with primary polyuria (e.g., hypercalcemia and mediastinal lymphadenopathy in dogs with lymphoma or increased serum alkaline phosphatase activity in dogs with hyperadrenocorticism). Frequently, further specific tests are necessary to confirm a diagnosis (e.g., lymph node aspiration or biopsy for lymphoma and an ACTH-stimulation test for hyperadrenocorticism [Table 41-4]).
 TABLE 41-4 Ancillary Diagnostic Tests that May Be Used to Evaluate Dogs and Cats with Polydipsia and Polyuria
TABLE 41-4 Ancillary Diagnostic Tests that May Be Used to Evaluate Dogs and Cats with Polydipsia and Polyuria
| SUSPECTED DISORDER | FURTHER DIAGNOSTIC TESTS |
|---|---|
| Primary polydipsia | Plasma osmolality, modified water deprivation, rule out hepatic insufficiency or PSS |
| Pituitary diabetes insipidus | Plasma osmolality, modified water deprivation test, response to exogenous antidiuretic hormone |
| Nephrogenic diabetes insipidus Renal insufficiency or failure | Serum urea nitrogen and creatinine concentrations, creatinine clearance, electrolyte fractional clearance, biopsy |
| Hyperadrenocorticism | ACTH-stimulation test, dexamethasone-suppression test, urine cortisol/creatinine ratio |
| Hypoadrenocorticism | Serum sodium/potassium ratio, ACTH-stimulation test |
| Hepatic insufficiency or PSS | Serum bile acids preprandially and postprandially, abdominal ultrasonography ± Doppler, 99Tc scan (enema), portal angiography, biopsy |
| Pyometra | Abdominal radiography or ultrasonography, vaginal cytology |
| Hypercalcemia | Serum calcium concentrations (total and ionized), radiography, lymph node cytology or biopsy, bone marrow cytology, PTH/PTHrp assays |
| Hypokalemia | Serum potassium concentration, potassium fractional clearance |
| Glucosuria | Obtain concurrent serum glucose concentration |
| Hyperthyroidism | Serum total and free thyroxine concentrations, triiodothyronine-suppression test, cardiac evaluation, 99Tc scanning |
| Renal medullary solute washout | Repeat water deprivation and exogenous ADH testing after gradual water restriction and dietary salt and protein supplementation for 10 to 14 days |
ACTH, Adrenocorticotropic hormone; ADH, antidiuretic hormone; PSS, portosystemic shunt; PTH, parathyroid hormone; PTHrp, parathyroid hormone–related peptide.
The urine specific gravity may also be helpful in determining the underlying cause of the syndrome and in confirming whether the pet is actually polyuric. Urine specific gravity is usually divided into four ranges: hyposthenuric urine has a specific gravity of between 1.001 and 1.007; isosthenuric urine has the same specific gravity as plasma, 1.008 to 1.012; minimally concentrated urine has a specific gravity of between 1.013 and 1.030 in dogs and 1.013 and 1.035 in cats; and hypersthenuric urine has a specific gravity of more than 1.030 in dogs and more than 1.035 in cats. The animal’s hydration status, serum urea nitrogen and creatinine concentrations, and current medications must be known in order to interpret random urine specific gravity values. For example, a normally hydrated dog may have a urine specific gravity in the isosthenuric range and a cat receiving furosemide may be somewhat dehydrated and still have minimally concentrated urine; however, normal dogs and cats should produce hypersthenuric urine in response to clinically detectable dehydration.
It is unusual for dogs and cats with PD and/or PU to have a urine specific gravity consistently in the hypersthenuric range; this finding warrants the measurement of water consumption to confirm if the patient actually has either condition. Animals with primary polydipsia or with CDI usually have urine specific gravities in the hyposthenuric range, whereas animals with nephrogenic diabetes insipidus are most likely to be isosthenuric or to have minimally concentrated urine. If the history, physical examination, and minimal diagnostic workup findings are unrewarding, specialized diagnostic tests, including determination of the plasma osmolality, gradual water deprivation testing, and determination of the animal’s response to exogenous ADH, may be necessary to arrive at a diagnosis (see Chapter 42 and Fig. 41-9).
PROTEINURIA
Normally, the urine of dogs and cats contains only a small amount of protein because the selective permeability of the glomerular capillary wall restricts the filtration of most plasma proteins on the basis of protein weight and charge. Proteins with a molecular weight greater than 60,000 to 65,000 daltons are normally not present in large quantities in normal glomerular filtrate (Table 41-5). The negatively charged glomerular capillary wall further impedes the passage of negatively charged proteins such as albumin. In addition, smaller–molecular-weight proteins, as well as those positively charged proteins that do pass through the glomerular capillary wall, are largely resorbed by the proximal tubular epithelial cells. Such resorbed proteins may be broken down and used by the epithelial cells or returned to the bloodstream. Renal proteinuria most commonly arises because of glomerular capillary wall lesions that allow increased filtration of plasma proteins into the glomerular filtrate. Tubular lesions that result in decreased reabsorption of filtered proteins (primarily albumin) are another source of renal proteinuria. Although glomerular lesions result in greater magnitude of proteinuria compared with tubular lesions, proteinuria associated with both types of lesions tends to be persistent and serves as an important marker of kidney disease.
 TABLE 41-5 Approximate Molecular Weights of Various Plasma Proteins
TABLE 41-5 Approximate Molecular Weights of Various Plasma Proteins
| PLASMA PROTEIN | MOLECULAR WEIGHT (DALTONS) |
|---|---|
| Insulin | 6,000 |
| Parathyroid hormone | 9,000 |
| Lysozyme | 14,000 |
| Myoglobin | 17,000 |
| Growth hormone | 22,000 |
| Bence Jones proteins (monomer) | 22,000 |
| Amylase | 50,000 |
| Hemoglobin | 64,500 |
| Antithrombin | 65,000 |
| Albumin | 69,000 |
| Immunoglobulin G | 160,000 |
| Immunoglobulin A (dimer) | 300,000 |
| Fibrinogen | 400,000 |
| Immunoglobulin M | 900,000 |
Proteinuria is routinely detected by semiquantitative methods, including the dipstick colorimetric test and the sulfosalicylic turbidimetric test. The dipstick test is inexpensive and easy to use; amino groups of proteins bind to the indicator incorporated in the filter paper on the dipstick and cause a color change. The color change is graded by comparing it to a standard, but the comparison is subjective. However, automated dipstick analyzers that use reflectance photometry to consistently read the color change and provide a printout of results are available (Idexx VetLab UA Analyzer, IDEXX Laboratories, Westbrook, Maine). The dipstick test is most sensitive to albumin because albumin has more free amino groups than globulins. False-positive results may be obtained if the urine is alkaline, if it has been contaminated with quaternary ammonium compounds, or if the dipstick is left in contact with the urine long enough to leach out the citrate buffer that is incorporated in the filter paper pad. False-negative results may occur in the setting of Bence Jones proteinuria or dilute or acidic urine. The dipstick test can detect approximately 30 to 1000 mg of protein per deciliter. The dipstick method is not affected by urine turbidity; however, the supernatant from centrifuged urine samples should ideally be used for all physiochemical analyses.
The sulfosalicylic acid test is performed by mixing equal quantities of urine supernate and 3% to 5% sulfosalicylic acid, and subjectively grading the turbidity that results from precipitation of protein on a 0 to 4 scale. This test is also more sensitive to albumin than globulins, but Bence Jones proteinuria can be detected. False-positive results may occur if the urine contains radiographic contrast agents, penicillin, cephalosporins, sulfisoxazole, or the urine preservative thymol. The protein content may be overestimated with the sulfosalicylic acid test if uncentrifuged urine or turbid urine is analyzed. False-negative results may occur if the urine is markedly alkaline or diluted. Because the varying degrees of turbidity are not standardized, results may also vary among laboratories. This test can detect approximately 5 to 5000 mg of protein per deciliter. Further information on such tests is contained in Chapter 42.
Proteinuria detected by these semiquantitative methods should always be interpreted in light of the urine specific gravity and urine sediment. For example, a 2 proteinuria with a 1.010 urine specific gravity is suggestive of a much greater urine protein loss on a 24-hour basis than is a 2 proteinuria with a 1.040 urine specific gravity. Because the urine protein concentration is frequently increased in animals with LUTI or hemorrhage, proteinuria should also be assessed in the context of urine sediment changes indicative of inflammation or hemorrhage (e.g., bacteria and increased numbers of white and red blood cells and epithelial cells in the urine sediment). The evaluation of the animal with proteinuria is further discussed in Chapter 42.
Once persistent proteinuria has been documented, the next step is to identify its source. Proteinuria may be caused by physiologic or pathologic conditions (Table 41-6). Physiologic or benign proteinuria is often transient and abates when the underlying cause is corrected. Strenuous exercise, seizures, fever, exposure to extreme heat or cold, and stress are examples of conditions that may cause physiologic proteinuria. The pathophysiology of physiologic proteinuria is not completely understood; however, relative renal vasoconstriction, ischemia, and congestion are thought to be involved. Decreased physical activity may also affect urine protein excretion in dogs; one study showed that urinary protein loss is higher in dogs confined to cages than in dogs with normal activity. This is different from the postural or orthostatic proteinuria that occurs in people. In the latter condition, mild proteinuria occurs when the person is standing or active but diminishes when the person is recumbent.
 TABLE 41-6 Classification of Proteinuria
TABLE 41-6 Classification of Proteinuria
| TYPE | CAUSES |
|---|---|
| Physiologic | |
| Pathologic | |
| Nonurinary | Bence Jones proteinuria |
| Hemoglobinuria or myoglobinuria | |
| Congestive heart failure | |
| Genital tract inflammation | |
| Urinary | |
| Nonrenal | Cystourolithiasis |
| Bacterial cystitis | |
| Trauma or hemorrhage | |
| Neoplasia | |
| Drug-induced cystitis (e.g., cyclophosphamide) | |
| Renal | Glomerular lesions |
| Abnormal tubular resorption | |
| Renal parenchymal inflammation or hemorrhage |
Pathologic proteinuria may be caused by urinary or nonurinary abnormalities. Nonurinary disorders associated with proteinuria often involve the production of small–molecular-weight proteins that are filtered by the glomeruli and that subsequently overwhelm the resorptive capacity of the proximal tubule. Examples of this include the production of immunoglobulin light chains (Bence Jones proteins) by neoplastic plasma cells or lymphocytes and the release of hemoglobin from damaged red blood cells, which then exceeds the binding capacity of haptoglobin (in this case centrifuged urine would be discolored by the pigment). Renal congestion secondary to congestive heart failure can also result in pathologic nonurinary proteinuria, as can genital tract inflammation (e.g., prostatitis or metritis).
Pathologic urinary proteinuria may be renal or nonrenal in origin. Nonrenal proteinuria most frequently occurs in association with LUTI or hemorrhage. Changes seen in the urine sediment usually reflect the underlying cause (e.g., urolithiasis, neoplasia, trauma, bacterial cystitis). On the other hand, renal proteinuria is most often caused by glomerular lesions. Glomerulonephritis and amyloidosis alter the selective permeability of the glomerular capillaries and frequently result in a proteinuria greater than 50 mg/kg/24 h or urine protein : creatinine ratios greater than 2.0 (see Chapter 42). The occurrence of persistent proteinuria with a normal urine sediment or accompanied by hyaline cast formation is strongly suggestive of glomerular disease. Besides glomerular disease, renal proteinuria may be caused by inflammatory or infiltrative disorders of the kidney (e.g., neoplasia, pyelonephritis) or by tubular abnormalities that result in the decreased resorption of filtered protein (e.g., Fanconi’s syndrome and chronic kidney disease).
Prerenal (physiologic and pathologic—nonurinary) and postrenal (pathologic urinary—nonrenal) proteinuria, as well as inflammatory renal proteinuria, can usually be identified on the basis of history and physical examination findings and the urine sediment changes. Renal proteinuria caused by abnormal tubular resorption may be accompanied by normoglycemic glucosuria and an abnormal urinary loss of electrolytes, which can help differentiate tubular from glomerular proteinuria. It is important to identify the source of the proteinuria because the quantification of renal proteinuria can be a helpful prognostic tool, although it is not useful in animals with prerenal or postrenal proteinuria.
AZOTEMIA
Azotemia is defined as increased concentrations of urea and creatinine (and other nonproteinaceous nitrogenous substances) in the blood. The interpretation of serum urea nitrogen and creatinine concentrations as a measure of renal function requires a knowledge of the production and excretion of these substances. Urea is synthesized in the liver from ammonia, which is in turn generated from the catabolism of ingested and endogenous proteins. Urea production is increased in the settings of a high dietary protein intake, upper gastrointestinal tract hemorrhage, and catabolic states that result in the breakdown of body proteins (e.g., fever and corticosteroid administration). Conversely, urea production is decreased in the settings of a low dietary protein intake, use of anabolic steroids, decreased hepatic function, or decreased delivery of ammonia to the liver (e.g., portosystemic shunt). Urea has a small molecular weight (60 daltons) and is a permeate solute that readily diffuses throughout all body fluid compartments; its concentration is similar in intracellular and extracellular fluid and in plasma, serum, and blood. Urea that diffuses into the intestinal lumen is degraded by enteric organisms to ammonia, which is then reabsorbed into the portal circulation and again converted to urea by the liver. Urea is principally excreted by the kidneys; it is freely filtered through the glomeruli and passively resorbed by the renal tubules. The tubular resorption of urea is increased and the net excretion decreased when tubular flow rates and volumes are decreased, as it occurs in patients with dehydration. Conversely, the tubular resorption of urea is decreased and the excretion is increased in the presence of diuresis. Decreased renal blood flow (prerenal causes, such as dehydration or decreased cardiac output) and decreased excretion of urine (postrenal causes, such as urethral obstruction or ruptured bladder), as well as primary renal dysfunction, will result in decreased excretion of urea.
Creatinine is irreversibly formed by the nonenzymatic metabolism of creatine and phosphocreatine in muscle. Creatinine production is relatively constant and proportional to muscle mass; animals with a large muscle mass produce more creatinine each day than do animals with a small muscle mass. For example, serum creatinine concentration in Greyhounds is higher than in dogs of other breeds. Muscle trauma and inflammation do not increase the production of creatinine. In comparison with the urea nitrogen concentration, the creatinine concentration is relatively unaffected by the dietary protein level; however, serum creatinine concentrations can increase after the ingestion of meat and the subsequent increased absorption of creatinine from the gastrointestinal tract. The molecular weight of creatinine is 113 daltons; therefore it diffuses throughout body fluid compartments more slowly than urea does. Some creatinine diffuses into the intestinal lumen, is degraded by enteric bacteria, and is excreted from the body in the feces; however, most creatinine is excreted by the kidneys. Creatinine is freely filtered by the glomeruli and is not significantly resorbed or secreted by the renal tubules. Because the production of creatinine is relatively constant, an increase in the serum creatinine concentration is indicative of decreased renal excretion. It is important to remember, however, that prerenal and postrenal factors influence renal function and, therefore, the excretion of creatinine. Disproportionate increases in blood urea nitrogen (BUN) relative to creatinine can be caused by high-protein diets, upper gastrointestinal hemorrhage, and increased tubular reabsorption of urea nitrogen associated with prerenal azotemia. Conversely, a disproportionately low BUN can be observed with decreased liver function, portosystemic shunts, low-protein diets, and prolonged diuresis.
Rule outs for azotemia include prerenal, renal, and postrenal causes. Any condition that causes a decrease in renal blood flow may result in prerenal azotemia, and this includes hypovolemia (e.g., dehydration, hypoadrenocorticism), hypotension (e.g., anesthesia, cardiomyopathy), and aortic or renal arterial thrombus formation. Initially, the kidneys are structurally and functionally normal in dogs and cats with prerenal azotemia, and they respond to the decreased renal blood flow by conserving water and sodium. Hypersthenuric urine (i.e., specific gravity greater than 1.030 in dogs and greater than 1.035 in cats) with a relatively low concentration of sodium and a high concentration of creatinine is produced (Table 41-7). Elimination of the underlying disorder (e.g., fluid therapy to correct hypovolemia) results in rapid resolution of the azotemia unless the underlying disorder has persisted long enough or is severe enough to have caused renal parenchymal damage.
 TABLE 41-7 Differentiation of Prerenal Azotemia from Acute Renal Failure
TABLE 41-7 Differentiation of Prerenal Azotemia from Acute Renal Failure
| INDICES | PRERENAL AZOTEMIA | ACUTE RENAL FAILURE |
|---|---|---|
| Urine specific gravity | Hypersthenuric | Isosthenuric or minimally concentrated |
| Fractional clearance of sodium (UrineNa × SerumCr/(UrineCr × SerumNa) | <1% | >2% |
| Urine creatinine-to-serum creatinine ratio | >20:1 | <10:1 |
Postrenal azotemia is usually caused by an obstruction to urine outflow or a rupture of the urine outflow tract. Similar to prerenal azotemia, in postrenal azotemia the kidneys are initially normal; however, the urine specific gravity varies depending on the animal’s hydration status. In patients with urethral obstruction, catheterization is difficult and dysuria and stranguria are common clinical signs. Rupture of the urinary tract that results in azotemia usually involves the bladder or urethra, is more common in male than female animals, and frequently results in abdominal effusion or subcutaneous fluid accumulation. Fluid obtained by abdominocentesis is usually sterile and contains a higher concentration of creatinine than the serum does. Even though creatinine is a small molecule and equilibrates rapidly, the concentration of creatinine in the abdominal fluid is higher than that of serum if the kidneys are producing urine that is draining into the abdomen. Positive contrast-enhanced urethrography or cystography is the best way to confirm a rupture of the urethra or bladder.
Renal azotemia occurs as a result of nephron loss or damage. A diagnosis of renal azotemia is confirmed if the azotemia is persistently associated with isosthenuria or minimally concentrated urine (see Table 41-7). Inasmuch as urine is usually stored in the bladder for several hours, it is important not to evaluate the specific gravity of urine produced before the onset of the azotemia. For example, prerenal azotemia may occur in response to acute, severe dehydration; however, the animal may appear to have renal azotemia if the hypersthenuric urine being produced in response to the dehydration is diluted by a larger volume of previously formed, less concentrated urine. The differentiation of prerenal from renal azotemia can be a diag-nostic challenge in some animals. Prerenal dehydration causing azotemia and accompanied by a decreased urine-concentrating ability can be confused with renal azotemia. Examples of conditions that can cause this syndrome include furosemide treatment, which causes dehydration, and hypercalcemia, which compromises the urine-concentrating ability and results in dehydration secondary to vomiting. Although fluid therapy is often implemented initially in animals with either prerenal or renal azotemia to manage the dehydration, the prognosis is quite different. Frequently, the response to fluid therapy is the best way to differentiate prerenal from renal azotemia; renal azotemia does not completely resolve in response to fluid therapy alone.
Renal failure is a state of decreased renal function in which azotemia and the inability to produce hypersthenuric urine persist concurrently. The treatment and prognosis vary for animals with acute renal failure and chronic kidney disease; therefore it is important to distinguish between these two entities. Acute renal failure (ARF) develops within hours or days. Unique clinical signs and clinicopathologic findings often associated with ARF include enlarged or swollen kidneys, hemoconcentration, good body condition, an active urine sediment, relatively severe hyperkalemia and metabolic acidosis, and relatively severe clinical signs for the degree of azotemia (Box 41-3). Chronic kidney disease (CKD) develops over a period of weeks, months, or years, and the clinical signs are often relatively mild for the magnitude of azotemia. Unique signs of CKD often include a history of weight loss and PD/PU, poor body condition, nonregenerative anemia, small and irregular kidneys, and osseous fibrodystrophy caused by secondary renal hyperparathyroidism (see Box 41-3).
RENOMEGALY
Renal enlargement is usually detected by physical examination or by abdominal imaging. A quick rule of thumb is that the kidney length on abdominal radiographs should be approximately equivalent to 2.5 to 3 times the length of the second lumbar vertebra in cats and 2.5 to 3.5 times the length of the second lumbar vertebra in dogs. Enlarged kidneys with a normal shape can be caused by edema, acute inflammation, diffusely infiltrating neoplastic disease, unilateral compensatory hypertrophy, trauma (intracapsular hemorrhage), perirenal cysts, or hydronephrosis. Enlarged, abnormally shaped kidneys may be caused by renal neoplasia, cysts, abscesses, hydronephrosis, or hematomas. Ultrasonography, intravenous urography, and advanced imaging (CT scan or magnetic resonance imaging) can be used to further define kidney shape and reveal internal details. Ultrasonography is particularly useful for evaluating enlarged kidneys associated with fluid accumulation (e.g., hydronephrosis, abscesses, and perirenal and parenchymal cysts) and can also be used to guide fine-needle aspiration or needle biopsy of the affected kidney. Kidney biopsy is often necessary to confirm the cause of the renomegaly; however, biopsy is contraindicated if only one kidney is present or if a bleeding disorder, hydronephrosis, a cyst, or an abscess is suspected.
Bartges JW. Discolored urine. In Ettinger SJ, Feldman EC, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
DiBartola SP. Renal disease: Clinical approach and laboratory evaluation. In Ettinger SJ, Feldman EC, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: Elsevier/Saunders, 2005.
Fischer JR, Lane IF. Incontinence and urine retention. In Elliott JA, Grauer GF, editors: BSAVA manual of canine and feline nephrology and urology, ed 2, Gloucester, England: British Small Animal Veterinary Association, 2007.
German A. Abnormal renal palpation. In Elliott JA, Grauer GF, editors: BSAVA manual of canine and feline nephrology and urology, ed 2, Gloucester, England: British Small Animal Veterinary Association, 2007.
Lees GE, et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM Forum Consensus Statement (Small Animal). J Vet Intern Med. 2005;19:377.
Syme HM. Polyuria and polydipsia. In Elliott JA, Grauer GF, editors: BSAVA manual of canine and feline nephrology and urology, ed 2, Gloucester, England: British Small Animal Veterinary Association, 2007.
Watson ADJ. Dysuria and hematuria. In Elliott JA, Grauer GF, editors: BSAVA manual of canine and feline nephrology and urology, ed 2, Gloucester, England: British Small Animal Veterinary Association, 2007.
Wilson HM, et al. Clinical signs, treatments, and outcome in cats with transitional cell carcinoma of the urinary bladder: 20 cases (1990-2004). J Am Vet Med Assoc. 2007;231:101.