CHAPTER 55 Electrolyte Imbalances
HYPERNATREMIA
Etiology
Hypernatremia exists if the serum sodium concentration exceeds 160 mEq/L, although reference ranges may vary between laboratories. It most commonly develops after water loss exceeds sodium loss (Box 55-1). The water loss may be pure (i.e., not accompanied by a loss of electrolytes, such as that which occurs with diabetes insipidus), or it may be hypotonic (i.e., loss of both water and sodium but with the water loss predominating, such as that which occurs with gastrointestinal fluid loss and renal failure). Insufficient water intake or an abnormal thirst mechanism are usually facets of an excessive water loss. Rarely, hypernatremia may occur in animals with hypodipsia caused by neurologic disease, an abnormal thirst mechanism, or defective osmoregulation of vasopressin release.
 BOX 55-1 Causes of Hypernatremia in Dogs and Cats
BOX 55-1 Causes of Hypernatremia in Dogs and Cats
Modified from DiBartola SP: Disorders of sodium and water: hypernatremia and hyponatremia. In DiBartola SP, editor: Fluid, electrolyte and acid-base disorders in small animal practice, ed 3, St Louis, 2006, Saunders/Elsevier.
* Common causes.
Less commonly, hypernatremia develops after sodium retention, such as that which occurs with iatrogenic sodium overload or primary hyperaldosteronism. Primary hyperaldosteronism is caused by an aldosterone-secreting adrenal tumor or idiopathic bilateral adrenal hyperplasia but is rare in dogs and cats. Increased serum aldosterone concentrations cause variable hypernatremia, hypokalemia, and systemic hypertension.
Clinical Features
Clinical signs of hypernatremia originate in the central nervous system (CNS) and include lethargy, weakness, muscle fasciculations, disorientation, behavioral changes, ataxia, seizures, stupor, and coma. Clinical signs typically become apparent when the plasma osmolality exceeds 350 mOsm/kg (serum sodium concentration of greater than 170 mEq/L). Clinical signs are caused by neuronal dehydration. Hypernatremia and hyperosmolality cause fluid to shift from the intracellular to the extracellular space. As the brain shrinks, meningeal vessels are damaged and torn, causing hemorrhage, hematoma, venous thrombosis, infarction of cerebral vessels, and ischemia. This gradient flow of water from the intracellular to the extracellular compartment often maintains adequate skin turgor and gives a false impression of hydration, even though the animal has experienced a detrimental loss of fluid.
The severity of clinical signs is related to the absolute increase in serum sodium concentration and especially the rapidity of onset of hypernatremia and hyperosmolality. Clinical signs usually do not develop until the serum sodium concentration approaches 170 mEq/L. If hypernatremia is rapid in onset, clinical signs may develop at a lower sodium concentration, and vice versa. With a gradual increase in the serum sodium concentration, the cells in the CNS can produce osmotically active solutes (idiogenic osmoles) intracellularly to reestablish osmotic equilibration between the extracellular and intracellular compartments, thereby minimizing cell shrinkage.
Diagnosis
Measurement of the serum sodium concentration identifies hypernatremia. After it has been identified, the underlying cause should be sought. Careful evaluation of the history, physical examination findings, and results of routine clinical pathologic tests (i.e., complete blood count [CBC], serum biochemistry panel, urinalysis) usually yields clues to the cause. Evaluation of the urine specific gravity is especially helpful. Hypernatremia and hyperosmolality stimulate the release of vasopressin, resulting in hypersthenuria. A urine specific gravity of less than 1.008 in a dog or cat with hypernatremia is consistent with central or nephrogenic diabetes insipidus. A urine specific gravity of more than 1.030 in a dog and 1.035 in a cat implies a normal vasopressin–renal tubular axis and indicates the existence of sodium retention, primary hypodipsia-adipsia, or gastrointestinal or insensible water loss. A urine specific gravity of between 1.008 and 1.030 (dog) or of 1.035 (cat) indicates the presence of partial vasopressin deficiency or an impaired renal tubular response to vasopressin, most likely secondary to a primary renal disorder.
Treatment
The goal in treating hypernatremia is to restore the extracellular fluid (ECF) volume to normal and correct water deficits at a fluid rate that avoids significant complications and to identify and correct the underlying cause of the hypernatremia. The initial priority is to restore ECF volume to normal. In animals with modest volume contraction (e.g., tachycardia, dry mucous membranes, slow skin turgor), fluid deficits should be corrected with 0.45% saline supplemented with an appropriate amount of potassium (Table 55-1). With severe dehydration 0.9% saline solution, plasma, or whole blood should be used to expand vascular volume. In deficit replacement rapid administration of fluids is contraindicated unless there are signs of significant hypovolemia. Any fluid should be administered in a volume only large enough to correct hypovolemia. Worsening neurologic status or sudden onset of seizures during fluid therapy is generally indicative of cerebral edema and the need for hypertonic saline solution or mannitol therapy. Once ECF deficits have been replaced, the serum sodium (Na) concentration should be reevaluated and water deficits corrected if hypernatremia persists. An approximation of the water deficit in liters may be calculated using the following formula:
 TABLE 55-1 Guidelines for Potassium Supplementation in IV Fluids
TABLE 55-1 Guidelines for Potassium Supplementation in IV Fluids
| SERUM K+ (mEq/L) | TOTAL mEq OF K+ /LITER OF FLUIDS | MAXIMUM FLUID INFUSION RATE (ml/kg/h)* |
|---|---|---|
| >3.5 | 20 mEq | 25 |
| 3.0-3.5 | 30 mEq | 16 |
| 2.5-3.0 | 40 mEq | 12 |
| 2.0-2.5 | 60 mEq | 8 |
| <2.0 | 80 mEq | 6 |
* Total hourly potassium administration should not exceed 0.5 mEq/kg body weight.
Because the brain adjusts to hypertonicity by increasing the intracellular solute content via the accumulation of “idiogenic osmoles,” the rapid repletion of body water with ECF dilution causes translocation of water into cells and may cause cerebral edema. If slower water repletion is undertaken, brain cells lose the accumulated intracellular solutes and osmotic equilibration can occur without cell swelling.
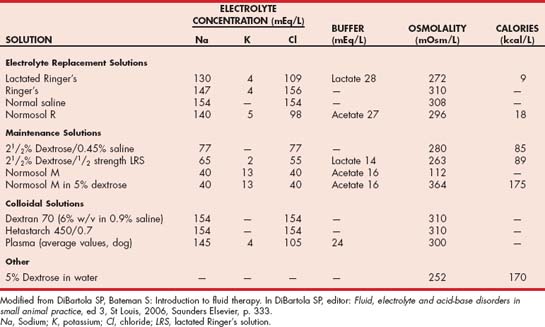
Maintenance crystalloid solutions (e.g., half-strength [0.45%] saline solution with 2.5% dextrose or half-strength lactated Ringer’s solution with 2.5% dextrose) should be used to correct the water deficit in hypernatremic animals with normal perfusion and hydration and should also be used in dehydrated animals with persistent hypernatremia after the correction of fluid deficits. D5W solution can be substituted for maintenance crystalloid solutions if the hypernatremia does not abate after 12 to 24 hours of fluid therapy.
Oral fluid administration is preferable for correcting water deficits, with fluid administered through an intravenous (IV) route if oral administration is not possible. The water deficit should be replaced slowly. Approximately 50% of the water deficit should be corrected in the first 24 hours, with the remainder corrected over the ensuing 24 to 48 hours. The serum sodium concentration should decline slowly, preferably at a rate of less than 1 mEq/L/hour. A gradual reduction in the serum sodium concentration minimizes the fluid shift from the extracellular to the intracellular compartment, thereby minimizing neuronal cell swelling and cerebral edema and increasing intracranial pressure. A deterioration in CNS status after the start of fluid therapy indicates the presence of cerebral edema and the immediate need to reduce the rate of fluid administration. Frequent monitoring of serum electrolyte concentrations, with appropriate adjustments in the type of fluid administered and rate of fluid administration, is important in the successful management of hypernatremia.
On rare occasions a hypernatremic animal presents with an increase in the ECF volume. Such animals are difficult to treat. The goal is to lower the serum sodium concentration without exacerbating an increase in the ECF volume and causing pulmonary congestion and edema. To slowly correct hypernatremia in these animals, the clinician should administer loop diuretics (e.g., furosemide, 1 to 2 mg/kg orally or intravenously q8-12h) to promote sodium loss in the urine, and this is done in conjunction with the judicious administration of D5W.
HYPONATREMIA
Etiology
Hyponatremia is present if the serum sodium concentration is less than 140 mEq/L, although reference ranges may vary between laboratories. It can result from excessive sodium loss, primarily through the kidney, or from increased water conservation, or both. The latter condition may be an appropriate response to a reduction in the ECF volume or may be inappropriate (e.g., syndrome of inappropriate antidiuretic hormone secretion). In most cases hyponatremia results from abnormalities in water balance (principally a defect in renal water excretion) rather than from abnormalities in sodium balance. Causes of hyponatremia in dogs and cats are listed in Box 55-2.
 BOX 55-2 Causes of Hyponatremia in Dogs and Cats
BOX 55-2 Causes of Hyponatremia in Dogs and Cats
Modified from DiBartola SP: Disorders of sodium and water: hypernatremia and hyponatremia. In DiBartola SP, editor: Fluid, electrolyte and acid-base disorders in small animal practice, ed 3, St Louis, 2006, Saunders/Elsevier.
Hyponatremia must be differentiated from pseudohyponatremia, which is a decrease in the serum sodium concentration as a result of laboratory methodology in the presence of normal plasma osmolality. Pseudohyponatremia occurs in the presence of hyperlipidemia or severe hyperproteinemia. An increase in the concentration of triglycerides or proteins in plasma reduces the sodium concentration in the total plasma volume, but the sodium concentration in plasma water remains the same. Methods that measure the amount of sodium in a specific volume of plasma (e.g., flame photometry) result in falsely low sodium values, whereas methodologies that determine the sodium concentration in the aqueous phase of plasma (e.g., direct potentiometry using ion-selective electrodes) yield an accurate sodium value. Pseudohyponatremia can usually be identified if the method used to measure the sodium concentration is known, a blood sample is examined for the presence of gross lipemia, and a CBC and serum biochemistry panel are performed.
Hyponatremia may also occur after there is an increase in the concentration of osmotically active solutes (e.g., glucose, mannitol) in the ECF. An increase in the concentration of osmotically active solutes in the ECF causes a fluid shift from the intracellular to the extracellular compartment and a corresponding decrease in the serum sodium concentration. For example, the serum sodium concentration decreases approximately 1.6 mEq/L for every 100 mg/dl increase in the serum glucose concentration, and this decrease may become more severe when the blood glucose concentration exceeds 500 mg/dl. Estimation of the plasma osmolality is helpful in differentiating the cause of hyponatremia. Hyponatremia is usually associated with hyposmolality (less than 290 mOsm/kg), whereas pseudohyponatremia is associated with normal plasma osmolality, and hyponatremia caused by an increase in osmotically active solutes in the ECF is associated with hyperosmolality. Plasma osmolality can be estimated using the following formula:
Normal plasma osmolality in dogs and cats is approximately 280 to 310 mOsm/kg.
Clinical Features
Clinical signs of hyponatremia include lethargy, anorexia, vomiting, weakness, muscle fasciculations, disorientation, seizures, and coma. CNS signs are the most worrisome and develop as changes in plasma osmolality cause fluid to shift from the extracellular to the intracellular space, resulting in neuronal swelling and lysis. The onset and severity of clinical signs depend on the rapidity with which the hyponatremia develops as well as on the degree of hyponatremia. The more chronic the hyponatremia and the more slowly it develops, the more capable the brain is of compensating for changes in osmolality through the loss of potassium and organic osmolytes from cells. Clinical signs develop when the decrease in plasma osmolality occurs faster than the brain’s defense mechanisms can counter the influx of water into the neurons.
Diagnosis
Hyponatremia is readily evident from measurement of serum electrolyte concentrations. However, hyponatremia must be differentiated from pseudohyponatremia (discussed in a previous section). Hyponatremia is not a diagnosis per se but rather a manifestation of an underlying disorder. As such, a diagnostic evaluation to identify the cause, as well as appropriate therapy to correct the hyponatremia, should be initiated. In most dogs and cats the cause of hyponatremia is readily apparent after evaluation of the history, physical examination findings, CBC, serum biochemistry panel, and urinalysis findings, but further diagnostic tests may be necessary. Careful assessment of the urine specific gravity; the hydration status of the animal; and, if necessary, the fractional excretion of sodium (FENa) help localize the problem (Fig. 55-1). The FENa can be determined by first measuring the urine (UNa) and plasma (PNa) sodium and urine (UCr) and plasma (PCr) creatinine concentrations and then applying the following formula:
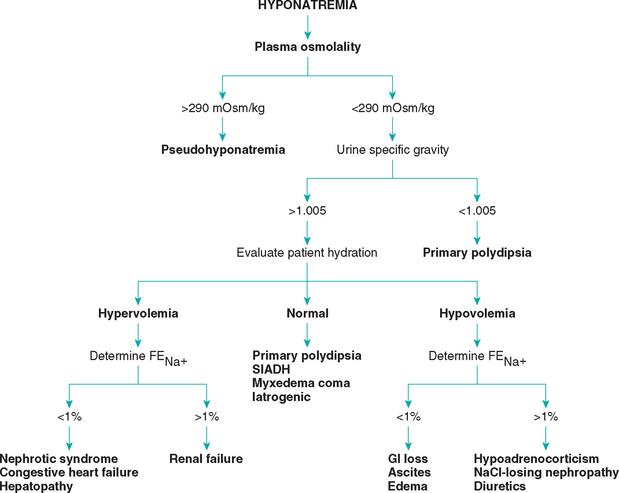
FIG 55-1 Diagnostic approach to hyponatremia. FENa, Fractional excretion of sodium.
(Adapted from DiBartola SP: Hyponatremia, Vet Clin North Am 19:215, 1989.)
Treatment
The goals of therapy are to treat the underlying disease and, if necessary, to increase the serum sodium concentration and plasma osmolality. The goal of treatment directed at the hyponatremia is to correct body water osmolality and restore cell volume to normal by raising the ratio of sodium to water in ECF using IV fluid therapy, water restriction, or both. The increase in ECF osmolality draws water from cells and therefore reduces their volume. The approach to treatment and the type of fluid used depend on the underlying etiology, the severity of the hyponatremia, and the presence or absence of clinical signs (Table 55-2). Chronic hyponatremia in an asymptomatic animal is best treated conservatively. Lactated Ringer’s or Ringer’s solution can be used for mild hyponatremia (serum sodium concentration of more than 135 mEq/L) and physiologic saline solution for more severe hyponatremia (serum sodium concentration of less than 135 mEq/L). Physiologic saline solution is typically used in symptomatic animals with severe hyponatremia.
Fluid and electrolyte balance should gradually be restored over 24 to 48 hours, with periodic assessment of serum electrolyte concentrations. The more acute and severe the hyponatremia, the more slowly the serum sodium concentration should be corrected. A rapid increase in the serum sodium concentration to levels greater than 125 mEq/L is potentially dangerous and should be avoided in animals with acute, severe hyponatremia (serum sodium concentration of less than 120 mEq/L) and neurologic signs. For these animals the serum sodium concentration should be gradually increased to 125 mEq/L or higher over 6 to 8 hours. Because loss of brain solute represents one of the compensatory mechanisms for preserving brain cell volume during dilutional states, an increase in serum sodium concentration toward normal is relatively hypertonic to brain cells that are partially depleted of solute as a result of hyponatremia. Consequently, raising the serum sodium concentration rapidly to greater than 125 mEq/L can cause CNS damage. Dietary sodium restriction (e.g., Prescription Diet h/d, Hill’s Pet Products) and diuretic therapy should be considered in edematous animals.
HYPERKALEMIA
Etiology
Hyperkalemia is present if the serum potassium concentration exceeds 5.5 mEq/L, although reference ranges may vary between laboratories. Hyperkalemia can develop after an increased potassium intake (uncommon), after a compartmental shift in potassium from the intracellular to extracellular space (uncommon), or as a result of impaired potassium excretion in the urine (common; Box 55-3). Impaired urinary excretion of potassium is usually caused by renal dysfunction or hypoadrenocorticism. Iatrogenic-induced hyperkalemia is also common in dogs and cats. Pseudohyperkalemia refers to an increase in potassium in vitro and can occur in the setting of severe hypernatremia (if dry reagent methodologies are used), leukocytosis (white blood cell count of more than 100,000/μl), and thrombocytosis (more than 1 × 106/μl); if the blood specimen has been obtained from fluid lines or catheters contaminated with potassium-containing fluids; and in the setting of hemolysis in Akitas (and possibly Shiba Inus and Kindos) and in English Springer Spaniels with phosphofructokinase deficiency.
 BOX 55-3 Causes of Hyperkalemia in Dogs and Cats
BOX 55-3 Causes of Hyperkalemia in Dogs and Cats
Modified from DiBartola SP, Autran de Morais H: Disorders of potassium: hypokalemia and hyperkalemia. In DiBartola SP, editor: Fluid, electrolyte and acid-base disorders in small animal practice, ed 3, St Louis, 2006, Saunders Elsevier. ICF, Intracellular fluid; ECF, extracellular fluid; DKA, diabetic ketoacidosis.
Iatrogenic†
Clinical Features
The clinical manifestations of hyperkalemia reflect changes in cell membrane excitability and the magnitude and rapidity of onset of hyperkalemia. Mild-to-moderate hyperkalemia (serum potassium concentration of less than 6.5 mEq/L) is typically asymptomatic. Generalized skeletal muscle weakness develops as the hyperkalemia worsens. Weakness occurs after a hyperkalemia-induced decrease in the resting cell membrane potential to the level of the threshold potential, thereby impairing repolarization and subsequent cell excitation. The most prominent manifestations of hyperkalemia are cardiac in nature. Hyperkalemia causes decreased myocardial excitability, an increased myocardial refractory period, and slowed conduction, effects that may cause potentially life-threatening cardiac rhythm disturbances (Box 55-4).
Diagnosis
Measurement of the serum potassium concentration or electrocardiography can identify hyperkalemia. Once it has been identified, a careful review of the history, physical findings, CBC, serum biochemistry panel, and urinalysis usually yields clues to the cause. The most common causes for hyperkalemia in the dog and cat are iatrogenic, most notably excessive potassium administration in IV fluids; renal dysfunction, especially acute oliguric-anuric renal failure, urethral obstruction (tomcats), and rupture within the urinary system leading to uroabdomen; and hypoadrenocorticism. It can be a diagnostic challenge to differentiate renal dysfunction from hypoadrenocorticism because both disorders can result in a similar clinical picture. An adrenocorticotropic hormone (ACTH) stimulation test is needed to confirm hypoadrenocorticism. Small rents in the urinary bladder can be difficult to identify, and contrast-enhanced radiographic studies or surgical exploration is frequently necessary to confirm their presence.
Treatment
For most animals therapy for hyperkalemia is directed at treating the underlying cause. Symptomatic therapy for hyperkalemia should be initiated if the serum potassium concentration is greater than 7 mEq/L or if pronounced cardiac toxicity (i.e., complete heart block, premature ventricular contractions, arrhythmias) is identified on an electrocardiogram (ECG; Table 55-3). The rapid institution of therapy in animals with marked hyperkalemia could mean the difference between life and death. The goal of symptomatic therapy is to reverse the cardiotoxic effects of hyperkalemia and, if possible, reestablish normokalemia. Asymptomatic animals with normal urine output and chronic hyperkalemia of less than 7 mEq/L may not require immediate treatment, but a search for the underlying cause should be initiated.
IV fluid administration in amounts designed to correct fluid deficits and cause volume expansion rehydrates the animal, improves renal perfusion and potassium excretion, and dilutes the blood potassium concentration. Physiologic saline solution is the fluid of choice for this purpose. Potassium-containing fluids (e.g., lactated Ringer’s solution) can be used if physiologic saline solution is not available because the low potassium concentration in these fluids (see Table 55-2) in relation to that in blood will still have a dilutional effect on the blood potassium concentration. Dextrose can be added to the fluids to make a 5% to 10% dextrosecontaining solution, or 1 to 2 ml/kg of 50% dextrose can be administered by slow IV bolus. Dextrose stimulates insulin secretion, which in turn promotes the movement of glucose and potassium from the extracellular to the intracellular space. Fluids containing more than 5% dextrose should be given into a central vein to minimize the risk of phlebitis.
Rarely, additional therapy may be required to block the cardiotoxic effects of hyperkalemia (see Table 55-3). Sodium bicarbonate and regular insulin given with dextrose act to shift potassium from the extracellular to the intracellular space. IV calcium infusions block the effects of hyperkalemia on cell membranes but do not lower the blood potassium concentration. These therapies constitute aggressive, short-term, life-saving measures that can reestablish normal cardiac conduction until more conventional therapy (i.e., IV fluids) has the time to become effective.
HYPOKALEMIA
Etiology
Hypokalemia is present when the serum potassium concentration is less than 4.0 mEq/L, although reference ranges may vary between laboratories. Hypokalemia can develop after decreased dietary potassium intake (uncommon), translocation of potassium from the ECF to the intracellular fluid (common), or increased potassium loss in urine or gastrointestinal secretions (common; Box 55-5). Iatrogenic hypokalemia is also common in dogs and cats. Pseudohypokalemia is uncommon and depends on the method used to measure the serum potassium concentration. Hyperlipidemia, hyperproteinemia (more than 10 g/dl), hyperglycemia (more than 750 mg/dl), and azotemia (urea nitrogen concentration of more than 115 mg/dl) can potentially cause pseudohypokalemia.
 BOX 55-5 Causes of Hypokalemia in Dogs and Cats
BOX 55-5 Causes of Hypokalemia in Dogs and Cats
Modified from DiBartola SP, Autran de Morais H: Disorders of potassium: hypokalemia and hyperkalemia. In DiBartola SP, editor: Fluid, electrolyte and acid-base disorders in small animal practice, ed 3, St Louis, 2006, Saunders/Elsevier. ECF, Extracellular fluid; ICF, intracellular fluid.
Iatrogenic*
* Common causes.
Clinical Features
Most dogs and cats with mild to moderate hypokalemia (i.e., 3.0 to 4.0 mEq/L) are asymptomatic. Clinically severe hypokalemia primarily affects the neuromuscular and cardiovascular systems, owing to the hypokalemia-induced initial hyperpolarization followed by hypopolarization of cell membranes. The most common clinical sign of hypokalemia is generalized skeletal muscle weakness. In cats ventroflexion of the neck (see Chapter 72), forelimb hypermetria, and a broad-based hindlimb stance may be observed. The timing of the onset of hypokalemia-induced weakness is extremely variable among animals. Cats seem more susceptible than dogs to the deleterious effects of hypokalemia. In dogs signs may not be evident until the serum potassium concentration is less than 2.5 mEq/L, whereas in cats signs can be seen when the serum potassium concentration is between 3 and 3.5 mEq/L.
Cardiac consequences of hypokalemia include decreased myocardial contractility, decreased cardiac output, and disturbances in cardiac rhythm. Cardiac disturbances assume a variable clinical expression, often evidenced only by electrocardigraphy (see Box 55-4). Other metabolic effects of hypokalemia include hypokalemic nephropathy, which is characterized by chronic tubulointerstitial nephritis, impaired renal function, and azotemia and manifested clinically as polyuria, polydipsia, and impaired urine concentrating capability; hypokalemic polymyopathy, which is characterized by increased serum creatine kinase activity and electromyographic abnormalities; and paralytic ileus, manifested clinically as abdominal distention, anorexia, vomiting, and constipation. Hypokalemic nephropathy and polymyopathy are most notable in cats.
Diagnosis
Measurement of the serum potassium concentration identifies hypokalemia. Once it has been identified, a careful review of the history, physical findings, CBC, serum biochemistry panel, and urinalysis findings usually provides clues to the cause (see Box 55-5). If the cause is not readily apparent after review of this information, other, less likely causes for hypokalemia should be considered, such as renal tubular acidosis or another renal potassium-wasting disorder, primary hyperaldosteronism, and hypomagnesemia. To help differentiate renal and nonrenal sources of potassium loss, the clinician may need to determine the fractional excretion of potassium determined on the basis of a single urine and serum potassium and creatinine concentration or determine 24-hour urine potassium excretion (see Chapter 42).
Treatment
Therapy is indicated if the serum potassium concentration is less than 3 mEq/L, if clinical signs related to hypokalemia are present, or if a serum potassium loss is anticipated (e.g., insulin therapy in diabetic ketoacidosis [DKA]) and the animal’s ability to compensate for the loss is impaired. The goal of therapy is to reestablish and maintain normokalemia without inducing hyperkalemia.
Potassium supplements should be given orally whenever possible. Oral potassium supplements come in the form of elixirs, wax-matrix tablets, and microencapsulated slow- release formulations. Problems with oral preparations include poor palatability, which can be minimized by mixing them with food, and gastrointestinal tract irritation, which may cause vomiting, diarrhea, and melena. Two products that are well accepted by most dogs and cats and that have minimal gastrointestinal tract side effects are potassium gluconate elixir (Kaon Elixir, Adria Laboratories) and potassium gluconate prepared in a palatable protein base (Tumil-K, Virbac). The recommended dose for these products is 2.2 mEq of potassium per 100 calories of required energy intake per day or 2 mEq of potassium per 4.5 kg of body weight twice a day. Subsequent adjustments in dosage are made on the basis of clinical response and serum potassium concentrations. Bananas are also a good source of potassium. Ten inches (25 cm) of banana contains approximately 10 mEq of potassium.
Parenteral potassium supplementation is indicated if oral administration is not possible (e.g., vomiting, anorexia). Potassium chloride is the compound most commonly used, in part to help promote chloride as well as potassium repletion. IV administration is preferred, although potassium chloride can be given subcutaneously as long as the concentration of potassium does not exceed 30 mEq/L. In dogs and cats with normal renal function, the maintenance amount of potassium supplementation is approximately 20 mEq/L of fluids. The initial amount of potassium added to fluids depends on the animal’s serum potassium concentration (see Table 55-1) and the amount of potassium already present in the fluids (see Table 55-2). The rate of IV potassium administration should not exceed 0.5 mEq/kg/hour.
It is difficult to estimate the amount of potassium required to reestablish normal potassium balance on the basis of the serum potassium concentration because potassium is primarily an intracellular cation. As such, serial measurement of the serum potassium concentration is important during treatment and should initially be done every 6 to 12 hours depending on the severity of the hypokalemia and the rate of potassium administration. Adjustments in potassium therapy should be made accordingly, with the goal of establishing a normal serum potassium concentration and then maintaining the serum potassium concentration in the normal range as treatment is withdrawn. Clinical signs of hypokalemia usually resolve within 1 to 5 days after correction of hypokalemia. Depending on the underlying cause, long-term oral potassium supplementation may be required to prevent recurrence of hypokalemia.
HYPERCALCEMIA
Identification
Hypercalcemia is present if the serum calcium concentration is greater than 12 mg/dl or the serum ionized calcium concentration is greater than 1.45 mmol/L, although reference ranges may vary between laboratories. The serum total and ionized calcium concentration is higher in puppies than in adult dogs. A mild increase in the serum total calcium (i.e., less than 13 mg/dl), ionized calcium (i.e., less than 1.55 mmol/L), and phosphorus (i.e., less than 10 mg/dl) concentrations in a clinically healthy puppy, together with an increase in the serum alkaline phosphatase activity and normal urea nitrogen and creatinine concentrations, should be considered normal. The serum total calcium concentration does not fluctuate with age in cats, but the serum ionized calcium concentration may be higher (less than 0.1 mmol/L) in cats younger than 2 years of age compared with results in older cats.
Most automated and in-house serum chemistry analyzers measure the total serum calcium concentration, which consists of biologically active, ionized calcium (55%); protein-bound calcium (35%); and calcium complexes (10%). A drawback to this is that alterations in the plasma protein concentration may alter the total serum calcium concentration, yet the ionized calcium concentration remains normal. For this reason the serum albumin and total protein concentrations should be measured when determining the total serum calcium concentration in the dog. Simple quantitative changes in the albumin and total plasma proteins do not cause hypocalcemia or hypercalcemia in dogs, even though the total serum calcium levels may appear to be low or high on the biochemistry panel. The following formulas have been used to estimate the total serum calcium concentration in dogs with hypoalbuminemia or hypoproteinemia:
The formulas are not used in dogs younger than 24 weeks of age, because high values may be obtained, nor are they used in cats, because there is no linear relationship between serum total calcium and serum albumin and total protein concentration in cats. These formulas yield a rough estimate of the total serum calcium concentration and were developed without verification by serum ionized calcium measurements. Subsequent studies identified a poor correlation between the adjusted total calcium results and the corresponding serum ionized calcium concentration, suggesting that adjusted total serum calcium concentrations are not reliable indicators of calcium homeostasis.
The biologically active, ionized fraction of calcium can be determined directly, which bypasses the influence of plasma proteins on the total serum calcium concentration. Ionized calcium measurements are generally superior to serum total calcium measurements for assessing calcium in dogs and cats. Automated equipment that uses a calcium ion-selective electrode allows accurate measurement of ionized calcium in blood, plasma, or serum. Ionized calcium results can be affected by many variables, including method of sample collection (samples collected anaerobically provide more precise results); the amount and type of heparin, if used (may underestimate or overestimate ionized calcium results); and change in sample pH (ionized calcium increases as pH decreases). Protocols established by the clinical chemistry laboratory for submitting blood samples for ionized calcium determination should be followed to ensure accurate results.
Etiology
Hypercalcemia is uncommon in dogs and cats. Persistent hypercalcemia usually results from increased calcium resorption from bone or kidney or increased calcium absorption from the gastrointestinal tract. Humoral hypercalcemia of malignancy (HHM), the most common cause of hypercalcemia, occurs when the tumor produces substances that promote osteoclastic activity and renal calcium reabsorption. These substances include parathyroid hormone (PTH); parathyroid hormone–related peptide (PTHrP); 1,25dihydroxyvitamin D; cytokines, such as interleukin-1 and tumor necrosis factor; prostaglandins; and humoral factors that stimulate renal 1-α-hydroxylase. Tumors may also induce hypercalcemia by local osteolytic activity after they metastasize to bone. Less commonly, hypercalcemia develops from impaired loss of calcium from the serum (e.g., reduced glomerular filtration) or reduced plasma volume (e.g., dehydration).
The list of differential diagnoses for hypercalcemia is relatively short in dogs and cats (see Table 50-2). In the dog HHM (especially lymphoma), hypoadrenocorticism, chronic renal failure, hypervitaminosis D, and primary hyperparathyroidism are the most common diagnoses. In the cat idiopathic hypercalcemia, hypercalcemia of malignancy (especially lymphoma and squamous cell carcinoma), chronic renal failure, and primary hyperparathyroidism are the most common diagnoses. Calcium oxalate urolithiasis and consumption of acidifying diets are commonly identified in cats with hypercalcemia, but their role, if any, in causing the disorder is unknown.
Hypercalcemia can develop in dogs and cats with chronic and, less commonly, acute renal failure. The pathogenesis of hypercalcemia associated with renal failure is complicated. The development of autonomously functioning parathyroid glands or an alteration of the set point for PTH secretion after the prolonged stimulation of renal secondary hyperparathyroidism, decreased PTH degradation by renal tubular cells, increased PTH-mediated intestinal absorption of calcium, increased PTH-mediated bone resorption, decreased renal excretion of calcium, and increased protein-bound or complexed fractions of calcium are believed to contribute to the hypercalcemia of renal failure. Prolonged hypercalcemia, especially in conjunction with concurrent high-normal to increased serum phosphorus concentration, can also cause renal insufficiency and azotemia. Determining whether the renal failure is primary or secondary in a dog with hypercalcemia, hyperphosphatemia, and azotemia poses an interesting diagnostic challenge (see the diagnosis section).
Clinical Features
Although all tissues can be affected by hypercalcemia, the neuromuscular, gastrointestinal, renal, and cardiac systems are the most important clinically. Secondary nephrogenic diabetes insipidus, loss of the renal concentration gradient, and metastatic mineralization of the kidney cause polyuria and polydipsia. Decreased excitability of the central and peripheral nervous systems occurring in conjunction with decreased excitability of gastrointestinal smooth muscle causes lethargy, anorexia, vomiting, constipation, weakness, and (rarely) seizures. In rare instances cardiac arrhythmias may develop in animals with severe hypercalcemia (i.e., more than 18 mg/dl). Prolongation of the PR interval and shortening of the QT interval may be found on electrocardiographic readings recorded in animals with milder hypercalcemia.
Clinical signs are often absent with mild increases in the serum calcium concentration, and hypercalcemia is discovered only after a serum biochemistry panel is performed, often for unrelated reasons. When clinical signs do develop, they initially tend to be insidious in onset. The severity of clinical signs depends in part on the severity, rate of onset, and duration of the hypercalcemia. Clinical signs become more severe as the magnitude of the hypercalcemia increases, regardless of the rate of onset or duration. Clinical signs are usually mild with serum calcium concentrations less than 14 mg/dl, are readily apparent with concentrations greater than 14 mg/dl, and become potentially life-threatening (i.e., cardiac arrhythmias) when the serum calcium concentration exceeds 18 to 20 mg/dl. Clinical signs resulting from the development of calcium uroliths may also occur.
Diagnosis
Hypercalcemia should always be reconfirmed, preferably from a nonlipemic blood sample obtained from the dog or cat following a 12-hour fast, before embarking on an extensive diagnostic evaluation. Results of a CBC, serum biochemistry panel, and urinalysis, in conjunction with the history and physical examination findings, often provide clues to the diagnosis (see Table 50-2). Special attention should be paid to the serum electrolytes and renal parameters. Hypoadrenocorticism-induced hypercalcemia typically occurs in conjunction with mineralocorticoid deficiency; hyponatremia, hyperkalemia, and prerenal azotemia should be present. The serum phosphorus concentration is in the lower half of the normal range or low with HHM and primary hyperparathyroidism (Fig. 55-2). If the serum phosphorus concentration is increased and renal function is normal, hypervitaminosis D and bone osteolysis from metastatic or primary bone neoplasia are the primary differentials.
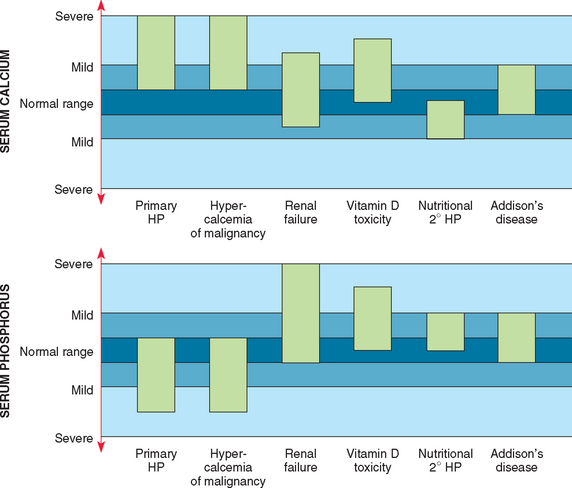
FIG 55-2 The range in serum calcium and phosphorus concentrations for the more common causes of hypercalcemia and/or hyperparathyroidism in the dog. HP, Hyperparathyroidism; 2° HP, secondary hyperparathyroidism.
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, Philadelphia, 2004, WB Saunders.)
Determining whether renal failure is primary or secondary to hypercalcemia caused by another disorder when hyperphosphatemia and hypercalcemia co-exist with azotemia can be difficult. Chronic and, less commonly, acute renal failure can cause hypercalcemia. Alternatively, disorders that cause persistent hypercalcemia with a concurrent high- normal to increased serum phosphorus concentration can cause progressive mineralization of the kidney and eventual renal failure. Measurement of the serum ionized calcium concentration may help identify dogs and cats with renal failure–induced hypercalcemia; serum ionized calcium concentrations are typically normal or decreased in renal failure and increased in hypercalcemia caused by other disorders.
Hypercalcemia of malignancy and primary hyperparathyroidism are the primary differentials when hypercalcemia and normal-to-low serum phosphorus concentrations are identified. The most common malignancy is lymphoma. A careful review of the history and physical examination findings may provide clues to the diagnosis. Systemic signs of illness suggest hypercalcemia of malignancy. Dogs and cats with primary hyperparathyroidism are usually healthy, and clinical signs are mild. The appendicular skeleton, peripheral lymph nodes, abdominal cavity, and rectum should be carefully palpated for masses, lymphadenopathy, hepatomegaly, splenomegaly, or pain on digital palpation of the long bones. Diagnostic tests that are helpful in identifying the underlying malignancy include thoracic and abdominal radiographs; abdominal ultrasound; cytologic evaluation of aspirates of the liver, spleen, lymph nodes, and bone marrow; determination of the serum ionized calcium, PTH, and PTHrP concentrations; and cervical ultrasound.
Sternal and hilar lymphadenopathy is common with lymphoma-induced hypercalcemia and can be readily identified with thoracic radiographs. Radiographs of the thorax and abdomen can also be used to evaluate bones; discrete lytic lesions in the vertebrae or long bones suggest multiple myeloma. Hyperproteinemia, proteinuria, and plasma cell infiltration in the bone marrow suggest multiple myeloma. Cytologic evaluation of peripheral lymph node, bone marrow, and splenic aspirates can be helpful in identifying lymphoma; involvement of the peripheral lymph nodes or spleen by lymphoma can be present without causing their enlargement. Ideally, the largest lymph node should be evaluated. Normal lymph node, bone marrow, and splenic aspirates do not rule out lymphoma.
Measurement of the serum ionized calcium, PTH, and PTHrP levels from the same blood sample is helpful in differentiating primary hyperparathyroidism from HHM. Excessive secretion of biologically active PTHrP plays a central role in the pathogenesis of hypercalcemia in most forms of HHM. An increased serum ionized calcium concentration, a detectable serum PTHrP concentration, and a nondetectable serum PTH concentration are diagnostic for HHM. Lymphoma is the most common cause of detectable PTHrP concentrations, but other tumors, including apocrine gland adenocarcinoma and various carcinomas (e.g., mammary gland, squamous cell, bronchogenic), can also cause hypercalcemia by this mechanism. In contrast, an increased serum ionized calcium concentration, a normal to increased serum PTH concentration, and a nondetectable PTHrP concentration are diagnostic of primary hyperparathyroidism. Ultrasonographic examination of the thyroparathyroid complex may reveal enlargement of one or more parathyroid glands. Most parathyroid adenomas measure 4 to 10 mm in greatest diameter, although parathyroid adenomas can exceed 2 cm. In contrast, the parathyroid glands will be small or undetectable with hypercalcemia of malignancy.
Evaluation of the change in the serum calcium concentration following l-asparaginase administration should be considered for the animal with hypercalcemia of undetermined etiology to rule out occult lymphoma. For the l-asparaginase trial 20,000 IU/m2 of the drug is administered intravenously, and the serum calcium concentration is measured before and every 12 hours after administration for as long as 72 hours. A decline in the serum calcium level, usually into the normal range, is strongly suggestive of occult lymphoma. Hypersensitivity reactions are the most common adverse effect associated with l-asparaginase administration; pretreatment with an antihistamine is recommended.
Idiopathic hypercalcemia is a common diagnosis in young and middle-aged cats that is established by ruling out the other causes of hypercalcemia. Hypercalcemia is usually mild (less than 13 mg/dl), and cats are usually asymptomatic. The serum phosphorus concentration and renal parameters are normal. The etiology is unknown. The results of a complete diagnostic evaluation, as described previously, are unremarkable. Serum PTH concentrations are in the normal range or low; primary hyperparathyroidism has not been confirmed in any of these cats. Excessive serum PTHrP, 25-hydroxyvitamin D or calcitriol concentrations have not been identified. Nephrocalcinosis and urolithiasis may develop, presumably secondary to increased urinary calcium excretion. Effective treatment has not been identified primarily because the pathogenesis of this problem remains unknown. Serum calcium concentrations have decreased in some cats after a change to a high-fiber diet or renal diets containing low calcium and phosphorus content or after prednisone treatment (initial dose, 5 mg q24h) was initiated, but the response has been unpredictable and often short-lived. Preliminary trials with oral biphosphonates (e.g., alendronate) have been promising in some cats with idiopathic hypercalcemia (see treatment section). Serum calcium, phosphorus, and renal parameters should be monitored periodically in affected cats and appropriate therapy initiated if renal insufficiency begins to develop (see Chapter 44).
Treatment
Medical therapy should be directed at eradicating the underlying cause of the hypercalcemia. Supportive therapy to decrease the serum calcium concentration to less toxic levels is indicated if clinical signs are severe, if the serum calcium concentration is greater than 16 mg/dl, if the calciumphosphorus solubility product ([Ca] × [Pi]) is greater than 60 to 70 (implying metastatic mineralization of soft tissues), or if azotemia is present. In dogs and cats correction of fluid deficits, saline diuresis, diuretic therapy with furosemide, and corticosteroids are the most commonly used modes of therapy (Box 55-6). Prerenal azotemia is common in dogs with hypercalcemia secondary to water restriction imposed by owners concerned about the polyuria and polydipsia. As such, diuretics should never be administered before volume replenishment is completed.
 BOX 55-6 Nonspecific Therapy for Control of Hypercalcemia
BOX 55-6 Nonspecific Therapy for Control of Hypercalcemia
IV, Intravenous; IM, intramuscular; PO, by mouth; SC, subcutaneous.
The supportive therapy implemented should not interfere with attempts to establish a definitive diagnosis. As a general rule, saline diuresis followed by diuretic therapy can be initiated without compromising the results of diagnostic tests. Because of the high incidence of lymphoma in animals with hypercalcemia, glucocorticoids should not be administered unless the cause of the hypercalcemia has been identified.
Calcitonin may be useful in the treatment of animals with severe hypercalcemia and could be used in lieu of prednisone for treating hypercalcemia in animals without a definitive diagnosis. Calcitonin inhibits osteoclast activity. It has been used most commonly to treat hypercalcemia in dogs with cholecalciferol rodenticide toxicosis. The decrease in the serum total calcium concentration after calcitonin administration is relatively small (3 mg/dl or less), and adverse reactions include anorexia and vomiting. Although the onset of action of calcitonin may be rapid, its effect may be short-lived (hours), and resistance often develops within a few days, presumably because of downregulation of calcitonin receptors. The transitory effect of calcitonin and its expense have limited its usefulness for treating hypercalcemia.
Bisphosphonates inhibit bone resorption by decreasing osteoclast activity and function and inducing osteoclast apoptosis and are used for maintenance treatment of hypercalcemia of malignancy, osteoporosis, and malignancy-induced bone pain in humans. Pamidronate (Aredia, Novartis) has been used to treat dogs and cats with a variety of disorders causing hypercalcemia, including cholecalciferol rodenticide toxicosis, hypercalcemia caused by lymphoma, myeloma, primary hyperparathyroidism, and nocardiosis. The IV administration of pamidronate has a rapid onset of action and is effective in lowering serum total and ionized calcium concentrations. The only adverse reaction reported with pamidronate is renal toxicity, which appears to be uncommon. Factors that affect onset of renal toxicity in humans include type of bisphosphonate administered, rate of infusion, and hydration status of the patient. Administration of pamidronate before a definitive diagnosis has been obtained should not adversely affect establishing the cause of the hypercalcemia. Unfortunately, expense limits the usefulness of pamidronate for treating hypercalcemia in animals. The reader is referred to Suggested Readings for more information on bisphosphonates.
The duration of therapy for hypercalcemia depends on the reversibility of the underlying cause. If prolonged supportive therapy is required (e.g., in an animal with cholecalciferol rodenticide toxicity or nontreatable malignancy), furosemide, corticosteroids, and a low-calcium diet (e.g., Prescription Diets u/d and s/d canned, Hill’s Pet Products) can be used to help control the hypercalcemia. Noncalcium-containing intestinal phosphorus binders (e.g., aluminum hydroxide) should be administered if hyperphosphatemia is present. Oral or IV administration of bisphosphonates, as needed to control hypercalcemia, may also be considered (see Suggested Readings).
HYPOCALCEMIA
Etiology
Hypocalcemia is present if the serum total calcium concentration is less than 9 mg/dl in adult dogs and less than 8 mg/dl in adult cats or if the serum ionized calcium concentration is less than 1.0 mmol/L, although reference ranges may vary between laboratories. Hypocalcemia develops after increased calcium loss in milk (e.g., puerperal tetany), decreased calcium resorption from bone or kidney (e.g., primary hypoparathyroidism), decreased calcium absorption from the gastrointestinal tract (e.g., malassimilation syndromes), or increased precipitation-chelation of serum calcium (e.g., ethylene glycol toxicity, acute pancreatitis). The acute onset of hyperphosphatemia can also cause hypocalcemia. The most common causes of hypocalcemia in dogs and cats are puerperal tetany, acute and chronic renal failure, malassimilation syndromes, and primary hypoparathyroidism (especially after thyroidectomy in hyperthyroid cats; see Table 50-3). The serum total calcium concentration is typically decreased in animals with concurrent hypoalbuminemia for reasons discussed in the section on hypercalcemia. Depending on the underlying etiology, the serum ionized calcium concentration may or may not be decreased. Measurement of serum ionized calcium should be done before rendering a diagnosis of hypocalcemia in an animal with decreased serum total calcium and albumin concentrations.
Clinical Features
Animals with hypocalcemia range from being asymptomatic to showing severe neuromuscular dysfunction. Serum total calcium concentrations between 7.5 and 9 mg/dl are often clinically silent; clinical signs usually occur if values are less than 7 mg/dl. The presence and severity of signs depend on the magnitude, rapidity of onset, and duration of hypocalcemia.
The most common clinical signs are directly attributable to a hypocalcemia-induced increase in neuronal excitability and include nervousness, behavioral changes, focal muscle twitching (especially ear and facial muscles), muscle cramping, stiff gait, tetany, and seizures. The seizures are not usually associated with loss of consciousness or urinary incontinence. Early indicators of hypocalcemia, especially in cats, include lethargy, anorexia, intense facial rubbing, and panting. Exercise, excitement, and stress may induce or worsen clinical signs. Additional physical examination findings may include fever, a “splinted” abdomen, cardiac abnormalities (e.g., weak femoral pulses, muffled heart sounds, tachyarrhythmias), and cataracts.
Diagnosis
Hypocalcemia should be confirmed before initiating diagnostic tests to identify the cause. The list of differential diagnoses for hypocalcemia is relatively short, and the history, physical examination findings, CBC, serum biochemistry panel, urinalysis, and tests for pancreatitis (e.g., pancreatic lipase immunoreactivity, abdominal ultrasound) usually provide the clues necessary to establish the diagnosis (see Table 50-3). Primary hypoparathyroidism is the most likely diagnosis in the nonazotemic, nonlactating dog or cat with clinical signs of hypocalcemia. The finding of a low or nondetectable baseline serum PTH concentration confirms this diagnosis.
Treatment
Therapy should be directed at eradicating the underlying cause of the hypocalcemia. Vitamin D, calcium, or both are indicated if clinical signs of hypocalcemia are present, if the serum calcium concentration is less than 7.5 mg/dl, or if the serum ionized calcium concentration is less than 0.8 mmol/L. If hypocalcemic tetany is present, calcium should be administered intravenously slowly to effect (Box 55-7). Calcium gluconate is the preferred agent because it is not caustic if administered outside of the vein, unlike calcium chloride. Auscultation and electrocardiographic monitoring is advisable during calcium administration; if bradycardia or shortening of the QT interval occurs, the IV infusion should be stopped briefly. Calcium-rich fluids should be infused with caution in dogs or cats with hyperphosphatemia because they can increase the probability of metastatic calcification of soft tissues, most notably in the kidney.
 BOX 55-7 Treatment Of Hypocalcemia in Dogs and Cats
BOX 55-7 Treatment Of Hypocalcemia in Dogs and Cats
IV, Intravenous; SC, subcutaneous.
Parenteral Treatment to Prevent Symptomatic Hypocalcemia
Oral Vitamin D and Calcium Treatment for Hypocalcemia
Once signs of hypocalcemic tetany have been controlled with IV calcium, oral vitamin D, oral or injectable calcium (or both) may be needed to prevent the recurrence of clinical signs. If the cause of hypocalcemia is readily reversible and the hypocalcemia is anticipated to be short-lived (e.g., weaning puppies from bitch with puerperal tetany), an injection of calcium gluconate subcutaneously may be all that is necessary to prevent the recurrence of clinical signs. The clinician can determine the dose of IV calcium gluconate required to control tetany originally, and this dose can then be administered subcutaneously after the calcium gluconate has been diluted at least 1 : 2 by volume with saline. Calcium chloride should never be administered subcutaneously because it is highly irritating to tissues and may cause sluffing of the skin.
In animals with disorders causing prolonged hypocalcemia (e.g., primary hypoparathyroidism), calcium gluconate should be administered by continuous IV infusion at an initial dosage of 60 to 90 mg of elemental calcium/kg/day. Ten milliliters of 10% calcium gluconate provides 93 mg of elemental calcium. Approximately 1, 2, and 3 mg/kg/hour elemental calcium is provided when 10, 20, or 30 ml of 10% calcium gluconate, respectively, is added to 250 ml of fluids and administered at a maintenance rate of 60 ml/kg/day (2.5 ml/kg/hour). Calcium salts should not be added to fluids that contain lactate, acetate, bicarbonate, or phosphates because calcium salt precipitates can result. The serum calcium concentration should be measured daily and calcium therapy gradually decreased and then discontinued once the serum total calcium concentration is consistently greater than 8 mg/dl or the serum ionized calcium concentration is greater than 0.9 mmol/L.
Long-term maintenance therapy may be necessary to control hypocalcemia. It is most commonly required for the control of primary hypoparathyroidism and hypoparathyroidism occurring after bilateral thyroidectomy in cats with hyperthyroidism. Oral vitamin D administration is the primary mode of treatment for the management of chronic hypocalcemia (see Box 55-7). Vitamin D works by stimulating intestinal calcium and phosphorus absorption and, together with parathyroid hormone, by mobilizing calcium and phosphorus from bone. Oral calcium supplements are needed early in maintenance therapy in addition to vitamin D.
The aim of maintenance therapy is to keep the serum calcium concentration between 9 and 10 mg/dl, which controls clinical signs, lessens the risk of hypercalcemia, and provides some stimulus for remaining or ectopic parathyroid tissue to become functional. The serum calcium concentration should be monitored closely (initially q24-48h) and adjustments in therapy made accordingly. Vitamin D therapy is required permanently in animals with primary hypoparathyroidism and in animals that have undergone total parathyroidectomy. Vitamin D therapy can usually be tapered and discontinued if there is only partial or transient parathyroid damage. Regardless, calcium supplementation often may be tapered and stopped. See Chapter 50 for more information on the treatment of hypocalcemia.
HYPERPHOSPHATEMIA
Etiology
Hyperphosphatemia is present when the serum phosphorus concentration is greater than 6.5 mg/dl in the adult dog and cat, although reference ranges may vary between laboratories. Serum phosphorus concentrations are highest (often greater than 6.5 mg/dl) in dogs and cats younger than 6 months of age and gradually decrease to adult values after 1 year of age. Bone growth and an increase in renal tubular reabsorption of phosphorus mediated by growth hormone are believed to contribute to this age effect. Hyperphosphatemia can result from increased intestinal phosphorus absorption, decreased phosphorus excretion in the urine, or a shift in phosphorus from the intracellular to the extracellular compartment. Translocation of phosphorus between the intracellular and extracellular compartment is similar to that of potassium. The most common cause of hyperphosphatemia in dogs and cats is decreased renal excretion secondary to renal failure (Box 55-8).
 BOX 55-8 Causes of Hyperphosphatemia in Dogs and Cats
BOX 55-8 Causes of Hyperphosphatemia in Dogs and Cats
Modified from DiBartola SD, Willard MD: Disorders of phosphorus: hypophosphatemia and hyperphosphatemia. In DiBartola SP, editor: Fluid, electrolyte and acid-base disorders in small animal practice, ed 3, St Louis, 2006, Saunders Elsevier. ICF, Intracellular fluid; ECF, extracellular fluid; IV, intravenous.
* Common causes.
Clinical Features
Hyperphosphatemia is a marker of underlying disease. By itself, hyperphosphatemia usually does not cause clinical signs. An acute increase in serum phosphorus may cause hypocalcemia and its associated neuromuscular signs. Sustained hyperphosphatemia can cause secondary hyperparathyroidism, fibrous osteodystrophy, and metastatic calcification in extraosseous sites. Fortunately, most causes of hyperphosphatemia cause a decrease in serum calcium concentration so that the calcium-phosphorus solubility product ([Ca] × [Pi]) remains less than 60. The risk of soft tissue mineralization increases when the [Ca] × [Pi] solubility product exceeds 60 to 70. Chronic renal failure is the most common cause of sustained hyperphosphatemia and an increase in the solubility product above 60 to 70.
Treatment
Hyperphosphatemia usually resolves with correction of the underlying disease. In dogs and cats with renal failure, hyperphosphatemia can initially be lowered with aggressive fluid therapy. Low-phosphorus diets and orally administered phosphate binders are the most effective way to treat sustained hyperphosphatemia caused by renal failure (see Chapter 44).
HYPOPHOSPHATEMIA
Etiology
Hypophosphatemia is present when the serum phosphorus concentration is less than 3 mg/dl in the dog and cat, although reference ranges may vary between laboratories. Hypophosphatemia is usually not clinically worrisome until the serum phosphorus concentration is less than 1.5 mg/dl. Hypophosphatemia results from decreased phosphorus absorption in the intestinal tract, increased urinary phosphorus excretion, or translocation from the extracellular to the intracellular compartment. The most common cause of clinically significant hypophosphatemia in the dog and cat occurs within the first 24 hours of therapy for diabetic ketoacidosis, when there is a shift of potassium and phosphorus from the extracellular to the intracellular compartment (Box 55-9). Translocation of phosphorus between the intracellular and extracellular compartments is similar to that seen with potassium. Factors that promote a shift of potassium into the intracellular compartment (e.g., alkalosis, insulin, glucose infusion) promote a similar shift in phosphorus. During therapy for diabetic ketoacidosis the serum phosphorus concentration can decline to severe levels (i.e., less than 1 mg/dl) as a result of the dilutional effects of fluid therapy and the intracellular shift of phosphorus after the initiation of insulin and bicarbonate therapy. Interestingly, the initial serum phosphorus concentration is usually normal or only mildly decreased because the metabolic acidosis of diabetic ketoacidosis results in a shift of phosphorus from the intracellular to the extracellular compartment.
 BOX 55-9 Causes of Hypophosphatemia in Dogs and Cats
BOX 55-9 Causes of Hypophosphatemia in Dogs and Cats
Modified from DiBartola SD, Willard MD: Disorders of phosphorus: hypophosphatemia and hyperphosphatemia. In DiBartola SP, editor: Fluid, electrolyte and acid-base disorders in small animal practice, ed 3, St Louis, 2006, Saunders Elsevier. DKA, Diabetic ketoacidosis.
* Common causes.
Clinical Features
Clinical signs may develop when the serum phosphorus concentration is less than 1.5 mg/dl, although signs are quite variable, and severe hypophosphatemia is clinically silent in many animals. Hypophosphatemia primarily affects the hematologic and neuromuscular systems in the dog and cat. Hemolytic anemia is the most common sequela to hypophosphatemia. Hypophosphatemia decreases the erythrocyte concentration of ATP, which increases erythrocyte fragility, leading to hemolysis. Hemolysis is usually not identified until the serum phosphorus concentration is 1 mg/dl or less. Hemolytic anemia can be life-threatening if not recognized and treated. Neuromuscular signs include weakness, ataxia, and seizures, as well as anorexia and vomiting secondary to intestinal ileus.
Treatment
For most dogs and cats hypophosphatemia resolves after correction of the underlying cause. Phosphate therapy is probably not indicated for asymptomatic animals in which the serum phosphorus concentration is greater than 1.5 mg/dl and is unlikely to decrease further. Phosphate therapy is indicated if clinical signs or hemolysis are identified or if the serum phosphorus concentration is less than 1.5 mg/dl, especially if a further decrease is possible. Phosphate supplementation is not indicated in dogs and cats with hypercalcemia, hyperphosphatemia, oliguria, or suspected tissue necrosis. If renal function is in question, phosphorus supplementation should not be done until the status of renal function and serum phosphorus concentration are known.
The goal of therapy is to maintain the serum phosphorus concentration greater than 2 mg/dl without causing hyperphosphatemia. Oral phosphate supplementation is preferred, using a buffered laxative (e.g., Phospho-Soda, Fleet Pharmaceuticals), balanced commercial diets, milk, or a combination of these. IV phosphate supplementation is usually required to correct severe hypophosphatemia, especially in animals with diabetic ketoacidosis. Potassium phosphate solutions are typically used. If potassium supplementation is contraindicated, sodium phosphate solutions can be substituted. Potassium and sodium phosphate solutions contain 3 mmol of phosphate per milliliter and either 4.4 mEq of potassium or 4 mEq of sodium per milliliter. The initial dosage of phosphate is 0.01 to 0.03 mmol/kg/hour, preferably administered by constant rate infusion in calcium-free IV fluids (i.e., 0.9% sodium chloride). In dogs and cats with severe hypophosphatemia, it may be necessary to increase the dosage to 0.03 to 0.12 mmol/kg/hour. Because the dose of phosphate necessary to replete an animal and the animal’s response to therapy cannot be predicted, it is important to initially monitor the serum phosphorus concentration every 8 to 12 hours and adjust the phosphate infusion accordingly. Adverse effects from overzealous phosphate administration include iatrogenic hypocalcemia and its associated neuromuscular signs, hypernatremia, hypotension, and metastatic calcification. Serum total or preferably ionized calcium concentration should be measured at the same time as serum phosphorus concentration and the rate of phosphate infusion decreased if hypocalcemia is identified.
HYPOMAGNESEMIA
Etiology
Hypomagnesemia is present if the serum total and ionized magnesium concentration are less than 1.5 mg/dl and 1.0 mg/dl, respectively, although reference ranges may vary between laboratories. Hypomagnesemia results from decreased oral intake or gastrointestinal tract absorption of magnesium (e.g., small intestinal disease causing malabsorption), increased gastrointestinal tract loss (e.g., protracted vomiting, diarrhea), increased urinary magnesium excretion (e.g., interstitial nephritis, diuretics), or translocation of the cation from the extracellular to the intracellular compartment. The most common causes of clinically significant hypomagnesemia in dogs and cats include disorders causing small intestinal malassimilation; renal disorders associated with a high urine output; the osmotic diuresis of diabetic ketoacidosis; and the shift of potassium, phosphorus, and magnesium from the extracellular to the intracellular compartment that occurs within the first 24 hours of therapy for diabetic ketoacidosis (Box 55-10). Magnesium is predominantly an intracellular cation. The nature of the translocation of magnesium between the intracellular and the extracellular compartments is similar to that of potassium in that factors that promote a shift of potassium into the intracellular compartment (e.g., alkalosis, insulin, glucose infusion) promote a similar shift in magnesium.
 BOX 55-10 Causes of Hypomagnesemia and Magnesium Depletion in Dogs and Cats
BOX 55-10 Causes of Hypomagnesemia and Magnesium Depletion in Dogs and Cats
Modified from Bateman S: Disorders of magnesium: magnesium deficit and excess. In DiBartola SP, editor: Fluid, electrolyte and acid-base disorders in small animal practice, ed 3, St Louis, 2006, Saunders/Elsevier.
* Common causes.
Clinical Features
Hypomagnesemia is reported to be the most common electrolyte disorder in critically ill dogs and cats, and magnesium deficiency may predispose animals to a variety of cardiovascular, neuromuscular, and metabolic complications. Clinical signs of hypomagnesemia do not usually occur until the serum total and ionized magnesium concentrations are less than 1.0 mg/dl and 0.5 mg/dl, respectively, and even at these low levels, many animals remain asymptomatic. A magnesium deficiency can result in several nonspecific clinical signs, including lethargy, anorexia, muscle weakness (including dysphagia and dyspnea), muscle fasciculations, seizures, ataxia, and coma. Concurrent hypokalemia, hyponatremia, and hypocalcemia occur in animals with hypomagnesemia, although the prevalence of these electrolyte abnormalities may differ between species. These electrolyte abnormalities may also contribute to the development of clinical signs. Magnesium is a cofactor for all enzyme reactions that involve ATP, most notably the sodium-potassium ATPase pump. Deficiencies in magnesium can lead to potassium wastage from the body, and the resultant hypokalemia may be refractory to appropriate potassium replacement therapy. Magnesium deficiency inhibits PTH secretion from the parathyroid gland, resulting in hypocalcemia. Magnesium deficiency causes the resting membrane potential of myocardial cells to be decreased and leads to increased Purkinje fiber excitability, with the consequent generation of arrhythmias. Electrocardiographic changes include a prolonged PR interval, widened QRS complex, depressed ST segment, and peaked T waves. Cardiac arrhythmias associated with magnesium deficiency include atrial fibrillation, supraventricular tachycardia, ventricular tachycardia, and ventricular fibrillation. Hypomagnesemia also predisposes animals to digitalis-induced arrhythmias.
Diagnosis
Measurement of serum total and ionized magnesium is indicated in those dogs and cats with disorders and predisposing factors that are associated with hypomagnesemia (see Box 55-10). Assessing an animal’s magnesium status is problematic, however, because there is no simple, rapid, and accurate laboratory test to gauge total body magnesium status. Serum total magnesium represents 1% of the body’s magnesium stores, and serum ionized magnesium represents 0.2% to 0.3% of total body magnesium stores. As a result, serum total and ionized magnesium concentrations do not always reflect total body magnesium status. A normal serum magnesium concentration may exist despite an intracellular magnesium deficiency. However, a low serum magnesium concentration would support the presence of a total body magnesium deficiency, especially when clinical signs or concurrent electrolyte abnormalities are consistent with hypomagnesemia.
Treatment
To date there are no clinical studies that have yielded guidelines for magnesium replacement in dogs and cats; currently, it is determined empirically. Hypomagnesemia is not generally a concern for dogs and cats eating commercial diets. Treatment of hypomagnesemia usually involves sick dogs and cats that are hospitalized and have problems with inappetence and/or excessive fluid loss from the gastrointestinal tract or kidneys. Treatment of hypomagnesemia may also be indicated during treatment of diabetic ketoacidosis in dogs and cats with refractory hypokalemia, hypocalcemia, or both and in dogs or cats in heart failure with concurrent ventricular arrhythmias that are being treated with loop diuretics, digitalis, or both.
Parenteral solutions of magnesium sulfate (8.12 mEq of magnesium per gram of salt) and magnesium chloride (9.25 mEq of magnesium per gram of salt) are available commercially. The IV dose for rapid and slow magnesium replacement is 0.75 to 1 mEq/kg/day and 0.3 to 0.5 mEq/kg/day, respectively, administered by constant-rate infusion in 5% dextrose in water. Magnesium is incompatible with solutions containing bicarbonate or calcium. Renal function should be assessed before the administration of magnesium and the magnesium dose reduced by 50% to 75% in azotemic animals. The use of magnesium with digitalis cardioglycosides may cause serious conduction disturbances. Serum magnesium, calcium, and potassium concentrations should be monitored daily. The goal of magnesium therapy is the resolution of clinical signs or refractory hypokalemia and hypocalcemia. The parenteral administration of magnesium sulfate may cause significant hypocalcemia such that calcium infusion may be necessary. Other adverse effects of magnesium therapy include hypotension; atrioventricular and bundle-branch blocks; and, in the event of overdose, respiratory depression and cardiac arrest. Overdoses are treated with IV calcium gluconate (see Box 55-7).
HYPERMAGNESEMIA
Etiology
Hypermagnesemia is present if the serum total and ionized magnesium concentration is greater than 2.5 mg/dl and 1.5 mg/dl, respectively, although reference ranges may vary between laboratories. It is an uncommon clinical problem, owing to the remarkable ability of the kidney to efficiently eliminate excessive magnesium. Hypermagnesemia may occur in dogs and cats with renal failure and postrenal azotemia and iatrogenically after an excessive intake of magnesium (e.g., IV administration). Because excess magnesium is rapidly excreted by the healthy kidney, iatrogenic hypermagnesemia usually occurs in animals with renal insufficiency. Hypermagnesemia has also been reported in cats with thoracic neoplasia and pleural effusion, although the mechanism involved with the development of hypermagnesemia in these cats is unknown.
Clinical Features
Clinical manifestations of hypermagnesemia include lethargy, weakness, and hypotension. Loss of deep tendon reflexes and electrocardiographic changes, consisting of prolonged PR intervals, widening QRS complexes, and heart block, occur at higher serum magnesium concentrations. Serious complications, including respiratory depression, apnea, cardiac arrhythmias, and cardiac arrest, occur when serum magnesium concentrations exceed 12 mg/dl. At these high levels magnesium acts as a nonspecific calcium-channel blocker.
Diagnosis
Measurement of the serum magnesium concentration identifies hypermagnesemia. Unlike magnesium depletion, serum concentrations cannot be normal if there is an increase in magnesium stores (see the section on hypomagnesemia). A correlation between increased serum magnesium concentrations and the severity of total body excess has not been reported.
Treatment
Treatment begins with the discontinuation of all exogenous sources of magnesium. Additional treatment depends on the severity of the hypermagnesemia, the clinical presentation, and the status of renal function. Most dogs and cats with healthy kidneys require only supportive care and observation. Treatment aimed at improving renal function is indicated in animals with concurrent renal insufficiency (see Chapter 44). Saline diuresis and administration of loop diuretics (e.g., furosemide) will accelerate renal magnesium excretion. Administration of IV calcium is indicated in dogs and cats with cardiac arrhythmias or significant hypotension (see Box 55-7).
Bissett SA, et al. Hyponatremia and hyperkalemia associated with peritoneal effusion in four cats. J Am Vet Med Assoc. 2001;218:1590.
Bolliger AP, et al. Detection of parathyroid hormone-related protein in cats with humoral hypercalcemia of malignancy. Vet Clin Path. 2002;31:3.
DiBartola SP, editor. Fluid, electrolyte and acid-base disorders in small animal practice, ed 3, St Louis: Saunders Elsevier, 2006.
Fan TM, et al. Evaluation of intravenous pamidronate administration in 33 caner-bearing dogs with primary or secondary bone involvement. J Vet Intern Med. 2005;19:74.
Fincham SC, et al. Evaluation of plasma ionized magnesium concentration in 122 dogs with diabetes mellitus: a retrospective study. J Vet Intern Med. 2004;18:612.
Hostutler RA, et al. Uses and effectiveness of pamidronate disodium for treatment of dogs and cats with hypercalcemia. J Vet Intern Med. 2005;19:29.
Khanna C, et al. Hypomagnesemia in 188 dogs: a hospital population–based prevalence study. J Vet Intern Med. 1998;12:304.
Kimmel SE, et al. Hypomagnesemia and hypocalcemia associated with protein-losing enteropathy in Yorkshire Terriers: five cases (1992–1998). J Am Vet Med Assoc. 2000;217:703.
Kimmel SE, et al. Incidence and prognostic value of low plasma ionized calcium concentration in cats with acute pancreatitis: 46 cases (1996–1998). J Am Vet Med Assoc. 2001;219:1105.
Midkiff AM, et al. Idiopathic hypercalcemia in cats. J Vet Intern Med. 2000;14:619.
Milner RJ, et al. Bisphosphonates and cancer. J Vet Intern Med. 2004;18:597.
Norris CR, et al. Serum total and ionized magnesium concentrations and urinary fractional excretion of magnesium in cats with diabetes mellitus and diabetic ketoacidosis. J Am Vet Med Assoc. 1999;215:1455.
Ramsey IK, et al. Hyperparathyroidism in dogs with hyperadrenocorticism. J Small Anim Pract. 2005;46:531.
Rumbeiha WK, et al. Use of pamidronate disodium to reduce cholecalciferol-induced toxicosis in dogs. Am J Vet Res. 2000;61:9.
Savary KCM, et al. Hypercalcemia in cats: a retrospective study of 71 cases (1991–1997). J Vet Intern Med. 2000;14:184.
Schenck PA, et al. Prediction of serum ionized calcium concentration by serum total calcium measurement in dogs. Am J Vet Res. 2005;66:1330.
Toll J, et al. Prevalence and incidence of serum magnesium abnormalities in hospitalized cats. J Vet Intern Med. 2002;16:217.
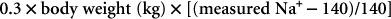

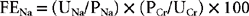
 BOX 55-4
BOX 55-4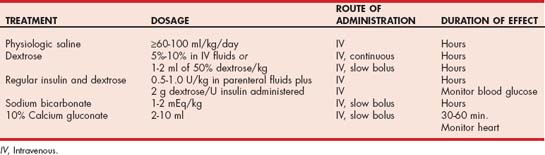
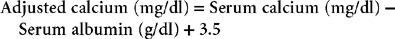
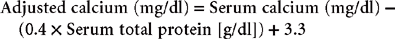
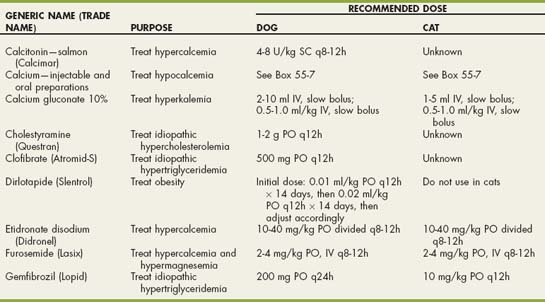
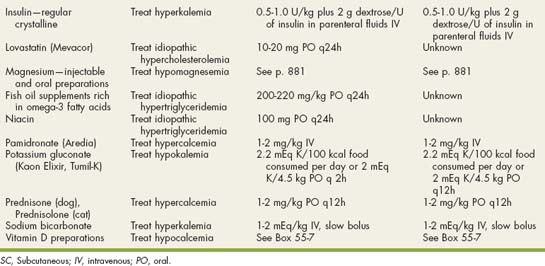
 Drugs Used in Electrolyte and Metabolic Disorders
Drugs Used in Electrolyte and Metabolic Disorders