CHAPTER 96 Polysystemic Rickettsial Diseases
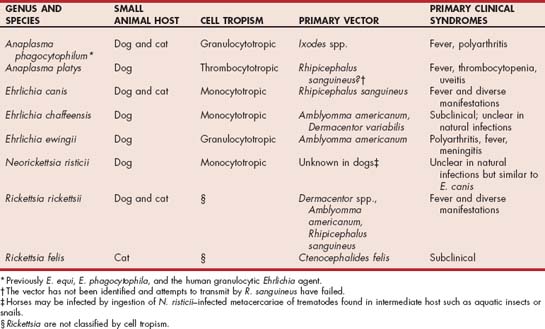
The organisms of the order Rickettsiales, in the families Rickettsiaceae and Anaplasmataceae, were reclassified in 2001 after phylogenetic analyses of the 16S rRNA and groESL gene sequences (Dumler et al., 2001). Some Ehrlichia spp. were transferred to the Neorickettsia genus (including E. risticii) and some Ehrlichia spp., including E. phagocytophila (also called E. equi and human granulocytic Ehrlichia) and E. platys were placed into the genus Anaplasma. The genera Ehrlichia and Neorickettsia were transferred to the family Anaplasmataceae; the genera of Rickettsia and Orientia remained in the Rickettsiaceae. The organisms in Ehrlichia, Anaplasma, and Neorickettsia are classified genetically and by cell tropism (monocytotropic, granulocytotropic, or thrombocytotropic). The organisms of most importance to dogs and cats discussed in this chapter include A. phagocytophilum, A. platys, Ehrlichia canis, E. chaffeensis, E. ewingii, Neorickettsia risticii, Rickettsia rickettsii, and R. felis (Table 96-1).
 TABLE 96-1 Ehrlichia spp., Anaplasma spp., Neorickettsia spp., and Rickettsia spp. of Primary Significance to Dogs or Cats
TABLE 96-1 Ehrlichia spp., Anaplasma spp., Neorickettsia spp., and Rickettsia spp. of Primary Significance to Dogs or Cats
CANINE GRANULOCYTOTROPIC ANAPLASMOSIS
Etiology and Epidemiology
Anaplasma phagocytophilum (previously known as E. equi, E. phagocytophila, canine granulocytic Ehrlichia, and human granulocytic ehrlichiosis agent) is known to infect a variety of animals, including small mammals, mountain lions, coyotes, sheep, cattle, deer, dogs, horses, and human beings (Dumler et al., 2001). Small mammals and deer are natural reservoirs. The distribution of A. phagocytophilum is defined by the range of Ixodes ticks and so is most common in California, Wisconsin, Minnesota, and the northeastern states as well as other areas of the world with this tick genus, including Europe, Asia, and Africa. Birds may play a role in spreading infected ticks and may also serve as a reservoir. In endemic areas, seroprevalence can be quite high; in one study of healthy dogs in California, 47.3% of the dogs tested in one county were seropositive (Foley et al., 2001). Borrelia burgdorferi is also transmitted by Ixodes ticks, so coinfections can occur (Jaderlund et al., 2007). The vector must be attached for approximately 24 to 48 hours to transmit the agent. Clinical signs usually develop approximately 1 to 2 weeks after infection. Neutrophils (and rarely other leukocytes) phagocytize the organism, and once intracellular A. phagocytophilum prevents phagolysosome fusion. This mechanism allows multiplication within the phagosome, which gives the appearance of morula in neutrophils under light microscopy. The exact pathogenesis of disease is still undetermined, and why some dogs but not others develop clinical signs of disease is unclear.
Clinical Features
A. phagocytophilum infection appears to be primarily an acute disease in dogs. It has been associated most commonly with nonspecific signs of fever, lethargy, and inappetence. Stiffness and lameness consistent with musculoskeletal pain are also common, and A. phagocytophilum has been associated with polyarthritis. Vomiting, diarrhea, difficult breathing, cough, lymphadenopathy, hepatosplenomegaly, and central nervous system signs (seizures and ataxia) have also been reported. Dogs can be chronic subclinical carriers, so exacerbation of disease could occur in some dogs. However, chronic disease syndromes such as those associated with E. canis infection have not been documented. In a recent study of dogs with neurologic diseases in Sweden, serologic evidence of exposure to A. phagocytophilum and B. burgdorferi was common, but neither organism was linked to the presence of neurologic disease (Jaderlund et al., 2007). In one study of valvular endocarditis, all dogs with Bartonella spp.–associated disease were also seropositive for A. phagocytophilum (MacDonald et al., 2004). Whether the coinfection potentiated the Bartonella-associated disease is unknown.
Diagnosis
Morula of A. phagocytophilum are commonly detected in neutrophils of most clinically affected dogs, so infection can be confirmed during performance of a complete blood cell count. Although thrombocytopenia and lymphopenia are common, neutrophil counts are usually normal. Hemolytic anemia and thrombocytopenia were thought to be from A. phagocytophilum infection in one dog in the United Kingdom (Bexfield et al., 2005). Reported biochemical panel and urinalysis abnormalities are mild and nonspecific. The morulae cannot be distinguished from those of E. ewingii, but the geographic range of the infections varies between the organisms; the travel history can help rank the differentials. Serologic test results (immunofluorescence assay [IFA] and enzyme-linked immunosorbent assay [ELISA]) can be used if morulae are not identified. A point of care assay that detects antibodies against A. phagocytophilum is available (SNAP 4Dx, IDEXX, Westbrook, Maine). Antibody assay results can be falsely negative in acute cases, so a convalescent test 2 to 3 weeks later may be required to confirm exposure. This assay also detects antibodies against A. platys. Because A. phagocytophilum infections are limited geographically, this antibody test result is not needed in the majority of the United States. Polymerase chain reaction assays performed on blood collected in ethylenediamine tetraacetic acid can be used to confirm infection and differentiate A. phagocytophilum infection from other infections, but microbial DNA can also be amplified from healthy dogs (Henn et al., 2007). Most dogs infected by A. phagocytophilum have subclinical infections, most infected dogs only have an acute phase, exposure rates in endemic areas are high, and the disease syndromes associated with infection have multiple other causes. Thus antibody test results and polymerase chain reaction (PCR) assay results alone cannot be used to prove clinical disease associated with A. phagocytophilum infection. For example, although A. phagocytophilum is known to cause thrombocytopenia and polyarthritis in some dogs, a recent study failed to show an association between A. phagocytophilum PCR assay or serologic test results in dogs with polyarthritis or thrombocytopenia (Foley et al., 2007).
Treatment
Several antibiotics are effective against A. phagocytophilum in vitro (Maurin et al., 2003). Doxycycline administered at 5-10 mg/kg PO q12-24h for at least 10 days is recommended by most clinicians. Whether a 28-day course of doxycycline therapy as recommended for E. canis is needed is unknown (Neer et al., 2002). If tetracyclines are used, 22 mg/kg PO q8h for 2 to 3 weeks is recommended. Most dogs respond to therapy within hours to days of initiating therapy.
Zoonotic Aspects and Prevention
A. phagocytophilum infects people as well as dogs and so is zoonotic. Human infections are most likely acquired by direct tick transmission, but handling infected blood and carcasses can also lead to infection. Care should also be taken when handling ticks. No vaccine for A. phagocytophilum infection is currently available. Infection can be avoided by tick control or prophylactic use of tetracyclines when visiting endemic areas. In one study, application of imidacloprid-permethrin prevented transmission of A. phagocytophilum from naturally infected Ixodes scapularis ticks to dogs (Blackburn et al., 2004). Dogs appear to be susceptible to reinfection, so tick control should be maintained at all times in endemic areas. Dogs used for blood donors that reside in endemic areas should be screened for A. phagocytophilum infections by serology or PCR.
FELINE GRANULOCYTOTROPIC ANAPLASMOSIS
Etiology and Epidemiology
Cats have shown to be susceptible to A. phagocytophilum infection after experimental inoculation (Lewis et al., 1975; Foley et al., 2003). In naturally exposed cats, DNA of A. phagocytophilum has been amplified from several countries, including Sweden, Denmark, Ireland, and the United States (Bjoersdorff et al., 1999; Shaw et al., 2001; Lappin et al., 2004). Morulae consistent with A. phagocytophilum have been detected cytologically in neutrophils of naturally infected cats in other countries, including Brazil, Kenya, and Italy (Almosny et al., 1998; Buoro, 1989; Tarello et al., 2005). Cats living in endemic areas are commonly seropositive (Magnarelli et al., 2005; Billeter et al., 2007). As in dogs, A. phagocytophilum is transmitted by Ixodes ticks, so infections of cats are likely to be most common in these areas. Although rodents are commonly infected with A. phagocytophilum, whether ingestion or direct contact with rodents plays a role in A. phagocytophilum infection of cats is currently unknown. Although the pathogenesis of disease associated with A. phagocytophilum in cats is unknown, some cats experimentally inoculated with A. phagocytophilum developed antinuclear antibodies and increased interferon-γ mRNA, suggesting that an immune pathogenesis of disease may contribute to the clinical findings (Foley et al., 2003).
Clinical Features
Fever, anorexia, and lethargy were the most common clinical abnormalities. Tachypnea has also been detected. Ticks may or may not currently be infesting infected cats. Overall, clinical signs associated with A. phagocytophilum infection in cats were mild and resolved quickly after initiating tetracycline therapy.
Diagnosis
Approximately 50% of cats with proven clinical infections induced by A. phagocytophilum have a mild thrombocytopenia (66,000 to 118,000/μL). Neutrophilia with a left shift, lymphocytosis, lymphopenia, and hyperglobulinemia have been detected in some cats. Morulae are less commonly detected than in dogs. The abnormalities resolved quickly after doxycycline treatment was initiated (Bjoersdorff et al., 1999; Lappin et al., 2004). Biochemical and urinalysis abnormalities are unusual. Some commercial laboratories offer serologic testing. Infected cats are negative for antibodies against E. canis, so A. phagocytophilum IFA slides should be used. Approximately 30% of cats with proven clinical infections induced by A. phagocytophilum are seronegative when first assessed serologically, but all proven cases to date have ultimately seroconverted. Some mountain lions with A. phagocytophilum DNA amplified from blood have been serum antibody negative (Foley et al., 1999), so a single negative antibody result in an acutely infected cat does not exclude infection. Therefore cats with suspected anaplasmosis may need convalescent serum samples to prove infection. Alternately, antibody testing could be combined with PCR testing of whole blood in acute cases (Lappin et al., 2004).
Therapy
Supportive care should be administered as needed. Several antibiotics have been administered to naturally infected cats, but all cats in two studies became clinically normal within 24 to 48 hours after initiation of tetracycline or doxycycline administration and recurrence was not reported (Bjoersdorff et al., 1999; Lappin et al., 2004). Although clinically normal, two cats were still PCR positive 17 days and 90 days after treatment (of 21 to 30 days’ duration), respectively, which suggests that treatment with tetracyclines for 21 to 30 days may be inadequate for eliminating the organism from the body (Lappin et al., 2004).
Zoonotic Aspects and Prevention
See the section on canine granulocytic anaplasmosis for a discussion of zoonotic aspects. To prevent A. phagocytophilum infection in cats, ascaricidal products approved for use on cats should be used. A. phagocytophilum can likely be transmitted by blood; therefore cats used as blood donors in endemic areas should be screened for infection by serum antibody tests or PCR assay, and positive cats should be excluded as donors.
CANINE THROMBOCYTOTROPIC ANAPLASMOSIS
Etiology and Epidemiology
Anaplasma platys was formerly classified as Ehrlichia platys (Dumler et al., 2001). The organism forms morulae in circulating platelets and has been called canine infectious cyclic thrombocytopenia. Infected dogs have been detected primarily in the south and southeastern United States, Western Europe, South America, Australia, Africa, and the Middle East. Inclusions morphologically similar to A. platys have been detected in one cat in Brazil, but attempts to transmit the organism from a dog to a cat failed (Harvey, 2006). A tick vector is suspected because A. platys DNA has been amplified from ticks. However, attempts to transmit infection by Rhipicephalus sanguineous have failed. After intravenous inoculation the incubation period is 8 to 15 days. Although cyclic thrombocytopenia and parasitemia can occur at 10- to 14-days intervals, organism numbers and severity of thrombocytopenia may lessen over time. Later in infection thrombocytopenia can be severe, but the organism may not be recognized cytologically or by PCR with blood (Eddlestone et al., 2007). In these experimentally infected dogs microbial DNA could be amplified from bone marrow and splenic aspirates. Anemia and thrombocytopenia in dogs experimentally infected with either A. platys and/or E. canis were more persistent in the coinfected dogs (Gaunt et al., 2007).
Clinical Features
Dogs with A. platys infections in the United States are usually subclinically infected or have mild fever. More severely affected dogs have exhibited fever, uveitis, and clinical evidence of bleeding, including ecchymosis, petechia, epistaxis, melena, gingival bleeding, retinal hemorrhage, and hematoma formation. Coinfection with other tick borne agents such as E. canis is common and may potentiate clinical disease (Kordick et al, 1999; Gaunt et al, 2007).
Diagnosis
Anemia, thrombocytopenia, and neutrophilic leukocytosis can occur. Morulae may or may not be present within platelets. In endemic areas A. platys infection, alone or in combination with other tick-borne agents, should be suspected in dogs with anemia or thrombocytopenia. Serum antibodies can be detected by IFA. Cross-reactivity with E. canis is thought to be minimal but A. platys antibodies may be detected in serologic assays for A. phagocytophilum. Antibody assay results can be falsely negative in acute cases, so a convalescent test 2 to 3 weeks later may be required to confirm exposure. PCR assays performed on blood collected in ethylenediamine tetraacetic acid (EDTA) can be used to confirm infection and differentiate A. platys infections from other infections and microbial DNA can also be amplified from healthy dogs (Kordick et al., 1999) and can be negative in clinically ill dogs (Eddlestone et al., 2007). Most dogs infected by A. platys have subclinical infections, most infected dogs only have an acute phase, exposure rates in endemic areas are high, and the disease syndromes associated with infection have multiple other causes. Thus antibody test results and PCR assay results alone cannot be used to prove clinical disease associated with A. platys infection.
Treatment
The doxycycline and tetracycline treatment protocols discussed for A. phagocytophilum infections of dogs should also be effective for A. platys infections. If coinfection with E. canis exists, treatment duration should be at least 4 weeks (Neer et al., 2002).
CANINE MONOCYTOTROPIC EHRLICHIOSIS
Etiology and Epidemiology
Organisms that are associated with monocytotropic ehrlichiosis in naturally infected dogs include Ehrlichia canis, E. chaffeensis, and Neorickettsia risticii var atypicalis. An individual dog can be infected by more than one ehrlichial agent, and coinfection with other tick-borne pathogens is common (Kordick et al., 1999).
E. canis is the most common of these agents and causes the most severe clinical disease; it is maintained in the environment from passage from ticks to dogs. Rhipicephalus sanguineus and Dermacentor variabilis are the known vectors. The organism is not passed transovarially in the tick, so unexposed ticks must feed on a rickettsemic dog in the acute phase to become infected and perpetuate the disease. Male R. sanguineus can take multiple feedings and can both acquire and transmit E. canis in the absence of female ticks (Bremer et al., 2005). Dogs seropositive for E. canis have been identified in many regions of the world and most of the United States, but the majority of cases occur in areas with high concentrations of R. sanguineus, such as the Southwest and Gulf Coast.
E. chaffeensis is a cause of human mononuclear ehrlichiosis. White-tailed deer, voles, coyotes, and opossums are reservoirs, and Amblyomma americanum, D. variabilis, and some Ixodes ticks are vectors. Infections by E. chaffeensis are detected primarily in the southeastern United States. Clinical manifestations in dogs are currently being detailed (Breitschwerdt et al., 1998; Zhang et al., 2003) and appear to be rare. N. risticii var atypicalis has been detected only in the United States to date and causes similar clinical signs as E. canis (Kokoma et al., 1991). Bats and swallows may be the natural reservoirs of this organism. Trematodes of snails and water insects are thought to be the vectors (Pusterla et al., 2003).
Ehrlichia canis infection causes acute, subclinical, and chronic phases of disease. Infected mononuclear cells marginate in small vessels or migrate into endothelial tissues, inducing vasculitis during the acute phase. The acute phase begins 1 to 3 weeks after infection and lasts 2 to 4 weeks; most immunocompetent dogs survive. The subclinical phase lasts months to years in naturally infected dogs. Although some dogs clear the organism during the subclinical phase, the organism persists intracellularly in some, leading to the chronic phase of infection. Many of the clinical and clinicopathologic abnormalities that develop during the chronic phase are from immune reactions against the intracellular organism. The variable duration of the subclinical phase of disease explains why E. canis infection does not have a distinct seasonal incidence as does Rocky Mountain spotted fever (RMSF). However, acute-phase disease is recognized most frequently in the spring and summer when the tick vectors are most active.
Clinical Features
Clinical disease from ehrlichial infection can occur in any dog, but its severity varies depending on the organism, host factors, and presence of coinfections. Virulence is thought to vary with different field strains of E. canis. Dogs with depressed cell-mediated immunity develop severe disease. However, E. canis itself did not cause immunosuppression in young, experimentally infected dogs within the first several months of infection (Hess et al., 2006).
Clinical findings in dogs with E. canis infections vary with the timing of infection (Table 96-2). The clinical manifestations of acute-phase disease are quite similar to those of RMSF as a result of the development of vasculitis. Ticks are most commonly found on dogs during the acute phase of infection. Fever can occur in both clinical phases of infection but is more common in dogs with acute ehrlichiosis. Petechiae or other evidence of bleeding noted during the acute phase is generally caused by a combination of mild thrombocytopenia (consumption or immune-mediated destruction) and vasculitis; thrombocytopenia (consumption, immune-mediated destruction, sequestration, decreased production), vasculitis, and platelet function abnormalities (Brandao et al., 2006) occur in the chronic phase. The thrombocytopenia in the acute phase is generally not severe enough to result in spontaneous bleeding, so bleeding may be primarily from vasculitis and decreased platelet function.
 TABLE 96-2 Clinical Abnormalities Associated with Ehrlichia canis Infection in Dogs
TABLE 96-2 Clinical Abnormalities Associated with Ehrlichia canis Infection in Dogs
| STAGE OF INFECTION | ABNORMALITIES |
|---|---|
| Acute | |
| Subclinical | |
| Chronic |
Pale mucous membranes usually only occur in the chronic phase during the development of pancytopenia. Hepatomegaly, splenomegaly, and lymphadenopathy are from chronic immune stimulation (i.e., lymphoreticular hyperplasia) and are detected most frequently in dogs in the chronic phase. Interstitial or alveolar edema secondary to vasculitis or inflammation, pulmonary parenchymal hemorrhage secondary to vasculitis or thrombocytopenia, or secondary infections from neutropenia are mechanisms resulting in dyspnea or cough in some dogs with ehrlichiosis. Polyuria, polydipsia, and proteinuria are reported in some dogs that develop renal insufficiency.
Stiffness, exercise intolerance, and swollen, painful joints occur in some dogs with suppurative polyarthritis. Most dogs with polyarthritis from which the organism has been demonstrated have been infected with E. ewingii or A. phagocytophilum. Ophthalmic manifestations of disease are common; tortuous retinal vessels, perivascular retinal infiltrates, retinal hemorrhage, anterior uveitis, and exudative retinal detachment occur (Komnenou et al., 2007). Central nervous system signs can include depression, pain, ataxia, paresis, nystagmus, and seizures.
Diagnosis
Clinicopathologic and radiographic abnormalities consistent with E. canis infection are summarized in Table 96-3. Neutropenia is common during acute-phase vasculitis and after bone marrow suppression in the chronic phase. Chronic immune stimulation causes monocytosis and lymphocytosis; lymphocytes often have cytoplasmic azurophilic granules (i.e., large granular lymphocytes). Regenerative anemia is from blood loss (acute and chronic phases); normocytic, normochromic nonregenerative anemia is from bone marrow suppression or anemia of chronic disease (chronic phase). Thrombocytopenia can occur with either acute or chronic ehrlichiosis but is generally more severe with chronic phase disease. Thrombocytopathies from hyperglobulinemia potentiate bleeding in some dogs with chronic ehrlichiosis. Chronic ehrlichiosis is classically associated with pancytopenia, but any combination of neutropenia, thrombocytopenia, and anemia can occur. Changes in bone marrow cell lines associated with ehrlichiosis vary from hypercellular (acute phase) to hypocellular (chronic phase). Bone marrow plasmacytosis is common in dogs with subclinical and chronic ehrlichiosis, and the disease can be confused with multiple myeloma, particularly in dogs with monoclonal gammopathies. Dogs with ehrlichiosis are usually not hypercalcemic and do not have lytic bone lesions.
 TABLE 96-3 Clinicopathologic Abnormalities Associated with Ehrlichia canis Infection in Dogs
TABLE 96-3 Clinicopathologic Abnormalities Associated with Ehrlichia canis Infection in Dogs
| STAGE OF INFECTION | ABNORMALITIES |
|---|---|
| Acute | |
| Subclinical | |
| Chronic |
PCR, Polymerase chain reaction.
Hypoalbuminemia in the acute phase is probably caused by third spacing of albumin in tissues because of vasculitis, whereas in chronic-phase disease it is caused by glomerular loss from immune complex deposition or chronic immunostimulation (i.e., monoclonal or polyclonal gammopathy). Prerenal azotemia can occur with acute or chronic disease; renal azotemia develops in some dogs with severe glomerulonephritis from chronic ehrlichiosis. The combination of hyperglobulinemia and hypoalbuminemia is consistent with subclinical or chronic ehrlichiosis. Polyclonal gammopathies are most common, but monoclonal (e.g., immunoglobulin G) gammopathies can also occur.
Aspirates of enlarged lymph nodes and spleen reveal reactive lymphoreticular and plasma cell hyperplasia. Nondegenerate neutrophils are the primary cells in synovial fluid from dogs with polyarthritis caused by any Ehrlichia spp.; E. ewingii and A. phagocytophilum morulae can be identified in synovial neutrophils from some dogs. Bone marrow aspirates in dogs with chronic ehrlichiosis typically reveal myeloid, erythroid, and megakaryocytic hypoplasia in association with lymphoid and plasma cell hyperplasia. Morulae from E. canis are rarely detected in the cytoplasm of mononuclear cells. Ehrlichiosis generally causes mononuclear pleocytosis and increased protein concentrations in cerebrospinal fluid. Antiplatelet antibodies, antinuclear antibodies, antierythrocyte antibodies (by direct Coombs test), and rheumatoid factors are detected in some dogs with ehrlichiosis, leading to an inappropriate diagnosis of primary immune-mediated disease (Smith et al, 2004).
No pathognomonic radiographic signs appear in dogs with ehrlichiosis. The polyarthritis is nonerosive, and dogs with respiratory signs most commonly have increased pulmonary interstitial markings, but alveolar patterns can occur.
Identification of morulae in cells documents Ehrlichia infection, but it is uncommon with monocytotropic strains. Examination of buffy coat smears or blood smears made from blood collected from an ear margin vessel may increase the chances of finding morulae. Some Ehrlichia spp. can be cultured, but the procedure is low yield and expensive and so is not clinically useful.
Most commercial laboratories (using IFAs) and one point-of-care diagnostic test (SNAP 4Dx) use reagents that detect antibodies against E. canis in serum. These tests are generally used as the first screening procedures in dogs suspected to have ehrlichiosis. The American College of Veterinary Internal Medicine (ACVIM) Infectious Disease Study Group suggests that E. canis IFA antibody titers between 1 : 10 and 1 : 80 be rechecked in 2 to 3 weeks because of the potential for false-positive results at these titer levels (Neer et al., 2002). At low titers, agreement between IFA and ELISA can be poor (O’Connor et al., 2006).
If serum antibodies against E. canis are detected in a dog with clinical findings consistent with ehrlichiosis, a presumptive diagnosis of canine ehrlichiosis infection should be made and appropriate treatment begun. However, detection of antibodies alone is not diagnostic of ehrlichiosis because of the existence of cross-reactive antibodies among E. canis, N. helminthoeca, and Cowdria ruminantium and because some dogs are subclinically infected. In addition, negative test results do not totally exclude ehrlichiosis from the list of differential diagnoses because clinical disease can be detected before seroconversion and not all Ehrlichia spp. induce antibodies that are consistently detected in E. canis assays.
PCR assays are now available commercially and can be used to detect organism-specific DNA in peripheral blood. It can be performed on joint fluid, aqueous humor, cerebrospinal fluid, and tissues. Blood PCR results can be positive before seroconversion in some experimentally inoculated dogs and positive results document infection, whereas positive serologic tests only document exposure. However, as for serology, no standardization among laboratories currently exists, and insufficient quality control can lead to falsepositive or false-negative results. Until more information is available, the ACVIM Infectious Disease Study Group suggests using PCR with serology, not in lieu of it. Because antibiotic treatment rapidly induces negative blood PCR results, the clinician should draw the blood sample for testing and place it in an EDTA tube before treatment. In one recent study tissues (lymph nodes, spleen, liver, bone marrow, and blood) from naturally infected dogs were assayed by PCR. Blood and lymph nodes were the most likely to be positive but were falsely negative in approximately 30% of the samples (Gal et al., 2007).
Treatment
Supportive care should be provided as indicated. Several different tetracycline, doxycycline, chloramphenicol, and imidocarb diproprionate protocols have been used. The ACVIM Infectious Disease Study Group currently recommends doxycycline (10 mg/kg PO q24h for at least 28 days). In one study of experimentally infected dogs, ticks still could acquire E. canis from feeding on dogs previously treated with doxycycline for 14 days (Schaefer et al., 2007). Clinical signs and thrombocytopenia should rapidly resolve. If clinical abnormalities are not resolving within 7 days, other differential diagnoses should be considered. Results of studies that used imidocarb diproprionate (5 to 7 mg/kg IM or SQ repeated in 14 days) to treat canine ehrlichiosis have been variable. In one recent study thrombocytopenia persisted and infection was not cleared in experimentally inoculated dogs (Eddlestone et al., 2006). Some patients develop pain at the injection site, salivation, oculonasal discharge, diarrhea, tremors, and dyspnea after administration of this drug. Quinolones are not effective for the treatment of E. canis infections in dogs. Although coinfections common occur, the presence of agents such as A. phagocytophilum, A. platys, and Leishmania infantum did not adversely affect the response to therapy (Mylonakis et al., 2004).
Positive antibody titers have been detected for up to 31 months after therapy in some naturally infected dogs. Dogs with low (less than 1 : 1024) antibody titers generally revert to negative by 1 year after therapy. Dogs with antibody titers greater than 1 : 1024 often maintain positive antibody titers after therapy. Whether these dogs are persistent carriers of the organism is undetermined. On the basis of these findings antibody titers are considered to be ineffective for monitoring response to therapy. The ACVIM Infectious Disease Study Group recommends monitoring resolution of thrombocytopenia and hyperglobulinemia as markers of therapeutic elimination of the organism.
Whether ehrlichial infections are cleared by treatment is currently unknown. If PCR is to be used to monitor treatment, the ACVIM Infectious Disease Study Group recommends the following steps be taken. The PCR test should be repeated 2 weeks after stopping treatment. If still positive, treatment should be reinstituted for 4 weeks and retesting performed. If PCR results are still positive after two treatment cycles, an alternate anti-Ehrlichia drug should be used. If PCR results are negative the test should be repeated in 8 weeks, and if still negative therapeutic elimination is assumed to be likely. In one study PCR assay performed on splenic aspirates was superior to blood PCR to document elimination of infection (Harrus et al., 2004).
Whether to treat seropositive healthy dogs is controversial. Arguments for and against testing or treating healthy dogs were reviewed by the ACVIM Infectious Disease Study Group (Neer et al., 2002). The primary reason to treat a seropositive healthy dog is to try to eliminate infection before development of chronic-phase disease. However, treatment of healthy dogs is controversial for at least six reasons: (1) whether treatment halts progression to the chronic phase is unknown; (2) not all seropositive dogs are infected; (3) not all seropositive dogs progress to the chronic phase; (4) whether treatment eliminates infection is unknown; (5) even if infection is eliminated, reinfection can occur; and (6) treatment of healthy carriers may result in antimicrobial resistance.
Because further data are needed to make definitive recommendations, owners should be given the pros and cons and asked to make treatment decisions.
The prognosis is good for dogs with acute ehrlichiosis, and it is variable to guarded for those with chronic ehrlichiosis. Fever, petechia, vomiting, diarrhea, epistaxis, and thrombocytopenia often resolve within days after initiation of therapy in acute cases. Bone marrow suppression from chronic-phase ehrlichiosis may not respond for weeks to months, if at all. Anabolic steroids and other bone marrow stimulants can be administered but are unlikely to be effective because precursor cells are often lacking. Immunemediated events resulting in the destruction of red blood cells or platelets are likely to occur with ehrlichiosis, leading to the recommendation to administer antiinflammatory or immunosuppressive doses of glucocorticoids to acutely affected animals. Prednisone (2.2 mg/kg PO divided q12h during the first 3 to 4 days after diagnosis) may be beneficial in some cases.
Zoonotic Aspects and Prevention
Dogs and human beings are both infected by E. canis, E. ewingii, and E. chaffeensis (Buller et al., 1999). Although people cannot acquire ehrlichiosis from handling an infected dog, dogs may be reservoirs for these agents and may play a role in the human disease by bringing vectors into the human environment. Ticks should be removed and handled with care.
Tick control should be maintained at all times; administration of fipronil was shown to lessen transmission in one study (Davoust et al., 2003). Because E. canis is not passed transovarially in the tick, it can be eliminated in the environment by tick control or by treating all dogs through a generation of ticks. Rhipicephalus can only transmit E. canis for approximately 155 days; if tick control is not feasible tetracycline can be administered (6.6 mg/kg PO daily for 200 days). During this time infected dogs will not infect new ticks and previously infected ticks will lose the ability to transmit the organism. Doxycycline given at 100 mg/dog/day was used successfully as a chemopreventative (Davoust et al., 2005). Dogs used as blood donors should be screened serologically yearly and seropositive dogs should not be used.
FELINE MONOCYTOTROPIC EHRLICHIOSIS
Etiology and Epidemiology
Ehrlichia-like bodies or morulae have been detected in peripheral lymphocytes or monocytes of naturally exposed cats in a number of countries, including the United States, Kenya, France, Brazil, and Thailand (Bouloy et al., 1994; Buoro et al., 1994; Beaufils et al., 1995; Beaufils et al, 1999; Almosny et al, 1998; Jittapalapong, 1993). Two studies of naturally infected cats have amplified DNA consistent with E. canis (Breitschwerdt et al., 2002; Beaufils et al., 2002). Other studies of cats in endemic areas (Florida and Arizona) have failed to amplify Ehrlichia spp. DNA from the blood of cats (Luria et al., 2004; Eberhardt et al., 2006). To our knowledge, only two experimental inoculation studies of cats with monocytotropic Ehrlichia spp. have been performed (Dawson et al., 1988; Lappin and Breitschwerdt, unpublished observations, 2007). Morulae of N. risticii were detected in mononuclear cells from two of six cats inoculated intravenously but not subcutaneously; diarrhea developed in one cat and depression, anorexia, and lymphadenomegaly developed in the other. When cats were inoculated subcutaneously with an E. canis strain (North Carolina State University canine isolate) maintained in cell culture, microbial DNA or antibodies that reacted to E. canis morulae were not detected in an 8-week follow-up period (Lappin and Breitschwerdt, unpublished observations, 2007). These results indicate the E. canis–like DNA amplified from naturally infected cats may be from a different Ehrlichia spp. more infective to cats, not all E. canis stains will infect cats, not all cats are susceptible to infection by E. canis, or subcutaneous inoculation is not an effective method for infecting cats with E. canis.
Sera from cats have been assessed for Ehrlichia spp. antibodies by using IFA or Western immunoblot. However, standardization of methods among laboratories has not been performed, the most appropriate cutoff values have not been determined, and variable serologic cross-reactivity has occurred among Ehrlichia spp., Neorickettsia spp., and Anaplasma spp. Therefore results of serologic studies should be interpreted cautiously. Serum antibodies that react with E. canis morulae have been detected by IFA in cats from multiple states in the United States, France, Italy, and Kenya (Bouloy et al., 1994; Matthewman et al., 1996; Peavy et al., 1989; Beaufils et al., 1999; Stubbs et al., 2000). Although antibodies have been commonly detected in naturally exposed cats, DNA of Ehrlichia spp. is rarely amplified from blood. When taken together these results suggest that cats are less susceptible to monocytotropic ehrlichial infections than are dogs.
How cats are exposed to monocytotropic ehrlichial agents is currently unknown. Documentation of arthropod exposure in proven cases has been variable. Pathogenesis of disease associated with monocytotropic ehrlichiosis in cats is unknown but is likely to be similar to that for E. canis infection of dogs.
Clinical Features
All ages of cats have been infected; most cats were domestic short haired, and both males and females have been affected. Anorexia, fever, inappetence, lethargy, weight loss, hyperesthesia or joint pain, pale mucous membranes, splenomegaly, dyspnea, and lymphadenomegaly were the most common historic and physical examination abnormalities. Dyspnea, petechiae, retinal detachments, vitreous hemorrhages, and pale mucous membranes were other reported physical examination abnormalities. Concurrent diseases are rarely reported but have included hemoplasmas (previously Haemobartonella felis), Cryptococcus neoformans, feline leukemia virus and feline immunodeficiency virus infections, and lymphoma.
Diagnosis
Anemia is common and usually nonregenerative. Leukopenia; leukocytosis characterized by neutrophilia, lymphocytosis, and monocytosis; and intermittent thrombocytopenia have been reported in some cats. Bone marrow evaluation of cats with cytopenias has revealed primarily hypoplasia of the effected cell line. However, one cat had bone marrow cytologic characteristics consistent with myeloid leukemia (Breitschwerdt et al., 2002). Hyperglobulinemia was reported in multiple cats; protein electrophoresis usually reveals a polyclonal gammopathy. An epidemiologic link has been made between the presence of Ehrlichia spp. antibodies in serum and monoclonal gammopathy (Stubbs et al., 2000). On the basis of the cases reported to date, ehrlichiosis should be considered on the differential list for cats with unexplained leukocytosis, cytopenias, and hyperglobulinemia. Biochemical abnormalities were infrequently reported in cats with suspected monocytotropic ehrlichiosis and were nonspecific. The three cats with E. canis–like DNA in the blood also had antinuclear antibodies, similar to results reported for infected dogs (Breitschwerdt et al., 2002).
Some cats with suspected clinical ehrlichiosis seroreacted to E. canis or N. risticii morulae. Antibodies that seroreact to more than one Ehrlichia spp. are sometimes detected. Some cats with E. canis – like DNA in blood were seronegative (Breitschwerdt et al., 2002). In contrast, most A. phagocytophilum –infected cats have strongly positive antibody test results. Positive serologic test results occur in both healthy and clinically ill cats, so a diagnosis of clinical ehrlichiosis should not be based on serologic test results alone. A tentative diagnosis of clinical feline ehrlichiosis can be based on the combination of positive serologic test results, clinical signs of disease consistent with Ehrlichia infection, exclusion of other causes of the disease syndrome, and response to anti-rickettsial drugs. Ehrlichia spp. have been cultured from some cats on monocyte cell cultures. PCR and gene sequencing can also be used to confirm infection and should be considered the tests of choice at this time. However, no standardization for dogs exists among laboratories providing Ehrlichia spp. PCR assays.
Treatment
Clinical improvement after therapy with tetracycline, doxycycline, or imidocarb dipropionate was reported for most cats. However, for some cats a positive response to therapy was a criterion for the diagnosis of ehrlichiosis. The current recommendation of the ACVIM Infectious Disease Study Group is to give doxycycline (10 mg/kg PO q24h for 28 days). For cats with treatment failure or those intolerant of doxycycline, imidocarb diproprionate can be given safely (5 mg/kg IM or SQ twice, 14 days apart). Salivation and pain at the injection site are the common adverse effects, and imidocarb efficacy is in question for the treatment of canine monocytotropic ehrlichiosis (Eddlestone et al., 2007).
CANINE GRANULOCYTOTROPIC EHRLICHIOSIS
Etiology and Epidemiology
Ehrlichia ewingii forms morulae in neutrophils and eosinophils and has been detected in dogs and human beings that reside in the southern and southeastern United States. Although one canine case was reported in New York, A. phagocytophilum is more likely in this region (see sections on canine and feline granulocytotropic anaplasmosis). E. ewingii has been detected in a number of ticks, but A. americanum is the only proven vector to date (Murphy et al., 1998). Deer are infected and serve as a reservoir (Yabsley et al., 2002). The incubation period after tick exposure is approximately 13 days. Pathogenesis of disease is unknown but is likely be to similar to other Ehrlichia spp. In general, clinical signs of E. ewingii infection are less severe that those of E. canis. Concurrent disease or infections may play a significant role in the pathogenesis of E. ewingii infection.
Clinical Features
Nonspecific signs of E. ewingii infection include fever, lethargy, anorexia, depression, and signs consistent with polyarthritis, such as stiffness. Other clinical signs include vomiting, diarrhea, and peripheral edema and neurologic signs such as ataxia, paresis, and vestibular disease. Clinical signs can be mild, self-limited, or inapparent (Goodman et al., 2003). Similar to R. rickettsii, acute disease seems to be most common, so E. ewingii infection should be highest on the list of differential diagnoses from the spring through autumn when A. americanum is most active.
Diagnosis
Suppurative polyarthritis is most common. Other clinicopathologic findings typically associated with acute E. canis infection (Table 96-3), such as mild to moderate thrombocytopenia and anemia, also occur. Morulae can be detected in neutrophils and eosinophils in peripheral blood and in neutrophils from synovial fluid. However, presence of morulae is transient and so easily missed cytologically. The organism has not been cultured to date, so a specific serologic test is not available. However, because the organism is closely related to E. canis, antibodies against E. ewingii can often be detected in E. canis IFA assays. However, E. ewingii antibodies do not bind to the E. canis peptide used in a point-of-care diagnostic assay in the United States (SNAP 4Dx), so this assay cannot be used to screen dogs for E. canis infection (Daniluk et al., 2007). PCR assays are now used to differentiate between members of the Ehrlichia, Anaplasma, and Neorickettsia genera and should be performed on blood collected in EDTA before administration of antibiotics.
Treatment
Supportive care should be provided as indicated. The tetracycline, doxycycline, and chloramphenicol protocols recommended for E. canis infections are generally effective. The ACVIM Infectious Disease Study Group currently recommends doxycycline (10 mg/kg PO q24h for at least 28 days) for Ehrlichia spp. infections of dogs (Neer et al., 2002).
Zoonotic Aspects and Prevention
Dogs and human beings are both infected by Ehrlichia canis, E. ewingii, and E. chaffeensis (Buller et al., 1999). Although people cannot acquire ehrlichiosis from handling an infected dog, dogs may be reservoirs for these agents and may play a role in the human disease by bringing vectors into the human environment. Ticks should be removed and handled with care. Dogs used as blood donors should be screened serologically with E. canis IFA tests yearly, and seropositive dogs should not be used.
ROCKY MOUNTAIN SPOTTED FEVER
Etiology and Epidemiology
RMSF is caused by R. rickettsii. Other members of the genus also infect dogs in the United States; however, they are not associated with clinical disease but can induce antibodies that cross-react with R. rickettsii (see Diagnosis below). For example, 17 of 22 canine sera submitted for R. akari (rickettsialpox in human beings) IFA testing cross-reacted serologically with R. rickettsii (Comer et al., 2001). In another study of dogs coinfected with several tick-borne pathogens, infection with an uncharacterized rickettsial agent commonly induced cross-reacting antibodies to R. rickettsii (Kordick et al., 1999). Canine RMSF is recognized predominantly in the southeastern states from April through September when the tick vectors are most active. From 1993 to 1996, 52% of human cases of RMSF were reported from the south Atlantic region (Treadwell et al., 2000). Dermacentor andersoni (American wood tick), Dermacentor variabilis (American dog tick), and A. americanum (Lone Star tick) are the principal vectors, host, and reservoir of R. rickettsii. A reemergence of RMSF in the southwestern states has recently occurred, and R. sanguineous ticks are the vector (Demma et al., 2005, 2006; Nicholson et al., 2006). The organism has also been detected in R. sanguineous in California (Wikswo et al., 2007). Strains of R. rickettsii that infect dogs and human beings are closely related genetically (Kidd et al., 2006). Seroprevalence rates are high in endemic areas. In one study of dogs in the southeastern United States 14.1% and 29.7% of healthy and clinically ill dogs, respectively, had detectable R. rickettsii serum antibody titers (Solano-Gallego et al., 2004).
The organism is maintained in nature in a cycle between ticks and small mammals such as voles, ground squirrels, and chipmunks, and it is transmitted transovarially in ticks, so nymphs and larvae can be infected without feeding. R. rickettsii replicates in endothelial tissues (causing vasculitis) and so can lead to diverse and sometimes severe clinical manifestations of disease as soon as 2 to 3 days after exposure. Antiplatelet antibodies can be detected in many infected dogs, suggesting an immune-mediated component to the thrombocytopenia that is frequently present (Grindem et al., 1999). Although seropositive cats have been detected, whether clinical illness occurs is unclear (Case et al., 2006).
Clinical Features
Any dog not previously exposed to R. rickettsii can develop RMSF. The tick frequently feeds on and leaves the dog before the development of clinical signs. In one study only five of 30 owners knew their dogs had been infested by ticks (Gasser et al., 2001). After infection the majority of dogs are subclinical; some develop acute disease with a clinical course of approximately 14 days. No age or sex predilection exists.
Fever and depression are the most common clinical signs. Interstitial pulmonary disease, dyspnea, and cough occur in some dogs, and gastrointestinal signs occur in some acutely infected dogs. Because the disease is generally acute, lymphadenopathy and splenomegaly are not as common as in dogs with ehrlichiosis. Petechiae, epistaxis, subconjunctival hemorrhage, hyphema, anterior uveitis, iris hemorrhage, retinal petechiae, and retinal edema occur frequently. Cutaneous manifestations can include hyperemia, petechiae, edema, and dermal necrosis. Hemorrhage results from vasculitis, thrombocytopenia from consumption of platelets at sites of vasculitis, thrombocytopenia from immune destruction and, in some dogs, disseminated intravascular coagulation. Central nervous system signs include vestibular lesions (nystagmus, ataxia, head tilt), seizures, paresis, tremors, changes in mentation, and hyperesthesia (Mikszewski et al., 2005). Fatal RMSF is generally secondary to cardiac arrhythmias and shock, pulmonary disease, acute renal failure, or severe central nervous system disease.
Diagnosis
Clinicopathologic and radiographic abnormalities are common but do not definitively document RMSF. Neutrophilic leukocytosis, with or without a left shift and toxic cells, is found in most clinically affected dogs. Platelet counts are variable, but in one study 14 of 30 dogs had less than 75,000 platelets/μL without evidence of disseminated intravascular coagulation (Gasser, 2001). In other dogs hemostatic abnormalities consistent with disseminated intravascular coagulation occur. Anemia occurs in some dogs, primarily from blood loss. Increased activities of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase, as well as hypoalbuminemia from blood loss or third spacing of albumin in tissues secondary to vasculitis, occur frequently. Because R. rickettsii does not result in chronic intracellular infection as does ehrlichiosis, hyperglobulinemia is rare. Renal insufficiency in some dogs causes azotemia and metabolic acidosis. Serum sodium, chloride, and potassium concentrations decrease in many dogs with gastrointestinal tract signs or renal insufficiency. Compared with dogs with chronic ehrlichiosis, chronic proteinuria from glomerulonephritis is rare. Positive direct Coombs test results occur in some dogs.
Nonseptic, suppurative polyarthritis occurs in some dogs. CNS inflammation usually causes increased protein concentrations and neutrophilic pleocytosis in cerebrospinal fluid; some dogs may have mononuclear cell pleocytosis or mixed inflammation. No pathognomonic radiographic abnormalities are associated with RMSF, but both experimentally and naturally infected dogs commonly develop unstructured pulmonary interstitial patterns.
A presumptive diagnosis of canine RMSF can be based on the combination of appropriate clinical, historic, and clinicopathologic evidence of disease; serologic test results; exclusion of other causes of the clinical abnormalities; and response to anti-rickettsial drugs. Documentation of seroconversion or an increasing titer 2 to 3 weeks after initial serologic testing suggests recent infection. Diagnostic criteria used in one study included a fourfold rise in antibody titer or a single titer of greater than 1 : 1024 if the initial titer was submitted 1 week or more after initial onset of clinical abnormalities (Gasser et al., 2001). Positive serum antibody test results alone do not prove RMSF because subclinical infection is common. In addition, positive serum antibody tests do not document infection by R. rickettsii because infection with nonpathogenic spotted fever group agents can induce cross-reacting antibodies. Demonstration of R. rickettsii by inoculating affected tissues or blood into susceptible laboratory animals or by documenting the organism in endothelial cells by using direct fluorescent antibody staining leads to a definitive diagnosis of RMSF but is not clinically practical. PCR can be used to document the presence of rickettsial agents in blood, other fluids, or tissues and document infection. However, some apparently healthy dogs have had Rickettsia spp. DNA amplified from blood, so positive PCR assay results may not always correlate to RMSF (Kordick et al., 1999).
Treatment
Supportive care for gastrointestinal tract fluid and electrolyte losses, renal disease, disseminated intravascular coagulation, and anemia should be provided as indicated. Overzealous fluid therapy may worsen respiratory or central nervous system manifestations of disease if vasculitis is severe.
Tetracycline derivatives, chloramphenicol, and enrofloxacin are the antirickettsial drugs used most frequently. Trovafloxacin and, to a lesser extent, azithromycin were beneficial for treatment of RMSF in experimentally inoculated dogs (Breitschwerdt et al., 1999). Tetracycline (22 mg/kg PO q8h for 14 to 21 days) has been commonly used historically. Doxycycline (5 to 10 mg/kg PO q12h for 14 to 21 days) is an alternative to tetracyclines; gastrointestinal absorption and central nervous system penetration are superior to tetracycline because of increased lipid solubility. Chloramphenicol (22 to 25 mg/kg PO q8h for 14 days) can be used in puppies younger than 5 months to avoid dental staining associated with tetracyclines. Enrofloxacin (3 mg/kg PO q12h for 7 days) is as effective as tetracycline or chloramphenicol. In one study of 30 dogs with RMSF, all dogs survived and no apparent differences in response rate occurred among tetracycline, doxycycline, chloramphenicol, or enrofloxacin (Gasser et al., 2001). Fever, depression, and thrombocytopenia often begin to resolve within 24 to 48 hours after starting therapy. Administration of prednisolone at antiinflammatory or immunosuppressive doses in combination with doxycycline did not potentiate RMSF in experimentally infected dogs. The prognosis for canine RMSF is fair; death occurs in less than 5% of affected dogs.
Zoonotic Aspects and Prevention
Because RMSF has not been reported twice in the same dog, permanent immunity is likely. Infection can be prevented by providing strict tick control. Human beings probably do not acquire R. rickettsii from contact with dogs, but dogs may increase human exposure to RMSF by bringing ticks into the human environment. People can also be infected when removing ticks with activated R. rickettsii from the dog by hand. Two dogs and the owner all died of RMSF in one study (Elchos and Goddard, 2003). As in dogs, RMSF in people is most commonly recognized from April to September when the tick vectors are most active. Untreated RMSF is fatal in approximately 20% of infected people.
OTHER RICKETTSIAL INFECTIONS
Rickettsia felis was originally detected in a commercial cat flea (Ctenocephalides felis) colony and has been shown to belong in the spotted fever group. Fever, headache, myalgia, and macular rash in human beings have been attributed to R. felis infection around the world. In addition, one person in Mexico developed neurologic symptoms after R. felis infection, suggesting that the organism may be the cause of severe debilitating disease in some people. The organism has been detected in C. felis, C. canis, and Pulex irritans; these fleas have a worldwide distribution. C. felis is a biologic vector for R. felis; the organism can be transmitted transovarially and transtadially within the flea. Rickettsia felis DNA has been amplified from C. felis collected from cats in the United Kingdom, France, Israel, New Zealand, Australia, Thailand, and the United States (Hawley et al., 2006).
In a recent study in our laboratory we assayed 92 pairs of cat blood and flea extracts from Alabama, Maryland, and Texas by using PCR assays that amplify a region of the citrate synthase gene (gltA) and the outer membrane protein B gene (ompB). Of the 92 pairs, 62 (67.4%) of flea extracts and none of the cat blood samples were positive for R. felis DNA (Hawley et al., 2006). In another study we showed R. felis and R. rickettsii antibody prevalence rates in cats with fever to be 5.6% and 6.6%, respectively, but neither organism was amplified from blood (Bayliss et al., 2007). These results prove that cats are sometimes exposed, but further data are needed to determine significance of diseases associations. Because clinical illness in cats has not been documented, optimal treatment is unknown. However, on the basis of results in dogs, doxycycline or a fluoroquinolone would be logical choices. Prevention in cats and people should include flea control.
Neorickettsia helminthoeca (salmon poisoning) causes enteric signs of disease in dogs from the Pacific Northwest. Coxiella burnetii infection is associated with parturient or aborting cats and is primarily a zoonotic disease (see Chapter 100). H. felis has been reclassified as a Mycoplasma.
Canine Granulocytotropic Anaplasmosis
Bexfield NH, et al. Immune-mediated haemolytic anaemia and thrombocytopenia associated with Anaplasma phagocytophilum in a dog. J Small Anim Pract. 2005;46:543.
Blagburn BL, et al. Use of imidacloprid-permethrin to prevent transmission of Anaplasma phagocytophilum from naturally infected Ixodes scapularis ticks to dogs. Vet Ther. 2004;5:212.
Chen SM, et al. Identification of a granulocytic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589.
Dumler JS, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145.
Foley JE, et al. Spatial distribution of seropositivity to the causative agent of granulocytic ehrlichiosis in dogs in California. Am J Vet Res. 2001;62:1599.
Foley J, et al. Association between polyarthritis and thrombocytopenia and increased prevalence of vector borne pathogens in Californian dogs. Vet Rec. 2007;160:159.
Henn JB, et al. Gray foxes (Urocyon cinereoargenteus) as a potential reservoir of a Bartonella clarridgeiae—like bacterium and domestic dogs as part of a sentinel system for surveillance of zoonotic arthropod-borne pathogens in northern California. J Clin Microbiol. 2007;45:2411.
Jaderlund KH, et al. Seroprevalence of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in dogs with neurological signs. Vet Rec. 2007;160:825.
Kirtz G, et al. Anaplasma phagocytophilum infection in a dog: identifying the causative agent using PCR. J Small Anim Pract. 2005;46:300.
Lewis GE, et al. Experimentally induced infection of dogs, cats, and nonhuman primates with Ehrlichia equi, etiologic agent of equine ehrlichiosis. J Am Vet Med Assoc. 1975;36:85.
MacDonald KA, et al. A prospective study of canine infective endocarditis in northern California (1999-2001): emergence of Bartonella as a prevalent etiologic agent. J Vet Intern Med. 2004;18:56.
Maurin M, et al. Antibiotic susceptibilities of Anaplasma (Ehrlichia) phagocytophilum strains from various geographic areas in the United States. Antimicrob Agents Chemother. 2003;47:413.
Mylonakis ME, et al. Chronic canine ehrlichiosis (Ehrlichia canis): a retrospective study of 19 natural cases. J Am Anim Hosp Assoc. 2004;40:174.
Feline Granulocytotropic Anaplasmosis
Almosny NRP, et al. Ehrlichiose clinica em gato (Felis catus). R Bras Ci Vet. 1998;5:82.
Artursson K, et al. Diagnosis of borreliosis and granulocytic ehrlichiosis of horses, dogs, and cats in Sweden. Svensk Veterinartidning. 1994;45:331.
Billeter SA, et al. Prevalence of Anaplasma phagocytophilum in domestic felines in the United States. Vet Parasitol. 2007;147:194.
Bjoersdorff A, et al. Feline granulocytic ehrlichiosis—a report of a new clinical entity and characterization of the new infectious agent. J Sm Anim Pract. 1999;40:20.
Buoro IBJ, et al. Feline anaemia associated with Ehrlichia-like bodies in three domestic short-haired cats. Vet Rec. 1989;125:434.
Foley JE, et al. Evidence for modulated immune response to Anaplasma phagocytophila sensu lato in cats with FIV-induced immunosuppression. Comp Immunol Microbiol Infect Dis. 2003;26:103.
Lappin MR, et al. Molecular and serologic evidence of Anaplasma phagocytophilum infection in cats in North America. J Am Vet Med Assoc. 2004;225:893.
Lappin MR, et al. Feline granulocytotropic anaplasmosis. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:227.
Lewis GE, et al. Experimentally induced infection of dogs, cats, and nonhuman primates with Ehrlichia equi, etiologic agent of equine ehrlichiosis. J Am Vet Med Assoc. 1975;36:85.
Magnarelli LA, et al. Seroprevalence of antibodies against Borrelia burgdorferi and Anaplasma phagocytophilum in cats. Am J Vet Res. 2005;66:1895.
Shaw SE, et al. Molecular evidence of tick-transmitted infections in dogs and cats in the United Kingdom. Vet Rec. 2005;157:645.
Tarello W. Microscopic and clinical evidence for Anaplasma (Ehrlichia) phagocytophilum infection in Italian cats. Vet Rec. 2005;256:772.
Canine Thrombocytotropic Anaplasmosis
Eddlestone SM, et al. PCR detection of Anaplasma platys in blood and tissue of dogs during acute phase of experimental infection. Exp Parasitol. 2007;115:205.
Gaunt SD et al: Potentiation of thrombocytopenia and anemia in dogs experimentally co-infected with Anaplasma platys and Ehrlichia canis, Proceedings of the ACVIM Forum, Seattle WA, June 2007.
Harrus S, et al. Clinical manifestations of infectious canine cyclic thrombocytopenia. Vet Rec. 1997;141:247.
Harvey JW. Thrombocytotropic anaplasmosis (A. platys [E. platys] infection). In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:229.
Canine Monocytotropic Ehrlichiosis
Anderson BE, et al. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838.
Bartsch RC, et al. Post-therapy antibody titers in dogs with ehrlichiosis: follow-up study on 68 patients treated primarily with tetracycline and/or doxycycline. J Vet Intern Med. 1996;10:271.
Brandao LP, et al. Platelet aggregation studies in acute experimental canine ehrlichiosis. Vet Clin Pathol. 2006;35:78.
Branger S, et al. Evaluation of antibiotic susceptibilities of Ehrlichia canis, Ehrlichia chaffeensis, and Anaplasma phagocytophilum by real-time PCR. Antimicrob Agents Chemother. 2004;48:4822.
Breischtwerdt E, et al. Monoclonal gammopathy associated with naturally occurring canine ehrlichiosis. J Vet Intern. 1987;1:2.
Breitschwerdt EB, et al. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645.
Bremer WG, et al. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus. Vet Parasitol. 2005;131:95.
Bulla C, et al. The relationship between the degree of thrombocytopenia and infection with Ehrlichia canis in an endemic area. Vet Rec. 2004;35:141.
Davoust B, et al. Validation of chemoprevention of canine monocytic ehrlichiosis with doxycycline. Vet Microbiol. 2005;107:279.
Davoust B, et al. Assay of fipronil efficacy to prevent canine monocytic ehrlichiosis in endemic areas. Vet Parasitol. 2003;112:91.
Dawson JE, et al. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am J Vet Res. 1996;57:1175.
Dawson JE, Ewing SA. Susceptibility of dogs to infection with Ehrlichia chaffeensis, causative agent of human ehrlichiosis. Am J Vet Res. 1992;53:1322.
de Castro MB, et al. Experimental acute canine monocytic ehrlichiosis: clinicopathological and immunopathological findings. Vet Parasitol. 2004;119:73.
Eddlestone SM, et al. Failure of imidocarb dipropionate to clear experimentally induced Ehrlichia canis infection in dogs. J Vet Intern Med. 2006;20:849.
Gal A, et al. Detection of Ehrlichia canis by PCR in different tissues obtained during necropsy from dogs surveyed for naturally occurring canine monocytic ehrlichiosis. Vet J. 2007. Mar 15; [Epub ahead of print]
Harrus S, et al. Comparison of simultaneous splenic sample PCR with blood sample PCR for diagnosis and treatment of experimental Ehrlichia canis infection. Antimicrob Agents Chemother. 2004;48:4888.
Hess PR, et al. Experimental Ehrlichia canis infection in the dog does not cause immunosuppression. Vet Immunol Immunopathol. 2006;109:117.
Iqbal Z, et al. Comparison of PCR with other tests for early diagnosis of canine ehrlichiosis. J Clin Microbiol. 1994;32:1658.
Iqbal Z, et al. Reisolation of Ehrlichia canis from blood and tissues of dogs after doxycycline treatment. J Clin Microbiol. 1994;32:1644.
Kakoma I, et al. Serologically atypical canine ehrlichiosis associated with Ehrlichia risticii “infection,”. J Am Vet Med Assoc. 1991;199:1120.
Kakoma I, et al. Cultural, molecular, and immunological characterization of the etiologic agent for atypical canine ehrlichiosis. J Clin Microbiol. 1994;32:170.
Komnenou AA, et al. Ocular manifestations of natural canine monocytic ehrlichiosis (Ehrlichia canis): a retrospective study of 90 cases. Vet Ophthalmol. 2007;10:137.
Mylonakis ME, et al. Chronic canine ehrlichiosis (Ehrlichia canis): a retrospective study of 19 natural cases. J Am Anim Hosp Assoc. 2004;40:174.
Neer TM, et al. Consensus statement on ehrlichial disease of small animals from the Infectious Disease Study Group of the ACVIM. J Vet Int Med. 2002;16:309.
O’Connor TP, et al. Comparison of an indirect immunofluorescence assay, western blot analysis, and a commercially available ELISA for detection of Ehrlichia canis antibodies in canine sera. Am J Vet Res. 2006;67:206.
Pusterla N, et al. Digenetic trematodes, Acanthatrium sp. and Lecithodendrium sp., as vectors of Neorickettsia risticii, the agent of Potomac horse fever. J Helminthol. 2003;77:335.
Ristic M, et al. Susceptibility of dogs to infection with Ehrlichia risticii, causative agent of equine monocytic ehrlichiosis (Potomac horse fever). Am J Vet Res. 1988;49:1497.
Schaefer JJ, et al. Tick acquisition of Ehrlichia canis from dogs treated with doxycycline hyclate. Antimicrobiol Agents Chemother. 2007;51:3394.
Smith BE, et al. Antinuclear antibodies can be detected in dog sera reactive to Bartonella vinsonii subsp. berkhoffii, Ehrlichia canis, or Leishmania infantum antigens. J Vet Intern Med. 2004;18:47.
Steiert JG, Gilfoy F. Infection rates of Amblyomma americanum and Dermacentor variabilis by Ehrlichia chaffeensis and Ehrlichia ewingii in southwest Missouri. Vector Borne Zoonotic Dis. 2002;2:53.
Weiser MG, et al. Granular lymphocytosis and hyperproteinemia in dogs with chronic ehrlichiosis. J Am Anim Hosp Assoc. 1991;27:84.
Zhang XF, et al. Experimental Ehrlichia chaffeensis infection in beagles. J Med Microbiol. 2003;52:1021.
Feline Monocytotropic Ehrlichiosis
Almosny NRP, et al. Ehrlichiose clinica em gato (Felis catus). R Bras Ci Vet. 1998;5:82.
Beaufils JP, et al. Ehrlichia infection in cats: a review of three cases. Pratique Medicale Chirurgicate de l’Animale de Compagnie. 1995;30:397.
Beaufils JP, et al. Ehrlichiosis in cats. A retrospective study of 21 cases. Pratique Medicale Chirurgicale de l’Animal de Compagnie. 1999;34:587.
Bouloy RP, et al. Clinical ehrlichiosis in a cat. J Am Vet Med Assoc. 1994;204:1475.
Breitschwerdt E, et al. Molecular evidence of Ehrlichia canis infection in cats from North America. J Vet Int Med. 2002;16:642.
Buoro IBJ, et al. Feline anaemia associated with Ehrlichia-like bodies in three domestic short-haired cats. Vet Rec. 1989;125:434.
Charpentier F, et al. Probable case of ehrlichiosis in a cat. Bull Acad Vet Fr. 1986;59:287.
Dawson JE, et al. Susceptibility of cats to infection with E. risticii, causative agent of equine monocytic ehrlichiosis. Am J Vet Res. 1988;49:2096.
Eberhardt JE, et al. Prevalence of select infectious disease agents in cats from Arizona. J Fel Med Surg. 2006;8:164.
Jittapalapong S, et al. Preliminary survey on blood parasites of cats in Bangkhen District Area. Kasetsart J Nat Sci. 1993;27:330.
Lappin MR, et al. Effects of imidocarb diproprionate in cats with chronic haemobartonellosis. Vet Ther. 2002;3:144.
Lappin MR, Breitschwerdt EB. Feline mononuclear ehrlichiosis. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:224.
Luria BJ, et al. Prevalence of infectious diseases in feral cats in Northern Florida. J Fel Med Surg. 2004;6:287.
Matthewman LA, et al. Antibodies in cat sera from southern Africa react with antigens of Ehrlichia canis. Vet Rec. 1996;138:364.
Peavy GM, et al. Suspected ehrlichial infection in five cats from a household. J Am Vet Med Assoc. 1997;210:231.
Stubbs CJ, et al. Feline ehrlichiosis; literature review and serologic survey. Compend Contin Educ. 2000;22:307.
Canine Granulocytotropic Ehrlichiosis
Anderson BE, et al. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int J System Bacteriol. 1992;42:299.
Buller RS, et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148.
Breitschwerdt EB, et al. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol. 1998;36:2645.
Daniluk D et al: Preliminary evaluation of a peptide-based assay for detection of Ehrlichia ewingii antibodies in experimentally and naturally-infected dogs, Proceedings of the ACVIM Forum, Seattle, WA, June 2007.
Goodman RA, et al. Molecular identification of Ehrlichia ewingii infection in dogs: 15 cases (1997-2001). J Am Vet Med Assoc. 2003;222:1102.
Liddell AM, et al. Predominance of Ehrlichia ewingii in Missouri dogs. J Clin Microbiol. 2003;41:4617.
Murphy GL, et al. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol. 1998;79:325.
Paddock CD, et al. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin Infect Dis. 2001;33:1586.
Yabsley MJ, et al. Ehrlichia ewingii infection in white-tail deer (Odocoileus virginian us). Emerg Infect Dis. 2002;8:668.
Bayliss D et al: Prevalence Rickettsia species infections in cats with and without fever, Proceedings of the ACVIM Forum, Seattle WA, June 2007.
Breitschwerdt EB, et al. Efficacy of chloramphenicol, enrofloxacin, and tetracycline for treatment of experimental Rocky Mountain spotted fever in dogs. Antimicrob Agents Chemother. 1991;35:2375.
Breitschwerdt EB, et al. Prednisolone at anti-inflammatory or immunosuppressive dosages in conjunction with doxycycline does not potentiate the severity of Rickettsia rickettsii infection in dogs. Antimicrob Agents Chemother. 1997;41:141.
Breitschwerdt EB, et al. Efficacy of doxycycline, azithromycin, or trovafloxacin for treatment of experimental Rocky Mountain spotted fever in dogs. Antimicrob Agents Chemother. 1999;43:813.
Case JB, et al. Serological survey of vector-borne zoonotic pathogens in pet cats and cats from animal shelters and feral colonies. J Feline Med Surg. 2006;8:111.
Comer JA, et al. Serologic evidence of Rickettsia akari infection among dogs in a metropolitan city. J Am Vet Med Assoc. 2001;218:1780.
Davidson MG, et al. Identfication of Rickettsiae in cutaneous biopsy specimens from dogs with experimental Rocky Mountain spotted fever. J Vet Int Med. 1989;3:8.
Davidson MG, et al. Ocular manifestations of Rocky Mountain spotted fever in dogs. J Am Vet Med Assoc. 1989;194:777.
Demma LJ, et al. Serologic evidence for exposure to Rickettsia rickettsii in eastern Arizona and recent emergence of Rocky Mountain spotted fever in this region. Vector Borne Zoonotic Dis. 2006;6:423.
Demma LJ, et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587.
Drost WT, et al. Thoracic radiographic findings in dogs infected with Rickettsia rickettsii. Vet Radiol Ultrasound. 1997;38:260.
Elchos BN, Goddard J. Implications of presumptive fatal Rocky Mountain spotted fever in two dogs and their owner. J Am Vet Med Assoc. 2003;223:1450.
Gasser AM, et al. Canine Rocky Mountain spotted fever: a retrospective study of 30 cases. J Am Anim Hosp Assoc. 2001;37:41.
Greene CE, Breitschwerdt EB. Rocky Mountain spotted fever, murine typhuslike disease, rickettsialpox, typhus, and Q fever. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:232.
Grindem CB, et al. Platelet-associated immunoglobulin (antiplatelet antibody) in canine Rocky Mountain spotted fever and ehrlichiosis. J Am Anim Hosp Assoc. 1999;35:56.
Hawley JR, et al. Prevalence of Rickettsia felis DNA in the blood of cats and their fleas in the United States. J Feline Med Surg. 2007;9:258.
Helmick CG, et al. Rocky Mountain spotted fever: clinical, laboratory, and epidemiological features of 262 cases. J Infect Dis. 1984;150:480.
Kenny MJ, et al. Rickettsia felis in the United Kingdom. Emerg Infect Dis. 2003;9:1023.
Kidd L, et al. Molecular characterization of Rickettsia rickettsii infecting dogs and people in North Carolina. Ann NY Acad Sci. 2006;1078:400.
Kordick SK, et al. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631.
Mikszewski JS, Vite CH. Central nervous system dysfunction associated with Rocky Mountain spotted fever infection in five dogs. J Am Anim Hosp Assoc. 2005;41:259.
Nicholson WL, et al. Spotted fever group rickettsial infection in dogs from eastern Arizona: how long has it been there? Ann NY Acad Sci. 2006;1078:519.
Rolain JM, et al. Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerg Infect Dis. 2003;9:338.
Solano-Gallego L, et al. Bartonella henselae IgG antibodies are prevalent in dogs from southeastern USA. Vet Res. 2004;35:585.
Suksawat J, et al. Serologic and molecular evidence of coinfection with multiple vector-borne pathogens in dogs from Thailand. J Vet Intern Med. 2001;15:453.
Weiser IB, et al. Dermal necrosis associated with Rocky Mountain spotted fever in four dogs. J Am Vet Med Assoc. 1989;195:1756.
Wikswo ME, et al. Detection of Rickettsia rickettsii and Bartonella henselae in Rhipicephalus sanguineus ticks from California. J Med Entomol. 2007;44:158.