CHAPTER 78 Complications of Cancer Chemotherapy
GENERAL CONSIDERATIONS
Because most anticancer agents are relatively nonselective, they kill not only rapidly dividing neoplastic tissues but also some of the rapidly dividing normal tissues in the host (e.g., villus epithelium, bone marrow cells). In addition, similar to other commonly used agents (e.g., digitalis glycosides), most anticancer agents have low therapeutic indices (i.e., narrow therapeutic : toxic ratios).
Because anticancer agents follow first-order kinetic principles (i.e., the fraction of cells killed is directly proportional to the dose used), increasing the dose of a particular drug increases the proportion of the neoplastic cells killed, but it also enhances its toxicity. This is commonly seen when a tumor relapses and higher doses of a previously prescribed chemotherapeutic agent are administered.
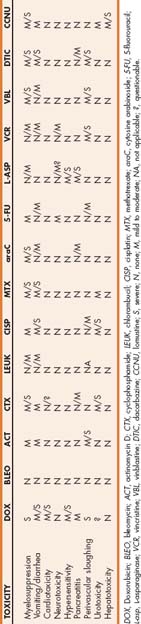
Because toxicity generally tends to affect rapidly dividing tissues, given the short doubling times of the bone marrow and villal epithelial cells, myelosuppression and gastrointestinal signs are the most common toxicities encountered in practice. Other rare complications of chemotherapy include anaphylactoid (or anaphylactic) reactions, dermatologic toxicity, pancreatitis, cardiotoxicity, pulmonary toxicity, neurotoxicity, hepatopathies, and urotoxicity. Table 78-1 lists anticancer drugs commonly used in small animals and their toxicities.
Several factors can potentiate the effects of anticancer agents and thereby enhance their toxicity. For example, drugs that are excreted primarily through the kidneys (e.g., cisplatin, carboplatin, methotrexate) are more toxic to animals with renal disease; thus a dose reduction or the use of an alternative drug is usually recommended in such cases.
In addition to the direct effects of some drugs on different organ systems, rapid killing of certain neoplastic cells (i.e., lymphoma cells) can lead to sudden metabolic derangements that result in acute clinical signs mimicking those of drug toxicity (i.e., depression, vomiting, diarrhea). This syndrome is referred to as acute tumor lysis syndrome (ATLS) (see p. 1167).
In general, cats appear to be more susceptible than dogs to some of the adverse effects of chemotherapy (e.g., anorexia, vomiting) but not to others (e.g., myelosuppression). Certain breeds of dogs, including Collies and Collie crosses, Old English Sheepdogs, Cocker Spaniels, and West Highland White Terriers, also appear to be more prone to some of the acute adverse reactions to chemotherapy (i.e., gastrointestinal signs, myelosuppression) than the general dog population.
The overall prevalence of toxicity of different chemotherapy protocols is considerably lower in dogs and cats (approximately 5% to 40%) than in humans (75% to 100%) treated with similar drugs or combinations. A recent survey of owners whose pets had been treated with a variety of chemotherapy protocols at The Ohio State University Veterinary Teaching Hospital revealed that more than 80% considered their pets’ quality of life to be equal to or better than that before the start of chemotherapy.
HEMATOLOGIC TOXICITY
The high mitotic rate and growth fraction (i.e., 40% to 60%) of the bone marrow cells predispose this organ to relevant toxicity from anticancer drugs. Hematologic toxicity constitutes the most common complication of chemotherapy, and often the severe and potentially life-threatening cytopenias that occur necessitate the temporary or permanent discontinuation of the offending agent or agents. Table 78-1 lists agents commonly implicated in this type of toxicity.
It is easy to anticipate the cell line that will be affected on the basis of the bone marrow transit times and circulating half-lives of blood-formed elements. For example, the bone marrow transit time and circulating half-life of red blood cells in the dog are approximately 7 and 120 days, those of the platelets are 3 days and 4 to 6 days, and those of granulocytes are 6 days and 4 to 8 hours, respectively. On the basis of this, neutropenia usually occurs first, followed by thrombocytopenia. Chemotherapy-induced anemia is rare in dogs and cats and, if it occurs, is of late onset (3 to 4 months after initiation of therapy). Other patient-related factors (e.g., malnutrition, old age, concurrent organ dysfunction, prior extensive chemotherapy) and tumor-related factors (e.g., bone marrow infiltration, widespread parenchymal organ metastases) can also affect the degree of myelosuppression.
Although thrombocytopenia is probably as common as neutropenia, it is rarely severe enough to cause spontaneous bleeding, and therefore it is not discussed at length here. In general, in most dogs with chemotherapy-induced thrombocytopenia, the platelet counts remain above 50,000 cells/μl. Spontaneous bleeding usually does not occur until platelet counts are below 30,000/μl. Some drugs and protocols are associated with predictable thrombocytopenia, including doxorubicin and dacarbazine (ADIC), D-MAC (see the table on cancer chemotherapy protocols at the end of Part 11), lomustine, and melphalan in dogs; platelet counts associated with these protocols are usually less than 50,000/μl. Chemotherapy-induced thrombocytopenia is extremely rare in cats. Thrombocytosis is common in cats and dogs receiving vincristine.
Neutropenia usually constitutes the dose-limiting cytopenia and occasionally leads to life-threatening sepsis in dogs; although neutropenia does occur in cats receiving chemotherapy, it rarely leads to the development of clinically recognizable sepsis. The nadir of neutropenia for most drugs (i.e., lowest point in the curve) usually occurs 5 to 7 days after treatment, and the neutrophil counts return to normal within 36 to 72 hours of the nadir. With certain drugs the nadir of neutropenia is delayed (i.e., approximately 3 weeks for carboplatin in dogs and cats). Dogs with neutrophil counts less than 2000 cells/μl should be closely monitored for the development of sepsis, although overwhelming sepsis rarely occurs in animals with neutrophil counts of more than 1000 cells/μl. The development of sepsis in neutropenic cats is extremely rare, or it goes unrecognized.
The pathogenesis of sepsis in neutropenic animals is as follows: First, the chemotherapy-induced death and desquamation of gastrointestinal crypt epithelial cells occur simultaneously with myelosuppression; next, enteric bacteria are absorbed through the damaged mucosal barrier into the systemic circulation (bacterial translocation); and, finally, because the number of neutrophils in the circulation is not sufficient to phagocytose and kill the invading organisms, multiple organs become colonized with the bacteria and death ensues, unless the animal is treated appropriately.
It is important to identify the septic neutropenic animal using laboratory means because the cardinal signs of inflammation (i.e., redness, swelling, increased temperature, pain, abnormal function) may be absent because there are not enough neutrophils to participate in the inflammatory process. The same holds true for radiographic changes compatible with inflammation; for example, dogs with neutropenia and bacterial pneumonia diagnosed on the basis of cytologic and microbiologic findings in transtracheal wash material often have normal thoracic radiographic findings (Fig. 78-1). As a general rule, if a severely neutropenic animal (neutrophil count <500/μl) is evaluated because of pyrexia (>104° F [>40° C]), the fever should be attributed to bacterial pyrogens until proved otherwise and the patient should be treated aggressively with antimicrobial therapy (see following paragraphs). Neutropenic septic patients can also be hypothermic.
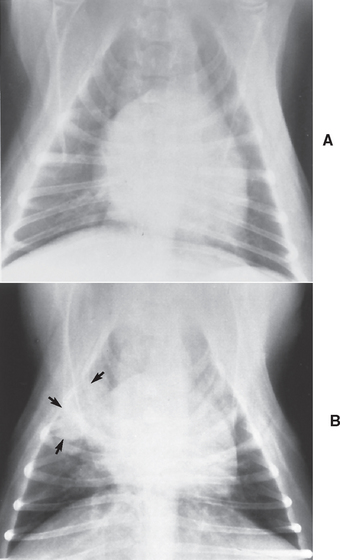
FIG 78-1 Thoracic radiographs from a 5-year-old male, castrated Boston Terrier with multicentric lymphoma treated with doxorubicin and dacarbazine (ADIC) chemotherapy. This dog presented as an emergency because of depression, fever, and mild bilateral nasal discharge. The neutrophil count on admission was 1500/μl. A, Thoracic radiograph findings were considered normal at the time, but a transtracheal wash specimen contained bacteria. B, Two days later, when the neutrophil count increased to 16,300/μl, focal areas of pneumonia became evident.
(From Couto CG: Management of complications of cancer chemotherapy, Vet Clin North Am 20:1037, 1990.)
All dogs and cats undergoing chemotherapy should be up to date on their vaccines; it is controversial whether the use of modified-live vaccines should be avoided because of the potential for inducing illness in immunosuppressed animals. Recent evidence suggests that dogs with cancer undergoing chemotherapy have protective serum antibody titers for commonly used vaccines.
Hematologic monitoring of the patient receiving chemotherapy constitutes the most effective way to prevent (or anticipate) severe, life-threatening sepsis or bleeding secondary to myelosuppression. Complete blood counts (CBCs) should be obtained weekly or every other week (depending on the treatment protocol), and the myelosuppressive agent or agents should be temporarily discontinued (or the dose decreased) if the neutrophil count decreases to less than 2000 cells/μl or if the platelet count decreases to less than 50,000 cells/μl. Discontinuing the offending agent or agents for two or three administrations usually allows sufficient time for the cell counts to return to normal. When therapy is reinstituted, it is recommended that only 75% of the initial dose be given and the doses increased during the next 2 to 3 weeks until the initially recommended dose (or a dose that does not produce marked cytopenias) is reached. Obviously, the drawback of discontinuing chemotherapy is the potential for tumor relapse, so the clinician and owner must weigh the pros and cons of temporarily discontinuing treatment.
Clinically, neutropenic animals can be classified as febrile or afebrile. Neutropenic, febrile animals should be managed aggressively because they are usually septic. Thus fever in a neutropenic patient constitutes a medical emergency. The following protocol is the one currently used in such patients at our clinic. First, a thorough physical examination is performed to search for a septic focus, an indwelling intravenous (IV) catheter is placed aseptically, and IV fluids are administered as required. All anticancer agents are discontinued immediately, with the exception of corticosteroids, which should be discontinued gradually, if at all, because acute hypoadrenocorticism can develop in animals receiving steroid therapy if the drug is abruptly discontinued. Blood samples for a CBC and serum biochemical profile are obtained immediately. A urine sample for urinalysis and bacterial culture is also obtained, unless the patient is thrombocytopenic, in which case cystocentesis should be avoided to prevent intravesical bleeding. Two or three sets of aseptically collected blood samples can be obtained at 30-minute intervals for aerobic and anaerobic bacterial cultures and antibiotic susceptibility tests, although this is usually not necessary because the bacterial isolates are quite predictable (see following paragraph) and because the results of these tests will not be available for several days. After the second set of samples for blood cultures is collected, therapy with an empirical bactericidal antibiotic combination is instituted. We use a combination of enrofloxacin (5 to 10 mg/kg IV q24h) and ampicillin (22 mg/kg IV q8h) because most bacterial isolates in such animals are Enterobacteriaceae and staphylococci, organisms commonly susceptible to these agents. Once the neutrophil count returns to normal and the animal’s condition is clinically normal (usually within 72 to 96 hours), the antibiotic combination is discontinued and the animal is allowed to go home, with instructions to the owner to administer sulfadiazine-trimethoprim (ST) at a dosage of 13 to 15 mg/kg by mouth (PO) q12h or enrofloxacin (5 to 10 mg/kg PO q24h) for 5 to 7 days. When the patient returns for additional chemotherapy, the dose of the offending agent or agents should be decreased by 15% to 20%.
At our clinic the yield for three sets of blood cultures in dogs with cancer, fever, and normal-to-high neutrophil counts is approximately 40%, whereas it is approximately 30% in dogs with cancer, fever, and neutropenia. Isolates in the former group usually include Streptococcus spp., Staphylococcus spp., Enterobacter spp., Klebsiella spp., and Escherichia coli, in decreasing order of frequency. In neutropenic, febrile dogs the isolates include mainly Klebsiella spp. and E. coli; Staphylococcus spp. is isolated in fewer than 20% of the dogs.
Neutropenic, afebrile, asymptomatic patients can be treated as outpatients by discontinuing the drug or drugs as described earlier and administering ST (13 to 15 mg/kg PO q12h). The patient that is afebrile but has constitutional signs should be considered to be septic and treated as described in previous paragraphs. If the neutropenia is not severe (i.e., >2000 cells/μl), no therapy is required and the animal should only be observed by the owner. Owners should be instructed to take their pet’s rectal temperature twice daily and to call the veterinarian if pyrexia develops, in which case the animal is treated as neutropenic and febrile. ST eliminates the aerobic intestinal florae but preserves the anaerobic bacteria, which are an important component of the local defense system because of their ability to produce local antibiotic factors. In addition, ST is active against many pathogens isolated from animals with cancer, and it achieves therapeutic blood and tissue concentrations and also high intragranulocytic concentrations.
Myelosuppression may be alleviated through the use of lithium carbonate (10 mg/kg PO q12h) in dogs or recombinant human granulocyte colony–stimulating factor (G-CSF; Neupogen; 5 μg/kg subcutaneously [SC] q24h) in dogs and cats. Although several studies have reported the beneficial role of G-CSF or granulocyte-macrophage colony–stimulating factor (GM-CSF) in dogs and cats, it is unlikely that these agents will find their way into the clinic owing to their high cost (approximately $50 to $150/day) and the fact that dogs and cats can mount an antibody response to this protein of human origin and inactivate it; moreover, in dogs with chemotherapy-induced neutropenia the activity of endogenous G-CSF is extremely high, and neutrophil counts return to normal within 36 to 72 hours, the same interval reported for “response” to G-CSF. In our clinic G-CSF is typically reserved for patients that received accidental chemotherapy overdoses and in which the predicted duration of neutropenia is unknown.
GASTROINTESTINAL TOXICITY
Although less common than myelosuppression, gastrointestinal toxicity is a relatively common complication of cancer chemotherapy in pets. From a clinical standpoint, two major types of gastrointestinal complications can occur: gastroenterocolitis and the combination of anorexia, nausea, and vomiting.
Although results of controlled studies are not available, nausea and vomiting are not apparently as common in pets as they are in humans receiving similar drugs and dosages. Drugs associated with nausea and vomiting in dogs or cats include dacarbazine (DTIC), cisplatin, doxorubicin (primarily in cats), methotrexate, actinomycin D, cyclophosphamide, and 5-fluorouracil (5-FU; see Table 78-1).
Acute anorexia, nausea, and vomiting caused by injectable drugs are usually prevented by administering the offending agents by slow IV infusion. If these problems persist despite this tactic, antiemetics such as metoclopramide can be given at a dosage of 0.1 to 0.3 mg/kg IV, SC, or PO q8h, or prochlorperazinecan be administered intramuscularly at a dosage of 0.5 mg/kg q8-12h. Other antiemetics that may be effective in dogs with chemotherapy-induced emesis are butorphanol (Torbugesic; Fort Dodge Labs, Fort Dodge, Iowa) at a dosage of 0.1 to 0.4 mg/kg intramuscularly or intravenously every 6 to 8 hours and IV ondansetron (Zofran; Glaxo, Research Triangle Park, N.C.) at a dosage of 0.1 mg/kg immediately before chemotherapy and every 6 hours thereafter, or maropitant (Cerenia, Pfizer Animal Health, Kalamazoo, MI) at a dosage of 2 mg/kg, PO q24h. (For additional information on this subject, see Chapter 30.) Methotrexate and cyclophosphamide, two drugs that are commonly administered PO, can also cause anorexia, nausea, and vomiting. Methotrexate commonly causes anorexia and vomiting 2 or 3 weeks after the start of therapy in dogs; these adverse effects are usually controlled with metoclopramide given at the dosage just described. If these problems persist, it may be necessary to discontinue methotrexate treatment. Cyclophosphamide tends to induce anorexia or vomiting in cats. Cyproheptadine (Periactin; Merck Sharp & Dohme, West Point, Pa) at a dosage of 1 to 2 mg (total dose) PO q8-12h is quite effective as an appetite stimulant and antinausea agent in cats.
Gastroenterocolitis is uncommon in animals receiving anticancer agents. Drugs that occasionally cause mucositis include methotrexate, 5-FU, actinomycin D, and doxorubicin. It occurs rarely in association with other alkylating agents, such as cyclophosphamide. Of the drugs mentioned in the previous paragraphs, only doxorubicin and methotrexate appear to be of clinical relevance. On the basis of our experience, Collies and Collie crosses, Old English Sheepdogs, Cocker Spaniels, and West Highland White Terriers appear to be extremely susceptible to doxorubicin-induced enterocolitis.
Doxorubicin-induced enterocolitis is characterized by the development of hemorrhagic diarrhea (with or without vomiting), primarily of the large bowel type, 3 to 7 days after the administration of the drug. Supportive fluid therapy (if necessary) and treatment with therapeutic doses of bismuth subsalicylate–containing products (Pepto-Bismol, 3 to 15 ml or 1-2 tabs PO q8-12h) are generally effective in controlling the clinical signs in dogs, which usually resolve in 3 to 5 days. The administration of Pepto-Bismol from days 1 to 7 of the treatment may alleviate or prevent these signs in dogs at risk for gastroenterocolitis (i.e., one of the breeds mentioned, an animal with a history of this toxicity). The use of bismuth subsalicylate should be avoided in cats. Gastroenteritis associated with the PO administration of methotrexate usually occurs a minimum of 2 weeks after the animal has been receiving this drug; the treatment is the same as that used for doxorubicin-induced enterocolitis.
HYPERSENSITIVITY REACTIONS
Acute type I hypersensitivity reactions occasionally occur in dogs receiving parenteral l-Asparaginase or doxorubicin and are common in dogs treated with IV etoposide or taxol derivatives; in the latter two, there is a reaction to the solubilizing agent (Tween 80). The reaction to doxorubicin does not appear to be a true hypersensitivity reaction, however, because this agent can induce direct mast cell degranulation independently of immunoglobulin E (IgE) mediation. Etoposide can be safely administered to dogs PO. Hypersensitivity reactions to anticancer agents are extremely rare in cats and thus are not discussed.
Clinical signs in dogs with hypersensitivity reactions to anticancer agents are similar to those in dogs with other types of hypersensitivity reactions (i.e., they are primarily cutaneous and gastrointestinal). Typical signs appear during or shortly after administration of the agent and include head shaking (caused by ear pruritus), generalized urticaria and erythema, restlessness, occasionally vomiting or diarrhea, and rarely collapse caused by hypotension.
Most systemic anaphylactic reactions can be prevented by pretreating the patient with H1 antihistamines (i.e., IM diphenhydramine, 1 to 2 mg/kg 20 to 30 minutes before administration of the drug) and by administering certain drugs (e.g., l-Asparaginase) subcutaneously or intramuscularly rather than through an IV route. If the agent cannot be given by any other routes (i.e., doxorubicin), it should be diluted and administered by slow IV infusion.
The treatment of acute hypersensitivity reactions includes immediate discontinuation of the agent and the administration of H1 antihistamines (i.e., diphenhydramine, 0.2 to 0.5 mg/kg by slow IV infusion), dexamethasone sodium phosphate (1 to 2 mg/kg IV), and fluids if necessary. If the systemic reaction is severe, epinephrine (0.1 to 0.3 ml of a 1 : 1000 solution IM or IV) should be used. Once the reaction subsides (and if it was mild), the administration of certain drugs, such as doxorubicin, may be continued. Injectable H1 antihistamines should be used with caution in cats (if at all), because they can cause acute central nervous system depression leading to apnea.
DERMATOLOGIC TOXICITY
It is rare for anticancer agents to cause dermatologic toxicity in small animals. However, three types of dermatologic toxicities can occur: local tissue necrosis (caused by extravasation), delayed hair growth and alopecia, and hyperpigmentation.
Local tissue necrosis resulting from the extravasation of vincristine, vinblastine, actinomycin D, or doxorubicin is occasionally seen in dogs receiving these drugs but is extremely rare in cats. Indeed, according to anecdotal reports, cats have accidentally received entire doses of doxorubicin perivascularly without developing tissue necrosis. The pathogenesis of this toxicity is poorly understood, but it is thought to be mediated by release of free radicals; however, some of these drugs are also directly caustic if given perivascularly, causing moderate to severe tissue necrosis. As a consequence, every effort should be made to ensure that these drugs are administered intravascularly. In addition to this complication, some retrievers (e.g., Labrador and Golden Retrievers) appear to experience pruritus or discomfort around the site of the IV injection even when the drug is known to have been administered intravascularly. This pain and discomfort frequently lead to licking and the development of a pyotraumatic dermatitis (“hot spot”) within hours of the injection. In these dogs applying a bandage over the injection site or placing an Elizabethan collar prevents this type of reaction.
To prevent or minimize the probability of extravascular injection of caustic drugs, they should be administered through small-gauge (22- to 23-gauge), indwelling, IV, over-the-needle catheters or through 23- to 25-gauge butterfly catheters. We use the former to administer doxorubicin and the latter to administer the vinca alkaloids and actinomycin D. Caustic drugs should be properly diluted before administration (i.e., vincristine to a final concentration of 0.1 mg/ml and doxorubicin to a concentration of 0.5 mg/ml) and the patency of the intravascular injection site ensured by intermittently aspirating until blood appears in the catheter. In our clinic, we do not administer doxorubicin by IV constant-rate infusion because such patients may be more likely to undergo extravasation. If the site is not patent, the catheter should be placed in another vein. Recommendations for the management of extravascular injections are listed in Box 78-1.
 BOX 78-1 Recommendations for the Management of Perivascular Injections of Caustic Anticancer Drugs in Cats and Dogs*
BOX 78-1 Recommendations for the Management of Perivascular Injections of Caustic Anticancer Drugs in Cats and Dogs*
IV, Intravenous.
* Please see text for additional information.
If, despite these precautions, a local tissue reaction occurs, it develops approximately 1 to 7 days after the perivascular injection of vinca alkaloids or actinomycin D and 7 to 15 days after doxorubicin extravasation. Tissue necrosis resulting from doxorubicin extravasation is far more severe than that associated with the extravasation of other agents because the drug is extremely caustic and persists in tissues for up to 16 weeks. If perivascular administration of doxorubicin has occurred (and the clinician has recognized it during or immediately after the administration), dexrazoxane (Zinecard, Pfizer) can be administered at 5 to 10 times the dose of doxorubicin given (i.e., for 30 mg of doxorubicin, 150-300 mg of dexrazoxane should be given). Dexrazoxane is rather expensive, so it is not routinely used in small animal patients.
We recently evaluated carvedilol (Coreg, Glaxo Smith Kline) in a limited number of dogs that received perivascular doxorubicin. In three dogs that received treatment immediately after drug extravasation (at a dosage of 0.1 to 0.4 mg/kg q12-24h), there were no visible signs of necrosis. In three dogs that developed necrosis after perivascular doxorubicin administration, carvedilol resulted in rapid healing of the area (i.e., within 2-3 weeks).
Clinical signs include pain, pruritus, erythema, moist dermatitis, and necrosis of the affected area; severe tissue sloughing may occur (Fig. 78-2).
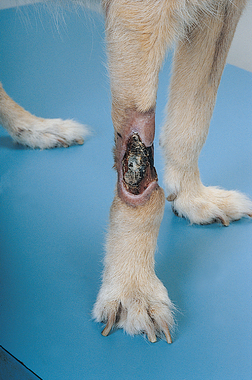
FIG 78-2 Tissue necrosis after extravascular injection of doxorubicin in a dog. Note the full-thickness sloughing of the area.
If local tissue reactions develop, they can be treated as shown in Box 78-2.
 BOX 78-2 Treatment of Local Tissue Reactions
BOX 78-2 Treatment of Local Tissue Reactions
In dogs and cats undergoing chemotherapy delayed hair growth is more common than alopecia. This is in contrast to the situation in human patients, in whom severe scalp alopecia is a predictable complication of therapy. Because most chemotherapeutic agents affect rapidly dividing tissues, cells in the anagen (growth) phase of the hair cycle are usually affected. Therefore hair is slow to regrow in areas that were clipped or shaved before or during chemotherapy. Excessive shedding is also common.
Alopecia occurs predominantly in woolly-haired (coarse-haired) dogs, such as Poodles, Schnauzers, and Kerry Blue Terriers (Fig. 78-3). It affects primarily the tactile hairs in short-haired dogs and cats. Although the exact reason that chemotherapy-induced alopecia occurs in woolly-haired dogs is unknown, a prolonged anagen phase and synchronous hair growth, comparable to those occurring in human scalp hair, may make these dogs prone to this toxic effect. Drugs commonly associated with delayed hair growth and alopecia include cyclophosphamide, doxorubicin, 5-FU, 6-thioguanine, and hydroxyurea (Hydrea; E.R. Squibb & Sons, Princeton, N.J.). Alopecia and delayed hair growth usually resolve shortly after discontinuation of the offending agent.
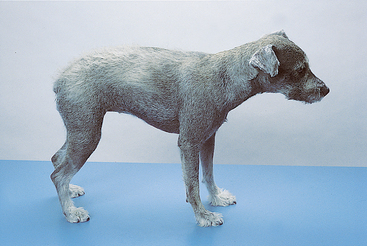
FIG 78-3 Alopecia in a 7-year-old Schnauzer undergoing doxorubicin and dacarbazine (ADIC) chemotherapy. Note the short and light-colored haircoat.
Hyperpigmentation is uncommon in dogs and extremely rare in cats receiving chemotherapy. Cutaneous hyperpigmentation affecting the face, ventral abdomen, and flanks is common in dogs receiving doxorubicin- and bleomycin-containing protocols.
PANCREATITIS
Pancreatitis is a well-recognized entity in human patients undergoing chemotherapy. Offending drugs in humans include corticosteroids, azathioprine, 6-mercaptopurine, l-Asparaginase, cytosine arabinoside, and combination chemotherapy. Sporadic reports of pancreatitis in dogs (but not in cats) receiving chemotherapeutic and immunosuppressive agents have also appeared in the literature.
We have documented acute pancreatitis in several dogs receiving l-Asparaginase or combination chemotherapy. Dogs in the latter group were receiving COAP (cyclophosphamide, vincristine, cytosine arabinoside, prednisone), ADIC (doxorubicin, DTIC); or VAC (vincristine, doxorubicin, cyclophosphamide) chemotherapy. Clinical signs developed 1 to 5 days after the start of chemotherapy and consisted of anorexia, vomiting, and depression. Physical examination findings in these dogs were unremarkable, and abdominal pain was rare. Serum lipase and amylase activities were high in all the animals, and ultrasonographic evidence of pancreatitis was detected in approximately one half of the dogs. The animals were treated with IV fluids, and the clinical signs resolved within 3 to 10 days in most dogs.
It is difficult to prevent chemotherapy-induced pancreatitis because it is not a predictable complication. As a general precaution, we refrain from using l-Asparaginase in dogs at high risk for pancreatitis (i.e., overweight middle-age to older female dogs). As a further precaution, dogs receiving drugs with the potential to cause pancreatitis should be fed a low-fat diet.
CARDIOTOXICITY
Cardiotoxicity is a relatively uncommon complication of doxorubicin therapy in dogs; it is extremely rare in cats. Two types of doxorubicin-induced cardiac toxicity are observed in dogs: an acute reaction occurring during or shortly after administration and a chronic cumulative toxicity. Acute doxorubicin toxicity is characterized by cardiac arrhythmias (mainly sinus tachycardia) that develop during or shortly after administration. This phenomenon is thought to stem from doxorubicin-induced, histamine-mediated catecholamine release because the sinus tachycardia and hypotension can be prevented by pretreatment with H1 and H2 antihistamines. Several weeks or months after repeated doxorubicin injections, persistent arrhythmias, including ventricular premature contractions, atrial premature contractions, paroxysmal ventricular tachycardia, second-degree atrioventricular blocks, and intraventricular conduction defects, develop. These rhythm disturbances are usually associated with the development of a dilated cardiomyopathy, similar to that which occurs spontaneously in Doberman Pinschers and Cocker Spaniels.
The hallmark of chronic doxorubicin toxicity is a dilated cardiomyopathy that develops after a total cumulative dose of approximately 240 mg/m2 is exceeded in the dog. The histologic lesions seen in dogs with doxorubicin-induced cardiomyopathy consist of vacuolation of myocytes, with or without myofibril loss. Clinical signs of toxicity in dogs are those of congestive heart failure (usually left-sided). Therapy consists of discontinuation of the offending drug and the administration of cardiac drugs such as digitalis glycosides or nonglycoside inotropic agents. Once cardiomyopathy develops, the prognosis is poor because the myocardial lesions are irreversible.
It is critical to monitor patients receiving doxorubicin to prevent fatal cardiomyopathy. In this respect, dogs (and possibly) cats with underlying rhythm disturbances or impaired myocardial contractility, as shown by decreased fractional shortening on M-mode or Doppler echocardiograms, should not receive doxorubicin. It is also recommended that animals receiving doxorubicin undergo echocardiographic evaluation every three doxorubicin cycles (9 weeks) to assess myocardial contractility and that the drug be discontinued if decreased fractional shortening occurs. Endomyocardial biopsy specimens are commonly obtained in people receiving doxorubicin in an effort to detect submicroscopic lesions, but this is impractical in dogs. The value of serum cardiac troponin I concentrations to detect early myocardial damage from doxorubicin is currently being evaluated in dogs.
Several protocols have been devised in an attempt to minimize doxorubicin-induced cardiomyopathy in dogs. Unfortunately, only two have shown promise in minimizing or preventing cardiomyopathy. Of these, weekly low-dose doxorubicin therapy in humans has been found to be associated with a significantly lower frequency of histologic changes than the conventional 3-week schedule has been. I have been able to administer total cumulative doses of 500 mg/m2 to two dogs using a 10 mg/m2 weekly protocol. However, recent reports describe a loss of antitumor activity when using weekly low-dose doxorubicin in dogs with lymphoma. A new compound, dexrazoxane (Zinacard, Upjohn-Pharmacia, Kalamazoo, Mich.), offers a promising means of reducing the chronic cardiotoxicity induced by doxorubicin; doses in excess of 500 mg/m2 have been administered to dogs receiving the agent without causing significant cardiotoxicity. Recently, carvedilol (0.1-0.4 mg/kg, PO, q12-24h) has been used successfully to prevent or decrease the probability of developing doxorubicin-associated cardiomyopathy in people (Kalay et al, 2006); we have successfully used carvedilol in dogs with subclinical myocardial dysfunction that needed doxorubicin.
UROTOXICITY
The urinary tract in small animals is rarely affected by adverse reactions to anticancer agents. Only two specific complications are of clinical importance in pets with cancer: nephrotoxicity and sterile hemorrhagic cystitis. Transitional cell carcinomas of the urinary bladder associated with chronic cyclophosphamide therapy have also been reported in dogs.
Nephrotoxicity is rarely observed in dogs and cats undergoing chemotherapy. Although several potentially nephrotoxic drugs are commonly used in these species, only doxorubicin (primarily in cats), cisplatin (in dogs), and intermediate to high doses of methotrexate (in dogs) are of concern to clinicians. In our clinic we do not use cisplatin frequently on account of its potential to induce nephrotoxicity.
Doxorubicin may be a nephrotoxin in cats, and the limiting cumulative toxicity in this species may be renal rather than cardiac. Doxorubicin may cause nephrotoxicosis in dogs with preexisting renal disease and in those concomitantly receiving other nephrotoxins, such as aminoglycoside antibiotics or cisplatin. The administration of cisplatin using forced diuresis protocols minimizes the prevalence of nephrotoxicity in dogs.
Sterile hemorrhagic cystitis is a relatively common complication of long-term cyclophosphamide therapy in dogs; rarely, it may also occur acutely after a single dose of cyclophosphamide. This toxicity is not clinically relevant in cats. Acute clinical signs and urinalysis changes compatible with sterile hemorrhagic cystitis developed after the first injection in three dogs treated at our clinic with IV cyclophosphamide, 100 mg/m2, and four dogs receiving PO cyclophosphamide, 300 mg/m2. Sterile cystitis results from the irritating effects of one of the cyclophosphamide metabolites (acrolein). It develops in approximately 5% to 25% of dogs and 1% to 3% of cats treated with cyclophosphamide, usually after an average of 18 weeks of therapy. Furosemide or prednisone administered concomitantly with cyclophosphamide appears to decrease the prevalence of cystitis.
Forced diuresis appears to minimize the severity of this complication or prevent it. I usually recommend administering the cyclophosphamide in the morning, allowing the pet to urinate frequently (if it is an indoor dog), salting the food, and administering prednisone on the same day that the animal receives the cyclophosphamide (if the protocol calls for prednisone administration).
Clinical signs of sterile hemorrhagic cystitis are similar to those of other lower urinary tract disorders and include pollakiuria, hematuria, and dysuria. Urinalysis typically reveals blood and mildly to moderately increased numbers of white blood cells but no bacteria. Treatment of this complication consists of discontinuing the cyclophosphamide, forcing diuresis, diminishing the inflammation of the bladder wall, and preventing secondary bacterial infections. The cystitis resolves in most dogs within 1 to 4 months after the cyclophosphamide is discontinued. I administer furosemide (Lasix) at a dosage of 2 mg/kg PO every 12 hours for its diuretic effects, prednisone at a dosage of 0.5 to 1 mg/kg PO every 24 hours for its antiinflammatory (and diuretic) effect, and an ST combination at a dose of 13 to 15 mg/kg PO every 12 hours to prevent secondary bacterial contamination. If the clinical signs worsen despite this approach, the instillation of 1% formalin solution in water into the bladder can be attempted. Gross hematuria resolved within 24 hours and did not recur in two dogs thus treated. The intravesical infusion of a 25% to 50% dimethylsulfoxide solution may also alleviate the signs of cystitis in dogs.
HEPATOTOXICITY
Chemotherapy-induced hepatotoxicity is extremely rare in dogs and cats. With the exception of the hepatic changes induced by corticosteroids in dogs, to my knowledge only methotrexate, cyclophosphamide, lomustine, and azathioprine (Imuran; Burroughs Wellcome, Research Triangle Park, N.C.) have been implicated as or confirmed to be hepatotoxins in dogs. In my experience, the hepatotoxicity caused by anticancer drugs in small animals is of little or no clinical relevance, with the exception of lomustine.
A recent report describes a low prevalence of hepatotoxicity (<10%) in dogs receiving lomustine (CCNU) for lymphoma or mast cell tumors. In our clinic we have documented marked increases in alanine transaminase (ALT) activities (>1000 IU/L) and mild increases in alkaline phosphatase (ALP) activities (<500 IU/L) within 3 weeks of starting lomustine therapy in several dogs with mast cell tumors or granulomatous meningoencephalitis. Most dogs had decreases in the ALT and ALP concentrations after lengthening the dosing interval, decreasing the individual dosage, or both. In my experience, hepatoprotectors appear to be of no benefit in preventing CCNU-induced hepatotoxicity.
Dogs with immune-mediated disorders receiving chronic azathioprine therapy rarely develop increases in liver enzyme activities that respond to discontinuation of the drug.
NEUROTOXICITY
Anticancer agent–induced neurotoxicity is also extremely rare in dogs and cats. Neurotoxicosis occurs infrequently in dogs receiving 5-FU, although it is common in cats (for this reason, this drug should not be used in cats). Neurotoxicity can also occur in dogs and cats that ingest 5-FU intended for human use (i.e., prescribed for the owners). Clinical signs occur shortly (3 to 12 hours) after ingestion of the drug and consist primarily of excitation and cerebellar ataxia, resulting in death in approximately one third of the dogs and in most cats. Neurotoxicity was also documented in 25% of dogs receiving a combination of actinomycin D, 5-FU, and cyclophosphamide (the CDF protocol) for the management of metastatic or nonresectable carcinomas at our clinic. This prevalence is considerably higher than that seen in association with the use of 5-FU in combination with other drugs and may be a result of drug interactions.
PULMONARY TOXICITY
Pulmonary toxicity is extremely rare in dogs and cats receiving chemotherapy. To my knowledge, only cisplatin has been documented as a cause of pulmonary toxicity in cats. Acute signs of dyspnea leading to death occur within 48 to 96 hours of the administration of cisplatin in this species. Necropsy findings consist of pulmonary and mediastinal edema and microangiopathic changes in the pulmonary vasculature. Because of the risk of this serious toxicity, cisplatin should not be used in cats; carboplatin, a cisplatin derivative, does not cause pulmonary toxicity in this species.
ACUTE TUMOR LYSIS SYNDROME
In human patients the rapid lysis of certain tumor cells (e.g., lymphoma cells) shortly after chemotherapy may lead to a syndrome of hyperuricemia, hyperphosphatemia, and hyperkalemia, either singly or in combination. This clinical entity is referred to as acute tumor lysis syndrome and is thought to be secondary to the release of high quantities of intracellular phosphate, uric acid, and nucleic acid metabolites. The intracellular concentration of phosphorus in human lymphoma and leukemic cells is four to six times higher than that in normal lymphocytes, and the same appears to be true for dogs.
In dogs ATLS has been reported to occur only in association with lymphomas treated with chemotherapy, radiation therapy, or both and is characterized by hyperphosphatemia, with or without azotemia, hyperkalemia, hypocalcemia, metabolic acidosis, and hyperuricemia. It is rare in cats. Clinical signs include depression, vomiting, and diarrhea and occur within hours of the start of chemotherapy.
We have documented clinically evident ATLS after chemotherapy in 10 dogs with lymphoma, during a period in which approximately 2000 dogs with lymphoma were treated with chemotherapy. In most dogs the pretreatment serum creatinine concentrations or the tumor burden was high; one of the dogs had high liver enzyme activities. Within 1 to 7 days of the start of chemotherapy, lethargy, vomiting, and bloody diarrhea developed in affected dogs and the serum phosphorus concentrations increased markedly (Fig. 78-4). Aggressive fluid therapy and the correction of acid-base and electrolyte disturbances resulted in resolution of the clinical signs within 3 days in six dogs; the remaining two dogs died as a result of ATLS.
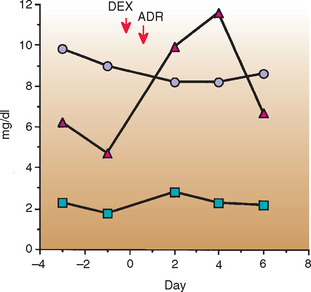
FIG 78-4 Serum phosphorus (δ), calcium ( ), and creatinine (
), and creatinine ( ) concentrations in a dog with acute tumor lysis syndrome after chemotherapy for a primary pulmonary lymphoma. Note the increase in the serum phosphorus concentrations, with a mild decrease in the calcium concentrations and minor increases in the serum creatinine concentrations. DEX, Dexamethasone; ADR, doxorubicin.
) concentrations in a dog with acute tumor lysis syndrome after chemotherapy for a primary pulmonary lymphoma. Note the increase in the serum phosphorus concentrations, with a mild decrease in the calcium concentrations and minor increases in the serum creatinine concentrations. DEX, Dexamethasone; ADR, doxorubicin.
(From Couto CG: Management of complications of cancer chemotherapy, Vet Clin North Am 20:1037, 1990.)
Charney SC, et al. Risk factors for sterile hemorrhagic cystitis in dogs with lymphoma receiving cyclophosphamide with or without concurrent administration of furosemide: 216 cases (1990–1996). J Am Vet Med Assoc. 2003;222:1388.
Couto CG. Management of complications of cancer chemotherapy. Vet Clin N Am. 1990;20:1037.
Crow SE, et al. Cyclophosphamide-induced cystitis in the dog and cat. J Am Vet Med Assoc. 1977;171:259.
Harvey HJ, et al. Neurotoxicosis associated with use of 5-fluorouracil in five dogs and one cat. J Am Vet Med Assoc. 1977;171:277.
Kalay N, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258.
Knapp DW, et al. Cisplatin toxicity in cats. J Vet Intern Med. 1988;1:29.
Kristal O, et al. Hepatotoxicity associated with CCNU (lomustine) chemotherapy in dogs. J Vet Intern Med. 2004;18:75.
Laing EJ, et al. Acute tumor lysis syndrome following treatment of canine lymphoma. J Am Anim Hosp Assoc. 1988;24:691.
Laing EJ, et al. Treatment of cyclophosphamide-induced hemorrhagic cystitis in five dogs. J Am Vet Med Assoc. 1988;193:233.
Peterson JL, et al. Acute sterile hemorrhagic cystitis after a single intravenous administration of cyclophosphamide in three dogs. J Am Vet Med Assoc. 1992;201:1572.
Thamm DH, Vail DM. Aftershocks of cancer chemotherapy: managing adverse effects. J Am Anim Hosp Assoc. 2007;43:1.
Weller RE. Intravesical instillation of dilute formalin for treatment of cyclophosphamide-induced cystitis in two dogs. J Am Vet Med Assoc. 1978;172:1206.