CHAPTER 50 Disorders of the Parathyroid Gland
CLASSIFICATION OF HYPERPARATHYROIDISM
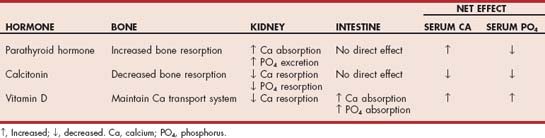
Hyperparathyroidism is a sustained increase in parathyroid hormone (PTH) secretion. Chief cells located within the parathyroid gland synthesize and secrete PTH—a peptide hormone that controls the minute-to-minute concentration of ionized calcium in the blood and extracellular fluid (ECF). The major regulator of PTH secretion is the concentration of ionized calcium in the blood. Decreased serum ionized calcium increases PTH secretion, and vice versa. PTH stimulates calcium reabsorption and inhibits phosphate reabsorption by the kidney, stimulates synthesis of the active form of vitamin D in the kidney, and stimulates bone resorption. The net effect is to increase serum ionized and total calcium concentration and decrease serum phosphorus concentration.
Hyperparathyroidism can result from a normal physiologic response to decreased serum ionized calcium concentrations (renal, nutritional, and adrenal secondary hyperparathyroidism) or a pathologic condition resulting from excessive synthesis and secretion of PTH by abnormal, autonomously functioning parathyroid chief cells (i.e., primary hyperparathyroidism [PHP]). In PHP increased secretion of PTH is maintained regardless of the serum ionized calcium concentration.
Hypercalcemia and hypophosphatemia develop as a result of the physiologic actions of PTH. In renal secondary hyperparathyroidism renal failure causes retention of phosphate and development of hyperphosphatemia. Hyperphosphatemia decreases serum ionized calcium concentration by the mass law effect ([Ca] × [Pi] = constant). The decrease in serum ionized calcium, in turn, stimulates PTH secretion. The net effect is increased serum phosphate, normal-to-low serum ionized calcium, increased serum PTH concentration, and diffuse parathyroid gland hyperplasia. The etiogenesis of hyperparathyroidism is similar in nutritional secondary hyperparathyroidism, except the decrease in calcium results from feeding diets containing low calcium-to-phosphorus ratios, such as beef heart or liver. Dietary calcium deficiency or phosphorus excess decreases serum calcium concentration, inducing increased PTH secretion and parathyroid gland hyperplasia. An increase in serum PTH has been documented in dogs with hyperadrenocoricism and is believed to be a compensatory response to increased calcium loss and/or increased serum phosphate concentrations—hence the term adrenal secondary hyperparathyroidism. Serum phosphate and PTH decrease and serum calcium increases after successful treatment of hyperadrenocorticism.
PRIMARY HYPERPARATHYROIDISM
Etiology
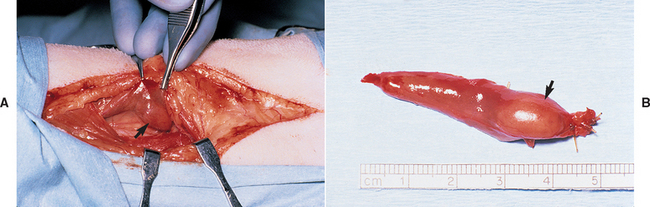
PHP is a disorder resulting from the excessive, relatively uncontrolled secretion of PTH by one or more abnormal parathyroid glands. The physiologic actions of PTH ultimately cause hypercalcemia and hypophosphatemia (Table 50-1). It is an uncommon disorder in the dog and rare in the cat. Parathyroid adenoma is the most common histologic finding; parathyroid carcinoma and parathyroid hyperplasia have also been described in dogs and cats but are uncommon. Parathyroid adenomas are typically small, well-encapsulated, light brown to red tumors located in close apposition to the thyroid gland (Fig. 50-1). The remaining parathyroid glands are normal, atrophied, or not visible at surgery. Parathyroid carcinomas grossly appear similar to adenomas; the diagnosis of carcinoma is based on finding certain histologic features such as capsular or vascular invasion by the tumor. The biologic behavior of parathyroid carcinoma is not well characterized in dogs and cats. Similarly, the histologic criteria for differentiating between adenoma and hyperplasia is not well established. Although involvement of multiple parathyroid glands suggests hyperplasia, adenoma involving two glands and hyperplasia involving only one gland have been identified in dogs with PHP. In addition, hyperplasia caused by renal and nutritional secondary hyperparathyroidism may not cause uniform enlargement of the parathyroid glands even though the stimulus for enlargement is the same for each gland. Differentiating hyperplasia from adenoma has important prognostic implications. The surgical removal of parathyroid adenoma(s) results in a cure, assuming at least one normal parathyroid gland remains to prevent hypoparathyroidism. In contrast, hypercalcemia caused by parathyroid hyperplasia may persist or recur weeks to months after surgery if the remaining grossly normal-appearing parathyroid tissue is hyperplastic at the time of surgery or becomes hyperplastic in the future.
SIGNALMENT
The age at which clinical signs of PHP appear in dogs ranges from 4 to 16 years, with a mean age of 10 years. There is no sex-related predilection. Any breed of dog can be affected, although PHP is most commonly diagnosed in the Keeshond and is an autosomal dominant, genetically transmitted disease in this breed. The age at the time of diagnosis of PHP in cats has ranged from 8 to 20 years, with a mean age of 13 years. The majority of cats have been mixed breed and Siamese. There is no apparent sex predisposition.
CLINICAL SIGNS
Clinical signs of PHP result from the physiologic actions of excessive PTH secretion rather than from the space-occupying nature of the tumor. Clinical signs are caused by hypercalcemia, which is the hallmark of this disorder, and by the presence of cystic calculi and lower urinary tract infections, which are consequences of the hypercalcemia. Clinical signs are absent in most dogs and cats with the mildest form of PHP, and hypercalcemia is discovered only after a serum biochemistry panel is performed, often for unrelated reasons. When clinical signs do develop, they initially tend to be nonspecific and insidious in onset. The clinical signs in dogs are typically renal, gastrointestinal, and neuromuscular in origin (Box 50-1). The most common clinical signs in cats with PHP are lethargy, anorexia, and vomiting. Less common clinical signs in cats include constipation, polyuria, polydipsia, and weight loss.
PHYSICAL EXAMINATION
The physical examination is usually normal, which is an important diagnostic finding when differentiating dogs with PHP from dogs with hypercalcemia of malignancy (see Chapter 55). Lethargy, generalized muscle atrophy, weakness, and cystic calculi (calcium phosphate, calcium oxalate, or both types) may be noted in some dogs with PHP. The severity of weakness is variable but usually subtle. Cervical palpation of a parathyroid mass is rare in dogs with PHP. If a mass is palpated in the neck of a dog with hypercalcemia, thyroid gland carcinoma; squamous cell carcinoma; lymphoma; and, least likely, parathyroid gland carcinoma should be considered. In contrast, cats with PHP often have a palpable parathyroid mass that is typically located in the region of the thyroid gland. As such, a palpable mass in the ventral cervical region of the neck should raise suspicion for hyperthyroidism (common) as well as PHP (rare) in cats.
Diagnosis
PHP should be suspected in a dog or cat with persistent hypercalcemia and normophosphatemia to hypophosphatemia. The serum calcium concentration is typically 12 to 15 mg/dl but can exceed 16 mg/dl. The serum ionized calcium concentration is typically 1.4 to 1.8 mmol/L but can exceed 2.0 mmol/L. The serum phosphorus concentration is typically less than 4 mg/dl, unless concurrent renal insufficiency is present. Although hypercalcemia in dogs and cats has several causes (Table 50-2), the primary differential diagnoses for hypercalcemia and hypophosphatemia are humoral hypercalcemia of malignancy (most notably lymphoma in dogs and carcinomas in cats) and PHP (see Chapter 55). The history, findings on physical examination, results of routine blood and urine tests, thoracic radiographs, abdominal and cervical ultrasound, and measurement of PTH and parathyroid hormone–related peptide (PTHrp) will usually establish the diagnosis. With PHP clinical signs are usually mild to absent, the physical examination is normal, and results of routine blood work, thoracic and abdominal radiography, and abdominal ultrasonography are unremarkable, except for hypercalcemia, hypophosphatemia, and cystic calculi. Additional tests used to identify lymphoma as the cause of hypercalcemia (i.e., cytologic evaluations of bone marrow and lymph node, liver, and splenic aspirates and PTHrp concentrations) are normal in dogs with PHP.
 TABLE 50-2 Causes of Hypercalcemia in Dogs and Cats
TABLE 50-2 Causes of Hypercalcemia in Dogs and Cats
| DISORDER | TESTS TO HELP ESTABLISH THE DIAGNOSIS |
|---|---|
| Primary hyperparathyroidism | Serum PTH concentration, cervical ultrasound, surgery |
| Physical examination, thoracic and abdominal radiography, abdominal ultrasonography, aspiration of lymph nodes, liver, spleen and bone marrow, serum PTHrp | |
| History, serum biochemistry panel, serum vitamin D concentration | |
| Hypoadrenocorticism | Serum electrolytes, ACTH stimulation test |
| Renal failure | Serum biochemistry panel, urinalysis |
| Idiopathic—cats | Rule out by exclusion |
| Thoracic radiography, abdominal ultrasonography, fundic examination, cytologic studies of pulmonary wash samples or intestinal biopsy specimens, serum fungal titers | |
| Radiography of peripheral skeleton | |
| History | |
| Dehydration (mild hyercalcemia) | — |
| — | |
| Laboratory error | Repeat calcium measurement |
PTH, Parathyroid hormone; LSA, lymphosarcoma; PTHrp, parathyroid hormone–related peptide; ACTH, adrenocorticotropic hormone; FIP, feline infectious peritonitis.
Renal failure in a dog with hypercalcemia can create a diagnostic dilemma. Fortunately, development of hypercalcemia-induced renal failure rarely occurs in dogs with PHP. Prolonged severe hypercalcemia may cause progres-sive nephrocalcinosis, renal damage, and azotemia, but most dogs with PHP have mild hypercalcemia and concurrent hypophosphatemia; the latter protects the kidney by keeping the calcium × phosphorus product less than 50. Measurement of serum ionized calcium concentration will help identify the etiology of hypercalcemia in dogs with concurrent renal failure. Serum ionized calcium concentration is typically normal in dogs with renal failure–induced hypercalcemia and increased in dogs with PHP and concurrent renal failure. Urine specific gravity is usually not helpful when assessing renal function in dogs with hypercalcemia because of the interference of calcium with the actions of vasopressin on renal tubular cells. Urine specific gravities less than 1.015 are common in dogs with PHP. Hematuria, pyuria, bacteriuria, and crystalluria may be identified if cystic calculi and secondary bacterial cystitis develop. Hypercalciuria, proximal renal tubular acidosis with impaired bicarbonate resorption, and the production of alkaline urine may predispose dogs to the development of cystic or renal calculi and bacterial cystitis. In one study urinary tract infection was identified in 29% and cystic calculi in 31% of 210 dogs with PHP (Feldman et al., 2005). Uroliths are typically composed of calcium phosphate, calcium oxalate, or mixtures of the two salts.
Cervical ultrasound should identify one or more enlarged parathyroid glands in dogs and cats with PHP (Fig. 50-2). The parathyroid glands of healthy dogs are typically 3 mm or less in maximum width when visualized ultrasonographically. The maximum width of the abnormal parathyroid glands ranged from 3 to 23 mm (median 6 mm) in 130 dogs with PHP (Feldman et al., 2005). A solitary parathyroid mass was identified in 89%, and two parathyroid masses were identified in 10% of the dogs.
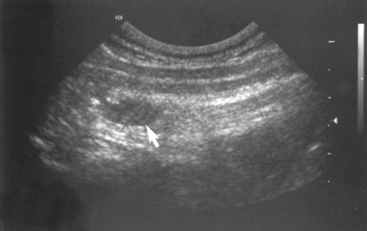
FIG 50-2 Ultrasound image of the right thyroid lobe of a 13-year-old Labrador Retriever with hypercalcemia and primary hyperparathyroidism. A hypoechoic mass is seen in the region of the parathyroid gland (arrow). Hypercalcemia resolved following heat ablation of the parathyroid mass.
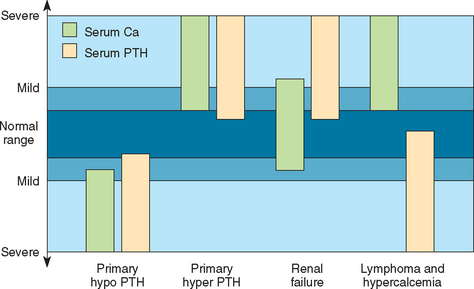
Measurement of baseline serum PTH concentration is used to establish the diagnosis of PHP. The two-site immunoradiometric (IRMA) assay system is currently used by most veterinary laboratories and is considered the most reliable assay system for PTH quantification in dogs and cats. Most laboratories have a similar PTH reference range for dogs (2 to 13 pmol/L) and cats (0.8 to 4.6 pmol/L). The major regulator of PTH secretion is the concentration of ionized calcium in the blood. Decreased serum ionized calcium increases PTH secretion, and vice versa. Serum PTH test results should always be interpreted in conjunction with serum calcium or, preferably, serum ionized calcium measured from the same blood sample. If the parathyroid gland is functioning normally, the serum PTH concentration should be below the reference range or undetectable in the face of hypercalcemia because of the inhibitory effects of an increased serum calcium concentration on parathyroid gland function. Dogs with nonparathyroid-induced hypercalcemia should also have low to undetectable serum PTH concentrations. Serum PTH concentration within or above the reference range is inappropriate in the face of hypercalcemia and indicative of an autonomously functioning parathyroid gland (Fig. 50-3). In 185 dogs with PHP none had serum PTH concentration below the reference range, 45% were in the lower half of the reference range (2.3 to 7.9 pmol/L), 28% were in the upper half of the reference range (8.0 to 13.0 pmol/L), and 27% had increased serum PTH concentrations (13 to 121 pmol/L; Feldman et al., 2005).
Treatment
Surgical removal of the abnormal parathyroid tissue is the treatment of choice. Slatter (2003) and Fossum (2007) have adequately described the surgical techniques for the thyroparathyroid complex (see Suggested Readings). Almost all dogs and cats with PHP have a solitary, easily identified parathyroid adenoma (see Fig. 50-1). Enlargement of more than one parathyroid gland indicates the presence of either multiple adenomas or parathyroid hyperplasia. If none of the parathyroid glands appear enlarged or if all appear small, the diagnosis of PHP must be questioned and hypercalcemia stemming from occult neoplasia or PTH production by a parathyroid tumor in an ectopic site (e.g., cranial mediastinum) or by a nonparathyroid tumor should be considered.
Chemical (i.e., ethanol) and heat ablation of abnormal parathyroid tissue performed under ultrasound guidance are also effective treatments for PHP (Fig. 50-4). Surgery is avoided, anesthetic time is significantly reduced, and there are no incisions or issues related to wound healing. However, the management of the dog after chemical or heat ablation is identical to the management after surgical removal of the parathyroid mass. In a recent retrospective study surgical removal, heat ablation, and chemical ablation of the parathyroid mass were successful in controlling hypercalcemia in 94%, 90%, and 72% of dogs treated for PHP, respectively (Rasor et al., 2007). Not all dogs are candidates for chemical or heat ablation. Surgery is indicated if more than one parathyroid mass is identified with cervical ultrasound, the parathyroid mass is less than 4 mm or greater than 15 mm in maximum width, a parathyroid mass is not identified, the parathyroid mass is too close to the carotid artery, or cystic calculi are identified with abdominal radiographs or ultrasound.
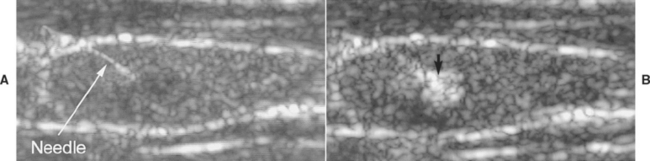
FIG 50-4 A, Ultrasound image of the left thyroid lobe of an 12-year-old Keeshond with hypercalcemia. A mass is in the region of the parathyroid gland (arrow), and a needle has been inserted into the mass using ultrasound guidance before heat ablation of the mass. B, Heat is being administered to the mass, causing hyperechogenicity of the mass (arrow).
An attempt must be made to ensure that at least one parathyroid gland remains intact to maintain calcium homeostasis and prevent permanent hypocalcemia. Removal or ablation of the parathyroid tumor results in a rapid decline in circulating PTH and a decrease in serum calcium. In the early stages of PHP the remaining parathyroid glands may secrete PTH in response to the decrease in serum calcium, thereby preventing development of severe hypocalcemia. In dogs with more advanced PHP, atrophy of the normal parathyroid glands may prevent a response to the decrease in serum calcium, leading to severe hypocalcemia and clinical signs within 7 days of surgery or ablation. In these dogs intravenous and oral calcium and oral vitamin D therapy must be initiated to correct and/or prevent hypocalcemia.
There are two approaches for managing the dog (and cat) once the parathyroid tumor has been removed with surgery or ablation. One approach is to arbitrarily treat all dogs with oral calcium and vitamin D at the time the parathyroid tumor is removed, and another approach is to withhold calcium and vitamin D therapy until the serum calcium concentration decreases below a safe concentration, typically a serum calcium or ionized calcium concentration of 9.0 mg/dl and 0.9 mmol/L, respectively, and before clinical signs of hypocalcemia develop. Regardless of which approach is taken, serum total or ionized calcium should be monitored once or twice a day until the serum calcium concentration is stable and in the reference range. I prefer to withhold calcium and vitamin D therapy in dogs in which I suspect parathyroid gland atrophy is mild and calcium and vitamin D therapy may not be needed. The higher the preoperative serum calcium concentration or the more chronic the hypercalcemic condition, or both, the more likely the dog will become clinically hypocalcemic after removal of the abnormal parathyroid gland or glands. As a general rule, I do not initially treat hyperparathyroid dogs with oral calcium and vitamin D if the serum calcium or ionized calcium concentration before surgery or ablation is less than 14 mg/dl or 1.6 mmol/L, respectively, and hypercalcemia has been present for less than 6 months. Serum calcium or ionized calcium concentrations greater than 14 mg/dl and 1.6 mmol/L, respectively, and hypercalcemia that has been present for greater than 6 months suggest the existence of significant atrophy of the remaining parathyroid glands and a high probability for the development of signs of hypocalcemia after surgery or ablation. In these dogs oral calcium and vitamin D therapy is started at the time PHP is treated. In dogs with severe hypercalcemia (total calcium or ionized calcium>18 mg/dl and 2.0 mmol/L, respectively), vitamin D therapy can be initiated 24 to 36 hours before surgery or ablation because of the known delay in the onset of vitamin D’s action.
Therapy for hypocalcemia includes the administration of intravenous calcium to control immediate clinical signs and the long-term oral administration of calcium and vitamin D supplements to maintain low-normal blood calcium concentrations while the parathyroid gland atrophy resolves. (See Chapter 55 and Box 55-7 for details about the management of hypocalcemia.) The goal of calcium and vitamin D therapy is to maintain the serum calcium concentration within the low to low-normal range (9 to 10 mg/dl). Maintaining the serum calcium concentration in the low-normal range prevents development of clinical signs of hypocalcemia, minimizes the risk of hypercalcemia, and stimulates a return of function in the remaining atrophied parathyroid glands. Once the parathyroid glands regain control of calcium homeostasis and the serum calcium concentration is stable in the dog or cat in the home environment, the calcium and vitamin D supplements can be gradually withdrawn over a period of 3 to 6 months. This gradual withdrawal allows time for the parathyroid glands to become fully functional and thereby prevents hypocalcemia. Vitamin D therapy is withdrawn by gradually increasing the number of days between administrations. The dosing interval should be increased by 1 day every 2 to 3 weeks, after the serum calcium concentration has been measured and found to be 9 mg/dl or greater. Vitamin D therapy can be discontinued once the dog or cat is clinically normal, the serum calcium concentration is stable between 9 and 11 mg/dl, and the vitamin D dosing interval is every 7 days.
Prognosis
The prognosis for dogs and cats undergoing surgical or ablation therapy for PHP is excellent, assuming severe hypocalcemia is avoided postoperatively and PHP is caused by a parathyroid adenoma. Hypercalcemia may recur weeks to months after surgery in dogs and cats with PHP caused by parathyroid hyperplasia if one or more parathyroid glands have been left in situ.
PRIMARY HYPOPARATHYROIDISM
Etiology
Primary hypoparathyroidism develops as a result of an absolute or relative deficiency in the secretion of PTH. This deficiency ultimately causes hypocalcemia and hyperphosphatemia because of a loss of the effects of PTH on bone, kidney, and intestine (see Table 50-1). The major signs of hypoparathyroidism are directly attributable to the decreased concentration of ionized calcium in the blood, which leads to increased neuromuscular activity.
Spontaneous primary hypoparathyroidism is uncommon in dogs and cats. Most cases are classified as idiopathic (i.e., there is no evidence of trauma, malignant or surgical destruction, or other obvious damage to the neck or parathyroid glands). The glands are difficult to locate visually and show microscopic evidence of atrophy. Histologic evaluation of the parathyroid gland may reveal a diffuse lymphocytic, plasmacytic infiltration and fibrous connective tissue, suggesting an underlying immune-mediated cause of the disorder.
Iatrogenic hypoparathyroidism after performance of bilateral thyroidectomy for the treatment of hyperthyroidism is common in cats. The parathyroid tissue in such animals may be excised or traumatized, or its blood supply may be compromised during surgery. This form of hypoparathyroidism may be transient or permanent, depending on the viability of the parathyroid gland or glands saved at the time of surgery. Only one viable parathyroid gland is needed to maintain a normal serum calcium concentration.
Transient hypoparathyroidism may develop secondary to severe magnesium depletion (serum magnesium concentration <1.2 mg/dl). Severe magnesium depletion may suppress PTH secretion without parathyroid destruction, increase end-organ resistance to PTH, and impair the synthesis of the active form of vitamin D (i.e., calcitriol). The end result is mild hypocalcemia and hyperphosphatemia. Magnesium repletion reverses the hypoparathyroidism. Serum magnesium concentrations in dogs and cats with spontaneous primary hypoparathyroidism usually have been normal when measured. (See Chapter 55 for more information on magnesium.)
SIGNALMENT
The age at which the clinical signs of hypoparathyroidism appear in dogs ranges from 6 weeks to 13 years, with a mean of 4.8 years. There may be a sex-related predisposition in female dogs. There is no apparent breed-related predisposition, although Toy Poodles, Miniature Schnauzers, Labrador Retrievers, German Shepherd Dogs, and Terriers are commonly affected breeds. However, this increased prevalence may merely reflect the popularity of these breeds. Only a few cases of naturally acquired primary hypoparathyroidism in cats have been reported. To date, these cats have been young to middle-aged (6 months to 7 years), of several breeds, and usually male.
CLINICAL SIGNS
The clinical signs and physical examination findings in dogs and cats with primary hypoparathyroidism are similar. The major clinical signs are directly attributable to hypocalcemia, most notably its effects on the neuromuscular system. Neuromuscular signs include nervousness, generalized seizures, focal muscle twitching, rear-limb cramping or tetany, ataxia, and weakness (Box 50-2). Additional signs include lethargy, inappetence, intense facial rubbing, and panting. The onset of clinical signs tends to be abrupt and severe and to occur more frequently during exercise, excitement, and stress. Clinical signs also tend to occur episodically. Episodes of clinical hypocalcemia are interspersed with relatively normal periods, lasting minutes to days. Interestingly, hypocalcemia persists during these clinically “normal” periods.
PHYSICAL EXAMINATION
The most common physical examination findings are related to muscular tetany and include a stiff gait; muscle rigidity; a tense, splinted abdomen; and muscle fasciculations. Fever, panting, and nervousness, often so pronounced that they interfere with the examination, are also common. Potential cardiac abnormalities include bradycardia, paroxysmal tachyarrhythmias, muffled heart sounds, and weak femoral pulses. Cataracts have been noted in a few dogs and cats with primary hypoparathyroidism. Cataracts were small, punctate-to-linear, white opacities that were randomly distributed in the anterior and posterior cortical subcapsular region of the lens; there was no loss of vision. The physical examination is occasionally normal, despite the previous history of neuromuscular disorders.
Diagnosis
Primary hypoparathyroidism should be suspected in a dog or cat with persistent hypocalcemia, hyperphosphatemia, and normal renal function. The serum calcium concentration is usually less than 7 mg/dl, the serum ionized calcium is usually less than 0.8 mmol/L, and the serum phosphorus is usually greater than 6 mg/dl. Low serum calcium and high serum phosphorus concentrations can also be encountered during nutritional and renal secondary hyperparathyroidism, after phosphate-containing enema, and during tumor lysis syndrome. The diagnosis of primary hypoparathyroidism is established by identifying an undetectable serum PTH concentration in the face of severe hypocalcemia in a dog or cat in which other causes of hypocalcemia have been ruled out (Table 50-3). Most causes of hypocalcemia can be identified after evaluation of the history, findings on physical examination, and results of routine blood and urine tests and an abdominal ultrasound. The history and physical examination findings are essentially unremarkable in dogs and cats with primary hypoparathyroidism, other than those findings caused by hypocalcemia. The only relevant abnormalities identified on routine blood and urine tests are severe hypocalcemia and, in most dogs and cats, hyperphosphatemia. The serum total protein, albumin, urea nitrogen, creatinine, and magnesium concentrations are normal. Abdominal ultrasound is also normal.
 TABLE 50-3 Causes of Hypocalcemia in Dogs and Cats
TABLE 50-3 Causes of Hypocalcemia in Dogs and Cats
| DISORDER | TESTS TO HELP ESTABLISH THE DIAGNOSIS |
|---|---|
| History, serum PTH concentration, rule out other causes | |
| Puerperal tetany | History |
| Serum biochemistry panel, urinalysis | |
| Ethylene glycol toxicity | History, urinalysis |
| Acute pancreatitis | Physical findings, abdominal ultrasound, serum PLI |
| Intestinal malabsorption syndromes | History, digestion and absorption tests, intestinal biopsy |
| Hypoproteinemia or hypoalbuminemia | Serum biochemistry panel |
| Hypomagnesemia | Serum total and ionized Mg |
| Nutritional secondary hyperparathyroidism | Dietary/History |
| Tumor lysis syndrome | History |
| Phosphate-containing enemas | History |
| Anticonvulsant medications | History |
| NaHCO3 administration | History |
| Laboratory error | Repeat calcium measurement |
PTH, parathyroid hormone; PLI, pancreatic lipase immunoreactivity; Mg, magnesium.
Measurement of serum PTH concentration helps confirm a diagnosis of primary hypoparathyroidism. Blood for PTH determination should be obtained before the initiation of calcium and vitamin D therapy while the animal is still hypocalcemic. The two-site IRMA assay system is currently used by most veterinary laboratories and is considered the most reliable assay system for PTH quantification in dogs and cats. Interpretation of the serum PTH concentration must be done in conjunction with the serum calcium concentration. If the parathyroid gland is functioning normally, the serum PTH concentration should be increased in the face of hypocalcemia because of the stimulatory effects of a decreased serum ionized calcium concentration on parathyroid gland function. A low-to-undetectable serum PTH concentration in a hypocalcemic dog or cat is strongly suggestive of primary hypoparathyroidism (see Fig. 50-3). Dogs and cats with nonparathyroid-induced hypocalcemia should have normal or high serum PTH concentrations; the exceptions are those disorders causing severe hypomagnesemia.
Treatment
The therapy for primary hypoparathyroidism involves the administration of vitamin D and calcium supplements (see Chapter 55 and Box 55-7). Therapy is typically divided into two phases. The first phase (i.e., acute therapy) should initially control hypocalcemic tetany and involves the slow administration of calcium gluconate (not calcium chloride) intravenously, to effect. Once clinical signs of hypocalcemia are controlled, calcium gluconate should then be administered by continuous intravenous infusion until orally administered calcium and vitamin D therapy (i.e., second phase of therapy) becomes effective. Calcium gluconate is initially administered at a dose of 60 to 90 mg/kg per day (approximately 2.5 ml/kg of 10% calcium gluconate added to the infusion solution and administered every 6 to 8 hours). Calcium should not be added to solutions containing lactate, bicarbonate, acetate, or phosphates because of the potential for precipitation problems. Serum calcium concentrations should be monitored twice a day and the rate of infusion adjusted as needed to control clinical signs and maintain the serum calcium concentration greater than 8 mg/dl.
The second phase of therapy (i.e., maintenance therapy) should maintain the blood calcium concentration between 8 and 10 mg/dl through the daily administration of vitamin D and calcium. These calcium concentrations are above the level at which there is a risk for clinical hypocalcemia and below the level at which hypercalciuria (risk of calculi formation) or severe hypercalcemia and hyperphosphatemia (risk of nephrocalcinosis and renal failure) may occur. Maintenance therapy should be initiated once the hypocalcemic tetany is controlled with intravenous calcium therapy. The onset of action of vitamin D varies depending on the formulation of vitamin D that is administered. In general, 1,25-dihydroxy-vitamin D3 (calcitriol) has the fastest onset of action and is preferred for treating hypoparathyroidism. The initial dosage of calcitriol is 0.02 to 0.03 μg/kg/day. Dogs and cats should ideally remain hospitalized until their serum calcium concentration remains between 8 and 10 mg/dl without parenteral support. Serum calcium concentrations should be monitored weekly, with the vitamin D dose adjusted to maintain a concentration of 8 to 10 mg/dl. The aim of therapy is to prevent hypocalcemic tetany and not induce hypercalcemia. Serum calcium concentrations of more than 10 mg/dl are unnecessary to prevent tetany and only increase the likelihood of unwanted hypercalcemia.
Once the serum calcium concentration has stabilized, attempts can be made to slowly taper the dose of oral calcium and then vitamin D to the lowest dose that maintains the serum calcium concentration between 8 and 10 mg/dl. Vitamin D is critical for establishing and maintaining a normal blood calcium concentration. Most dogs and cats with primary hypoparathyroidism require permanent vitamin D therapy. The calcium supplement can often be gradually tapered over a period of 2 to 4 months and then stopped once the animal’s serum calcium concentration is stable between 8 and 10 mg/dl. Calcium in the diet is often sufficient for maintaining the calcium needs of the animal. Supplementing the diet with calcium-rich foods (e.g., dairy products) helps ensure an adequate source of dietary calcium. Once the animal’s serum calcium concentration is stable and maintenance therapy has become established, reevaluation of the serum calcium concentration every 3 to 4 months is advisable.
Prognosis
The prognosis depends on the dedication of the client. The prognosis is excellent if proper therapy is instituted and timely reevaluations are performed. Proper management requires close monitoring of the serum calcium concentration. The more frequent the rechecks, the better the chance of preventing extremes in the concentration and the better the chance of a normal life expectancy.
Feldman EC, Nelson RW. Canine and feline endocrinology and reproduction, ed 3. St Louis: WB Saunders, 2004.
Fossum TW. Small animal surgery, ed 3. St Louis: Mosby, 2007.
Slatter D. Textbook of small animal surgery, ed 3. Philadelphia: WB Saunders, 2003.
Bolliger AP, et al. Detection of parathyroid hormone-related protein in cats with humoral hypercalcemia of malignancy. Vet Clin Path. 2002;31:3.
Feldman EC, et al. Pretreatment clinical and laboratory findings in dogs with primary hyperparathyroidism: 210 cases (1987-2004). J Am Vet Med Assoc. 2005;227:756.
Gear RNA, et al. Primary hyperparathyroidism in 29 dogs: diagnosis, treatment, outcome and associated renal failure. J Small Anim Pract. 2005;46:10.
Goldstein RE, et al. Inheritance, mode of inheritance, and candidate genes for primary hyperparathyroidism in Keeshonden. J Vet Intern Med. 2007;21:199.
Long CD, et al. Percutaneous ultrasound-guided chemical parathyroid ablation for treatment of primary hyperparathyroidism in dogs. J Am Vet Med Assoc. 1999;215:217.
Pollard RE, et al. Percutaneous ultrasonographically guided radiofrequency heat ablation for treatment of primary hyperparathyroidism in dogs. J Am Vet Med Assoc. 2001;218:1106.
Rasor L, et al. Retrospective evaluation of three treatment methods for primary hyperparathyroidism in dogs. J Am Anim Hosp Assoc. 2007;43:70.
Tebb AJ, et al. Canine hyperadrenocorticism: effects of trilostane on parathyroid hormone, calcium and phosphate concentration. J Small Anim Pract. 2005;46:537.
 TABLE 50-1
TABLE 50-1