CHAPTER 30 General Therapeutic Principles
FLUID THERAPY
Fluid therapy is primarily used to treat shock, dehydration, and electrolyte and acid-base disturbances. Accurately predicting the nature of electrolyte and acid-base changes on the basis of clinical parameters is impossible; therefore serum electrolyte concentrations must be measured. Vomiting gastric contents inconsistently produces a classic hypokalemic, hypochloremic metabolic alkalosis. The loss of intestinal contents classically produces hypokalemia, with or without acidosis, but a hypokalemic, metabolic alkalosis may occur. Vomiting animals are often assumed to be hypokalemic; however, animals with hypoadrenocorticism or anuric renal failure may be hyperkalemic. If electrolytes have not been measured or if fluid therapy must be started before they are available, physiologic saline solution plus 20 mEq potassium chloride per liter is a reasonable therapeutic choice (see Table 30-1), assuming that the fluids are administered at one to two times the maintenance requirement. A lead II electrocardiographic (ECG) tracing may be evaluated to ensure that moderate to severe hyperkalemia is unlikely (see Chapter 55).
 TABLE 30-1 General Guidelines for Potassium Supplementation of IV Fluids
TABLE 30-1 General Guidelines for Potassium Supplementation of IV Fluids
| PLASMA POTASSIUM CONCENTRATION (mEq/L) | AMOUNT OF KCl TO ADD TO FLUIDS GIVEN AT MAINTENANCE RATES* (mEq/L) |
|---|---|
| 3.7-5.0 | 10-20 |
| 3.0-3.7 | 20-30 |
| 2.5-3.0 | 30-40 |
| 2.0-2.5 | 40-60 |
| <2.0 | 60-70 |
* Do not exceed potassium, 0.5 mEq/kg/hr, except in animals in hypokalemic emergencies and then only with constant, close electrocardiogram (ECG) monitoring. Be sure to routinely monitor plasma potassium concentrations whenever administering fluids with more than 30 to 40 mEq of potassium per liter.
It is rarely necessary or appropriate to administer bicarbonate because reexpanding the vascular compartment and improving peripheral perfusion will alleviate lactic acidosis. Bicarbonate is primarily administered in patients with extreme acidosis (e.g., pH < 7.05 or bicarbonate <10 mEq/L) that are in imminent danger of dying. Bicarbonate or lactated Ringer’s solution should not be used if alkalosis seems likely (e.g., vomiting of gastric origin).
Parenteral fluid administration is indicated if the animal is significantly hypovolemic or if the absorption of enteral fluids is questionable (e.g., severe intestinal disease, obstruction, vomiting, or ileus). Subcutaneous (SC) fluid administration is acceptable if the animal is not in shock, absorbs the fluids, and accepts repeated SC administration. Multiple SC depots of 10 to 50 ml each are given, depending on the animal’s size. Dependent areas should be checked for the presence of unabsorbed fluids before administering more fluid. Severely dehydrated animals may not absorb SC fluids as rapidly as desired, making initial intravenous (IV) administration more effective. IV fluid administration is required in patients that are severely dehydrated or are in shock, even if a venous cutdown is necessary. Intramedullary administration may be used if IV administration is desired but a catheter cannot be established. To do this, a large-bore hypodermic needle or a bone marrow aspiration needle (preferable) can be inserted through the femur (trochanteric fossa), the tibia, the wing of the ilium, or the humerus. Fluids can be administered by the intramedullary route at a maintenance rate or faster. Intraperitoneal administration is acceptable but repletes the intravascular compartment more slowly than IV or intramedullary techniques.
Dogs in shock (e.g., those with tachycardia, poor peripheral perfusion, cool extremities, a prolonged capillary refill time, a weak femoral pulse, and/or tachypnea) may receive 88 ml of isotonic crystalloids per kilogram or more intravenously during the first hour. This “maximum” rate may be exceeded if necessary to reestablish adequate peripheral perfusion; the patient must be closely monitored to determine whether the fluids are being administered appropriately. It is also important to remember that dogs with systemic inflammatory response syndrome (SIRS) initially have brick-red oral mucous membranes; warm extremities; and a strong, bounding femoral pulse before the signs of classic shock occur. Large dogs in severe shock, such as those with a gastric volvulus, may require two simultaneous 16- to 18-gauge cephalic catheters and IV bags placed in pneumatic compression devices to achieve an adequate flow rate. It is easier to overhydrate cats; the clinician should therefore monitor cats carefully when rapidly administering fluids. In general, the clinician should not exceed 55 ml/kg during the first hour for cats in shock. Lactated Ringer’s solution or physiologic saline solution is commonly used for treating shock. However, the clinician must be sure that fluids that are to be administered rapidly for shock do not contain too much potassium because cardiotoxicity can occur.
Hypertonic saline solution (i.e., 7%) may be used to treat severe hypovolemic or endotoxic shock. Relatively small volumes (i.e., 4 to 5 ml/kg delivered over 10 minutes) seem to be as effective as larger volumes of isotonic crystalloids. Hypertonic solutions shift fluid from the intracellular and interstitial compartments into the intravascular compartment and stimulate vascular reflexes. Hypertonic solutions generally should not be used in animals with hypernatremic dehydration, cardiogenic shock, or renal failure. Uncontrolled hemorrhage may also be a contraindication to their use. The clinician may readminister hypertonic saline solution in 2 ml/kg aliquots until a total of 10 ml/kg has been given or until the serum sodium concentration is 160 mEq/L or more. After administering hypertonic saline solution, the clinician may continue to administer other fluids but at a reduced rate (e.g., 10 to 20 ml/kg/hr) until shock is controlled. A mixture of 7% saline solution plus dextran 70 has a longer duration of action than hypertonic saline solution alone. This combination may be administered at a rate of 3 to 5 ml/kg over 5 minutes. Dextran is rarely associated with allergic reactions or renal failure but should be used carefully or not at all in animals with coagulopathies.
Colloids (e.g., hetastarch) are also useful in treating shock. Like hypertonic saline solution, colloids draw water from the interstitial compartment into the vascular compartment; however, their effects last longer and do not increase the total body sodium load. Relatively small volumes can be administered quickly (i.e., 5 to 10 ml/kg, maximum of 20 ml/kg in 1 day), and the clinician must reduce the subsequent rate of IV fluid administration to prevent hypertension. Colloids should be used with caution in animals with bleeding tendencies.
If it is difficult to maintain peak systolic blood pressures above 80 to 90 mm Hg, vasopressors may be needed. Constant rate infusion of vasopressin has been very effective for this purpose, even when dobutamine and dopamine were unsuccessful.
Approximately 44 to 66 ml of fluid per kilogram of body weight is required daily for maintenance for dogs weighing between 10 and 50 kg, with larger dogs needing less than smaller dogs. Dogs weighing less than 5 kg may need 80 ml/kg/day. It is important to choose the correct fluid to prevent electrolyte imbalances, especially hypokalemia. In general, potassium should be supplemented if the animal is anorec-tic or vomiting, has diarrhea, or is receiving prolonged or intense fluid therapy (see guidelines for administration in Table 30-1). The animal should be monitored for the development of iatrogenic hyperkalemia (e.g., ECG or plasma potassium determinations), and no more than 0.5 mEq/kg/h should generally be administered. Oral (PO) potassium supplementation is often more effective than parenteral supplementation if the animal is not vomiting. Cats receiving IV fluids often show an initial decrease in their serum potassium concentrations, even if the fluids contain 40 mEq or more of potassium chloride per liter.
Dehydrated animals not in shock are treated by replacing the estimated fluid deficit. To do this, first the degree of dehydration must be estimated. Prolonged skin tenting is usually first noted at 5% to 6% dehydration. However, any dog or cat that has lost weight may show skin tenting, whereas obese animals and those with peracute dehydration often do not show skin tenting, regardless of the severity of dehydration. Dry, tacky oral mucous membranes usually indicate 6% to 7% dehydration. However, dehydrated, nauseated animals may have moist oral mucous membranes, whereas well-hydrated, panting, or dyspneic animals have dry mouths. Multiplying the estimated percentage of dehydration by the animal’s weight (in kilograms) determines the liters required to replace the deficit. This amount is typically replaced over 2 to 8 hours, depending on the animal’s condition. Fluid delivery rate should generally not exceed 88 ml/kg/hr. In general, it is better to slightly overestimate rather than underestimate the fluid deficit, unless the animal has congestive heart failure, anuric or oliguric renal failure, severe hypoproteinemia, severe anemia, or pulmonary edema. It is usually easier to harm cats than dogs by excessive fluid administration.
Ongoing losses are typically estimated from the observation of vomiting, diarrhea, and urination; however, it is common to underestimate losses. Weighing the animal regularly is one way to estimate the adequacy of maintenance fluid therapy. A progressive weight loss suggests inadequate fluid therapy. The same scale should always be used to ensure consistent results. A change of 1 lb (0.45 kg) represents approximately 500 ml of water.
The development of inspiratory pulmonary crackles, a gallop rhythm, or edema (especially cervical) indicates that the animal is probably overhydrated. A new heart murmur is not always a sign of overhydration; severely dehydrated dogs with valvular insufficiency may not have an audible murmur until they are volume replete. The central venous pressure is excellent for detecting excessive fluid administration; however, it is rarely necessary to measure it, except in animals with severe cardiac or renal failure and those receiving aggressive fluid therapy. The central venous pressure (CVP) is normally less than 4 cm H2O and generally should not exceed 10 to 12 cm H2O, even during aggressive fluid therapy. Poor technique will often give falsely high CVP readings.
Oral rehydration therapy makes use of the facilitated intestinal absorption of sodium. The co-administration of a monosaccharide (e.g., dextrose) or amino acid with sodium speeds up sodium absorption and subsequent water uptake. This approach works if the animal can ingest oral fluids (i.e., it is not vomiting) and the intestinal mucosa is functional (i.e., there is reasonable villus function). Absorption primarily occurs in the mature epithelium near the villus tip. Various products for use in people are commercially available, and there are also recipes for making these solutions. Failure to monitor the patient or follow instructions may lead to the development of severe hypernatremia. Some dogs and cats with acute enteritis not caused by severe parvoviral enteritis can receive rehydration fluids orally.
The type of fluid therapy used in hypoproteinemic animals depends on the degree of hypoalbuminemia. Excessive fluids can dilute the serum albumin concentration, causing ascites, edema, diminished peripheral perfusion, or a combination of these. Careful calculation of the fluid needs and ongoing losses is therefore necessary. In animals with severe hypoalbuminemia (e.g., serum albumin of 1.5 g/dl or less), a plasma transfusion (6 to 10 ml/kg of plasma initially) may be considered to improve the oncotic pressure. A common mistake is to give inadequate amounts of plasma. Therefore the serum albumin concentration should be measured 8 to 12 hours after the transfusion to ensure that sufficient plasma was administered. Further, animals with severe protein-losing enteropathies and protein-losing nephropathies rapidly excrete the supplemented protein, making repeated transfusions necessary if the plasma albumin concentration is to be maintained. It can therefore be very expensive to replenish albumin in large, hypoalbuminemic dogs. Human albumin has been used instead of canine plasma and appears efficacious although side effects have been reported. Hetastarch (5 to 20 ml/kg/day) and dextran 70 may be used in place of plasma or albumin. Hetastarch (supplied as a 6% solution) is larger than albumin and therefore may persist in the intravascular space longer than albumin, thereby helping maintain the plasma oncotic pressure in animals with severe protein-losing enteropathies. If hetastarch is used, the clinician should decrease the rate of fluid administration to prevent hypertension. Sometimes, administering hetastarch results in massive fluid retention and substantial worsening of ascites.
DIETARY MANAGEMENT
Symptomatic or specific dietary therapy is often important in animals with gastrointestinal tract problems. Symptomatic therapy usually involves the use of bland, easily digested diets, whereas specific therapy typically involves the use of elimination or hypoallergenic diets, diets with a highly restricted fat content, fiber-supplemented diets, or a combination of these.
Bland, easily digested diets are indicated in animals with acute gastritis or enteritis. Such diets are available commercially (Box 30-1). Homemade versions usually consist of boiled poultry or lean hamburger, low-fat cottage cheese, boiled rice, and/or boiled potatoes in some combination. Boiled chicken, turkey, or fish and green beans may be useful in cats. A typical mixture is one part boiled chicken or cottage cheese and two parts boiled potato. The restricted-fat content facilitates digestion. These diets also tend to be low in lactose, which helps prevent maldigestion. Frequent, small amounts of these foods are usually fed until the diarrhea resolves, and then the diet is gradually changed back to the routine one. This diet may be continued after the event is over; however, if a homemade diet is used long-term, it must be nutritionally balanced (especially for puppies and kittens).
 BOX 30-1 Examples of Commercial Bland* Diets
BOX 30-1 Examples of Commercial Bland* Diets
This list is a partial list for the purpose of showing examples of such diets. It is not an all-inclusive list of such diets.
Iams Eukanuba Low-Residue-Adult
Royal Canin Intestinal HE Formula (dogs)
Royal Canin Intestinal HE 30 Formula (cats)
* “Bland” refers to easily digestible diets that often contain less fat than is found in many pet foods.
These easily digested diets usually also help prevent vomiting because they are low in fat and fiber (both delay emptying) and high in complex carbohydrates. Extremely hyperosmolar diets should be avoided (e.g., do not use concentrated sugar solutions or honey) because they also may delay gastric emptying.
Elimination diets are indicated if a dietary allergy (i.e., an immune-mediated hypersensitivity to a dietary component) or intolerance (i.e., a nonimmune-mediated problem) is sus pected. There is also evidence that such diets may help treat and control antibiotic-responsive enteropathies. These diets may be composed of the same ingredients found in bland diets; however, they must be formulated so that the animal is fed food that it has not eaten before (and hence could not be responsible for causing allergy or intolerance) or food that is very unlikely to provoke allergy or intolerance (e.g., potatoes). Excellent commercial elimination diets are available, or the clinician may suggest a homemade diet. Examples of homemade elimination diets are described in Box 30-2.
 BOX 30-2 Examples of Homemade, Hypoallergenic* Diets
BOX 30-2 Examples of Homemade, Hypoallergenic* Diets
1 part boiled white chicken or turkey meat without the skin; 2 parts boiled or baked potato (without the skin)
1 part boiled or broiled white fish without the skin; 2 parts boiled or baked potato (without the skin)
1 part boiled mutton, venison, or rabbit without the skin; 2 parts boiled or baked potato (without the skin)
1 part drained, low-fat cottage cheese; 2 parts boiled or baked potato (without the skin)
* Hypoallergenic refers to a diet specially formulated for a given animal, one that does not expose the animal to potential allergens that it has eaten in the past. Therefore the clinician must obtain a careful dietary history to determine what will or will not constitute a hypoallergenic diet for a particular animal.
Elimination diets that are going to be effective are usually effective within 3 to 4 weeks, although in rare cases patients may require 6 or more weeks before clinical efficacy is evident. It is critical that no other foods or treats be given to the animal during this time (e.g., flavored pills, toys, medications). If the signs resolve during this time, the diet should be continued for at least 4 to 6 more weeks to ensure that it is the diet that is responsible for the animal’s improvement and not a spontaneous fluctuation of the disease. If a homemade diet was used, the clinician should try to gradually switch the animal to a commerical diet or balance the homemade diet with appropriate vitamins, minerals, and fatty acids.
Partially hydrolyzed diets (Purina HA; Nestle Purina, Hill’s z/d; Hill’s Pet Products, Hypoallergenic HP19 Formula [dogs] and Hypoallergenic HP23 Formula [cats]; Royal Canin) have been formulated in an attempt to eliminate proteins large enough to cause immunologic reactions (i.e., make a diet that is hypoallergenic for all animals). Although these diets are not uniformly effective, many dogs and cats with gastrointestinal diseases will have clinical improvement when eating these diets exclusively. The partially hydrolyzed proteins may also make such diets easier for diseased alimentary tracts to digest and absorb.
Elemental diets (e.g., Vivonex TEN; Novartis Nutrition) are diets in which the nutrients are supplied as amino acids and simple sugars. These diets are hypoallergenic, but more important, they are extremely easy to digest and absorb when there is major small intestinal disease. Diseased intestines have increased permeability, which allows luminal contents to leak into the mucosa. Such leakage may be an important mechanism perpetuating intestinal inflammation. Because the amino acids and simple sugars found in elemental diets do not elicit an inflammatory reaction when they enter the interstitium, they do not contribute to perpetuation of the inflammatory response in the intestines. The elemental diets prepared for people (e.g., Vivonex TEN) typically have less protein than desired for veterinary patients. Therefore protein supplements are usually given when preparing this diet by adding 350 ml of water plus 250 ml of 8.5% amino acids (for injection) instead of 600 ml of water. Adding 1 to 2 ml of a flavored vitamin syrup often makes it palatable. If the animal will not drink this formulation, it may be administered via nasoesophageal tube. These diets are generally reserved for patients that are extremely ill from severe intestinal disease.
Ultra–low-fat diets are indicated in animals with intestinal lymphangiectasia. Because long-chain fatty acids enter lacteals and are reesterified, removing them from the diet therefore prevents the dilation and rupture of lacteals and the subsequent intestinal lymphatic loss. Medium-chain triglycerides (MCTs) were once recommended as supplements to such diets at a dose of 1 to 2 ml/kg of body weight. MCTs appear to be absorbed into the portal blood without going through the lacteals and thoracic duct. They have an unpleasant taste, so very small amounts (e.g., 1 tsp/lb of food) should be added to the diet initially. Otherwise, the animal may refuse to eat the food. Using a highly digestible, ultra–low-fat diet usually eliminates the need for supplementing MCTs; however, MCTs have been used to help very thin animals with severe gastrointestinal disease absorb nutrients and gain weight.
Fiber supplementation may help many dogs and cats with large (and rarely small) intestinal diseases. Although fiber is generally classified as soluble or insoluble, many fibers have characteristics of both. Insoluble fiber is poorly digested or metabolized by bacteria and ultimately produces more stool bulk. Some insoluble fibers apparently normalize colonic myoelectrical activity and help prevent spasms. Soluble fiber can be metabolized by bacteria into short-chain volatile fatty acids, which are trophic to colonic mucosa; it may also slow the absorption of nutrients by the small intestine.
Fiber-enriched diets may ameliorate diarrhea in many animals with large bowel disease (especially those with minimal inflammation) and lessen constipation not caused by obstruction or pain. Such a diet should be fed for at least 2 weeks before assessing efficacy, although most animals that respond do so within the first week. A commercial high-fiber diet may be used, or fiber may be added to the current diet. Psyllium hydrocolloid (e.g., Metamucil) or coarse, unprocessed wheat bran may be added to the pet’s diet in the amount of 1 to 2 teaspoons or 1 to 4 tablespoons per can of food, respectively. Some cats will not eat these diets or fiber supplements; however, canned pumpkin pie filling is effective and usually acceptable to cats; 1 to 3 tablespoons may be given daily. It is important that the animal maintain adequate water intake, lest the increased dietary fiber produce obstipation. If too much soluble fiber is fed, there may be excessive stool, which the owner then mistakes for continued large bowel disease.
SPECIAL NUTRITIONAL SUPPLEMENTATION
If the animal refuses to ingest adequate calories, special nutritional supplementation is necessary. Daily nutritional requirements should be calculated to avoid underfeeding. Approximately 60 kcal/kg/day is reasonable for the maintenance needs of mature dogs and cats that are not lactating or losing a significant amount of energy or protein. More exact calculations are recommended if the animal has severe disease or ongoing fluid and nutritional losses (Box 30-3).
 BOX 30-3 Calculation of Nutritional Needs and Formulations of Total Parenteral Nutrition Solution
BOX 30-3 Calculation of Nutritional Needs and Formulations of Total Parenteral Nutrition Solution
For partial (also called peripheral) parenteral nutrition formulation, see Zsombor-Murray et al: Peripheral parenteral nutrition, Comp Cont Educ 21:512, 1999.
Actual body weight = __________ kg
Protein Requirement
6 g/kg in cats and hypoproteinemic dogs
If there is renal failure, use 1.5 g/kg in dogs or 3 g/kg in cats
__________ g of protein necessitates __________ ml of an 8.5% or 10% amino acid solution (85 or 100 mg of protein/ml, respectively).
Determine the calories derived from the protein (4 kcal/g of protein), and subtract this from the daily caloric needs. Supply the remaining calories with glucose and lipid. __________ kcal needed.
Provide at least 10%, and preferably 40%, of caloric needs with lipid emulsion. A 20% lipid emulsion has 2 kcal/ml. Do not use in lipemic animals; use with caution in animals with pancreatitis. __________ ml needed. Provide remainder of calories with 50% dextrose, which has 1.7 kcal/ml. __________ ml needed.
Use one half the calculated amount of solution on the first day, and increase it to the calculated amount on the second day, if hyperglycemia, lipemia, azotemia, or hyperammonemia does not occur.
Either use amino acid solution with electrolytes or add electrolytes so that the solution has sodium, 35 mEq/L; chloride, 35 mEq/L; potassium, 42 mEq/L; magnesium, 5 mEq/L; and phosphate, 15 mmol/L. These concentrations may be adjusted as needed, depending on the animal’s serum electrolyte concentrations. Add multiple vitamins and trace elements (especially zinc and copper) that are formulated for parenteral nutrition solutions.
In some cases, simply sending the animal home, warming the food, or feeding the animal a palatable diet (e.g., chicken baby food for dogs) ensures adequate caloric intake. The clinician can attempt force-feeding by manually placing food in the animal’s mouth, although this seldom works in severely anorectic animals. Cyproheptadine (2 to 4 mg per cat) stimulates some cats to eat, especially those with mild anorexia. However, cyproheptadine seldom induces a severely anorectic cat (e.g., one with severe hepatic lipidosis) to ingest adequate calories. Diazepam rarely causes acute feline hepatic failure. Megestrol acetate is an excellent appetite stimulant but occasionally causes diabetes mellitus, reproductive problems, or tumors. Cobalamin injections have been noted to increase appetite in some dogs. Recently, mirtazapine has been used with anecdotal success in dogs (once daily) and cats (every three days). Appetite stimulants are usually less effective in dogs than in cats.
Tube feeding is a more reliable way to ensure that adequate calories are ingested. Intermittent orogastric tube feeding is useful for animals that need nutritional support for only a relatively short time, although it may be used for longer periods in orphaned puppies and kittens. It is typically done two or three times daily, using restraint and a mouth gag. A tube is measured and marked to correspond to the length from the tip of the nose to the midthoracic region. The tube is then carefully inserted through the mouth gag to the premarked point. If the animal coughs or is dyspneic, the tube may have entered the trachea and should be repositioned. To ensure safety, the clinician should flush the tube with water before the warmed gruel is administered. The gruel should be given over several seconds or 1 minute. Because relatively large-diameter tubes can be used, homemade gruels may be administered in this way. The major disadvantage is the need to physically restrain the animal. Placement of an indwelling tube (discussed in more detail later in this chapter) circumvents this problem.
Nasoesophageal tubes are indicated in animals that need nutritional support and have a functional esophagus, stomach, and intestines. They are easy to place, but they are difficult to maintain in animals that are vomiting. To place them, the clinician first anesthetizes the nose by instilling a few drops of lidocaine solution in one nostril. Then the clinician lubricates a sterile polyvinyl chloride, polyurethane, or silicone tube (the diameter depends on the animal’s size, but 5F to 12F is typical) with sterile, water-soluble jelly and inserts it into the ventromedial portion of the nostril. The animal’s head is restrained in its normal position, and the tube is inserted until the tip is just beyond the thoracic inlet. If the clinician encounters difficulty in passing the tube, the tip should be withdrawn, redirected, and advanced again. If the clinician is unsure whether the tube is in the esophagus, thoracic radiographs should be obtained or several milliliters of sterile saline solution should be instilled into the tube to see if this provokes coughing.
Tape is applied to the tube to secure it, and then the tape is glued or sutured as needed to the skin along the dorsal aspect of the nose. The tube must not be allowed to touch sensory vibrissae because the animal will not tolerate it. It may be necessary to place an Elizabethan collar on some animals to prevent them from pulling out the tube. Only small-diameter tubes (e.g., 5F) can be used in small dogs and cats, which limits the rate of administration and necessitates the use of commercial liquid diets (Table 30-2) instead of less expensive homemade gruels. The clinician should flush the tube with water after each feeding to prevent occlusion. Long-term acceptance is typical, but rhinitis occurs in some animals.
 TABLE 30-2 Selected Enteral Diets
TABLE 30-2 Selected Enteral Diets
| DIET | COMMENTS |
|---|---|
| Osmolite* | Polymeric diet; contains taurine, carnitine, and MCT |
| CliniCare† | Polymeric diet; contains taurine, but no lactose |
| Jevity* | Polymeric diet; contains taurine, fiber, carnitine, and MCT |
| Peptamen‡ | Oligomeric diet; contains taurine, carnitine, and MCT |
| Pulmocare* | Polymeric diet; contains taurine, carnitine, and MCT |
| Vital HN* | Oligomeric diet; contains MCT |
| Vivonex T.E.N.§ | Elemental diet; high in carbohydrates, low in protein and fat‖ |
MCT, Medium-chain triglyceride.
* Ross Laboratories, Columbus, Ohio.
† Abbott Animal Health, North Chicago, Ill.
‡ Nestle Nutrition, Deerfield, Ill.
§ Novartis Nutrition, Minneapolis, Minn.
‖ To increase protein content, reconstitute one packet of powder with 350 ml water plus 250 ml of 8.5% amino acids for injection.
Some dogs and cats do not tolerate nasoesophageal tubes and repeatedly pull them out. However, they are usually effective for short-term therapy (e.g., 1 to 10 days), and some animals tolerate them for weeks.
Pharyngostomy and esophagostomy tubes are indicated in animals with a functional esophagus, stomach, and intestines that require nutritional support but do not tolerate nasoesophageal or intermittent tube feeding. Vomiting may make it difficult to maintain these tubes, but they can be used for weeks to months.
To place a pharyngostomy tube, the clinician anesthetizes the animal and inserts a finger into the mouth so that the tip of the finger is caudal to the epihyoid bone and as dorsal and as close to the cricopharyngeal sphincter as possible. The tip of the finger is then pushed laterally, and a skin incision is made over this spot. Hemostats are used to bluntly dissect through to the pharynx. A soft latex or rubber catheter (18F to 22F, urinary) is then inserted into the opening and into the esophagus. In general, the tip of the catheter should end in the midthoracic esophagus. The tube is secured with traction sutures and the area bandaged. Some inflammation at the stoma is common, and routine cleansing and bandage changes are necessary. Systemic antibiotics are not typically needed. An Elizabethan collar may be used if the animal tries to remove the tube. To remove the tube, the clinician simply cuts the sutures and pulls it out. The opening will close spontaneously over the next 1 to 4 days. Pharyngostomy tubes effectively bypass oral lesions. Advantages of these tubes include easy placement, easy removal, and minimal complications if they have been properly inserted (i.e., they cannot cause peritonitis as gastrostomy or enterostomy tubes can). However, it is easy to place them such that they cause gagging and regurgitation (i.e., if they touch the larynx, especially in cats and small dogs). The clinician should take care not to disrupt vessels or nerves when using scissors or a scalpel during the dissection. Because pharyngostomy tubes are larger than nasoesophageal tubes, homemade gruels can be fed through them.
The placement of esophagostomy tubes is similar to that of pharyngostomy tubes. The animal is placed in right lateral recumbency, the mouth is held open, and a long right-angle hemostat is placed through the cricopharyngeal sphincter. The tip of the hemostat is then forced up to show where to make the incision in the left cervical region. The incision should be made midway between the cricopharyngeal sphincter and the thoracic inlet. The tip of the hemostat is forced up through the esophagus and the nick in the skin; the tip of a feeding tube is then grasped and pulled into the esophagus and out the mouth so that the flared end of the catheter (i.e., where the syringe will be attached) is left protruding from the neck. The distal end of the catheter is then redirected down the esophagus with a rigid colonoscope or other device. Esophagostomy tubes cannot cause gagging but are otherwise similar to pharyngostomy tubes.
Gastrostomy tubes bypass the mouth and esophagus in animals with a functional stomach and intestines. They can also be used when nasoesophageal, pharyngostomy, esophagostomy, or intermittent gastric tubing is unacceptable. Vomiting is not a contraindication. This technique requires surgery, endoscopy, or special devices for proper placement.
Endoscopy is the preferred and safest way to place these tubes percutaneously. The use of dedicated devices for the placement of gastrostomy tubes has made the procedure easier and readily available for clinicians without endoscopes (Fig. 30-1). To place gastrostomy tubes using these devices, the clinician positions the anesthetized animal in right lateral recumbency and surgically prepares the area behind the last rib on the left abdominal wall. The device is then blindly and carefully advanced down the esophagus until the tip is in the stomach and can be seen pushing against the skin behind the last rib. The plunger on the handle is advanced until the trocar in the tip penetrates the skin and can be seen. In the tip of the trocar is a hole in which a suture (e.g., No. 1 or 2 polyamide or other nonabsorbable material) is tied. The clinician then withdraws the device from the animal, bringing one end of the suture with it. In the meantime, another person grasps the other end of the suture firmly so that it cannot be pulled into the stomach. The end of the suture that was brought out through the mouth is now passed retrograde through the sheath of an 18-gauge over-the-needle IV catheter or a disposable pipette tip of similar diameter. Next, one can use either an umbrella catheter specifically designed for use as a gastrostomy tube, or one can modify a mushroom-type catheter (usually 18F to 24F). The latter is prepared by cutting off the syringe end. The clinician then attaches the suture that has been pulled out through the mouth to this end of the catheter, using a needle to pass the suture through the catheter and make a mattress suture pattern. The end of the mushroom catheter tip that has just been attached to the suture is inserted into the flared end of the IV catheter or disposable pipette tip. The other person then begins to pull on the end of the suture where it enters the abdominal wall, thus pulling the cut tip of the mushroom catheter into the stomach. The modified end of the mushroom catheter that was inserted into the disposable pipette tip is thereby pulled out of the stomach through the same hole previously made by the trocar. Traction is placed on the mushroom catheter until the mushroom head is securely placed against the gastric mucosa, which is pulled next to the abdominal wall. (The clinician should take care not to pull the mushroom tip of the catheter out of the stomach.) The catheter is held in place by an outer flange and/or traction sutures (excessive pressure on the gastric mucosa can cause avascular necrosis), and the area is bandaged lightly.
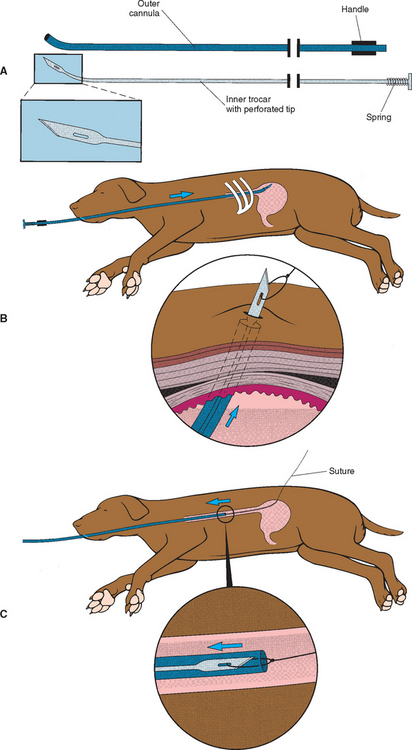
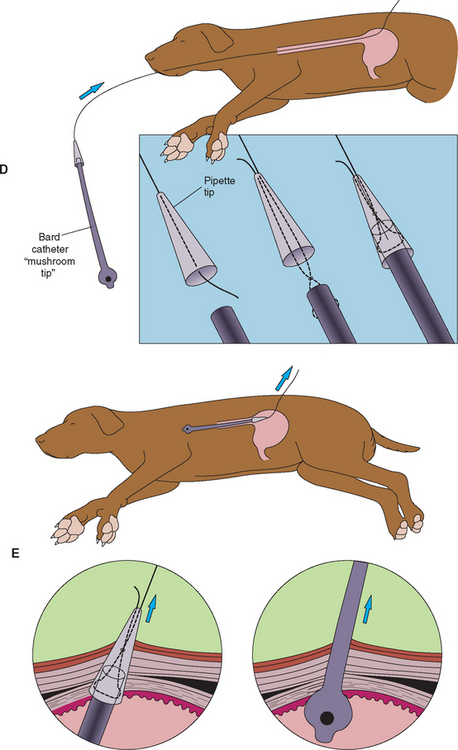
FIG 30-1 For legend see opposite. The proper way to use a dedicated device to place a gastrostomy tube. A, The device consists of a cannula with a handle and a trocar that is passed through the cannula once it is properly positioned. B, Proper placement of the device behind the last rib. The trocar is pushed through the cannula until the tip exits the skin such that a suture can be tied onto the tip. C, The device and the attached suture are withdrawn through the animal’s mouth. D, The proper way to use a dedicated device to place a gastrostomy tube. The tip of the suture that exits the mouth is attached to the cut end of a mushroom-tip catheter. E, The other end of the suture is pulled so that the tip of the catheter exits the skin. It is pulled until the mushroom tip is snugly against the gastric mucosa and the stomach is held against the abdominal wall.
(Reprinted with permission from Fossum T, editor: Small animal surgery, St Louis, 1997, Mosby.)
Gastrostomy tubes allow the administration of thick gruels and are often tolerated for weeks to years. Either a homemade gruel or a commercial liquid diet (see Table 30-2) may be used. These tubes must be left in place for at least 7 to 10 days to allow an adhesion to form between the stomach and the abdominal wall, which will prevent gastric leakage into the peritoneal cavity when the tube is removed. They are often used in cats that do not tolerate pharyngostomy, nasogastric, or esophagostomy tubes. The tube should be flushed with water and air after each feeding. Although the entire caloric requirement may be administered as soon as the tube is placed, it is often better to start with one half the daily requirement and work up to the complete nutritional needs over 1 to 3 days. If the tube becomes plugged, it can sometimes be unplugged by using flexible endoscopy forceps or by instilling a fresh carbonated beverage into the tube. When the tube is to be removed, sufficient traction is applied to the catheter so that the mushroom tip collapses and passes through the stomach and skin incision. The fistula usually closes spontaneously in 1 to 4 days. The major risk of using such tubes is leakage and peritonitis, which are rare but potentially catastrophic. In dogs larger than 20 to 25 kg, gastrostomy tubes are typically placed surgically or sutures are passed through the abdominal wall and into the gastric wall to ensure that the stomach and abdominal wall stay in apposition and form an adhesion that prevents leakage. Improper use of dedicated devices can result in malplacement of the tube and/or perforation of abdominal organs (e.g., spleen, omentum).
Low-profile gastrostomy tubes can be used if a stoma has been previously established by a routine gastrostomy tube. The major advantage of such tubes is that they may replace routine gastrostomy tubes that are disintegrating or have been inadvertently pulled out, and they may be placed without anesthesia or a surgical/endoscopic procedure. Typically, sedation is all that is needed. However, to use the preexisting stoma, the low-profile gastrostomy tube must be placed within 12 hours of removing the old gastrostomy tube, or another tube (e.g., a red latex male urinary catheter) must be inserted into the stoma as quickly as possible to prevent the old stoma from closing.
Enterostomy tubes are indicated in animals with functional intestines when the stomach must be bypassed (e.g., recent gastric surgery). Laparotomy or laparoscopy is generally needed to place these tubes. A 12-gauge needle is used to puncture the antimesenteric border of the intestine, and a sterile 5F plastic catheter is advanced aborally through the needle until approximately 15 cm extends into the intestinal lumen. The 12-gauge needle is removed, and a purse-string suture is placed to prevent the catheter from moving freely. The needle is then used in the same manner to make a pathway for the catheter to exit through the abdominal wall. The antimesenteric border of the intestine is sutured to the abdominal wall so that the sites where the tube enters the intestine and exits the abdomen are opposed. Traction sutures are used to secure the catheter.
The clinician may place a jejunostomy tube by first placing a gastrostomy tube and then inserting a jejunostomy tube through the gastrostomy tube. Next, the clinician directs the jejunostomy tube into the duodenum with a flexible endoscope. Alternatively, the clinician may use a guide wire placed in the duodenum via an endoscope to feed the jejunostomy tube through the gastrostomy tube and into the duodenum.
The small diameter of enterostomy tubes often necessitates the administration of commercial liquid diets (see Table 30-2), which are best infused at a constant rate. The rate necessary to administer daily caloric needs is calculated. A one half–strength feeding solution is administered at one half the calculated rate on the first day. On the second day the rate of administration is increased to the calculated rate, but a one half–strength solution is still used. On the third day a full-strength solution is administered at the calculated rate. If diarrhea occurs, the rate of administration can be decreased or fiber (e.g., psyllium) can be added to the liquid diet. The tube should be left in place for 7 to 10 days, if possible, to allow adhesions to develop around the area and prevent leakage. When enteral feeding is no longer necessary, the clinician simply removes the sutures and pulls out the catheter.
DIETS FOR SPECIAL ENTERAL SUPPORT
Commercial diets (see Table 30-2) may be used for enteral support. If the feeding tube diameter is sufficient, blended commercial diets, which are less expensive and still effective, can be used. A gruel made by blending one can of feline p/d (Hill’s Pet Products) plus 11/2 C (0.35 L) of water provides approximately 0.9 kcal/ml and is useful for dogs and cats. Elemental diets may be better than blended gruels in animals with intestinal disease. However, some elemental diets (e.g., Vivonex, Novartis Nutrition) do not have as much protein as desired for dogs and cats (see Table 30-2); therefore the clinician may replace some of the water used in mixing the elemental diet with 8.5% amino acids for injection (e.g., 350 ml water +250 ml 8.5% amino acids). When feeding cats, the clinician must be sure that sufficient taurine is present in the diet.
Nasoesophageal, pharyngostomy, esophagostomy, and gastrostomy tubes are usually used for bolus feeding. Animals that have been anorectic for days to weeks should usually start by receiving small amounts (e.g., 3 to 5 ml/kg) every 2 to 4 hours. The amount is gradually increased and the frequency decreased until the animal is receiving its caloric needs in three or four daily feedings. The clinician should expect to ultimately administer at least 22 to 30 ml/kg at each feeding to most dogs and cats. Larger volumes may be given if they do not cause vomiting or distress.
Enterostomy tubes are usually used for constant-rate feeding, which best involves the use of an enteral feeding pump. The clinician should begin by feeding the animal a one half–strength diet at one half the rate that will ultimately be needed to meet the animal’s caloric needs. If diarrhea does not result after 24 to 36 hours, the clinician increases the flow rate to what will ultimately be needed. If diarrhea still does not occur, the diet may then be changed from one half strength to full strength. Constant infusion of these same diets may be done through gastrostomy and esophagostomy tubes in animals that readily vomit when fed food in boluses (e.g., some cats with severe hepatic lipidosis). Animals that are critically ill and vomit readily are believed to potentially benefit from “microalimentation,” in which very small amounts of liquid diet (e.g., 1 to 2 ml/h in 30 to 40 kg dogs) are infused into nasoesophageal tubes in an effort to get some nutrition to the intestinal mucosa and prevent bacterial translocation and, ultimately, sepsis.
PARENTERAL NUTRITION
Parenteral nutrition is indicated if the animal’s intestines cannot reliably absorb nutrients. It is the most certain method of supplying nutrition to such animals; however, it is expensive and can be associated with metabolic and infectious complications. There are two types of parenteral nutrition: total parenteral nutrition (TPN) and partial (also called peripheral) parenteral nutrition (PPN). In general, PPN is much more convenient and less expensive than TPN. For TPN a central IV line is dedicated to the administration of the TPN solution only (i.e., the piggybacking of other solutions and the obtaining of blood samples are forbidden). Double-lumen jugular catheters allowing the administration of parenteral nutrition and fluids through the same catheter are optimal. The aseptic placement and management of the catheter are the best protection against catheter-related sepsis. Antibiotic prophylaxis does not replace proper management and is ineffective in preventing infections. The daily caloric and protein requirements are determined (see Box 30-3), and the customized solution is administered by constant IV infusion. The clinician must routinely monitor the animal’s weight; rectal temperature; and serum sodium, chloride, potassium, phosphorus, and glucose concentrations (in addition to the urine for glucosuria). The feeding solution is adjusted to prevent or correct serum imbalances. PPN is similar but (1) supplies only approximately 50% of caloric needs, (2) has a lower osmolality than TPN solutions so that peripheral IV catheters are sufficient, and (3) is intended to be used for approximately a week with the goal to get a severely ill or emaciated patient “over the hump” before starting enteral nutrition. Regardless of whether TPN or PPN is used, the animal should also receive some feedings orally, if possible, to help prevent intestinal villous atrophy.
ANTIEMETICS
Antiemetics are indicated for symptomatic therapy in many animals with acute vomiting or those in which vomiting is contributing to morbidity (e.g., discomfort or excessive fluid and electrolyte losses). Peripherally acting drugs (Table 30-3) are less effective than centrally acting ones but may suffice in animals with minimal disease. Some of these drugs are given orally, but this is an unreliable route in nauseated animals. Parasympatholytics (e.g., atropine, aminopentamide) have been used extensively. Although they are given parenterally and may have some central activity, they are seldom effective in animals with severe vomiting.
 TABLE 30-3 Selected Antiemetic Drugs
TABLE 30-3 Selected Antiemetic Drugs
| DRUG | DOSAGE* |
|---|---|
| Peripherally Acting Drugs | |
| Kaopectate/bismuth subsalicylate (poorly effective)† | 1-2 ml/kg PO q8-24h (dogs only) |
| Anticholinergic drugs (modest efficacy) | |
| Propantheline (Pro-Banthine) | 0.25-0.5 mg/kg PO q8-12h |
| Aminopentimide (Centrine) | 0.01-0.03 mg/kg SC or IM q8-12h (dogs only) |
| 0.02 mg/kg SC or IM q8-12h (cats only) | |
| Centrally Acting Drugs | |
| Phenothiazine derivatives | |
| Chlorpromazine (Thorazine) | 0.3-0.5 mg/kg IM, IV, or SC q8h |
| Prochlorperazine (Compazine) | 0.1-0.5 mg/kg IM q8-12h |
| Metoclopramide (Region) | 0.25-0.5 mg/kg PO, IM, or IV q8-24h |
| 1-2 mg/kg/day, constant IV infusion | |
| Serotonin receptor antagonists | |
| Ondansetron (Zofran) | 0.1-0.2 mg/kg IV q8-24h |
| Dolasetron (Anzemet) | 0.3-1.0 mg/kg SC or IV q24h |
| Granisetron (Kytril) | 0.1-0.5 mg/kg PO (anecdotal, dogs only) |
| Neurokinin-1 receptor antagonist | |
| Maropitant (Cerenia) | 1 mg/kg SC q24h or 2 mg/kg PO q24h (dogs only) |
| Trimethobenzamide (Tigan) (poorly effective) | 3 mg/kg, IM q8h (dogs only) |
| Antihistamine | |
| Diphenhydramine (Benadryl) (poorly effective) | 2-4 mg/kg PO q8h |
| 1-2 mg/kg IV or IM q8-12h | |
PO, Orally; SC, subcutaneously; IM, intramuscularly.
* Dosages are for both dogs and cats unless otherwise specified.
† This drug contains salicylate and can be nephrotoxic if combined with other nephrotoxic drugs.
If it is important to halt the vomiting, a centrally acting drug should be administered parenterally. Suppositories are convenient, but their absorption is erratic.
Phenothiazine derivatives (e.g., prochlorperazine [Compazine]) are often effective. They inhibit the chemoreceptor trigger zone and, in higher doses, the medullary vomiting center. Antiemesis is usually achieved at doses that do not produce marked sedation. However, these drugs may cause vasodilation and can decrease peripheral perfusion in a dehydrated animal. Some data suggest that phenothiazines may lower the seizure threshold in animals with epilepsy, but this is uncertain.
Metoclopramide (Reglan) inhibits the chemoreceptor trigger zone and increases gastric tone and peristalsis, both of which inhibit emesis. Rarely, animals show unusual behavior after administration. The drug is excreted in the urine, and severe renal failure makes adverse effects more likely. It rarely worsens vomiting, perhaps because it causes excessive gastric contractions. The liquid form of metoclopramide given orally is often not accepted by cats. Because of its prokinetic activity, the drug is contraindicated in animals with a gastric or duodenal obstruction. Metoclopramide may be more effective in animals with severe vomiting if given intravenously at a dosage of 1 to 2 mg/kg/day by constant rate infusion.
Ondansetron (Zofran) and dolasetron (Anzemet) are serotonin receptor antagonists. Developed for use in people with vomiting resulting from chemotherapy, they are often effective in animals in which vomiting is not controlled with phenothiazines or metoclopramide (e.g., severe canine parvoviral enteritis). Granisetron (Kytril) has been used when an oral medication is required, but its efficacy is uncertain.
Maropitant (Cerenia) is a neurokinin-1 receptor antagonist that has recently been approved for use in dogs. Preliminary data suggest that this will be a useful drug in clinical practice.
Narcotics, such as fentanyl, oxymorphone, and butorphanol, may cause vomiting initially, but vomiting is usually inhibited once the drug penetrates to the medullary vomiting center. Trimethobenzamide (Tigan) and antihistamines are effective in some animals but generally are unreliable antiemetics in dogs and cats.
ANTACID DRUGS
Antacid drugs (Table 30-4) are indicated when appropriate to lessen gastric acidity (e.g., ulcer disease; acid hypersecretion resulting from renal failure, mast cell tumor, or gastrinoma). Although they are not antiemetics, they apparently may have an “antidyspepsic” effect due to diminishing gastric hyperacidity.
 TABLE 30-4 Selected Antacid Drugs
TABLE 30-4 Selected Antacid Drugs
| DRUG | DOSAGE* |
|---|---|
| Acid Titrating Drugs | |
| Aluminum hydroxide (many names) | 10-30 mg/kg PO q6-8h |
| Magnesium hydroxide (many names) | 5-10 ml PO q4-6h (dogs) q8-12h (cats) |
| Gastric Acid Secretion Inhibitors | |
| H2 receptor antagonists | |
| Cimetidine (Tagamet) | 5-10 mg/kg PO, IM, or IV q6-8h |
| Ranitidine (Zantac) | 1-2 mg/kg PO or IV q8-12h (dogs) |
| 2.5 mg/kg IV or 3.5 mg/kg PO q12h (cats) | |
| Nizatidine (Axid) | 2.5-5 mg/kg q24h PO (dogs) |
| Famotidine (Pepcid, Pepcid AC) | 0.5 mg/kg PO or IV q12-24h§ |
| Proton Pump Inhibitors | |
| Omeprazole (Prilosec) | 0.7-1.5 mg/kg PO q12-24h (dogs) |
| Lanosprazole (Prevacid) | 1 mg/kg IV q24h (dog)† |
| Pantoprazole (Protonix) | 1 mg/kg IV q24h (dog)† |
PO, Orally; SC, subcutaneously; IM, intramuscularly; IV, intravenously.
* Dosages are for both dogs and cats unless otherwise specified.
† Dosages based upon anecdotal reports. These drugs have not been used extensively, and their saftey and efficacy in dogs are not established.
§ Anecdotal reports suggest that higher doses may be necessary in severely ill or severely stressed patients.
Antacids, which titrate the gastric acidity, are over-the-counter preparations that are typically of limited efficacy because of the way they are administered. Compounds containing aluminum or magnesium tend to be more effective and do not cause the gastric acid rebound that sometimes occurs in response to calcium-containing antacids. Antacids should be administered orally every 4 to 6 hours to ensure continued control of gastric acidity; however, this may cause diarrhea, especially in animals receiving magnesium-containing compounds. Hypophosphatemia, although unlikely, is possible after extensive aluminum hydroxide administration. Hypermagnesemia, also unlikely, is possible in dogs and cats with renal failure that are given magnesium-containing compounds. These types of antacids may also interfere with the absorption of some other drugs (e.g., tetracycline, cimetidine).
Histamine2 (H2) receptor antagonists are indicated when controlling gastric acidity is important. They act by preventing histamine from stimulating the gastric parietal cell. Cimetidine (Tagamet) is effective but should be given three or four times per day to achieve best results; it inhibits hepatic cytochrome P-450 enzymes, thereby slowing the metabolism of some drugs. Famotidine (Pepcid) and nizatidine (Axid) are as or more effective than cimetidine when administered one or two times per day and do not affect hepatic enzyme activity as much as cimetidine does. It is not clear that ranitidine is effective in dogs. The H2 receptor antagonists are now available as over-the-counter preparations. The main indication for these drugs is the treatment of gastric and duodenal ulcers. Some clinicians use them prophylactically in an attempt to prevent ulceration associated with the use of some steroids and some nonsteroidal antiinflammatory drugs (NSAIDs), but they are most effective in treating existing ulcers after NSAID or steroid therapy has ceased. They are effective in lessening ulceration associated with submaximal exertion. Nizatidine and ranitidine have gastric prokinetic activity. Very rarely, these drugs may cause bone marrow suppression, central nervous system problems, or diarrhea. Parenteral administration, especially the rapid IV injection of ranitidine, may cause nausea, vomiting, or bradycardia. There is concern that severely ill or stressed animals may require larger than currently recommended doses in order to suppress gastric acid secretion; this is being investigated.
Proton pump inhibitors (i.e., Omeprazole [Prilosec], lansoprazole [Prevacid], and pantoprazole [Protonix]) block the final common pathway of gastric acid secretion. This is the most effective class of drugs for decreasing gastric acid secretion, but maximum suppression of acid secretion takes between 2 and 5 days when administered orally. Omeprazole is a noncompetitive inhibitor primarily used in animals with severe gastroesophageal reflux or gastrinomas (diseases in which H2 receptor-antagonists are often inadequate). It is uncertain whether most animals with gastric ulcers benefit from the enhanced blockade of gastric acid secretion that this drug provides, as compared with H2 receptor–antagonist therapy.
INTESTINAL PROTECTANTS
Intestinal protectants (Table 30-5) include drugs and inert adsorbents such as kaolin, pectin and barium sulfate contrast media. Many people believe that inert adsorbents hasten clinical relief in animals with minor inflammation, possibly because they coat the mucosa or adsorb toxins. They probably make fecal consistency more normal simply by increasing fecal particulate matter. Inert adsorbents do not have a proven efficacy in the treatment of gastritis or enteritis. It is inappropriate to rely on these drugs alone in very sick animals.
 TABLE 30-5 Selected Gastrointestinal Protectants and Cytoprotective Agents
TABLE 30-5 Selected Gastrointestinal Protectants and Cytoprotective Agents
| DRUG | DOSAGE* | COMMENT |
|---|---|---|
| Sucralfate (Carafate) | 0.5-1 g (dogs) or 0.25 g (cats) PO q6-8h, depending on animal’s size | Potentially constipating, absorbs some other orally administered drugs, primarily used to treat existing ulcers |
| Misoprostol (Cytotec) | 2-5 μg/kg PO q8h (dogs) | May cause diarrhea/abdominal cramps, primarily used to prevent ulcers, not for use in pregnant animals |
* Dosages are for both dogs and cats unless otherwise specified.
Sucralfate (Carafate) is principally indicated for animals with gastroduodenal ulceration or erosion but might also be useful for those with esophagitis (especially if administered as a slurry). It does not appear to effectively prevent NSAID-induced ulceration but may help prevent stress ulceration. Sucralfate is a nonabsorbable, sulfated sucrose complex that protects denuded mucosa by adhering tightly to it. It also inhibits peptic activity and may alter prostaglandin synthesis and the actions of endogenous sulfhydryl compounds. The dose is extrapolated from humans on the basis of the animal’s weight. Although no supportive data are available for dogs and cats, sucralfate and H2 receptor-antagonists are often used concurrently in animals with severe gastrointes tinal tract ulceration or erosion. However, because sucralfate may adsorb other drugs, other orally administered drugs should probably be given 1 to 2 hours before or after sucralfate administration. An acidic pH promotes optimal activity, and there is typically sufficient acid remaining after H2 receptor–antagonist therapy for sucralfate to be effective. There are no absolute contraindications to the use of sucralfate. The biggest disadvantage is that it must be given orally, and many animals that need it are vomiting. Sucralfate can cause constipation.
Misoprostol (Cytotec) is a prostaglandin E1 analog used to treat ulcers but especially to help prevent NSAID-induced gastroduodenal ulceration. The drug is primarily used in dogs that require NSAIDs but in which NSAIDs cause anorexia or vomiting. Use of NSAIDs that have a higher risk of causing gastrointestinal tract problems (e.g., piroxicam) might also be an indication. Misoprostol does not appear to be as effective in preventing NSAID-induced ulcers in dogs as it is in people. The major adverse effects of misoprostol seem to be abdominal cramping and diarrhea, which usually disappear after 2 to 3 days of therapy. Pregnancy may be a contraindication. There is evidence that misoprostol may have immunosuppressant properties, especially in combination with other drugs.
DIGESTIVE ENZYME SUPPLEMENTATION
Pancreatic enzyme supplementation is indicated to treat exocrine pancreatic insufficiency; however, it is often used empirically without justification in animals with diarrhea. There are many products that vary greatly in their potency. Although pills may work, powdered preparations tend to be more effective; enteric-coated pills are particularly ineffective. Viokase-V (A.H. Robins Co.) and Pancreazyme (Daniels Pharmaceuticals) seem to be particularly efficacious. The powder should be mixed with the food (approximately 1 to 2 teaspoons per meal), but allowing the mixture to “incubate” before feeding has not been found beneficial. Fat is the main nutrient that must be digested in animals with exocrine pancreatic insufficiency, and feeding them a low-fat diet may ameliorate diarrhea. Antacid or antibiotic therapy (or both) may occasionally help prevent gastric acidity or small intestinal bacteria from rendering the enzyme supplementation ineffective. Occasionally, a stomatitis or diarrhea develops in dogs receiving large amounts of enzyme supplementation.
MOTILITY MODIFIERS
Drugs that prolong the intestinal transit time are principally used to symptomatically treat diarrhea. Although infrequently needed, they are indicated if the diarrhea causes excessive fluid or electrolyte losses or owners demand control of the diarrhea at home. Opiates (Table 30-6) increase resistance to flow by augmenting segmental contraction. They tend to be more effective than parasympatholytics, which paralyze motility in the intestines (i.e., create ileus). Both classes of drugs have antisecretory effects. Because cats do not tolerate narcotics as well as dogs, opiates should not be used in this species, although loperamide may be used carefully.
 TABLE 30-6 Selected Drugs Used to Treat Diarrhea Symptomatically
TABLE 30-6 Selected Drugs Used to Treat Diarrhea Symptomatically
| DRUG | DOSAGE* |
|---|---|
| Intestinal Motility Modifiers | |
| Anticholinergic drugs | 0.3-1.0 mg/kg PO q8h (dog) |
| Methscopolamine (Pamine) | |
| Propantheline (Pro-Banthine) | 0.25-0.5 mg/kg PO q8-12h |
| Opiates | |
| Diphenoxylate (Lomotil) | 0.05-0.2 mg/kg PO q8-12h (dogs) |
| Loperamide (Imodium) | 0.1-0.2 mg/kg PO q8-12h (dogs) 0.08-0.16 mg/kg PO q12h (cats) |
| Paregoric | 0.05 mg/kg PO q12h (dogs) |
| Antiinflammatory/Antisecretory Drug | |
| Bismuth subsalicylate† (Pepto-Bismol, Kaopectate) | 1 ml/kg/day PO divided q8-12h (dogs) for 1-2 days |
PO, Orally.
* Dosages are for both dogs and cats unless otherwise specified.
† This drug contains salicylate and can be nephrotoxic if combined with other nephrotoxic drugs.
Loperamide (Imodium) is available as an over-the-counter drug. Use of loperamide theoretically increases the risk for bacterial proliferation in the intestinal lumen, thus potentially initiating or perpetuating disease; however, this is very rare in clinical practice. An overdose can cause narcotic intoxication (i.e., collapse, vomiting, ataxia, hypersalivation), which requires treatment with narcotic antagonists.
Diphenoxylate (Lomotil) is similar to loperamide but tends to be somewhat less effective. It has more potential for toxicity than loperamide. Rarely, a dog responds to it but not to loperamide. This drug should not be used in cats.
Drugs that shorten the transit time (prokinetic drugs) empty the stomach or increase intestinal peristalsis or both. Metoclopramide is a prokinetic drug that is effective only in the stomach and the proximal duodenum. However, it can be administered parenterally. Adverse effects are mentioned under the section on antiemetics. Cisapride stimulates normal motility from the lower esophageal sphincter to the anus. It is usually effective unless the tissue has been irreparably damaged (e.g., megacolon in cats). Primarily used for the treatment of constipation, it may also be used for the management of gastroparesis (in which it is usually more effective than metoclopramide) and small intestinal ileus. It has rarely been reported to be beneficial in dogs with megaesophagus. Cisapride is no longer available from human pharmacies but is generally available from veterinary pharmacies. It is available only as an oral preparation. It has few significant adverse effects, although intoxication with large doses may cause diarrhea, muscular tremors, ataxia, fever, aggression, and other central nervous system signs. Erythromycin stimulates motilin receptors and enhances gastric motility at doses less than required for antibacterial activity (i.e., 2 mg/kg). It may also increase intestinal motility. Nizatidine and ranitidine are H2 receptor antagonists that also have gastric prokinetic effects at routinely used doses. Bethanechol (Urecholine) is an acetylcholine analog that stimulates intestinal motility and secretion. It produces strong contractions that can cause pain or injure the animal; hence, it is infrequently used, except for increasing urinary bladder contractions. Obstruction of an outflow area can be a contraindication to the use of prokinetic drugs because vigorous contractions against such a lesion may cause pain or perforation. Obstruction of the urinary outflow tract is also a contraindication to the use of bethanechol. Tegaserod (Zelnorm) has prokinetic activity in the canine colon (0.05 to 0.1 mg/kg, q12h), but there is too little information regarding its effectiveness in clinical disease to make recommendations about its use.
Pyridostigmine (Mestinon) inhibits acetylcholinesterase and is used to treat myasthenia gravis (see Chapter 71). It is used for the treatment of acquired megaesophagus associated with the formation of antibodies to acetylcholine receptors. It must be used cautiously because overdose may cause toxicity accompanied by signs of parasympathetic overload (e.g., vomiting, miosis, diarrhea). Azathioprine (with or without steroids) may be a better long-term treatment for myasthenia gravis than pyridostigmine.
ANTIINFLAMMATORY AND ANTISECRETORY DRUGS
Intestinal antiinflammatory or antisecretory drugs (or both) are indicated for lessening the fluid losses resulting from diarrhea or for controlling intestinal inflammation that is unresponsive to dietary or antibacterial therapy.
Bismuth subsalicylate (Pepto-Bismol, Kaopectate) is an over-the-counter antidiarrheal agent that is effective in many dogs with acute enteritis (see Table 30-6), probably because of the antiprostaglandin activity of the salicylate moiety. The main disadvantages are that the salicylate is absorbed (warranting its cautious use in cats or in dogs receiving other nephrotoxic drugs), it turns stools black (which mimics melena), and it must be administered orally (many animals dislike its taste). Bismuth is bactericidal for certain organisms (e.g., Helicobacter spp.).
Octreotide (Sandostatin) is a synthetic analog of somatostatin that inhibits alimentary tract motility and the secretion of gastrointestinal hormones and fluids. It has had limited use in dogs and cats but might be helpful in a few animals with intractable diarrhea or pancreatitis.
Salicylazosulfapyridine (sulfasalazine [Azulfidine]) is indicated for animals with colonic inflammation. This drug is generally not beneficial in animals with small intestinal problems. It is a combination of sulfapyridine and 5-aminosalicylic acid. Colonic bacteria split the molecule, and the 5-aminosalicylic acid (probably the active moiety) is subsequently deposited on diseased colonic mucosa. Dogs generally receive 50 to 60 mg/kg, divided into three doses daily, but not to exceed 3 g daily. Sulfasalazine may be effective at lower-than-expected doses if used in combination with glucocorticoids. Empirically, 15 mg/kg/day, sometimes divided into twice-daily doses, is often tolerated by cats, but they must be closely observed for the development of salicylate intoxication (i.e., lethargy, anorexia, vomiting, hyperthermia, tachypnea). Some cats that vomit or become anorectic may tolerate the medication if it is given in enteric-coated tablets. Many dogs with colitis respond to therapy in 3 to 5 days. However, the drug should be given for 2 weeks before deciding that it is ineffective. If signs of colitis resolve, the dose of the drug should be gradually reduced. If the animal cannot be weaned off the drug entirely, the lowest effective dose should be used and the animal monitored regularly for the development of drug-induced adverse effects (especially those resulting from the sulfa drug). Sulfasalazine may cause transient or permanent keratoconjunctivitis sicca. Other possible complications include cutaneous vasculitis, arthritis, bone marrow suppression, diarrhea, and any other problem associated with sulfa drugs or NSAIDs.
Olsalazine and mesalamine contain or are metabolized to 5-aminosalicylic acid but do not have the sulfa, which is responsible for most of sulfasalazine’s adverse effects. In people they are as effective as sulfasalazine but safer. Olsalazine and mesalamine have been used effectively in dogs. They are given in a dose generally about one half that of sulfasalazine. Keratoconjunctivitis sicca has also been found in dogs receiving mesalamine.
Corticosteroids are specifically indicated in animals with chronic alimentary tract inflammation (e.g., moderate to marked inflammatory bowel diseases) that is not responsive to well-designed elimination diets. In cats prednisolone appears to have better activity than prednisone. Relatively high doses (i.e., prednisolone, 2.2 mg/kg/day) are often used initially, and the dose is tapered to find the lowest effective dose. Dexamethasone is sometimes effective when prednisolone is not, but dexamethasone has more adverse effects than prednisolone. If PO administration is a problem in a cat, long-lasting steroid injections (e.g., methylprednisolone acetate) may be tried.
Methyprednisolone appears to be more effective than prednisolone, requiring only 80% of the dose used for prednisolone. Budesonide (Entocort) is a steroid that is not more effective than prednisolone but is largely eliminated by first pass metabolism in the liver, which decreases its systemic effects. The response may be rapid or take weeks.
Corticosteroids are often beneficial in cats with inflammatory bowel disease, but they may worsen intestinal disease in some dogs and cats. Iatrogenic Cushing’s syndrome is more of a problem in dogs but can occur in cats that are grossly overdosed. It is important to have a histologically based diagnosis before using high-dose prednisolone therapy because some diseases that mimic steroid-responsive lymphocytic colitis (e.g., histoplasmosis) are absolute contraindications to corticosteroid therapy. Although more common in the southeastern United States and the Ohio River Valley, histoplasmosis has been found in unexpected states.
Retention enemas of corticosteroids or 5-aminosalicylic acid are sometimes indicated in animals with severe distal colitis. The dose is estimated from the human dose. These enemas place large doses of an antiinflammatory agent directly on the affected area while minimizing systemic effects. Although effective in controlling the clinical signs, their administration is unpleasant for both clients and animals. Further, the active ingredient may be absorbed if there is substantial inflammation and increased mucosal permeability (i.e., animals receiving corticosteroid enemas can become polyuric and polydipsic). Therapeutic retention enemas are typically used until the clinical signs are controlled and other therapy (e.g., sulfasalazine, diet) becomes effective. The contraindications to their use are the same as those to the systemic administration of the active ingredient of the enema.
Immunosuppressive therapy (e.g., azathioprine, chlorambucil, cyclosporine) is indicated in animals with severe inflammatory bowel disease that is unresponsive to corticosteroid and dietary therapy. It is also used in animals with severe disease in which it is in the animal’s best interest to use aggressive therapy initially. These drugs should be used only if the diagnosis has been confirmed histopathologically. Immunosuppressive therapy can be more efficacious than corticosteroid therapy alone and allows corticosteroids to be given at lower doses and for shorter periods, thereby decreasing their adverse effects. However, the possibility of adverse effects from these drugs usually limits their use to animals with severe disease. The reader is referred to Chapter 103 for additional information on immunosuppressive therapy.
Azathioprine (Imuran) is commonly used in dogs (50 mg/m2 daily or every other day) with severe alimentary tract inflammation. Azathioprine should not be used in cats because of the risk for myelotoxicity. For smaller dogs a 50-mg azathioprine tablet is typically crushed and suspended in a liquid (e.g., 15 ml of a vitamin supplement) to allow more accurate dosing. The suspension must be mixed well before each dosing. It may take 2 to 5 weeks before the beneficial effects of this drug are seen. Side effects in dogs may include hepatic disease, pancreatitis, and bone marrow suppression.
Chlorambucil is an alkylating agent that is used for the same reasons as azathioprine. Chlorambucil, however, appears to have fewer adverse effects than azathioprine. A reasonable starting dose in cats is 1 mg twice weekly for cats weighing less than 7 lb (3.5 kg) and 2 mg twice weekly for cats weighing more than that. Beneficial effects may not be seen for 4 to 5 weeks. If a response is seen, the dose should then be decreased very slowly over the next 2 to 3 months. The animal should be monitored for myelosuppression. Stronger alkylating agents, such as cyclophosphamide, are seldom used for the management of nonneoplastic gastrointestinal tract disease.
Cyclosporine (Atopica) is a potent immunosuppressive drug that is sometimes used in dogs with inflammatory bowel disease, lymphangiectasia, and perianal fistulas. The dose is 3 to 5 mg/kg q12h when given orally, but erratic bioavailability requires therapeutic drug monitoring and subsequent adjusting of the dose. There is considerable variation in the bioavailablity of different preparations of cyclosporine. It may be administered intravenously in vomiting patients, but then the initial dose should probably be decreased by 50%. Because of its considerable expense, it is sometimes administered with low doses of ketoconazole (3 to 5 mg/kg q12h), which inhibits metabolism of cyclosporine and in turn allows the use of lower doses at less expense to the client.
ANTIBACTERIAL DRUGS
In dogs and cats with gastrointestinal problems, antibiotics are primarily indicated if aspiration pneumonia, fever, a leukogram suggestive of sepsis, severe neutropenia, antibiotic-responsive enteropathy, clostridial colitis, symptomatic Helicobacter gastritis, or perhaps hematemesis or melena is found or suspected. Animals with an acute abdomen may reasonably be treated with antibiotics while the nature of the disease is being defined. Acute colitis is a reasonable indication for amoxicillin (22 mg/kg q12h) because clostridial colitis is reasonably common. However, most animals with acute enteritis or gastritis of unknown cause do not benefit from antibiotic therapy. In general, the routine use of antimicrobials in animals with alimentary tract disorders is not recommended, unless the animal is at high risk for infection or a specific disorder is being treated.
Nonabsorbable aminoglycosides (e.g., neomycin) are often used to “sterilize” the intestines. However, they do not kill anaerobic bacteria, which are the predominant type found there. Further, there are a plethora of viral and dietary causes of acute enteritis that are not responsive to antibio-tics. Thus aminoglycosides given orally are not indicated unless a specific infection (e.g., campylobacteriosis) is being considered.
Broad-spectrum antibiotics effective against aerobes and anaerobes may be used for the treatment of antibiotic-responsive enteropathy (ARE). Metronidazole (10 to 15 mg/kg q24h) may also be used for this purpose (see later discussion) but has not been as successful in this author’s experience. Tylosin (20 to 40 mg/kg q12h) is commonly used for this purpose. Tetracycline (22 mg/kg q12h) has also been used, and patients with severe disease believed to have ARE may be treated with combinations (e.g., metronidazole and enrofloxacin [7 mg/kg q24h]). Inappropriate antibiotic therapy may hypothetically eliminate enough resident bacteria that overgrowth of pathogenic bacteria in the colon occurs. However, this is rarely a clinical problem in dogs and cats. The clinician should treat the patient for at least 2 to 3 weeks before deciding that therapy for ARE has been unsuccessful.
Pets occasionally have enteritis caused by a specific bacterium. However, even this is not necessarily an indication for antibiotics. Clinical signs resulting from some bacterial enteritides (e.g., salmonellosis, enterohemorrhagic Escherichia coli) generally do not resolve more quickly when the animal is treated with antibiotics, even those to which the bacteria are sensitive.
Dogs and cats with viral enteritis but without obvious systemic sepsis may reasonably be treated with antibiotics if secondary sepsis is likely to occur (e.g., those with neutropenia or severe hemorrhagic diarrhea). First-generation cephalosporins (e.g., cefazolin) are often effective for such use.
If systemic or abdominal sepsis is suspected to have originated from the alimentary tract (e.g., septicemia caused by parvoviral enteritis, perforated intestine), broad-spectrum antimicrobial therapy is indicated. Antibiotics with a good aerobic gram-positive and anaerobic spectrum of action (e.g., ticarcillin plus clavulinic acid [Timentin], 50 mg/kg given intravenously three to four times daily, or clindamycin, 11 mg/kg given intravenously three times daily) combined with antibiotics with excellent activity against most aerobic bacteria (e.g., amikacin, 25 mg/kg given intravenously once daily or enrofloxacin, 15 mg/kg given intravenously once daily) are often effective. To improve the anaerobic spectrum, especially if a cephalosporin is used instead of ampicillin, the clinician may include metronidazole (10 mg/kg given intravenously two or three times daily). Alternatively, a second-generation cephalosporin (e.g., cefoxitin, 30 mg/kg given intravenously three or four times daily) may be used. In general, it takes at least 48 to 72 hours before the clinician can tell whether the therapy will be effective.
Helicobacter gastritis may be treated with various combinations of drugs. Currently, the combination of an antacid (i.e., famotidine or omeprazole; see Table 30-4) and a macrolide (i.e., erythromycin or azithromycin; see pp. 483–485) or amoxicillin seems to be very effective. Adding metronidazole and/or bismuth subsalicylate may enhance efficacy. However, some patients seem to respond to erythromycin or amoxicillin as a sole agent. If high doses of erythromycin (22 mg/kg given twice daily) cause vomiting, the dose may be lowered to 10 to 15 mg/kg given twice daily. A 10- to 14-day course of treatment appears to be adequate for most animals, although recurrence of infection is possible.
Metronidazole is a “miscellaneous” drug that is commonly used in animals with inflammatory bowel disease. It has antimicrobial activity against anaerobic bacteria (which predominate in the gastrointestinal tract) and protozoa (e.g., Giardia). It has been suggested to have some effect on the immune system, as shown by its apparent beneficial effects in people with Crohn’s disease. The usefulness of metronidazole in dogs and cats with inflammatory bowel disease (10 to 15 mg/kg given twice daily) is suspected but unproved. Adverse effects are uncommon but may include salivation (because of its taste), vomiting, central nervous system abnormalities (e.g., central vestibular signs), and perhaps neutropenia. These adverse effects usually resolve after withdrawing the drug. Cats sometimes accept oral suspensions better than the 250-mg tablets, which must be cut and have an unpleasant taste. Some cats diagnosed with inflammatory bowel disease respond to metronidazole better than they do to corticosteroids. Occasionally, dogs with colitis do likewise.
PROBIOTICS/PREBIOTICS
The administration of live bacteria or yeast in the food with the intent to produce a beneficial effect is called probiotic therapy. The administration of a specific dietary substance to specifically increase or decrease the numbers of specific bacteria is called prebiotic therapy. The concurrent use of probiotics and prebiotics is called symbiotic therapy. Although there is good evidence that these therapies are beneficial for specific conditions in people, there is currently no published work showing a clear benefit in clinically ill dogs or cats. However, this may change with time.
Lactobacillus, Bifidobacterium, and Enterococcus are the bacteria typically administered to dogs. These bacteria are believed to stimulate Toll-like receptors in the bowel and thereby benefit the patient. The beneficial effect seems to last only as long as the bacteria are being administered. There is no evidence that these bacteria become permanently established in the gastrointestinal microflora during administration. Not all probiotics sold in drug or grocery stores contain what the label states, which may be at least partially responsible for their failure to have demonstrated efficacy. In general, large numbers of bacteria appear to be necessary, which explains why feeding yogurt (which contains relatively modest numbers of Lactobacilli) is ineffective. At the time of this writing, there are two products marketed specifically for veterinary use (Fortiflora, Purina Co) that contains Enterococcus faecium and Proviable (Nutramax) that contains a mixture of several bacteria.
ANTHELMINTIC DRUGS
Anthelmintics are frequently prescribed for dogs and cats with alimentary tract disease, even if parasitism is not the primary problem. It is often reasonable to use these drugs empirically for the treatment of suspected parasitic infections in animals with acute or chronic diarrhea. Selected anthelmintics are listed in Table 30-7.
ENEMAS, LAXATIVES, AND CATHARTICS
Enemas are classified as either cleansing or retention.
Retention enemas are given so that the material administered stays in the colon until it exerts its desired effects (e.g., antiinflammatory retention enemas are used in animals with inflammatory bowel disease, water in obstipated animals). Obstipated animals may require frequent administrations of modest volumes of water (e.g., 20 to 200 ml, depending on the animal’s size) so that the water stays in the colon and gradually softens the feces. The clinician should avoid overdistending the colon or administering drugs that may be absorbed and produce undesirable effects. Suspected or pending colonic rupture is a contraindication to the use of enemas, but this outcome is difficult to predict. Animals that have undergone neurosurgery (e.g., those that have had a hemilaminectomy) and are receiving corticosteroids (e.g., dexamethasone) may be at increased risk for colonic perforation. Animals with colonic tumors or that have recently undergone colonic surgery or biopsy should not receive enemas either, unless there is an overriding reason.
Cleansing enemas are designed to remove fecal material. They involve the repeated administration of relatively large volumes of warm water. In dogs the water is administered by gravity flow from a bucket or bag held above the animal. The tube is gently advanced as far as it will easily go into the colon. Between 50 and 100 ml is tolerated by most small dogs, 200 to 500 ml by medium-size dogs, and 1 to 2 L by large dogs. Care should be taken to avoid overdistending or perforating the colon. Enemas are usually administered to cats with a soft canine male urinary catheter and a 50-ml syringe. If fluid is administered too quickly, however, the cat will usually vomit. A suspected or pending colonic perforation is also a contraindication to a cleansing enema.
Hypertonic enemas are potentially dangerous and should be used cautiously (if at all) because they can cause massive, fatal fluid and electrolyte shifts (i.e., hyperphosphatemia, hypocalcemia, hypokalemia, hyperkalemia). This is especially true for cats, small dogs, and any animal that cannot quickly evacuate the enema because of obstipation.
Cathartics and laxatives (Table 30-8) should be used only to augment defecation in animals that are not obstructed. They are not routinely indicated in small animals, except perhaps as part of lower bowel cleansing before contrast-enhanced abdominal radiography or endoscopy.
 TABLE 30-8 Selected Laxatives, Cathartics, Stool-Softening Agents, and Bulking Agents
TABLE 30-8 Selected Laxatives, Cathartics, Stool-Softening Agents, and Bulking Agents
| DRUG | DOSAGE (PO) | COMMENTS |
|---|---|---|
| Bisacodyl (Dulcolax) | 5 mg (small dogs and cats) | Do not break tablets |
| 10-15 mg (larger dogs) | ||
| Coarse wheat bran | 1-3 tbsp/454 g of food | |
| Canned pumpkin pie filling | 1-3 tbsp/day (cats only) | Principally for cats |
| Dioctyl sodium sulfosuccinate (Colace) | 10-200 mg q8-12h (dogs only) | Be sure animal is not dehydrated when treating |
| 10-25 mg q12-24h (cats only) | ||
| Lactulose (Cephulac) | 1 ml/5 kg q8-12h, then adjust dose as needed (dogs only) | Can cause severe osmotic diarrhea |
| 5 ml q8h, then adjust dose as needed (cats only) | ||
| Psyllium (Metamucil) | 1-2 tsp/454 g of food | Be sure animal has enough water, or constipation may develop |
PO, Orally.
Irritative laxatives (e.g., bisacodyl) stimulate defecation rather than soften feces. They are often used before colonoscopic procedures and in animals that are reluctant to defecate because of an altered environment. They are probably not appropriate for long-term use because of the dependence and colonic problems noted in people who have used them inappropriately. A glycerin suppository or a lubricated match stick is often an effective substitute for an irritative laxative. These objects are carefully placed in the rectum to stimulate defecation.
Bulk and osmotic laxatives include a variety of preparations: various fibers (especially the soluble ones); magnesium sulfate; lactulose; and, in milk-intolerant animals, ice cream or milk. They promote the fecal retention of water and are indicated in animals that have overly hard stools not caused by the ingestion of foreign objects. These laxatives are more appropriate for long-term use than the irritative cathartics are. Larger doses may be needed in cats because they retain fluids more effectively than dogs do.
Fiber is a bulking agent that is incorporated into the food and can be used indefinitely. Commercial diets relatively high in fiber may be used, or existing diets may be supplemented with fiber (see pp. 400–401). It is important to supply adequate amounts of water so that the additional fiber does not cause the formation of harder-than-normal stools. Too much fiber may cause excessive stool or inappetence resulting from decreased palatability (a danger for fat cats at risk for hepatic lipidosis). Fiber should not be given to animals with a partial or complete alimentary tract obstruction because impaction may occur.
Lactulose (Cephulac) was designed to control signs of hepatic encephalopathy, but it is also a very effective osmotic laxative. It is a disaccharide that is split by colonic bacteria into unabsorbed particles. Lactulose is particularly useful for animals that refuse to eat high-fiber diets. The dose necessary to soften feces must be determined in each animal, but 0.5 or 5 ml may be given two or three times daily to small and large dogs, respectively. Cats often need higher dosages (e.g., 5 ml three times daily). If gross overdosing occurs, so much water can be lost that hypernatremic dehydration ensues. There are no obvious contraindications to the use of lactulose.
Abood SK, et al. Enteral nutrition. In DiBartola SP, editor: Fluid, electrolyte, and acid-base disorders in small animal practice, ed 3, Philadelphia: WB Saunders, 2006.
Allenspach K, et al. Pharmacokinetics and clinical efficacy of cyclosporine treatment of dogs with steroid-refractory inflammatory bowel disease. J Vet Intern Med. 2006;20:239.
Berenas AM, et al. Effects of ranitidine, famotidine, pantoprazole, and omeprazole on intragastric pH in dogs. Am J Vet Res. 2005;66:425.
Boothe DM. Gastrointestinal pharmacology. In: Boothe DM, editor. Small animal clinical pharmacology and therapeutics. Philadelphia: WB Saunders, 2001.
Chan DL, et al. Retrospective evaluation of partial patenteral nutrition in dogs and cats. J Vet Intern Med. 2002;16:440.
Day TK, et al. Shock syndromes. In DiBartola SP, editor: Fluid, electrolyte, and acid-base disorders in small animal practice, ed 3, Philadelphia: WB Saunders, 2006.
Freeman LM, et al. Total parenteral nutrition. In DiBartola SP, editor: Fluid, electrolyte, and acid-base disorders in small animal practice, ed 3, Philadelphia: WB Saunders, 2006.
Hall EJ, et al. Diseases of the small intestine. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Hughes D, et al. Fluid therapy with macromolecular plasma volume expanders. In DiBartola SP, editor: Fluid, electrolyte, and acid-base disorders in small animal practice, ed 3, Philadelphia: WB Saunders, 2006.
Lloyd S, et al. Activity of toltrazuril and diclazuril against Isospora species in kittens and puppies. Vet Rec. 2001;148:509.
Marshall-Jones ZV, et al. Effects of Lactobacillus acidophilus DSM13241 as a probiotic in healthy adult cats. Am J Vet Res. 2006;67:1005.
Puente-Redondo VA, et al. The anti-emetic efficacy of maropitant (Cerenia) in the treatment of ongoing emesis caused by a wide range of underlying clinical aetiologies in canine patients in Europe. J Small Anim Pract. 2007;48:93.
Remillard RL, et al. Assisted feeding in hospitalized patients: enteral and parenteral nutrition. In Hand MS, et al, editors: Small animal clinical nutrition, ed 4, Topeka, Kan: Mark Morris Institute, 2000.
Rosado TW, et al. Neurotoxicosis in 4 cats receiving ronidazole. J Vet Intern Med. 2007;21:328.
Simpson KW, et al. Fluid and electrolyte disorders in gastrointestinal and pancreatic disease. In DiBartola SP, editor: Fluid, electrolyte, and acid-base disorders in small animal practice, ed 3, Philadelphia: WB Saunders, 2006.
Tumulty JW, et al. Clinical effects of short-term oral budesonide on the hypothalamic-pituitary-adrenal axis in dogs with inflammatory bowel disease. J Am Anim Hosp Assoc. 2004;40:120.
Washabau RJ. Update on antiemetic therapy. In August JR, editor: Consultations in feline internal medicine, ed 4, Philadelphia: WB Saunders, 2001.
Washabau RJ. Gastrointestinal motility disorders and gastrointestinal prokinetic therapy. Vet Clinics N Am. 2003;33:1007.
Washabau RJ, et al. Diseases of the large intestine. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 6, Philadelphia: WB Saunders, 2005.
Zsombor-Murray, et al. Peripheral parenteral nutrition. Comp Cont Educ. 1999;21:512.