CHAPTER 100 Zoonoses
Zoonotic diseases are defined as being common to, shared by, or naturally transmitted between humans and other vertebrates. Most of the agents discussed in this chapter can infect and cause disease in immunocompetent people, but disease is generally more prevalent or more severe in immunodeficient people. Immunosuppression is common in humans. People with acquired immunodeficiency syndrome (AIDS) are discussed most frequently, but the population also includes the very old, the very young, and those receiving chemotherapy for immune-mediated diseases, organ transplantation, or neoplasia. Immunosuppressed people are sometimes advised to give up their pets. However, humans are unlikely to contract zoonotic diseases from contact with their pets, so in most cases this is not necessary. The Centers for Disease Control and Prevention online publication Preventing Infections from Pets: A Guide for People with HIV Infection states, “You do not have to give up your pet” (www.cdc.gov/hiv/pubs/brochure/oi_pets.htm). I believe that all human and other animal health care providers should provide accurate information to pet owners concerning the risks and benefits of pet ownership so that an informed decision about acquiring and keeping pets can be made (Grant et al., 1999).
Many infectious agents can infect humans by direct contact with pets, their exudates, or their excrement. These agents are the most important to veterinary health care providers and to dog and cat owners and are discussed in this chapter by likely route of exposure. For some zoonoses, including Rickettsia rickettsii, Ehrlichia spp., Bartonella spp., and Borrelia burgdorferi, the pet brings the vector of the organism into the environment, resulting in exposure of the person. With other zoonoses, including Histoplasma capsulatum, Coccidioides immitis, Blastomyces dermatitidis, and Cryptococcus neoformans, the owner and pet are infected by shared environmental exposure to the agent.
Following is a brief description of the more common canine and feline zoonoses encountered in small animal practice. General guidelines for the avoidance of zoonotic transfer of disease for veterinarians and pet owners are listed in Boxes 100-1 and 100-2, respectively.
 BOX 100-1 General Guidelines for Veterinarians to Avoid Zoonotic Transfer of Disease
BOX 100-1 General Guidelines for Veterinarians to Avoid Zoonotic Transfer of Disease
 BOX 100-2 General Guidelines for Pet Owners to Avoid Zoonotic Transfer of Disease
BOX 100-2 General Guidelines for Pet Owners to Avoid Zoonotic Transfer of Disease
ENTERIC ZOONOSES
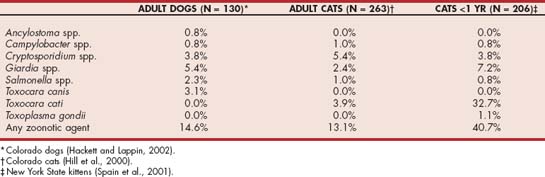
Multiple infectious agents of the gastrointestinal tract can be shared between animals and humans. Prevalences recently reported in two studies in cats and one in dogs are listed in Table 100-1. These findings emphasize that diagnostic workups for enteric infections are indicated because of potential human health risks. The minimal diagnostic plan to assess for enteric zoonoses includes a fecal flotation, fecal wet mount, rectal cytology, and Cryptosporidium spp. screening procedure. Fecal culture should be considered if infection with Salmonella spp. or Campylobacter spp. is on the list of differential diagnoses.
NEMATODES
Visceral larva migrans can be induced by infection of humans with Toxocara cati, Toxocara canis, or Baylisascaris procyonsis (Table 100-2). In the United States infection of humans is still common; an estimated 3 million to 6 million people in the United States are infected with Toxocara larva migrans each year, and the average seroprevalence of antibodies against Toxocara is 3.5% in the general human population (Schantz, 1989). These common roundworms are passed as eggs in feces. The eggs larvate and become infectious after 1 to 3 weeks and can survive in the environment for months. Humans are infected after ingesting larvated eggs. Dogs are considered more of a significant problem than cats for the spread of eggs. However, areas such as children’s sandboxes may be contaminated with T. cati because of the defecation habits of cats. Human infection after direct contact with dogs or cats is extremely unlikely because the eggs are not immediately infectious.
 TABLE 100-2 Characteristics of Common Enteric Zoonoses
TABLE 100-2 Characteristics of Common Enteric Zoonoses
| ORGANISM | SPECIES | INCUBATION |
|---|---|---|
| Bacterial | ||
| Campylobacter jejuni | B | Immediately infectious |
| Escherichia coli | B | Immediately infectious |
| Helicobacter spp.* | B | Immediately infectious |
| Salmonella spp. | B | Immediately infectious |
| Yersinia enterocolitica | B | Immediately infectious |
| Parasitic Amoeba | ||
| Entamoeba histolytica | D | Cysts are immediately infectious |
| Parasitic Cestodes | ||
| Echinococcus multilocularis | C | Ova are immediately infectious |
| Echinococcus granulosa | D | Ova are immediately infectious |
| Multiceps multiceps | D | Ova are immediately infectious |
| Parasitic Coccidians | ||
| Cryptosporidium spp. | B | Oocysts are immediately infectious |
| Toxoplasma gondii | C | Oocysts are infectious after 1-5 day incubation/exposure from environment |
| Parasitic Flagellates | ||
| Giardia spp. | B | Cysts are immediately infectious |
| Parasitic Helminths | ||
| Ancylostoma spp. | B | Larva infectious after a more than 3-day incubation/exposure from environment Skin penetration from larva in environment |
| Baylisascaris procyanosis | D | Larvated ova infectious after 1-3 week incubation/exposure from environment |
| Strongyloides stercoralis | B | Larvae immediately infectious |
| Toxocara canis | D | Larvated ova infectious after 1- to 3-week incubation/exposure from environment |
| Toxocara cati | C | As for T. canis |
| Uncinaria stenocephala | B | As for Ancylostoma spp. |
D, Dog; C, cat; B, dog and cat.
* Zoonotic potential undetermined; dogs are rarely infected by the ciliate Balantidium coli.
Dogs and cats can be subclinically affected or may develop poor haircoats, poor weight gain, and gastrointestinal signs. After ingestion of infectious eggs, larvae penetrate the intestinal wall and migrate through the tissues. Eosinophilic granulomatous reactions involving the skin, lungs, central nervous system (CNS), or eyes then occur, potentially leading to clinical signs of disease. Clinical signs and physical examination abnormalities in affected individuals include skin rash, fever, failure to thrive, CNS signs, cough, pulmonary infiltrates, and hepatosplenomegaly. Peripheral eosinophilia is common. Ocular larva migrans most commonly involves the retina and can cause reduced vision; uveitis and endophthalmitis can also occur. Visceral larva migrans is most common in children between 1 and 4 years of age, whereas ocular larva migrans is most common in older children. Diagnosis in human beings is confirmed by biopsy or can be presumed in cases with classic clinical manifestations, eosinophilia, and positive serology.
Ancylostoma caninum, Ancylostoma braziliense, Ancylostoma tubaeformis, Uncinaria stenocephala, and Strongyloides stercoralis have been associated with cutaneous larva migrans in the United States. After the passage of hookworm eggs into the environment in feces, infectious larvae are released after incubating for 1 to 3 days; humans are infected by skin penetration. In addition, eosinophilic enteritis in humans was reported after ingestion of larvated A. caninum eggs.
Animals are either subclinically ill or have nonspecific signs such as poor haircoats, failure to gain weight, vomiting, or diarrhea. Heavily infected puppies and kittens may have pale mucous membranes from blood loss anemia. In humans the larvae cannot penetrate the dermoepidermal junction and usually die in the epidermis. Clinical signs are related to migration of the larvae, which results in an erythematous, pruritic cutaneous tunnel. Cutaneous signs usually resolve within several weeks. Abdominal pain is the most common clinical sign in humans with A. caninum intestinal infection.
Trichuris vulpis, the dog whipworm, is most commonly associated with large-bowel diarrhea in dogs. The organism has been detected in feces in some people and has rarely been associated with gastrointestinal signs of disease (Dunn 2002).
Prevention of hookworms and roundworms is achieved by control of animal excrement in human environments. All puppies and kittens should have a fecal flotation performed and should be routinely treated with an anthelmintic that has efficacy against roundworms and hookworms. The Companion Animal Parasite Council (www.capcvet.org) recommends that puppies and their mothers be treated at 2, 4, 6, and 8 weeks of age and that kittens and their mothers be treated at 6, 8, and 10 weeks of age. These guidelines are particularly important for areas and animals with heavy parasite burdens. If the puppies and kittens are not presented to the veterinary clinic until vaccination age or are from areas with low prevalence rates for infection, I administer an appropriate anthelmintic such as pyrantel pamoate at each vaccination appointment. Roundworm and hookworm infections are occasionally occult, so all puppies or kittens should receive an anthelmintic whether or not eggs are detected on microscopic examination of feces. In most areas of the country monthly deworming should be considered. Administration of heartworm preventatives that also control hookworms and roundworms is an easy way to provide strategic deworming year-round.
CESTODES
Dipylidium caninum, Echinococcus granulosa, and Echinococcus multilocularis are cestodes that can infect humans. Wild carnivores are more common definitive hosts of Echinococcus spp. and shed infective eggs into the environment. E. granulosa eggs can be transmitted in feces of dogs after ingestion of infected sheep or rabbit tissues; E. multilocularis can be transmitted in feces of dogs or cats after ingestion of an infected rodent. Transmission to humans occurs after ingestion of the intermediate host (flea, Dipylidium) or eggs (Echinococcus spp.). Infection of dogs and cats with cestodes is generally subclinical. Dipylidium infection is most common in children and can lead to diarrhea and pruritus ani. In humans, after ingestion of eggs, which are immediately infectious, Echinococcus enters the portal circulation and spreads throughout the liver and other tissues. E. multilocularis is most common in the northern and central parts of North America but seems to be spreading with the fox population (most common definitive host). Prevention or control of cestodes is based on sanitation procedures and use of taeniacides. Praziquantel is labeled for the treatment of Echinococcus spp. Restricting hunting behavior of dogs and cats and feeding only processed or cooked foods should lessen potential exposure to Echinococcus spp. Monthly administration of praziquantel should be considered in dogs and cats allowed to hunt in endemic areas. Flea control should be maintained to lessen risk of D. caninum infection.
COCCIDIANS
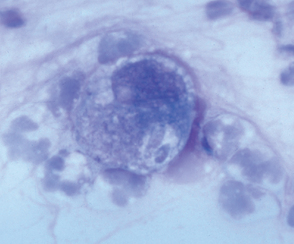
Cryptosporidium spp. inhabit the respiratory and intestinal epithelium of many vertebrates, including birds, mammals, reptiles, and fish. Once thought to be a commensal, Cryptosporidium spp. are now known to cause gastrointestinal tract disease in several mammalian species, including rodents, dogs, cats, calves, and humans. The organisms have an enteric life cycle similar to that of other coccidians that culminates in the production of thin-walled, autoinfective oocysts and thick-walled, environmentally resistant oocysts that are passed in feces (Fig. 100-1). Oocysts (4 to 6 μm in diameter) are passed sporulated and are immediately infectious to other hosts. Multiple species of Cryptosporidium spp. exist, including C. parvum, C. hominis, C. felis, and C. canis. Although some Cryptosporidium infect multiple animal species, others have a limited host range. However, strains that infect both pets and people cannot be differentiated by light microscopy from those that infect only pets, so all Cryptosporidium spp. should be considered potentially zoonotic. The most common Cryptosporidium spp. isolated from dogs and cats are the host-adapted C. canis and C. felis, respectively.
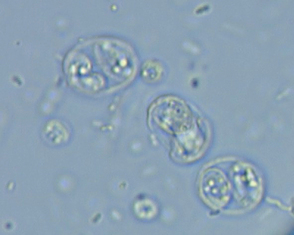
FIG 100-1 Cryptosporidium parvum and Toxoplasma gondii oocysts on a fecal fl otation. The C. parvum oocysts are approximately 4+5μ m, and the T. gondii oocysts are approximately 10+12 μ m.
The prevalence of Cryptosporidium spp. oocysts in dog and cat feces approximates that of Giardia (see Table 100-1), leading to the recommendation that all dogs or cats with diarrhea in the homes of immunosuppressed people be assessed for this infection. In the United States the seroprevalence of immunoglobulin G (IgG) antibodies in serum is 8.6% in cats and up to 58% in humans. In dogs and cats with diarrhea, Cryptosporidium spp. DNA was amplified from feces of 16.8% and 29%, respectively (Scorza et al., 2006). These results suggest that exposure to Cryptosporidium spp. is quite common in pets and people.
Person-to-person contact with oocysts by fecal-oral contamination and ingestion of contaminated water are the most likely routes of exposure. C. parvum infection of humans after exposure to infected calves has been recognized for years. Human infection associated with contact with infected dogs and cats has been reported but is thought to be unusual. In one study cat or dog ownership was not statistically associated with cryptosporidiosis in human immunodeficiency virus (HIV)-infected people (Glaser et al., 1999).
Infection of dogs and cats by Cryptosporidium spp. is usually subclinical, but small-bowel diarrhea occurs in some cases. Immunosuppression may potentiate disease; several dogs and cats had concurrent feline leukemia virus infection, canine distemper virus infection, or intestinal lymphoma. Clinical cryptosporidiosis is characterized by small-bowel diarrhea and is generally self-limiting in immunocompetent people, but fatal infection is common in those with AIDS. From 10% to 20% of humans with AIDS will be infected by C. parvum during the course of their illness.
The small size (approximately 4 to 6μm in diameter) of Cryptosporidium spp. oocysts leads to difficulty in diagnosis. Routine salt solution flotation and microscopic examination at ×100 magnification commonly lead to false-negative results. The combination of concentration techniques with fluorescent antibody staining or acid-fast staining appears to be more sensitive. Multiple enzyme-linked immunosorbent assays for the detection of C. parvum antigen in feces are commercially available but do not accurately detect C. felis or C. canis. Polymerase chain reaction (PCR) is the most sensitive test to date, but assays are not routinely available and are not standardized among laboratories. No drug has been shown to eliminate Cryptosporidium spp. from the gastrointestinal tract. However, clinical signs usually resolve when azithromycin is administered orally at 10mg/kg/day, tylosin is administered orally at 10 to 15mg/kg three times a day, or paromomycin is administered orally at 150mg/kg daily. Paromomycin has been associated with renal insufficiency and so should never be administered to animals with blood in the feces, which appears to allow for the absorption of this aminoglycoside. Optimal duration of therapy is unknown; some cases have required administration of azithromycin for several weeks before clinical signs resolve. Avoiding exposure is the most effective prevention. Routine disinfectants require extremely long contact with the organism to be effective. Drying, freeze thawing, and steam cleaning can inactivate the organism. Surface water collected in the field for drinking should be boiled or filtered.
Toxoplasma gondii is an ubiquitous coccidian with worldwide distribution. Most seroprevalence studies performed in the United States suggest that at least 30% of cats and humans have previously been exposed. Cats are the only known definitive host of the organism, and they complete the enteroepithelial cycle (sexual phase) that results in the passage of environmentally resistant unsporulated oocysts in feces. Oocyst sporulation occurs in 1 to 5 days in the presence of oxygen; sporulated oocysts are infectious to most warm-blooded vertebrates (see Fig. 100-1). After infection by T. gondii, an extraintestinal phase that ultimately leads to the formation of tissue cysts containing the organism develops. Infection by T. gondii occurs after ingestion of sporulated oocysts, after ingestion of tissue cysts, or transplacentally. Transplacental infection of humans and cats usually occurs only if the mother is infected for the first time during gestation.
In dogs and cats, clinical disease from T. gondii infection occurs occasionally and is manifested most commonly by fever, uveitis, pulmonary disease, hepatic disease, and CNS disease (see Chapter 99). Infected immunocompetent humans are generally asymptomatic; self-limiting fever, lymphadenopathy, and malaise occur occasionally. Transplacental infection of humans results in clinical manifestations, including stillbirth, hydrocephalus, hepatosplenomegaly, and retinochoroiditis. Presence of T. gondii antibodies has been associated with presence of behavioral abnormalities in people, but a direct cause and effect has not be established. Chronic tissue infection in humans can be reactivated by immunosuppression, leading to dissemination and severe clinical illness; this has been commonly associated with drug-induced immunosuppression and AIDS. Approximately 10% of humans with AIDS develop toxoplasmic encephalitis. Oocysts are most effectively demonstrated in cat feces after sugar solution centrifugation. Clinical toxoplasmosis is difficult to diagnose in humans, dogs, and cats but usually involves the combination of clinical signs, serologic test results, organism demonstration techniques, and response to anti-Toxoplasma drugs (see Chapter 99).
Although T. gondii is recognized as one of the most common zoonoses, humans are usually not infected by direct contact with cats. The oocyst shedding period usually lasts several days to several weeks (approximately 7 to 10 days if the cat was infected by tissue cyst ingestion). Because oocysts have to sporulate to be infectious, contact with fresh feces cannot cause infection. Cats are quite fastidious and usually do not allow feces to remain on their skin for periods long enough to lead to oocyst sporulation. Oocysts were not isolated from the fur of cats 7 days after completion of the oocyst shedding period. No association between cat ownership and T. gondii seroprevalence was demonstrated in a group of HIV-infected humans (Wallace et al., 1999). In most studies veterinary health care providers do not have an increased incidence of toxoplasmosis compared with the general population. Cats do not need to be removed from households with immunodeficient people or pregnant women because of the risk for acquiring toxoplasmosis (www.cdc.gov/ncidod/dpd/parasites/toxoplasmosis/ToxoWomen.pdf). Prevention of T. gondii infection is summarized in Box 99-1.
FLAGELLATES, AMOEBA, AND CILIATES
Giardia spp. (flagellate), Entamoeba histolytica (amoeba), and Balantidium coli (ciliate) are enteric protozoans that can be transmitted to humans by contact with feces; the cysts do not require an incubation period to become infectious. E. histolytica infection is extremely rare in dogs and cats; B. coli infection is rare in dogs and has never been reported in cats.
Giardia spp. infection of dogs and cats is common and can be detected in feces of normal dogs and cats and in those with small-bowel diarrhea (and occasionally mixed-bowel diarrhea in cats). Clinical signs of disease are generally more severe in immunodeficient individuals. Because the organism is immediately infectious when passed as cysts in stool, direct zoonotic transfer is possible. Genetic studies have detected multiple Giardia spp., and most dogs and cats are infected with the host-adapted assemblages C, D, and F. However, as is the case with Cryptosporidium, because determining zoonotic strains of Giardia spp. by microscopic examination is not possible, assume that feces from all dogs and cats infected with Giardia spp. are a potential human health risk.
Fecal examination should be performed on all dogs and cats at least yearly, and treatment with drugs with anti-Giardia activity, such as fenbendazole, metronidazole, or febantel/ praziquantel/pyrantel, should be administered if indicated (see Chapter 30). Centrifugation techniques (zinc sulfate or sugar) are considered by most parasitologists to be optimal for demonstration of cysts (see Fig. 92-3). If fresh stool is available from dogs or cats with diarrhea, examination of a wet mount to detect the motile trophozoites may improve sensitivity. Monoclonal antibody-based immunofluorescent antibody tests, fecal antigen tests, and PCR assays are available but should be used in addition to, not in lieu of, fecal flotation, which can also reveal other parasites.
Giardia vaccines for subcutaneous administration are now available for both dogs and cats (see Chapter 94). The Giardia vaccines are not currently recommended for routine prophylactic use in cats or dogs, but vaccination against Giardia could be considered in cats or dogs with recurrent infection as immunotherapy. Prevention of zoonotic giardiasis includes boiling or filtering surface water for drinking and washing hands that have handled fecally contaminated material, even if gloves were worn. In dogs and cats treated for giardiasis, infection can be documented again several weeks later in approximately 25% of animals. Thus the primary goal for the treatment of giardiasis is elimination of diarrhea. Whether these cases are a treatment failure or a reinfection is unknown.
BACTERIA
Salmonella spp., Campylobacter spp., Escherichia coli, Yersinia enterocolitica, and Helicobacter spp. each infect dogs and cats and can cause disease in humans. Transmission from animals to humans is by fecal-oral contact. Dogs can be subclinical carriers of Shigella spp., but humans are the natural hosts. Although Helicobacter pylori was isolated from a colony of cats, whether dogs and cats are a common source of Helicobacter infection in humans is unclear. However, on the basis of epidemiologic studies, it is unlikely. In three recent prevalence studies of enteric zoonoses, Salmonella spp. and Campylobacter spp. infections were uncommon in pet dogs and cats (see Table 100-1). The prevalence of Salmonella and Campylobacter infections is greater in young animals housed in unsanitary or crowded environments.
Gastroenteritis can occur in dogs or cats after infection by Salmonella spp., Campylobacter spp., or E. coli; Y. enterocolitica is probably a commensal agent in animals but causes fever, abdominal pain, polyarthritis, and bacteremia in humans. Helicobacter infections cause gastritis, which is commonly manifested as vomiting, belching, and pica. Salmonella spp. infection in dogs and cats is often subclinical. Approximately 50% of clinically affected cats have gastroenteritis; many are presented with signs of bacteremia. Salmonellosis of cats and humans has been associated with songbirds (songbird fever). Abortion, stillbirth, and neonatal death can result from in utero infection. Diagnosis of Salmonella spp., Campylobacter jejuni, E. coli, and Y. enterocolitica is based on culture of feces (see Chapter 92). A single negative culture may not rule out infection. Rectal cytology (see Chapter 92) should be performed on all animals with diarrhea. If neutrophils are noted, culture for enteric bacteria is indicated, particularly if the animal is owned by an immunodeficient individual.
Antibiotic therapy can control clinical signs of disease from infection by Salmonella spp. or Campylobacter spp. (see Chapter 30) but should not be administered orally to animals that are subclinical carriers of Salmonella because of the risk for antibiotic resistance. Strains of Salmonella resistant to most antibiotics have been detected in several cats. Prevention of enteric bacterial zoonoses is based on sanitation and control of exposure to feces. Immunodeficient people should avoid young animals and animals from crowded or unsanitary housing, particularly if clinical signs of gastrointestinal tract disease are occurring.
BITE, SCRATCH, OR EXUDATE EXPOSURE ZOONOSES
BACTERIA
Approximately 300,000 emergency room visits per year in the United States are made by people bitten by animals. Most of the aerobic and anaerobic bacteria associated with bite or scratch wounds cause only local infection in immunocompetent individuals. However, 28% to 80% of cat bites become infected, and severe sequelae, including meningitis, endocarditis, septic arthritis, osteoarthritis, and septic shock, can occur. The majority of the aerobic and anaerobic bacteria associated with dog or cat bite or scratch wounds lead only to local infection in immunocompetent individuals. Immunodeficient humans or those exposed to Pasteurella spp., Capnocytophaga canimorsus (DF-2), or Capnocytophaga cynodegmi more consistently develop systemic clinical illness. Splenectomized humans are at increased risk for developing bacteremia. Pasteurella multocida from a cat was cultured from the lungs of a man with AIDS who had only passive contact with the cat.
Dogs and cats are subclinical carriers of multiple bacteria in the oral cavity. After a person is bitten or scratched, local cellulitis is noted initially, followed by evidence of deeper tissue infection. Bacteremia and the associated clinical signs of fever, malaise, and weakness are common, and death can occur within hours after infection with Capnocytophaga spp. in immunodeficient or splenectomized humans. Diagnosis is confirmed by culture. Treatment of carrier animals is not needed. Treatment of clinically affected humans includes local wound management and parenteral antibiotic therapy. Penicillin derivatives are highly effective against most Pasteurella infections; penicillins and cephalosporins are effective against Capnocytophaga spp. in vitro.
Mycoplasma spp. infections of humans resulting from cat bites, one with cellulitis and one with septic arthritis, have been reported. l-form bacteria are cell wall–deficient organisms associated with chronic draining skin wounds in cats that are commonly resistant to cell wall-inhibiting antibiotics, such as penicillins and cephalosporins. Infection of a human being after a cat bite has been documented. Diagnosis can be confirmed only by histologic examination of tissue. Doxycycline has been used to treat cats and people successfully. Gloves should be worn when attending cats with draining tracts, and hands should be cleansed thoroughly.
Bartonella henselae can infect both dogs and cats and is the most common cause of cat scratch disease as well as bacillary angiomatosis and bacillary peliosis—common disorders in humans with AIDS (Table 100-3). Dogs and cats can also be infected with several other Bartonella spp., including B. clarridgeiae, B. koehlerae, B. vinsonii (dogs), and B. quintana (see Chapter 95). B. henselae has been isolated from the blood of subclinically ill, seropositive cats and also from some cats with a variety of clinical manifestations such as fever, lethargy, lymphadenopathy, uveitis, gingivitis, and neurologic diseases. Infection of dogs has also been associated with clinical illness. Seroprevalence in cats varies by region, but as many as 93% of cats in some geographic areas of the United States are Bartonella spp. seropositive. Both B. henselae and B. clarridgeiae are transmitted between cats by fleas, so the prevalence is greatest in cats from states where fleas are common (Lappin et al., 2006). Transmission to humans commonly occurs after cat bites or scratches; the disease appears to be transmitted most commonly from kittens. B. henselae survives in flea feces for days, so the cat’s claws and teeth are likely contaminated with B. henselae during grooming, which emphasizes the maintenance of flea control on dogs and cats.
 TABLE 100-3 Selected Canine or Feline Zoonoses Associated with Bites, Scratches, or Contact with Exudates
TABLE 100-3 Selected Canine or Feline Zoonoses Associated with Bites, Scratches, or Contact with Exudates
| ORGANISM | SPECIES | CLINICAL DISEASE |
|---|---|---|
| Bacterial | ||
| Bartonella spp. | C | |
| Capnocytophaga canimorsus | B | |
| Francisella tularensis | C | |
| Yersinia pestis | C | |
| Fungal | ||
| Dermatophytes | B | Dog, cat, and human: superficial dermatologic disease |
| Sporothrix schenkii | C | |
| Viral | ||
| Rabies | B | |
D, Dog; C, cat; B, dog and cat; CNS, central nervous system.
Humans with cat scratch disease develop a variety of clinical signs, such as lymphadenopathy, fever, malaise, weight loss, myalgia, headache, conjunctivitis, skin eruptions, and arthralgia. Bacillary angiomatosis is a diffuse disease resulting in vascular cutaneous eruptions. Bacillary peliosis is a diffuse systemic vasculitis of parenchymal organs, particularly the liver. The incubation period for cat scratch disease is approximately 3 weeks. Most cases of cat scratch disease are self-limiting but may take several months to completely resolve.
Blood culture, blood PCR, and serologic testing can be used to determine the risk of individual cats (see Chapter 95). However, although serologic testing can be used to determine whether an individual cat has been exposed, both seropositive and seronegative cats can be bacteremic, limiting the diagnostic utility of serologic testing. Thus testing healthy cats for Bartonella spp. infection is not currently recommended by the Centers for Disease Control and Prevention or the American Association of Feline Practitioners (Brunt et al., 2006). Testing should be reserved for cats with suspected clinical bartonellosis.
In experimental studies, administration of doxycycline, tetracycline, erythromycin, amoxicillin-clavulanate, or enrofloxacin can limit bacteremia but does not cure infection in all cats and has not been shown to lessen the risk of cat scratch disease. Azithromycin is commonly administered to cats with suspected clinical bartonellosis, but no published data document clearance of infection in cats. Thus antibiotic treatment of healthy bacteremic cats is controversial and not currently recommended by the Centers for Disease Control and Prevention or the American Association of Feline Practitioners (Brunt et al., 2006). Treatment should be reserved for cats with suspected clinical bartonellosis. Strict flea control should be maintained. Kittens should be avoided by immunodeficient people. Cat claws should be kept clipped, and cats should never be teased. Cat-induced wounds should immediately be cleansed, and medical advice sought.
Feline plague is caused by Yersinia pestis, a gram-negative coccobacillus found most commonly in Midwestern and far Western states, particularly New Mexico and Colorado. Rodents are the natural hosts for this bacterium; cats are most commonly infected by ingestion of bacteremic rodents or lagomorphs or by being bitten by Yersinia-infected rodent fleas. Dogs are more resistant to infection and have not been associated with zoonotic transfer. Humans are most commonly infected by rodent flea bites, but many cases of transmission by exposure to wild animals and infected domestic cats have been documented. From 1977 to 1998, 23 cases of human plague (7.7% of the total cases) resulted from contact with infected cats. Infection can be induced by inhalation of respiratory secretions of cats with pneumonic plague, through bite wounds, or by contamination of mucous membranes or abraded skin with secretions or exudates.
Bubonic, septicemic, and pneumonic plague can develop in cats and humans; each form has accompanying fever, headache, weakness, and malaise. Because cats are most commonly infected by ingestion of bacteremic rodents, suppurative lymphadenitis (buboes) of the cervical and submandibular lymph nodes is the most common clinical manifestation. Exudates from cats with lymphadenopathy should be examined cytologically for the presence of large numbers of the characteristic bipolar rods (see Fig. 95-2). The diagnosis is confirmed by fluorescent antibody staining of exudates; culture of exudates, the tonsillar area, and saliva; and documentation of increasing antibody titers. People who are exposed to infected cats should be urgently referred to physicians for antimicrobial therapy, and public health officials should be alerted. Doxycycline, enrofloxacin, chloramphenicol, or aminoglycosides can be used successfully for the treatment of plague. Parenteral antibiotics should be used during the bacteremic phase. Drainage of lymph nodes may be required. Cats with suppurative lymphadenitis should be considered plague suspects, and extreme caution should be exercised when handling exudates or treating draining wounds. Suspect animals should be treated for fleas and housed in isolation. Cats are generally not considered infectious to humans after 4 days of antibiotic treatment.
Francisella tularensis is the gram-negative bacillus found throughout the continental United States that causes tularemia. Dermacentor variabilis (American dog tick), Dermacentor andersoni (American wood tick), and Amblyomma americanum (Lone Star tick) are known vectors. Human tularemia occurs most commonly after exposure to ticks and less commonly from contact with infected animals. There have been at least 51 cases of human tularemia resulting from contact with infected cats. Dogs are not considered a source of human tularemia but may facilitate human exposure by bringing infected ticks into the environment. Cats are infected most frequently by tick bites or ingestion of infected rabbits or rodents. Most cases of feline tularemia have been documented in the Midwest, particularly Oklahoma. However, a recent study reported a seroprevalence of 12% in cats in a same sample set (n = 91) in the Northeast (Magnarelli et al., 2007)
Infected cats exhibit generalized lymphadenopathy and abscess formation in organs such as the liver and spleen, which leads to fever, anorexia, icterus, and death. Ulceroglandular, oculoglandular, glandular, oropharyngeal, pneumonic, and typhoidal forms have been described in humans and develop depending on the route of exposure. Unlike the situation with plague, the organism is not often recognized in exudates or lymph node aspirates from infected cats. Cultures and documentation of increasing antibody titers can be used to confirm the diagnosis in cats and humans. Most cases of tularemia in cats have been diagnosed at necropsy, so optimal treatment is unknown. Streptomycin and gentamicin are the drugs used most commonly to treat humans. Tetracycline or chloramphenicol can be used in cases not requiring hospitalization but may be associated with relapses. The disease is prevented by avoiding exposure to lagomorphs, ticks, and infected cats. All cats dying with bacteremia should be handled carefully.
FUNGI
Of the many fungal agents that infect both humans and animals, only Sporothrix schenckii and the dermatophytes have been shown to infect humans on direct exposure. Histoplasma, Blastomyces, Coccidioides, Aspergillus, and Cryptococcus infections of humans and animals can occur in the same household but generally result from a common environmental exposure (see Chapter 98).
Sporothrix is cosmopolitan in distribution, and the soil is believed to be the natural reservoir. Infection of cats and humans usually occurs after the organism contaminates broken skin. Cats are believed to be infected by scratches from contaminated claws of other cats; infection is most common in outdoor males. Humans can be infected by contamination of cutaneous wounds with exudates from infected cats. Sporothrix infection in cats can be cutaneolymphatic, cutaneous, or disseminated. Chronic draining cutaneous tracts are common. Cats commonly produce large numbers of the organism in feces, tissues, and exudates; thus veterinary care personnel are at high risk when treating infected cats (Fig. 100-2). The clinical disease in humans is similar to that in cats. Dogs generally do not produce large numbers of Sporothrix in exudates, so they are less of a zoonotic risk. The organism can be demonstrated by cytologic examination of exudates or culture. Fluconazole, itraconazole, or ketoconazole are effective treatments. Gloves should be worn when attending cats with draining tracts, and hands should be cleansed thoroughly.
VIRUSES
Rabies is still the only significant small animal viral zoonosis in the United States. See Chapter 69 for a discussion of this agent.
Pseudorabies is a herpesvirus that infects pigs; dogs and humans can develop self-limiting pruritic skin disease after exposure. Dogs occasionally develop CNS disease characterized by depression and seizures. Diagnosis is suspected on the basis of the exposure history, and prevention is by avoiding exposure.
Some authorities have been concerned whether the retroviruses of cats—feline leukemia virus (FeLV), feline immunodeficiency virus (FIV), and feline foamy virus (FeFV)—can infect humans because FeLV subtypes B and C can replicate in human cell lines. However, to date humans have not been shown to be infected with any of the feline retroviruses. In the most recent study 204 veterinarians and others potentially exposed to feline retroviruses were assessed for antibodies against FIV and FeFV, FeLV p27 antigen, and FeLV provirus; test results on all were negative (Butera et al., 2000). Because both FeLV and FIV can induce immune deficiency, infected cats should be considered more likely than retrovirus-naïve cats to be carrying other potential zoonotic agents, particularly if gastrointestinal tract signs are present.
RESPIRATORY TRACT AND OCULAR ZOONOSES
BACTERIA
Bordetella bronchiseptica is a bacterium that induces respiratory tract infections in dogs and cats (see Chapter 21). The classic clinical manifestation is tracheobronchitis, but the organism can also cause pneumonia, sneezing, and nasal discharge. Humans rarely develop clinical disease caused by B. bronchiseptica unless they are immunologically compromised (Table 100-4). Only 39 cases of B. bronchiseptica infection in humans had been reported by 1998; most of these patients were immunodeficient. Association with a cat has only been reported once, in a person coinfected with HIV and B. bronchiseptica. Amoxicillin-clavulanate, chloramphenicol, enrofloxacin, and tetracycline derivatives are all effective treatments. Animals with upper or lower respiratory tract inflammatory disease should be kept away from immunodeficient people until the animals are clinically normal. However, treated animals can still shed the organism.
 TABLE 100-4 Selected Canine or Feline Zoonoses Associated with Respiratory or Ocular Secretions of Dogs or Cats
TABLE 100-4 Selected Canine or Feline Zoonoses Associated with Respiratory or Ocular Secretions of Dogs or Cats
| ORGANISM | SPECIES | CLINICAL SIGNS |
|---|---|---|
| Bordetella bronchiseptica* | B | |
| Chlamydophyla felis* | C | |
| Francisella tularensis | C | |
| Streptococcus group A† | B | |
| Yersinia pestis | C |
D, Dog; C, cat; B, dog and cat.
* Zoonotic potential largely unknown.
Chlamydophila felis (formerly Chlamydia psittaci) causes mild conjunctival disease and rhinitis in cats (Table 100-4). In Japan the prevalence rates of antibodies against an isolate of C. felis were 51.1% in stray cats, 15.0% in pet cats, 3.1% in the general human population, and 5.0% in small animal clinic veterinarians, suggesting that transfer between cats and humans may occur (Yan et al., 2000). Conjunctivitis in humans after direct contact with ocular discharges from cats has been described. A human isolate of Chlamydia spp. was inoculated into cats, resulting in conjunctivitis and persistent infection, suggesting that the isolate was a feline strain. Occasionally the organism is associated with systemic disease; atypical pneumonia was diagnosed in an apparently immunocompetent 48-year-old man, malaise and cough were diagnosed in an immunosuppressed woman, and endocarditis and glomerulonephritis were diagnosed in a40-year- old woman. Diagnosis is based on organism demonstration by culture, cytologic documentation of characteristic inclusion bodies, or fluorescent antibody staining of conjunctival scrapings. Tetracycline or chloramphenicol-containing eye ointments generally are effective in the treatment of infection. Oral administration of doxycycline is still considered the optimal way to clear the carrier state. Care should be taken to avoid direct conjunctival contact with discharges from the respiratory or ocular secretions of cats, especially by immunosuppressed persons (see Box 100-2). Employees should be directed to wear gloves or wash hands carefully when attending cats with conjunctivitis.
Nosocomial spread of methicillin-resistant Staphylococcus aureus (MRSA) among veterinary or human patients and doctors was recently recognized as a significant problem in hospitals; MRSA should now also be considered a zoonotic agent (Weese et al., 2006).
Humans are the principal natural hosts for Streptococcus group A bacteria, Streptococcus pyogenes, and Streptococcus pneumoniae, which cause “strep throat” in humans. Dogs and cats in close contact with infected humans can develop transient, subclinical colonization of pharyngeal tissues and can transmit the infection to other humans. However, this is poorly documented and believed to be unusual. The organism can be cultured from the tonsillar crypts. Culture-positive animals should be treated with penicillin derivatives. If animals are to be treated in a household with chronic, recurrent strep throat, all humans should also be treated because they also could be chronic subclinical carriers.
Yersinia pestis and Francisella tularensis can be transmitted from cats to humans in respiratory secretions. In endemic areas, cats with clinical signs or radiographic abnormalities consistent with pneumonia should be handled as plague or tularemia suspects. Gloves, mask, gown, and eye protection should be worn while performing transoral airway washings in suspect cats.
VIRUSES
Avian influenza A (H5N1) virus has infected some cats after close exposure to infected birds. In studies of naturally exposed and experimentally infected cats, some cats developed respiratory disease (Thiry et al., 2007) and others have become asymptomatic carriers (Leschnik et al., 2007). Results of studies assessing transmission between infected cats have been variable. To date, transmission of the infection from cats to humans has not been documented.
GENITAL AND URINARY TRACT ZOONOSES
Coxiella burnetii is a rickettsial agent found throughout the world, including North America (Table 100-5). Many ticks, including Rhipicephalus sanguineus, are naturally infected with C. burnetii. Cattle, sheep, and goats are commonly subclinically infected and pass the organism into the environment in urine, feces, milk, and parturient discharges. Seropositive dogs have been detected, but zoonotic transfer to humans from dogs has not been documented. Infection of cats most commonly occurs after tick exposure, ingestion of contaminated carcasses, or aerosolization from a contaminated environment. Fever, anorexia, and lethargy developed in some experimentally infected cats. Infection has been associated with abortion in cats, but the organism can also be isolated from normal parturient cats. Infection of cats appears to be common; 20% of cats from a shelter in southern California and 20% of cats in Maritime Canada were seropositive, the organism was grown from the vagina of healthy cats in Japan (Nagaoka et al., 1998), and DNA of the organism was amplified from uterine tissues of cats in Colorado (Cairns et al., 2006).
 TABLE 100-5 Selected Canine and Feline Zoonoses Associated with Contact with Urine or Genital Secretions
TABLE 100-5 Selected Canine and Feline Zoonoses Associated with Contact with Urine or Genital Secretions
| ORGANISM | SPECIES | CLINICAL DISEASE |
|---|---|---|
| Bacterial | ||
| Bruceila canis | D | |
| Leptospira spp. | B* | |
| Rickettsial | ||
| Coxiella bumetii | C | |
D, Dog; C, cat; B, dog and cat, CNS, central nervous system.
* Cats of minimal significance.
Human illness associated with direct contact with infected cats occurs after aerosol exposure to the organism passed by parturient or aborting cats; clinical signs develop 4 to 30 days after contact. Humans commonly develop acute clinical signs similar to those associated with other rickettsial diseases, including fever, malaise, headache, pneumonitis, myalgia, and arthralgia. After primary infection, chronic Q fever develops in approximately 1% and can manifest as hepatic inflammation or valvular endocarditis. Tetracyclines, chloramphenicol, and quinolones are usually effective therapeutic agents in humans. Gloves and masks should be worn when attending to parturient or aborting cats. People who develop fever or respiratory tract disease after exposure to parturient or aborting cats should seek medical attention.
Leptospira spp. can be transmitted in urine from infected dogs and cats to humans, resulting in clinical disease. Host-adapted species cause subclinical infection; infection by non-host-adapted species commonly results in clinical illness. The organisms enter the body through abraded skin or intact mucous membranes. (See Chapter 95 for a detailed discussion of the clinical manifestations of this disease and its treatment in dogs and cats.) Human clinical syndromes vary with the serovar but are similar to those that occur in the dog. Animals with suspected leptospirosis should be handled while wearing gloves. Contaminated surfaces should be cleaned with detergents and disinfected with iodinecontaining products.
Brucella canis is a bacterium that preferentially infects the testicles, prostate, uterus, and vagina of dogs (see Chapter 57 and 62). The infection is maintained in dogs primarily by venereal transmission. Humans can be infected by direct contact with vaginal and preputial discharges from dogs. Clinical syndromes in dogs are diverse but commonly include abortion, stillbirth, failure to conceive, orchitis, epididymitis, vaginal discharge, uveitis, discospondylitis, and bacteremia. Intermittent fever, depression, and malaise are common in infected people. Diagnosis is based on serologic testing or demonstration of the organism by culture. Dogs with clinical signs of brucellosis should be evaluated serologically for Brucella infection with the 2-mercaptoethanol rapid slide agglutination card test. Seronegative dogs are unlikely to harbor Brucella unless the clinical syndrome is peracute. Seropositive dogs should have results confirmed by tube agglutination or agar gel immunodiffusion. Long-term antibiotic treatment (tetracyclines, aminoglycosides, quinolones) usually does not clear the infection. Ovariohysterectomy or castration will lessen contamination of the environment. Genital tract secretions should be avoided.
SHARED VECTOR ZOONOSES
Some zoonotic agents are transmitted between animals and humans by shared vectors such as fleas, ticks, or mosquitoes. Rickettsia rickettsii (ticks), Ehrlichia spp. (ticks), Anaplasma phagocytophilum (ticks), Borrelia burgdorferi (ticks), Rickettsia felis (fleas), Bartonella spp. (fleas and ticks), Dipylidium caninum (fleas), Dirofilaria immitis (mosquitoes), and West Nile virus (mosquitoes) are examples of vector-borne zoonoses common in the United States. For the flea- and tick-borne zoonoses, the pet brings the vector of the organism into the environment, resulting in exposure of the human being. Veterinary health care providers could have a slightly increased risk of exposure because they handle many animals infested with fleas and ticks. However, the vector, not direct contact with the infested animal, results in infection of the person. Flea and tick control should always be maintained animals, and infested animals that are seen in the clinic should be treated immediately. See other sections of this text for detailed discussions of these agents.
SHARED ENVIRONMENT ZOONOSES
Some agents that infect both animals and man are not commonly transmitted between the pet and the owner by direct contact but are acquired from the same environmental source. Notable examples include Histoplasma capsulatum, Coccidioides immitis, Blastomyces dermatitidis, Cryptococcus neoformans, and Aspergillus spp. See other sections of this text for detailed discussions of these agents.
Angulo FJ, et al. Caring for pets of immunocompromised persons. J Am Vet Med Assoc. 1994;205:1711.
Boost ME, et al. Characterisation of methicillin-resistant Staphylococcus aureus isolates from dogs and their owners. Clin Microbiol Infect. 2007;13:731.
Breitschwerdt EB, et al. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13:428.
Brunt J, et al. Association of Feline Practitioners 2006 Panel report on diagnosis, treatment, and prevention of Bartonella spp. infections. J Feline Med Surg. 2006;8:213.
Burton B. Pets and PWAs: Claims of health risk exaggerated. AIDS Patient Care. Feb 1989:34.
Butera ST, et al. Survey of veterinary conference attendees for evidence of zoonotic infection by feline retroviruses. J Am Vet Med Assoc. 2000;217:1475.
Capellan J, et al. Tularemia from a cat bite: case report and review of feline-associated tularemia. Clin Infect Dis. 1993;16:472.
Cairns K, et al. Prevalence of Coxiella burnetii DNA in vaginal and uterine samples from healthy cats of north-central Colorado. J Feline Med Surg. 2007;9:196.
Carmack B. The role of companion animals for persons with AIDS/HIV. Holist Nurs Pract. 1991;5:24.
Chomel BB, et al. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol. 1996;34:1952.
Croese J, et al. Occult enteric infection by Ancylostoma caninum: a previously unrecognized zoonosis. Gastroenterology. 1994;106:3.
De Santis AC, et al. Estimated prevalence of nematode parasitism among pet cats in the United States. J Am Vet Med Assoc. 2006;228:885.
De Santis-Kerr AC, et al. Prevalence and risk factors for Giardia and coccidia species of pet cats in 2003-2004. J Feline Med Surg. 2006;8:292.
Drabick JJ, et al. Pasteurella multocida pneumonia in a man with AIDS and nontraumatic feline exposure. Chest. 1993;103:7.
Dunn JJ, et al. Trichuris vulpis recovered from a patient with chronic diarrhea and five dogs. J Clin Microbiol. 2002;40:2703.
Dworkin MS, et al. Bordetella bronchiseptica infection in human immunodeficiency virus-infected patients. Clin Infect Dis. 1999;28:1095.
Dubey JP, et al. Toxoplasmosis and neosporosis. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:754.
Dunston RW, et al. Feline sporotrichosis: a report of five cases with transmission to humans. J Am Acad Dermatol. 1986;15:37.
Eidson M, et al. Clinical, clinicopathologic and pathologic features of plague in cats: 119 cases (1977–1988). J Am Vet Med Assoc. 1991;199:1191.
Gage KL, et al. Cases of cat-associated human plague in the Western US, 1977-1998. Clin Infect Dis. 2000;30:893.
Glaser CA, et al. Animal associated opportunistic infections among persons infected with the human immunodeficiency virus. Clin Infect Dis. 1994;18:14.
Goodman RA, Breitschweidt EB. Clinicopathologic findings in dogs seroreactive to Bartonella henselae antigens. Am J Vet Res. 2005;66:2060.
Grant S, et al. Preventing zoonotic diseases in immunocompromised persons: the role of physicians and veterinarians. Emerg Infect Dis. 1999;5:159.
Greene CE, et al. Bartonella henselae infection in cats: evaluation during primary infection, treatment, and rechallenge infection. J Clin Microbiol. 1996;34:1682.
Hackett T, Lappin MR. Prevalence of enteric pathogens in dogs of North-Central Colorado. J Am Anim Hosp Assoc. 2003;39:52.
Hartley JC, et al. Conjunctivitis due to Chlamydophila felis (Chlamydia psittaci feline pneumonitis agent) acquired from a cat: case report with molecular characterization of isolates from the patient and cat. J Infect. 2001;43:7.
Hinze-Selch D, et al. A controlled prospective study of Toxoplasma gondii infection in individuals with schizophrenia: beyond seroprevalence. Schizophr Bull. 2007;33:782.
Hill S, et al. Prevalence of enteric zoonotic agents in cats. J Am Vet Med Assoc. 2000;216:687.
Juranek DD. Cryptosporidiosis: sources of infection and guidelines for prevention. Clin Infect Dis. 1995;21(suppl):57.
Kordick DL, et al. Efficacy of enrofloxacin or doxycycline for treatment of Bartonella henselae or Bartonella clarridgeiae infection in cats. Antimicrob Agents Chemother. 1997;41:2448.
Lappin MR, et al. Enzyme-linked immunosorbent assay for the detection of Cryptosporidium spp. IgG in the serum of cats. J Parasitol. 1997;83:957.
Leschnik M, et al. Subclinical infection with avian influenza A (H5N1) virus in cats. Emerg Infect Dis. 2007;13:243.
Low JC, et al. Multiresistant Salmonella typhimurium DT104 in cats. Lancet. 1996;348:1391.
MacKenzie WR, et al. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161.
Magnarelli L, et al. Detection of antibodies to Francisella tularensis in cats. Res Vet Sci. 2007;82:22.
Marcus LC. Medical aspects of visceral and cutaneous larva migrans and hydatid disease in humans. Compend Contin Educ Pract Vet. 2001;23(suppl):11.
Marks SL, et al. Comparison of direct immunofluorescence, modified acid-fast staining, and enzyme immunoassay techniques for detection of Cryptosporidium spp in naturally exposed kittens. J Am Vet Med Assoc. 2004;225:1549.
Marrie TJ. Coxiella burnetii (Q fever) pneumonia. Clin Infect Dis. 1995;21(suppl):S253.
McReynolds C, et al. Regional seroprevalence of Cryptosporidium parvum IgG-specific antibodies of cats in the United States. Vet Parasitol. 1998;80:187.
Miro G, et al. Survey of intestinal parasites in stray dogs in the Madrid area and comparison of the efficacy of three anthelmintics in naturally infected dogs. Parasitol Res. 2007;100:317.
Morgan U, et al. Molecular characterization of Cryptosporidium isolates obtained from human immunodeficiency virus-infected individuals living in Switzerland, Kenya, and the United States. J Clin Microbiol. 2000;38:1180.
Nagaoka H, et al. Isolation of Coxiella burnetii from the vagina of feline clients at veterinary clinics. J Vet Med Sci. 1998;60:251.
Neiger R, et al. Helicobacter infection in dogs and cats: facts and fiction. J Vet Intern Med. 2000;14:125.
Olson ME, et al. Giardia vaccination. Parasitol Today. 2000;16:213.
Pieniazek NJ, et al. New Cryptosporidium genotypes in HIV-infected persons. Emerg Infect Dis. 1999;5:444.
Pinsky RL, et al. An outbreak of cat-associated Q fever in the United States. J Infect Dis. 1991;164:202.
Pretorius AM, et al. An update on human bartonelloses. Cent Afr J Med. 2000;46:194.
Prociv R, et al. Human enteric infection with Ancylostoma caninum: hookworms reappraised in the light of a “new” zoonosis. Acta Trop. 1996;62:23.
Sargent KD, et al. Morphological and genetic characterisation of Cryptosporidium oocysts from domestic cats. Vet Parasitol. 1998;77:221.
Schantz PM. Toxocaral larva migrans now. Am J Trop Med Hyg. 1989;41(Suppl):21.
Simpson K, et al. The relationship of Helicobacter spp. infection to gastric disease in dogs and cats. J Vet Intern Med. 2000;14:223.
Spain CV, et al. Prevalence of enteric zoonotic agents in cats less than 1 year old in central New York State. J Vet Intern Med. 2001;15:33.
Spencer L. Study explores health risks and the human animal bond. J Am Vet Med Assoc. 1992;201:1669.
Talan DA, et al. Bacteriologic analysis of infected dog and cat bites. N Engl J Med. 1999;340:84.
Tauni MA, et al. Outbreak of Salmonella typhimurium in cats and humans associated with infection in wild birds. J Small Anim Pract. 2000;41:339.
Thiry E, et al. Highly pathogenic avian influenza H5N1 virus in cats and other carnivores. Vet Microbiol. 2007;122:25.
Thompson RCA, et al. Nomenclature and genetic groupings of Giardia infecting mammals. Parasitol Today. 2000;16:210.
Valtonen M, et al. Capnocytophaga canimorsus septicemia: fifth report of a cat-associated infection and five other cases. Eur J Clin Microbiol Infect Dis. 1995;14:520.
Vasilopulos RJ, et al. Genotypic analysis of Giardia duodenalis in domestic cats. J Vet Intern Med. 2007;21:352.
Wallace M, et al. Cats and toxoplasmosis risk in HIV-infected adults. J Am Med Assoc. 1993;269:76.
Weese JS, et al. Suspected transmission of methicillin-resistant Staphylococcus aureus between domestic pets and humans in veterinary clinics and in the household. Vet Microbiol. 2006;6115:148.
Yan C, et al. Seroepidemiological investigation of feline chlamydiosis in cats and humans in Japan. Microbiol Immunol. 2000;44:155.
 Drugs Used to Treat Infectious Diseases of Dogs and Cats and General Dosing Guidelines*
Drugs Used to Treat Infectious Diseases of Dogs and Cats and General Dosing Guidelines*