CHAPTER 77 Practical Chemotherapy
CELL AND TUMOR KINETICS
To better understand the effects of chemotherapy on both neoplastic and normal tissues, it is necessary to have a basic understanding of cell biology and tumor kinetics. As a general rule, the biologic characteristics of neoplastic cells are similar to those of their normal counterparts, with the main difference being that neoplastic cells usually do not undergo terminal differentiation. Therefore the cell cycles of normal and neoplastic cells are similar.
The mammalian cell cycle has two apparent phases: mitosis and the resting phase. The resting phase is actually composed of four phases (Fig. 77-1):
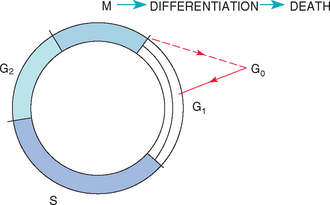
FIG 77-1 Mammalian cell cycle. Cells in mitosis (M) can differentiate and subsequently die (the rule in normal tissues); they can also progress to G0 (true resting phase), from which they can be recruited by a variety of stimuli (see text). G1, Gap 1; S, DNA synthesis; G2, gap 2.
The mitosis phase is termed the M phase.
Oncogenes serve as checkpoints between different phases of the cell cycle.
Several terms must be defined before chemotherapy is discussed. The mitotic index (MI) refers to the proportion of cells in the process of mitosis within a tumor; the pathologist often provides information about the mitotic activity in a given tumor sample, reported as the MI or as the number of mitoses per high-power field (or per X number of high-power fields). The growth fraction (GF) refers to the proportion of proliferating cells within a tumor and cannot be quantified in a patient. The doubling time (DT) refers to the time it takes for a tumor to double in size; it can be calculated by using sequential measurements of the tumor volume [V = π/6 × (mean diameter)3] seen on radiographs or ultrasonograms or determined by direct palpation. In dogs the DT ranges from 2 days (for metastatic osteosarcoma) to 24 days (for metastatic melanoma), whereas in humans it ranges from 29 days (for malignant lymphomas) to 83 days (for metastases from breast cancer). We recently evaluated the DT of pulmonary metastases in dogs with appendicular osteosarcoma treated with amputation and adjuvant chemotherapy; the median DT of the metastases was 13 days for Greyhounds and 21 days for nonGreyhounds. The DT depends on the time spent in mitosis, the cell cycle duration, the GF, and the cell loss resulting from death or metastasis. Given our knowledge of tumor kinetics, by the time a pulmonary metastatic nodule is visualized on radiographs, it consists of 200,000,000 cells, weighs less than 150 mg, and has already divided 25 to 35 times. A 1-cm palpable nodule has 109 tumor cells (1,000,000,000) and weighs 1 g (Fig. 77-2). As a general rule, most nonneoplastic tissues (with the exception of bone marrow stem cells and intestinal crypt epithelium) have a low GF, low MI, and prolonged DT, whereas most neoplastic tissues have a high MI, high GF, and short DT (at least initially; see Fig. 77-2).
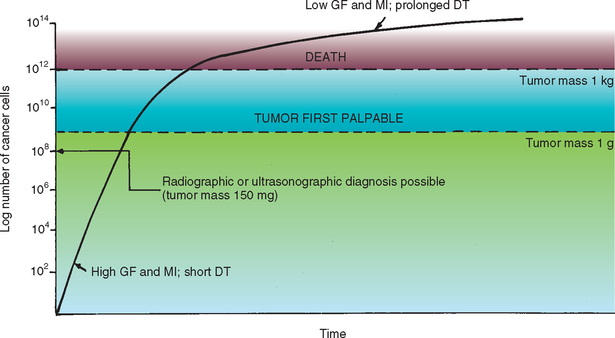
FIG 77-2 Tumor (cell) kinetics. Additional information on tumor kinetics can be found in the text. GF, Growth fraction; MI, mitotic index; DT, doubling time.
(From Couto CG: Principles of chemotherapy. In Proceedings of the Tenth Annual Kal Kan Symposium for the Treatment of Small Animal Diseases: Oncology, Kalkan Foods, Inc, Vernon, Calif, 1986, p. 37.)
Surgical cytoreduction (debulking) of a tumor that has reached a plateau of growth decreases the total number of cells, thus increasing the MI and GF and shortening the DT through yet unknown mechanisms (Fig. 77-3). In theory, this renders the neoplasm more susceptible to chemotherapy or radiotherapy.
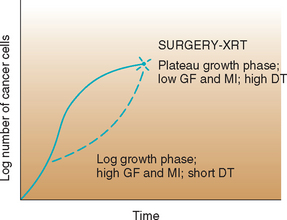
FIG 77-3 The effect of surgical or radiotherapeutic intervention on tumor kinetics. After cytoreduction, cells are recruited from the G0 phase and the tumor returns to the exponential phase. XRT, Radiation therapy; GF, growth factor; MI, mitotic index; DT, doubling time.
(From Couto CG: Principles of chemotherapy. In Proceedings of the Tenth Annual Kal Kan Symposium for the Treatment of Small Animal Diseases: Oncology, Kalkan Foods, Inc, Vernon, Calif, 1986, p. 37.)
BASIC PRINCIPLES OF CHEMOTHERAPY
Chemotherapeutic agents predominantly kill cells in rapidly dividing tissues. To exploit the tumoricidal effect of different chemotherapeutic drugs, it is common practice to combine three or more drugs to treat a given malignancy. These drugs are selected on the basis of the following principles: Each should be active against the given tumor type, each should act by a different mechanism of action, and they should not have superimposed toxicities. It is customary to name the protocol after the first letters of each drug in the combination (e.g., VAC for vincristine, doxorubicin [or Adriamycin], and cyclophosphamide). As a general rule, combination chemotherapy results in more sustained remissions and prolonged survival times, as compared with those achieved using single-agent chemotherapy; this is thought to result from the fact that multichemotherapy delays (or even prevents) the development of drug-resistant clones. However, some exceptions to this rule include the treatment of dogs with osteosarcoma using cisplatin, carboplatin, or doxorubicin as single agents; the treatment of dogs with chronic lymphocytic leukemia using chlorambucil alone; and the treatment of dogs with transmissible venereal tumors with vincristine alone.
Another general concept of chemotherapy from the standpoint of cell kinetics is that it is more effective in a relatively small tumor than in a large one, even though the inherent sensitivity to the drug or drugs may be the same. As can be seen in Fig. 77-3, a small tumor (e.g., 106 cells) is more likely than a larger one (e.g., 1011 cells) to be completely eradicated by the drugs because the smaller mass has a higher MI, a higher GF, and consequently a shorter DT than the larger mass (i.e., more cells are actively dividing at a given time).
Despite continued controversy, the doses of most chemotherapeutic agents are still determined on a body surface area (BSA) basis; exceptions will be listed later. This appears to provide a more constant metabolic parameter for comparing doses across species. It can be calculated using the following formula:
The constant is 10.1 for the dog and 10 for the cat. Table 77-1 is a conversion table of weight (in kilograms) to BSA (in squared meters) for dogs. Table 77-2 is a conversion table of pounds (and kilograms) to BSA for cats. When drugs such as doxorubicin are being used, doses determined on the basis of BSA usually lead to adverse effects in very small dogs (i.e., those under 10 kg) and cats. A dose determined on the basis of weight (e.g., 1 mg/kg) is more appropriate in such small animals.
 TABLE 77-1 Conversion of Body Weight to Body Surface Area in Dogs
TABLE 77-1 Conversion of Body Weight to Body Surface Area in Dogs
| BODY WEIGHT (kg) | BODY SURFACE AREA (m2) |
|---|---|
| 00.5 | 0.06 |
| 01 | 0.10 |
| 02 | 0.15 |
| 03 | 0.20 |
| 04 | 0.25 |
| 05 | 0.29 |
| 06 | 0.33 |
| 07 | 0.36 |
| 08 | 0.40 |
| 09 | 0.43 |
| 10 | 0.46 |
| 11 | 0.49 |
| 12 | 0.52 |
| 13 | 0.55 |
| 14 | 0.58 |
| 15 | 0.60 |
| 16 | 0.63 |
| 17 | 0.66 |
| 18 | 0.69 |
| 19 | 0.71 |
| 20 | 0.74 |
| 21 | 0.76 |
| 22 | 0.78 |
| 23 | 0.81 |
| 24 | 0.83 |
| 25 | 0.85 |
| 26 | 0.88 |
| 27 | 0.90 |
| 28 | 0.92 |
| 29 | 0.94 |
| 30 | 0.96 |
| 31 | 0.99 |
| 32 | 1.01 |
| 33 | 1.03 |
| 34 | 1.05 |
| 35 | 1.07 |
| 36 | 1.09 |
| 37 | 1.11 |
| 38 | 1.13 |
| 39 | 1.15 |
| 40 | 1.17 |
| 41 | 1.19 |
| 42 | 1.21 |
| 43 | 1.23 |
| 44 | 1.25 |
| 45 | 1.26 |
| 46 | 1.28 |
| 47 | 1.30 |
| 48 | 1.32 |
| 49 | 1.34 |
| 50 | 1.36 |
 TABLE 77-2 Conversion of Body Weight to Body Surface Area in Cats
TABLE 77-2 Conversion of Body Weight to Body Surface Area in Cats
| BODY WEIGHT (lb) | BODY WEIGHT (kg) | BODY SURFACE AREA (m2) |
|---|---|---|
| 5 | 2.3 | 0.165 |
| 6 | 2.8 | 0.187 |
| 7 | 3.2 | 0.207 |
| 8 | 3.6 | 0.222 |
| 9 | 4.1 | 0.244 |
| 10 | 4.6 | 0.261 |
| 11 | 5.1 | 0.278 |
| 12 | 5.5 | 0.294 |
| 13 | 6.0 | 0.311 |
| 14 | 6.4 | 0.326 |
| 15 | 6.9 | 0.342 |
| 16 | 7.4 | 0.356 |
| 17 | 7.8 | 0.371 |
| 18 | 8.2 | 0.385 |
| 19 | 8.7 | 0.399 |
| 20 | 9.2 | 0.413 |
INDICATIONS AND CONTRAINDICATIONS OF CHEMOTHERAPY
Chemotherapy is primarily indicated for animals with systemic (e.g., lymphoma, leukemias) or metastatic neoplasms, although it can also be used for the management of nonresectable, chemoresponsive neoplasms that have historically proved refractory to radiotherapy or hyperthermia (primary chemotherapy). It can also be used as an adjuvant treatment after partial surgical debulking of a neoplasm (e.g., partial excision of an undifferentiated sarcoma) and is indicated for the control of micrometastatic disease after the surgical excision of a primary neoplasm (e.g., cisplatin, carboplatin, or doxorubicin therapy after limb amputation in dogs with osteosarcoma; VAC after splenectomy for dogs with hemangiosarcoma). Chemotherapy can also be administered intracavitarily in dogs and cats with malignant effusions or neoplastic involvement of the cavity/area in question (e.g., intrapleurally administered cisplatin or 5-fluoruracil in dogs with pleural carcinomatosis). Finally, neoadjuvant, or primary, chemotherapy is the approach used in animals with bulky tumors not amenable to surgical excision. After the drugs cause the tumor to shrink, the tumor can be surgically excised; chemotherapy is then continued to eliminate any residual neoplastic cells (e.g., VAC chemotherapy for dogs with subcutaneous hemangiosarcomas).
As a general rule, chemotherapy should not be used as a substitute for surgery, radiotherapy, or hyperthermia; nor should it be used in animals with severe underlying multiple-organ dysfunction (or it should be used cautiously, with a dose modification) because this increases the risk of systemic toxicity.
MECHANISM OF ACTION OF ANTICANCER DRUGS
The effects of anticancer drugs on a neoplastic cell population follow first-order kinetic principles (i.e., the number of cells killed by a drug or drug combination is directly proportional to one variable: the dose used). These drugs kill a constant proportion of cells, rather than a constant number of cells. Therefore the efficacy of a drug or drug combination depends on the number of cells in a given tumor (e.g., a drug combination that kills 99% of the cells in a tumor containing 100,000,000 [109] cells leaves 1,000,000 [106] viable cells).
As discussed in the following paragraphs, different types of anticancer drugs kill tumor cells by different mechanisms. Drugs that kill only dividing tumor cells (i.e., that do not kill cells in the G0 phase) by acting on several phases of the cycle are termed cell cycle phase-nonspecific drugs. Alkylating agents belong to this group. Drugs that selectively kill tumor cells during a given phase of the cell cycle are termed cell cycle phase-specific drugs. Most antimetabolites and plant alkaloids are phase-specific drugs. Finally, drugs that kill neoplastic cells regardless of their cycle status (i.e., they kill both dividing and resting cells) are termed cell cycle-nonspecific drugs. These latter drugs are extremely myelosuppressive (e.g., nitrosoureas) and are infrequently used in veterinary medicine.
TYPES OF ANTICANCER DRUGS
Anticancer drugs are commonly classified into six categories (Box 77-1). Most of these drugs are currently available as generic products.
Alkylating agents cross-link DNA, thus preventing its duplication. Because they mimic the effects of radiotherapy, they are also referred to as radiomimetics. These drugs are active during several phases of the cell cycle (i.e., they are cell cycle phase-nonspecific) and are more active if given intermittently at high doses. The major toxicities of these drugs are myelosuppressive and gastrointestinal in nature. Alkylating agents commonly used in pets with cancer are listed in Box 77-1.
Antimetabolites exert their activity during the S phase of the cell cycle (cell cycle phase-specific) and are more active if given repeatedly at low doses or as continuous intravenous infusions. These drugs are structural analogs of naturally occurring metabolites (fake metabolites) that substitute for normal purines or pyrimidines. The major toxicities of these drugs are myelosuppressive and gastrointestinal. Box 77-1 lists the antimetabolites commonly used in small animals with cancer.
Antitumor antibiotics act by several mechanisms (i.e., cell cycle phase-nonspecific), the most important of which appears to be DNA damage produced by free radicals or by a topoisomerase-II–dependent mechanism. There are now several synthetic or semisynthetic antibiotics. The major toxicities of these drugs are myelosuppressive and gastrointestinal in nature; doxorubicin and actinomycin D are extremely caustic if given perivascularly, and the former has cumulative cardiotoxic effects. Antitumor antibiotics are listed in Box 77-1.
Plant alkaloids are derived from the periwinkle plant (Vinca rosea) and the May apple plant (Podophyllum peltatum). Vinca derivatives disrupt the mitotic spindle and are therefore cell cycle phase-specific (active during M phase), whereas Podophyllum derivatives cross-link DNA. The major toxicity is perivascular sloughing if the agent extravasates. Etoposide should not be administered intravenously because the vehicle (Tween 80) causes anaphylaxis. Box 77-1 lists commonly used plant alkaloids.
Hormones are commonly used for the treatment of hemolymphatic malignancies or endocrine-related tumors. Commonly used hormones are listed in Box 77-1.
With the exception of corticosteroids, hormones are not recommended as antineoplastics because they are associated with relevant adverse effects in animals.
Miscellaneous agents consist of drugs with a mechanism of action that is either unknown or differs from those of agents already described. Box 77-1 lists miscellaneous agents commonly used in small animals with cancer.
A novel approach to anticancer chemotherapy is to exploit the use of molecular targets. For example, c-KIT mutations are commonly identified in human with chronic myelogenous leukemia; imatinib (Gleevec, Novartis) selectively block this tyrosine kinase (TK) pathway and induces apoptosis of neoplastic (but not normal) cells. Mutations of c-KIT are also common in canine mast cell tumors, where small molecule TK inhibitors other than imatinib have been effective. A new TK inhibitor is now available for veterinary use (Palladia, Pfizer). In the dog imatinib appears to be hepatotoxic.
SAFE HANDLING OF ANTICANCER DRUGS
Cytotoxic drugs have very narrow therapeutic indices, with toxic effects very often noted at the standard therapeutic dosages. Occupational exposure, as might occur in personnel who commonly administer these drugs, has been documented in the literature; adverse effects, including headache, nausea, liver disease, and reproductive abnormalities, have been associated with this exposure. As such, no safe exposure level has been identified, and all possible measures to limit personnel exposure to cytotoxic drugs must be taken during their preparation and administration.
Reconstitution of cytotoxic drugs for administration must be performed in a biosafety level II vertical laminar airflow hood. Although the cost for this equipment is not prohibitively expensive for a large equine hospital (∼$6,000-$10,000), this cost is currently not justified by the frequency of use. A new closed system (PhaSealTM, Carmel Pharma, Columbus, OH) is practical and relatively inexpensive and limits operator and environmental drug exposure to almost zero. If containment devices are not available, cytotoxic drugs can be reconstituted at a human hospital or pharmacy or at a nearby small animal clinic with a sufficiently large oncology caseload. Care should be taken to respect the storage half-life of reconstituted drugs, and they should be administered to the patient as soon as possible after reconstitution. Drugs should be delivered in a clearly labeled, sealed plastic bag, and any handling of the drugs should be performed while wearing the appropriate personal protective gear.
Personal protective gear has been shown to all but eliminate detectable occupational exposure to cytotoxic drugs in human oncology nurses when combined with safe, conservative handling practices. All personnel present during chemotherapy administration to animal patients, including veterinarians, technicians, and ward staff, must wear thick latex chemotherapy gloves or two pairs of regular latex examination gloves. The thickness of the gloves is more important than the composition for barrier protection. Ideally, personnel should also wear impermeable disposable gowns, eye protection, and particle-filtering face masks. All fluid lines should be primed before addition of cytotoxic drugs to reduce environmental contamination, and all potentially contaminated supplies, including gowns, gloves, fluid bags, lines, and so forth, should be disposed of in properly labeled biohazard bags or plastic sharps containers. Disposal of material potentially contaminated with cytotoxic drugs may be arranged through a local human hospital; alternatively, an EPA-approved disposal facility should be located. Materials used in the preparation and administration of chemotherapy should not be reused. Patient waste, including urine and feces, should be disposed of similarly 24 to 48 hours after chemotherapy administration, and personnel involved in the husbandry of these patients should wear the above-recommended personal protective gear when attending patients.
Protocols for handling spills should be prepared in advance and posted in areas where patients may be receiving chemotherapy. This area should be a designated area of the hospital with low traffic and minimal drafts; a stall may be selected for this purpose in equine hospitals. Isolation stalls will minimize exposure of personnel to chemotherapeutic agents. Once the patient has received chemotherapy, its cage should be clearly identified with a notice that contains information about precautions to be taken during handling of the animal and its wastes.
Chabner BA, et al. Cancer chemotherapy and biotherapy: principles and practice, ed 3. Philadelphia: Lippincott, Williams and Wilkins, 2001.
Helfand SC. Principles and applications of chemotherapy. Vet Clin N Am. 1990;20:987.
London CA, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9:2755.
Moore AS. Recent advances in chemotherapy for non-lymphoid malignant neoplasms. Compend Contin Educ Pract Vet. 1993;15:1039.
Vail DM. Recent advances in chemotherapy for lymphoma in dogs and cats. Compend Contin Educ Pract Vet. 1993;15:1031.
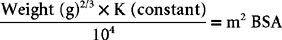
 BOX 77-1
BOX 77-1