CHAPTER 99 Polysystemic Protozoal Infections
BABESIOSIS
Etiology and Epidemiology
Babesiosis in dogs is most commonly associated with Babesia canis and B. gibsoni, protozoans that parasitize red blood cells (RBCs), leading to progressive anemia. B. canis has worldwide distribution including Africa, Asia, Australia, Europe, Central America, South America, Japan, and the United States. Three subspecies of B. canis have been proposed to be separate species. B. canis rossi is transmitted by Haemaphysalis leachi and is the most pathogenic; B. canis canis is transmitted by Dermacentor reticulatus and is moderately pathogenic; B. canis vogeli is the least pathogenic and is transmitted by Rhipicephalus sanguineus (brown dog tick). Babesia canis vogeli is the most common B. canis subspecies infecting dogs in the United States. B. gibsoni infects dogs in the United States, Japan, Sri Lanka, Korea, Malaysia, northern and eastern Africa, Australia, and southern Europe. B. gibsoni strains, of which there are at least three (Asia, California, and Theileria annae, a B. gibsoni-like organism common in dogs in northern Spain), vary genetically (Garcia 2006). Rhipicephalus sanguineus is a proposed vector for B. gibsoni in the United States. Presence of B. gibsoni DNA in dog blood has been associated with a history of a dog bite, especially by an American Pit Bull Terrier, suggesting that fighting is a route of transmission. Babesia spp. infections were detected in 29 states and Ontario (Birkenheuer et al., 2005). Other novel Babesia spp. that genetically vary considerably from other B. canis or B. gibsoni isolates have been described in the United States; however, the prevalence rates for these infections is unknown (Kocan et al., 2001; Meinkoth et al., 2002; Birkenheuer et al. 2004a). None of the Babesia spp. that infect cats (B. cati [India], B. felis [Africa, southern Asia, Europe], B. herpailuri [South America, Africa], B. canis presentii [Israel]) have been recognized in the United States. Babesia spp. can also be transmitted by blood transfusions.
After infection with pathogenic strains of B. canis or B. gibsoni, the incubation period varies from several days to several weeks. The degree of parasitemia varies by the organism studied but can be detected transiently in some dogs as soon as day 1 (Boozer & Macintire, 2003). The organisms replicate intracellularly in RBCs, resulting in intravascular or extravascular hemolytic anemia. Immune-mediated reactions against the parasites or altered self-antigens worsen the hemolytic anemia and commonly result in a positive direct Coombs test. Activation of macrophages leads to fever and hepatosplenomegaly. Severe hypoxia occurs because of rapid breakdown of RBCs. Disseminated intravascular coagulation occurs in some infected dogs during acute infection. The severity of disease depends on the species and strain of Babesia and the host’s immune status; chronic, subclinical infection can be common with some. Administration of glucocorticoids or splenectomy may activate chronic disease. Presence of coinfections, such as Bartonella spp., may increase the pathogenic potential (Kordick et al., 1999; Tuttle et al., 2003).
Clinical Features
In the United States, subclinical Babesia spp. infections are most common. Peracute or acute Babesia spp. infections result in anemia and fever, leading to pale mucous membranes, tachycardia, tachypnea, depression, anorexia, and weakness. Icterus, petechiae, and hepatosplenomegaly are present in some dogs depending on the stage of infection and the presence of disseminated intravascular coagulation. Severe anemia, disseminated intravascular coagulation, metabolic acidosis, and renal disease are most common during acute infection and are generally most severe with B. canis rossi infections in South Africa. The main differential diagnosis for acute babesiosis is primary immune-mediated hemolytic anemia. Chronically infected dogs commonly have weight loss and anorexia. Ascites, gastrointestinal signs, CNS disease, edema, and clinical evidence of cardiopulmonary disease occur in some dogs with atypical infection.
Diagnosis
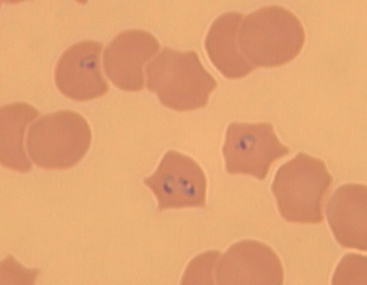
Regenerative anemia, hyperbilirubinemia, bilirubinuria, hemoglobinuria, thrombocytopenia, metabolic acidosis, azotemia, polyclonal gammopathy, and renal casts are common in dogs infected with pathogenic Babesia spp. Presence of the organism in RBCs detected by Wright’s or Giemsa stains on thin blood smears (see Chapter 92) can be used to support the diagnosis, but parasitemia can be intermittent, giving falsely negative results; capillary blood is the preferred source for blood smear evaluation. B. canis is typically found as paired, piriform bodies measuring 2.4 × 5.0 μm. B. gibsoni is typically found as single or paired annular bodies measuring 1.0 × 3.2 μm. Serologic and polymerase chain reaction (PCR) assays are also available to help aid in the diagnosis. Indirect fluorescent antibody tests for B. canis and B. gibsoni are available commercially. However, serologic cross-reactivity can exist between B. canis and B. gibsoni, so antibody test results cannot be used to determine the infective species definitively. Demonstration of increasing titers over 2 to 3 weeks is consistent with recent or active infection. No standardization between laboratories exists, so suggested positive cutoff titers vary. False-negative serologic test results can occur in some dogs, particularly those with peracute disease or concurrent immunosuppression. A titer above 1 : 320 is suggested as diagnostic for B. gibsoni, but not all infected dogs achieve this titer magnitude (Birkenheuer et al., 1999). Many dogs are seropositive but clinically normal, so serology alone cannot be used to make a definitive diagnosis of clinical babesiosis. Positive results in PCR assays performed on blood prove current infection but positive results do not always correlate with clinical illness.
Treatment
Supportive care, including blood transfusions, sodium bicarbonate therapy for acidosis, and fluid therapy, should be administered as indicated. A number of drugs, including diminazene aceturate, phenamidine, pentamidine isethionate, parvaquone, atovaquone, and niridazole, have also been used in an attempt to treat different Babesia spp. infections. In the United States, if clinical disease associated with B. canis is suspected, imidocarb diproprionate may be effective when administered (5 to 6.6 mg/kg SC or intramuscularly [IM]) twice, 14 days apart or (7.5 mg/kg, SC or IM) once. Adverse effects include transient salivation, diarrhea, dyspnea, lacrimation, and depression. Imidocarb is not as effective for the treatment of B. gibsoni infection. In the United States, if clinical disease associated with B. gibsoni is suspected, azithromycin (10 mg/kg PO q24h for a minimum of 10 days) or clindamycin hydrochloride (12.5 mg/kg PO q12h for at least 10 days) may lessen clinical disease if other drugs are not available. Azithromycin (as described) and atovaquone (13.3 mg/kg PO q8h for at least 10 days) is currently recommended for the treatment B. gibsoni infections, but this combination does not always result in elimination of infection (Birkenheuer et al., 2004b; Jefferies et al., 2007). Because no drugs are known to eliminate infection consistently, treatment of healthy, seropositive dogs is unlikely to be of benefit.
Zoonotic Aspects and Prevention
No evidence currently exists to suggest that Babesia spp. infecting dogs and cats can cause human disease. However, some Babesia spp. infections of people are genetically similar to those infecting dogs, and the organism should be considered important vector-borne diseases of people. Ticks should be controlled if possible. If controlling ticks is difficult in a B. canis–infected kennel, one dose of imidocarb at 7.5 mg/kg IM may eliminate the carrier state. Minimal cross-protection exists between species; a dog that has recovered from babesiosis may still become ill if infected with another species. Thus tick control must be maintained in endemic areas. Administration of immunosuppressive drugs and splenectomy should be avoided in previously infected dogs. Dogs used as blood donors should be assessed for infection by PCR or serologic screening and positive dogs excluded from the program. Dog bites should be avoided. A B. canis vaccine is available in Europe. For blood donor programs, high-risk breeds (Greyhound, American Pit Bull Terrier) or dogs from endemic areas should be screened for Babesia spp. infection by serology or PCR assays, and positive dogs should be excluded from the program (Wardrop et al., 2005).
CYTAUXZOONOSIS
Etiology and Epidemiology
Cytauxzoon felis is a protozoal disease of cats in the southeastern, mid-Atlantic, and south-central United States that is usually fatal. Large-scale prevalence rates have not been performed, but one study of 961 cats in Florida, North Carolina, and Tennessee showed a prevalence rate of 0.3% (Haber et al., 2007). Isolates from domestic cats have been genetically similar between studies (Birkenheuer et al., 2006b). Bobcats are usually subclinically affected and may therefore be the natural host of the organism. The organism can be passed experimentally from infected bobcats to domestic cats by Dermacentor variabilis (American dog tick); clinical illness occurs after an incubation period of 5 to 20 days. After infection, schizonts and macroschizonts form in mononuclear phagocytes. The infected macrophages line the lumen of veins throughout the body. Merozoites released from the infected macrophages infect erythrocytes. Clinical disease results from obstruction of blood flow through tissues by the mononuclear infiltrates and from hemolytic anemia. Domestic cats occasionally survive infection, suggesting that variants that are less virulent to cats also exist (Walker et al., 1995; Meinkoth et al., 2000; Haber et al., 2007).
Clinical Features
Most cases of cytauxzoonosis are in cats allowed to go outdoors. Fever, anorexia, dyspnea, depression, icterus, pale mucous membranes, and death are the most common clinical findings. A primary differential diagnosis is mycoplasmosis. Ticks are rarely identified on affected cats.
Diagnosis
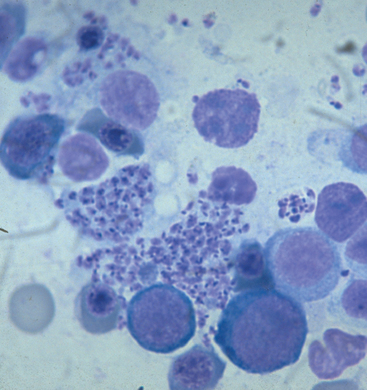
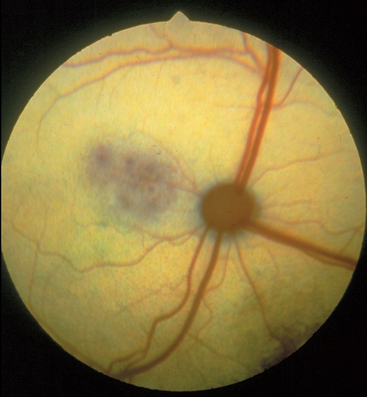
Regenerative anemia, pancytopenia, and neutrophilic leukocytosis are the most common hematologic findings; thrombocytopenia occurs in some cats. Hemoglobinemia, hemoglobinuria, hyperbilirubinemia, and bilirubinuria are uncommon. Antemortem diagnosis is based on demonstration of the erythrocytic phase on thin blood smears (Fig. 99-1) stained with Wright’s or Giemsa stains (see Chapter 92). Infected macrophages can be detected cytologically in bone marrow, spleen, liver, or lymph node aspirates. The organism is easily identified on histopathologic evaluation of most organs. Serologic testing is not commercially available. PCR can be used to amplify organism DNA from blood.
Treatment
Supportive care includes fluid therapy and blood transfusion administered as indicated. Diminazene (five cats) (2.0 mg/kg IM twice, 7 days apart) or imidocarb (one cat) (2 mg/kg IM twice, 14 days apart) was used in cats that survived infection (Greene et al., 1999). Historically, parvaquone (10 to 30 mg/kg IM or SC q24h) administered for 2 to 3 days, buparvaquone (10 mg/kg IM or SC q24h) administered for 2 to 3 days, or thiacetarsemide (0.1 mg/kg IV q12h) administered for 2 days have been attempted. Diminazene, parvaquone, and buparvaquone are not routinely available; thiacetarsemide is toxic for cats and should not be used in this species. If no other drug is available, enrofloxacin at 5.0 mg/kg PO or SC q12h for 7 to 10 days could be attempted. Azithromycin and atovaquone as used for B. gibsoni may be effective.
HEPATOZOONOSIS
Etiology and Epidemiology
Hepatozoonosis in dogs is caused by the protozoal agents Hepatozoon canis and H. americanum. In North America H. americanum predominates, is transmitted by Amblyomma maculatum (Gulf Coast tick), and is most common in the Texas Gulf Coast, Mississippi, Alabama, Georgia, Florida, Louisiana, and Oklahoma. In Africa, southern Europe, and Asia, H. canis predominates and is transmitted by Rhipicephalus sanguineus (brown dog tick). A Hepatozoon species is occasionally found in the blood of cats in Europe. Clinical disease associations are currently unclear, but the cats are commonly coinfected with feline leukemia virus or feline immunodeficiency virus. Vertebrate hosts develop macrogametes and microgametes in neutrophils and monocytes. The tick ingests the organism during a blood meal and oocysts develop. After a dog ingests an infected tick, sporozoites are released and infect mononuclear phagocytes and endothelial cells of the spleen, liver, muscle, lungs, and bone marrow and ultimately form cysts containing macromeronts and micromeronts. Micromeronts develop into micromerozoites, which infect leukocytes and develop into gamonts. Tissue phases induce pyogranulomatous inflammation, resulting in clinical disease. Glomerulonephritis or amyloidosis may occur as a result of chronic inflammation and immune complex disease. Infected dogs can serve as a source of infection for ticks for months to years (Ewing et al., 2003).
Clinical Features
H. americanum can be a primary pathogen, resulting in clinical illness without concurrent immune deficiency. Clinically affected dogs have been in all age groups, but disease is most commonly recognized in puppies. Fever, weight loss, and severe hyperesthesia over the paraspinal regions are common findings. Anorexia, pale mucous membranes from anemia, depression, oculonasal discharge, and bloody diarrhea occur in some dogs. Clinical signs can be intermittent and recurrent.
Diagnosis
Neutrophilic leukocytosis (20,000 to 200,000 cells/μL) with a left shift is the most common hematologic finding. Thrombocytopenia is unusual unless coinfection with Ehrlichia canis or Anaplasma spp. occurs. Normocytic, normochromic, nonregenerative anemia is common and is likely from chronic inflammation. Increased activity of alkaline phosphatase but not creatine kinase occurs in H. americanum–infected dogs. Hypoalbuminemia, hypoglycemia and, rarely, polyclonal gammopathy occur in some dogs. Periosteal reactions from the inflammatory reaction directed at tissue phases in muscle can occur in any bone except the skull, are most common in young dogs, do not occur in every case, and are not pathognomonic for hepatozoonosis. Definitive diagnosis is based on identification of gamonts in neutrophils or monocytes in Giemsa or Leishman’s stained blood smears or by demonstration of the organism in muscle biopsy sections. PCR assays may be used to aid in the diagnosis in the future.
Treatment
No therapeutic regimen has been shown to eliminate H. canis or H. americanum infection from tissues. However, clinical disease resolves rapidly with several drug protocols. For treatment of H. americanum, the combination of trimethoprim-sulfadiazine (15 mg/kg PO q12h), pyrimethamine (0.25 mg/kg PO q24h), and clindamycin (10 mg/kg PO q8h) for 14 days is highly successful in the acute stage (Macintire et al., 2001). Use of decoquinate (10 to 20 mg/kg q12h) with food lessens the likelihood of recurrence of clinical disease and prolongs survival time. Imidocarb dipropionate (5 to 6 mg/kg IM or SC) administered once or twice 14 days apart is the drug of choice for treatment of H. canis and may also be effective for H. americanum. Administration of nonsteroidal antiinflammatory agents may lessen discomfort for some dogs.
LEISHMANIASIS
Etiology and Epidemiology
Leishmania spp. are flagellates that cause cutaneous, mucocutaneous, and visceral diseases in dogs, human beings, and other mammals. Rodents and dogs are primary reservoirs of Leishmania spp., people and cats are probably incidental hosts, and sandflies are the vector in most endemic regions other than the United States. Leishmaniasis was considered unimportant in the United States until recently, with cases only reported occasionally. In 1999 L. donovani infection was confirmed in multiple dogs in a Foxhound kennel in New York state (Gaskin et al., 2002). Further investigation of more than 12,000 foxhounds and other canids documented L. donovani infection in 18 states and two Canadian provinces (Duprey et al., 2006) (Fig. 99-2). Infection of canids other than Foxhounds appears to be uncommon. In other countries flagellated promastigotes develop in the sandfly and are injected into the vertebrate host when the sandfly feeds. Promastigotes are engulfed by macrophages and disseminate through the body. After an incubation period of 1 month to 7 years, amastigotes (nonflagellate) form and cutaneous lesions develop; sandflies are infected during feeding. In Foxhounds in the United States transmission appeared to be primarily from dog to dog (Duprey et al., 2006). Transmission by fighting, shared needles, blood transfusions, breeding, and congenital transmission can occur (Duprey et al., 2006; de Freitas et al., 2006). The intracellular organism induces extreme immune responses; polyclonal gammopathies (and occasionally monoclonal); proliferation of macrophages, histiocytes, and lymphocytes in lymphoreticular organs; and immune complex formation resulting in glomerulonephritis and polyarthritis are common.
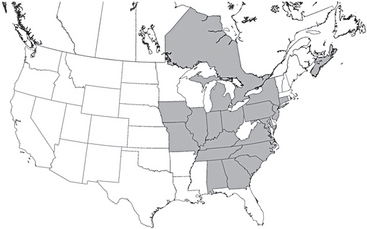
FIG 99-2 Distribution of hunt clubs with confirmed cases of visceral leishmaniasis, United States and Canada. States in which hunt clubs or kennels had 1 or more dogs infected with Leishmania infantum are shaded. Leishmania-positive Foxhounds were also found in Nova Scotia and Ontario.
(Reprinted from Duprey ZH et al: Canine visceral leishmaniasis, United States and Canada, 2000-2003, Emerg Infect Dis 12:440, 2006.)
Clinical Features
Dogs generally develop visceral leishmaniasis. A subclinical phase of infection may persist for months or years. Weight loss in the face of a normal to increased appetite, polyuria, polydipsia, muscle wasting, depression, vomiting, diarrhea, cough, petechiae, ecchymosis, epistaxis, sneezing, and melena are common presenting complaints. Splenomegaly, lymphadenopathy, facial alopecia, fever, rhinitis, dermatitis, increased lung sounds, icterus, swollen painful joints, uveitis, and conjunctivitis are commonly identified on physical examination. Cutaneous lesions are characterized by hyperkeratosis, scaling, thickening, mucocutaneous ulcers, and intradermal nodules on the muzzle, pinnae, ears, and foot pads. Bone lesions are detected in some dogs. Most dogs die or are euthanized as a consequence of chronic kidney disease. Cats are usually subclinically infected; one cat in Texas had cutaneous nodules on the pinna.
Diagnosis
The principal clinicopathologic abnormalities include hyperglobulinemia, hypoalbuminemia, proteinuria, increased liver enzyme activities, thrombocytopenia, azotemia, lymphopenia, and leukocytosis with left shift. The hyperglobulinemia is usually polyclonal, but an IgG monoclonal gammopathy was reported in a dog (Font et al., 1994). Neutrophilic polyarthritis occurs in some dogs as a manifestation of a type III hypersensitivity reaction. Demonstration of amastigotes (2.5 to 5.0 μm ×1.5 to 2.0 μm) in lymph node aspirates, bone marrow aspirates, or skin imprints stained with Wright’s or Giemsa stain gives a definitive diagnosis (Fig. 99-3). The organism can also be identified by histopathologic or immunoperoxidase evaluation of skin or organ biopsy, culture, inoculation of hamsters, or PCR. Antibodies against Leishmania can be detected in serum; IgG titers develop 14 to 28 days after infection and decline 45 to 80 days after treatment. Serologic cross-reactivity occurs between Trypanosoma cruzi and Leishmania. Because dogs are unlikely to eliminate infection spontaneously, most true-positive antibody test dogs are currently infected. PCR can be performed on ethylenediamine tetraacetic acid anticoagulated blood, bone marrow, or lymph node aspirates. Real-time PCR assays can be used to monitor response to therapy (Francino et al., 2006).
Treatment
Although clinical signs of disease often improve with drug administration, the prognosis for visceral leishmaniasis in dogs is variable; most cases are recurrent. No drug or drug combination has been used to clear Leishmania from the body successfully. The combination of antimony and allopurinol (15 mg/kg PO q12h) was superior to treatment with either drug alone (Denerolle et al., 1999), but even long-term therapy does not always eliminate infection (Manna et al., 2007). Because antimony drugs are not available in the United States, infected dogs should be started on allopurinol therapy initially. In one study, marbofloxacin was effective in vitro and may be considered for the treatment of infected dogs if other drugs are not available (Vouldoukis et al., 2006). Liposomal or lipid-emulsified amphotericin B at varying doses (0.8 to 3.3 mg/kg IV for varying numbers of treatments) has been prescribed with good clinical results, but recurrences can still occur (Oliva et al., 1995; Cortadella et al., 2003). Dogs with chronic kidney disease have a poor prognosis, but a recent study showed administration of allopurinol to be beneficial (Plevraki et al., 2006).
Zoonotic Aspects and Prevention
The primary zoonotic risk for canine leishmaniasis is from dogs acting as a reservoir host for the organism. Direct contact with amastigotes in draining lesions is unlikely to result in human infection. None of the 185 persons with potential exposure to infected Foxhounds had evidence of infection (Duprey et al., 2006). Avoidance of infected sandflies is the only means of prevention. If in endemic areas, house animals during night hours and control breeding places of sandflies. Use of 10% imidacloprid/50% permethrin may lessen transmission in sandfly-endemic areas (Otranto et al., 2007). A vaccine is available for use with dogs in some countries (Dantas-Torres, 2006). For blood donor programs, high-risk breeds (e.g., Foxhounds) or dogs from endemic areas should be screened for Leishmania spp. infection by serology or PCR assays, and positive dogs should be excluded from the program (Wardrop et al., 2005).
NEOSPOROSIS
Etiology and Epidemiology
Neospora caninum is a coccidian previously confused with T. gondii because of similar morphology. The sexual cycle is completed in the gastrointestinal tract of dogs and results in the passage of oocysts in feces. Oocyst shedding can continue for several months in some dogs (McGarry et al., 2003). Sporozoites develop in oocysts within 24 hours of passage. Tachyzoites (rapidly dividing stage) and tissue cysts containing hundreds of bradyzoites (slowly dividing stage) are the other two life stages. Dogs are infected by ingestion of bradyzoites but not tachyzoites. Infection has been documented after ingestion of infected bovine placental tissue. Dogs can become infected from ingesting intermediate hosts such as white-tailed deer (Gondim et al., 2004). Thus free-roaming dogs may be at increased risk of infection. Transplacental infection has been well documented; dams that give birth to infected offspring can repeat transplacental infection during subsequent pregnancies. Because repeated transplacental infections occur, puppies from a bitch who previously birthed infected puppies are at an increased risk. Canine neosporosis has been reported in many countries around the world. Seroprevalence of infection has varied from 0% to 100% depending on the country and lifestyle of the dog (Dubey et al., 2007a). The pathogenesis of the disease is primarily related to the intracellular replication of tachyzoites. Although organism replication occurs in many tissues, including the lungs, in dogs clinical illness is primarily neuromuscular.
Encephalomyelitis and myositis develop in experimentally infected kittens and seropositive, naturally exposed cats have been detected (Bresciani et al., 2007), but clinical disease in naturally infected cats has not been reported. N. caninum seropositive, nondomestic felids also have been reported (Spencer et al., 2003).
Administration of glucocorticoids may activate bradyzoites in tissue cysts, resulting in clinical illness.
Clinical Features
Ascending paralysis with hyperextension of the hindlimbs in congenitally infected puppies is the most common clinical manifestation of the disease. Muscle atrophy occurs in many cases. Polymyositis and multifocal CNS disease can occur alone or in combination. Clinical signs can be evident soon after birth or may be delayed for several weeks. Neonatal death is common. Although disease tends to be most severe in congenitally infected puppies, dogs as old as 15 years have been clinically affected. In one dog presented primarily for respiratory disease, cough was the principal sign. Myocarditis, dysphagia, ulcerative dermatitis, pneumonia, and hepatitis occur in some dogs. Whether clinical disease in older dogs is from acute, primary infection or exacerbation of chronic infection is unknown. Administration of glucocorticoids may activate bradyzoites in tissue cysts, resulting in clinical illness. Disease is caused by intracellular replication of Neospora caninum tachyzoites. Infection of CNS structures causes mononuclear cell infiltrates, which suggests an immune-mediated component to the pathogenesis of disease. Intact tissue cysts in neural structures are generally not associated with inflammation, but ruptured tissue cysts induce inflammation. Untreated disease generally results in death.
Diagnosis
Hematologic and biochemical findings are nonspecific. Myositis commonly results in increased creatine kinase and aspartate aminotransferase activities. CSF abnormalities include increased protein concentration (20 to 50 mg/dL) and a mild, mixed inflammatory cell pleocytosis (10 to 50 cells/μL) consisting of monocytes, lymphocytes, neutrophils and, rarely, eosinophils. Interstitial and alveolar patterns can be noted on thoracic radiographs.
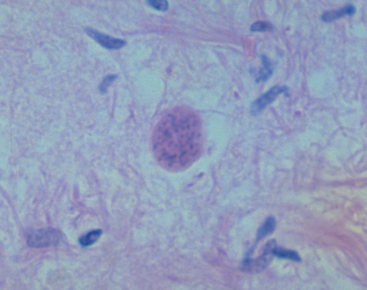
Definitive diagnosis is based on demonstration of the organism in CSF or tissues. Tachyzoites are rarely identified on cytologic examination of CSF, imprints of dermatologic lesions, and bronchoalveolar lavage. Mixed inflammation with neutrophils, lymphocytes, eosinophils, plasma cells, macrophages, and tachyzoites was noted on transthoracic aspirate of one dog with lung disease. Neospora caninum tissue cysts have a wall thicker than 1 μm; T. gondii tissue cysts have a wall thinner than 1 μm (Fig. 99-4). Oocysts can be detected in feces by microscopic examination after flotation or by PCR. The organism can be differentiated from T. gondii by electron microscopy, immunohistochemistry, and PCR. A multiplex PCR assay that detects both T. gondii and N. caninum for use with tissues or CSF has been reported (Schatzerg et al., 2003)
A presumptive diagnosis of neosporosis can be made by combining appropriate clinical signs of disease and positive serology or presence of antibodies in CSF with the exclusion of other etiologies inducing similar clinical syndromes, particularly T. gondii. Serologic cross-reactivity between T. gondii and N. caninum exist in some assays (Silva et al., 2007). IgG antibody titers of at least 1 : 200 have been detected in most dogs with clinical neosporosis; minimal serologic cross-reactivity occurs with T. gondii at titers of 1 : 50 or higher when using the immunofluorescent assay test.
Treatment
Although many dogs with neosporosis die, some have survived after treatment with trimethoprim-sulfadiazine combined with pyrimethamine; sequential treatment with clindamycin hydrochloride, trimethoprim-sulfadiazine, and pyrimethamine; or clindamycin alone. Administration of trimethoprim-sulfadiazine (15 mg/kg PO q12h) with pyrimethamine (1 mg/kg PO q24h) for 4 weeks or clindamycin (10 mg/kg PO q8h) for 4 weeks was recommended for the treatment of canine neosporosis. In one recent study of naturally infected beagle puppies, administration of clindamycin alone (75 mg/puppy at 9 weeks of age, PO, q12h [dose doubled at 13 weeks] for 6 months) lessened clinical signs of disease but did not eliminate the infection (Dubey et al., 2007b). Treatment of clinically affected dogs should be initiated before the development of extensor rigidity, if possible. The prognosis for dogs presented with severe neurologic involvement is grave.
Zoonotic Aspects and Prevention
Neospora caninum antibodies have been detected in people, but in one study no link was found to repeated abortion (Petersen et al., 1999). In addition, the organism has not been isolated from human tissues (Dubey et al., 2007a), so the zoonotic potential is still unproven. An epidemiologic link has been shown between dogs and cattle; efforts should be made to lessen dog fecal contamination of livestock feed, and dogs should not be allowed to ingest bovine placentas. Consuming raw meat is a risk factor for dogs and should be avoided (Reichel et al., 2007). Hunting behavior of dogs should be restricted if possible. Bitches that whelp clinically affected puppies should not be bred. Glucocorticoids should not be administered to seropositive animals, if possible, because a potential exists for activation of infection.
FELINE TOXOPLASMOSIS
Etiology and Epidemiology
Toxoplasma gondii is one of the most prevalent parasites infecting warm-blooded vertebrates. Only cats complete the coccidian life cycle and pass environmentally resistant oocysts in feces. Sporozoites develop in oocysts after 1 to 5 days of exposure to oxygen and appropriate environmental temperature and humidity. Tachyzoites disseminate in blood or lymph during active infection and replicate rapidly intracellularly until the cell is destroyed. Bradyzoites are the slowly dividing, persistent tissue stage that form in the extraintestinal tissues of infected hosts as immune responses attenuate tachyzoite replication. Tissue cysts form readily in the CNS, muscles, and visceral organs. Bradyzoites may persist in tissues for the life of the host.
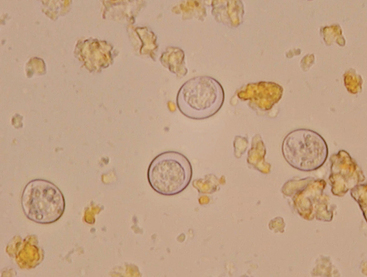
Infection of warm-blooded vertebrates occurs after ingestion of any of the three life stages of the organism or transplacentally. Most cats are not coprophagic and so are infected most commonly by ingesting T. gondii bradyzoites during carnivorous feeding; oocysts are shed in feces from 3 to 21 days. Sporulated oocysts can survive in the environment for months to years and are resistant to most disinfectants (Fig. 99-5). Results of a recent study confirm that the T. gondii oocyst shedding prepatent period is stage dependent (ingestion of bradyzoites has a shorted prepatent period than ingestion of sporozoites) and not dose dependent (Dubey, 2006). In addition, transmission of T. gondii is most efficient when cats consume tissue cysts (carnivorism) and when intermediate hosts consume oocysts (fecal-oral transmission). T. gondii infection of rodents changes the behavior of the prey species, making it less averse to cats, potentially increasing the likelihood the definitive host (felid) will become infected and potentiate the sexual phase of the organism (Vyas et al., 2007). Approximately 30% to 40% of cats and people in the United States are seropositive and are presumed to be infected. In a recent study of clinically ill cats, T. gondii antibodies were detected in 31.6% of the 12,628 cats tested (Vollaire et al., 2005).
Clinical Features
Approximately 10% to 20% of experimentally inoculated cats develop self-limiting, small-bowel diarrhea for 1 to 2 weeks after primary oral inoculation with T. gondii tissue cysts; this is presumed to be from enteroepithelial replication of the organism. However, detection of T. gondii oocysts in feces is rarely reported in studies of naturally exposed cats with diarrhea. T. gondii enteroepithelial stages were found in intestinal tissues from two cats with inflammatory bowel disease. Positive response to anti-Toxoplasma drugs in these two cats suggests that toxoplasmosis may occasionally induce inflammatory bowel disease. Eosinophilic fibrosing gastritis was recently described in a T. gondii–infected cat (McConnell et al., 2007).
Fatal extraintestinal toxoplasmosis can develop from overwhelming intracellular replication of tachyzoites after primary infection; hepatic, pulmonary, CNS, and pancreatic tissues are commonly involved. Kittens infected by the transplacental or transmammary routes develop the most severe signs of extraintestinal toxoplasmosis and generally die of pulmonary or hepatic disease. Common clinical findings in cats with disseminated toxoplasmosis include depression, anorexia, and fever followed by hypothermia, peritoneal effusion, icterus, and dyspnea. If a host with chronic toxoplasmosis is immunosuppressed, bradyzoites in tissue cysts can replicate rapidly and disseminate again as tachyzoites; this is common in people with acquired immunodeficiency syndrome (AIDS). Disseminated toxoplasmosis has been documented in cats concurrently infected with feline leukemia, feline immunodeficiency, or feline infectious peritonitis viruses as well as after cyclosporine administration for skin disease or after renal transplantation (Bernstein et al., 1999; Barrs et al., 2006).
Sublethal, chronic toxoplasmosis occurs in some cats. T. gondii infection should be on the differential diagnosis list for cats with anterior or posterior uveitis, cutaneous lesions, fever, muscle hyperesthesia, myocarditis with arrhythmias, weight loss, anorexia, seizures, ataxia, icterus, diarrhea, or pancreatitis (Fig. 99-6). Based on results of T. gondii–specific aqueous humor antibody and PCR studies, toxoplasmosis appears to be a common infectious cause of uveitis in cats. Kittens infected transplacentally or lactationally commonly develop ocular disease. Immune complex formation and deposition in tissues and delayed hypersensitivity reactions may be involved in chronic, sublethal clinical toxoplasmosis. Because none of the anti-Toxoplasma drugs totally clear the body of the organism, recurrence of disease is common.
Diagnosis
Cats with clinical toxoplasmosis can have a variety of clinicopathologic and radiographic abnormalities, but none documents the disease. Nonregenerative anemia, neutrophilic leukocytosis, lymphocytosis, monocytosis, neutropenia, eosinophilia, proteinuria, and bilirubinuria as well as increases in serum protein and bilirubin concentrations, creatinine kinase, alanine aminotransferase, alkaline phosphatase, and lipase activities occur in some cats. Pulmonary toxoplasmosis most commonly causes diffuse interstitial to alveolar patterns or pleural effusion. Mass lesions may be detected on computed tomography or magnetic resonance imaging examinations. CSF protein concentrations and cell counts are often higher than normal. The predominant white blood cells in CSF are small mononuclear cells, but neutrophils also are commonly found.
The antemortem definitive diagnosis of feline toxoplasmosis can be made if the organism is demonstrated; however, this is uncommon, particularly in association with sublethal disease. Bradyzoites or tachyzoites are rarely detected in tissues, effusions, bronchoalveolar lavage fluids, aqueous humor, or CSF. Detection of 10 × 12 μm oocysts in feces in cats with diarrhea suggests toxoplasmosis but is not definitive because Besnoitia and Hammondia infections of cats produce morphologically similar oocysts.
T. gondii–specific antibodies (IgM, IgG, IgA), antigens, and immune complexes can be detected in the serum of normal cats as well as in those with clinical signs of disease, so antemortem diagnosis of clinical toxoplasmosis is impossible based on these tests alone. Of the serum tests, IgM correlates the best with clinical feline toxoplasmosis because this antibody class is rarely detected in serum of healthy cats. The antemortem diagnosis of clinical toxoplasmosis can be tentatively based on the combination of the following:
Some cats with clinical toxoplasmosis will have reached their maximal IgG titer or have undergone antibody class shift from IgM to IgG by the time they are serologically evaluated, so the failure to document an increasing IgG titer or a positive IgM titer does not exclude the diagnosis of clinical toxoplasmosis. Because some healthy cats have extremely high serum antibody titers and some clinically ill cats have low serum antibody titers, the magnitude of titer is relatively unimportant in the clinical diagnosis of toxoplasmosis. Because the organism cannot be cleared from the body, most cats will be antibody positive for life, so repeating serum antibody titers after the clinical disease has resolved is not necessary.
The combination of aqueous humor or CSF T. gondii–specific antibody detection and organism DNA detection by PCR is the most accurate way to diagnose ocular or CNS toxoplasmosis (e.g., Diagnostic Laboratory, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins). Whereas T. gondii–specific IgA, IgG, and organism DNA can be detected in aqueous humor and CSF of both normal and clinically ill cats, T. gondii–specific IgM has only been detected in the aqueous humor or CSF of clinically ill cats and therefore may be the best indicator of clinical disease. Because T. gondii DNA can be detected in the blood of healthy cats, positive PCR results do not correlate to clinical disease (Burney et al., 1999).
Treatment
Supportive care should be instituted as needed. Clindamycin hydrochloride (10 to 12 mg/kg PO q12h) administered for 4 weeks or a trimethoprim-sulfonamide combination (15 mg/kg PO q12h) administered for 4 weeks has been used most frequently by the author for the treatment of clinical feline toxoplasmosis. Azithromycin (10.0 mg/kg PO q24h) has been used successfully in a limited number of cats, but the optimal duration of therapy is unknown. Pyrimethamine combined with sulfa drugs is effective for the treatment of human toxoplasmosis but commonly results in toxicity in cats. Cats with systemic clinical signs of toxoplasmosis, such as fever or muscle pain combined with uveitis, should be treated with anti-Toxoplasma drugs in combination with topical, oral, or parenteral corticosteroids to avoid secondary lens luxations and glaucoma. T. gondii–seropositive cats with uveitis that are otherwise normal can be treated with topical glucocorticoids alone unless the uveitis is recurrent or persistent. In these situations, administration of a drug with anti–T. gondii activity may be beneficial.
Clinical signs not involving the eyes or the CNS usually resolve within the first 2 to 3 days of clindamycin or trimethoprim-sulfonamide administration; ocular and CNS toxoplasmosis responds more slowly to therapy. If fever or muscle hyperesthesia does not decrease after 3 days of treatment, other causes should be considered. Recurrence of clinical signs may be more common in cats treated for less than 4 weeks. No evidence suggests that any drug can totally clear the body of the organism, so recurrences are common and infected cats will always be seropositive. The prognosis is poor for cats with hepatic or pulmonary disease caused by organism replication, particularly in those that are immunocompromised.
Zoonotic Aspects and Prevention
T. gondii is a major zoonosis. Primary infection of mothers during gestation can lead to clinical toxoplasmosis in the fetus; stillbirth, CNS disease, and ocular disease are common clinical manifestations. Primary infection in immunocompetent individuals results in self-limiting fever, malaise, and lymphadenopathy. As T-helper cell counts decline, approximately 10% of people with AIDS develop toxoplasmic encephalitis from activation of bradyzoites in tissue cysts.
People most commonly acquire toxoplasmosis transplacentally or by ingesting sporulated oocysts or tissue cysts. To prevent toxoplasmosis, avoid eating undercooked meats or ingesting sporulated oocysts (Box 99-1). In a recent study of 6282 meat samples from 698 retail meat stores, T. gondii was detected by bioassay in cats in none of the beef or chicken samples tested and only a small number of pork samples (Dubey et al., 2005). Although owning a pet cat was epidemiologically associated with acquiring toxoplasmosis in one study of pregnant women, touching individual cats is probably not a common way to acquire toxoplasmosis for the following reasons:
 BOX 99-1 Prevention of Human Toxoplasmosis
BOX 99-1 Prevention of Human Toxoplasmosis
Prevention of Oocyst Ingestion
Avoid feeding undercooked meats to cats.
Clean the litter box daily and incinerate or flush the feces.
Clean the litter box periodically with scalding water or use a litter box liner.
Wear gloves when working with soil.
Wash hands thoroughly with soap and hot water after gardening.
Wash fresh vegetables well before ingestion.
Keep children’s sandboxes covered.
Boil water for drinking that has been obtained from the general environment.
However, because some cats will repeat oocyst shedding when exposed a second time, feces should always be handled carefully. If a fecal sample from a cat is shown to contain oocysts measuring 10 × 12 μm, the organism is assumed to be T. gondii. The feces should be collected daily until the oocyst shedding period is complete; administration of clindamycin (25 to 50 mg/kg PO divided q12h) or sulfonamides (100 mg/kg PO divided q12h) can reduce levels of oocyst shedding.
Because human beings are not commonly infected with T. gondii from contact with individual cats, testing healthy cats for toxoplasmosis is not recommended. Fecal examination is an adequate procedure to determine when cats are actively shedding oocysts but cannot predict when a cat has shed oocysts in the past. No serologic assay accurately predicts when a cat shed T. gondii oocysts in the past, and most cats that are shedding oocysts are seronegative. Most seropositive cats have completed the oocyst shedding period and are unlikely to repeat shedding; most seronegative cats would shed the organism if infected. If owners are concerned that they may have toxoplasmosis, they should see their physician for testing.
CANINE TOXOPLASMOSIS
Etiology and Epidemiology
Dogs do not produce T. gondii oocysts like cats, but they can mechanically transmit oocysts after ingesting feline feces. The tissue phases of T. gondii infection occur in dogs and can induce clinical disease. Approximately 20% of dogs in the United States are seropositive for T. gondii antibodies. Before 1988 many dogs diagnosed with toxoplasmosis based on histologic evaluation were truly infected with Neospora caninum (see Neosporosis section).
Clinical Features
Respiratory, gastrointestinal, or neuromuscular infection resulting in fever, vomiting, diarrhea, dyspnea, and icterus occurs most commonly in dogs with generalized toxoplasmosis. Generalized toxoplasmosis is most common in immunosuppressed dogs, such as those with canine distemper virus infection or those receiving cyclosporine to prevent rejection of a transplanted kidney. Neurologic signs depend on the location of the primary lesions and include ataxia, seizures, tremors, cranial nerve deficits, paresis, and paralysis. Dogs with myositis present with weakness, stiff gait, or muscle wasting. Rapid progression to tetraparesis and paralysis with lower motor neuron dysfunction can occur. Some dogs with suspected neuromuscular toxoplasmosis probably had neosporosis. Myocardial infection resulting in ventricular arrhythmias occurs in some infected dogs. Dyspnea, vomiting, or diarrhea occurs in dogs with polysystemic disease. Retinitis, anterior uveitis, iridocyclitis, and optic neuritis occur in some dogs with toxoplasmosis, but they are less common than in cats. Cutaneous disease has also been detected (Webb et al., 2005).
Diagnosis
As in cats, hematologic, biochemical, urinalysis, and radiographic abnormalities are not specific. Increased protein concentrations and mixed inflammatory cell infiltrates occur in dogs with CNS toxoplasmosis.
Demonstration of the organism associated with inflammation in tissues or exudates can lead to a definitive diagnosis. More commonly an antemortem diagnosis is based on the combination of appropriate clinical signs, exclusion of other likely etiologies, positive serum antibody tests, exclusion of N. caninum infection by serologic testing, and response to an anti-Toxoplasma drug. Interpretation of serum, aqueous humor, and CSF antibody and PCR test results is as discussed for toxoplasmosis in cats.
Therapy
Clindamycin hydrochloride (10-12 mg/kg PO q12h) has been used most frequently for treatment of canine toxoplasmosis by the author. Trimethoprim-sulfa (15 mg/kg PO q12h) is an alternative protocol. Treatment should be continued for a minimum of 4 weeks. If uveitis occurs, topical glucocorticoid treatment should also be used.
Zoonotic Aspects and Prevention
Dogs do not complete the enteroepithelial phase of T. gondii but can mechanically transmit oocysts after ingesting feline feces. Like all other warm-blooded vertebrates, dogs are infected by the ingestion of sporulated oocysts or tissue cysts. Toxoplasmosis in dogs can be prevented by not allowing dogs to be coprophagic and to feed only cooked meat and meat byproducts.
AMERICAN TRYPANOSOMIASIS
Etiology and Epidemiology
Trypanosoma cruzi is a flagellate that infects many mammals and causes American trypanosomiasis. The disease is diagnosed primarily in South America, but several cases have been detected in dogs of North America. Infected reservoir mammals (dogs, cats, raccoons, opossums, armadillos) and vectors (reduviid [kissing] bugs) are found in the United States, but infection in dogs or people is rare; this may relate to differences in vector behavior and sanitation standards in the United States. In one study in Texas the number of serologically positive dogs increased between 1987 and 1996 (Meurs et al., 1998). Foxhounds infected with Leishmania spp. were recently shown to be coinfected with T. cruzi (Duprey et al., 2006) (Fig. 99-7). The organism has three life stages: trypomastigotes (flagellated stage found free in blood), amastigotes (nonflagellated intracellular form), and epimastigotes (flagellated form found in the vector). When infected kissing bugs defecate during feeding, epimastigotes enter the vertebrate host, infect macrophages and myocytes, and transform into amastigotes. Amastigotes divide by binary fission until the host cell ruptures, releasing trypomastigotes into the circulation. The vector is then infected by ingesting trypomastigotes during a blood meal. Transmission can also occur transplacentally by vector ingestion, blood transfusion, or ingestion of infected tissues or milk. Peak parasitemia occurs 2 to 3 weeks after infection, causing acute disease. Disease in dogs is primarily a cardiomyopathy that develops from parasite-induced damage to myocardial cells or immune-mediated reactions.
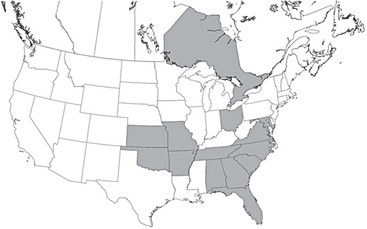
FIG 99-7 Distribution of hunt clubs with Trypanosoma cruzi–positive hounds, United States and Canada. States in which hunt clubs or kennels had 1 or more dogs infected with T. cruzi are shaded. A T. cruzi–positive hunt club was also found in Ontario.
(Reprinted from Duprey ZH et al: Canine visceral leishmaniasis, United States and Canada, 2000-2003, Emerg Infect Dis 12:440, 2006.)
Clinical Features
Exercise intolerance and weakness are nonspecific presenting complaints that relate to myocarditis or heart failure during acute infection. Generalized lymphadenopathy, pale mucous membranes, tachycardia, pulse deficits, hepatomegaly, and abdominal distension can be detected on physical examination. Anorexia, diarrhea, and neurologic signs occasionally occur. Dogs that survive acute infection can present for evaluation of chronic dilative cardiomyopathy. In one study of 11 dogs with chronic infection, right-sided cardiac disease, conduction disturbances, ventricular arrhythmias, and supraventricular arrhythmias were most common (Meurs et al., 1998).
Diagnosis
Common clinicopathologic abnormalities include lymphocytosis and increased activities of liver enzymes and creatine kinase. Thoracic radiographic, abdominal radiographic, and echocardiographic findings are consistent with cardiac disease and failure but are not specific for trypanosomiasis. The primary electrocardiographic findings are ventricular premature contractions, heart block, and T-wave inversion. Definitive diagnosis is based on organism demonstration. Trypomastigotes (one flagellum, 15 to 20 μm long) can be identified during acute disease on thick blood film (see Chapter 92) or buffy coat smears stained with Giemsa or Wright’s stain. The organism is sometimes detected in lymph node aspirates or abdominal effusions. Histopathologic evaluation of cardiac tissue may reveal amastigotes (1.5 to 4.0 μm). Trypomastigotes can also be cultured from blood or grown by bioassay in mice. PCR assays can also be used to prove infection (Nabity et al., 2006).
Treatment
Nifurtimox has been prescribed most frequently for Chagas disease but are toxic and not routinely available in the United States. In a recent study of allopurinol for the treatment of T. cruzi infection in an experimentally infected mouse model, a positive response was noted. Thus treating clinically affected dogs with allopurinol as described for Leishmania may be prudent. Glucocorticoid therapy may improve survival of infected dogs. Therapy for arrhythmias or heart failure should be instituted as needed. Most dogs that survive acute infection develop dilative cardiomyopathy. Survival time in 11 dogs ranged from 0 to 60 months (Meurs et al., 1998).
Zoonotic Aspects and Prevention
Infected dogs can serve as a reservoir of T. cruzi for vectors, and blood from infected dogs can be infectious to human beings. Vector control is the primary means of prevention. In one recent study use of deltamethrin-treated collars reduced Triatoma infestans feeding success on dogs (Reithinger et al., 2005). Dogs should be kept from other reservoir hosts, such as opossums, and should not be fed raw meat. Potential blood donors from endemic areas should be serologically screened. For blood donor programs, high-risk breeds (e.g., Foxhounds) or dogs from endemic areas should be screened for T. cruzi infection by serology or PCR assays, and positive dogs should be excluded from the program (Wardrop et al., 2005).
Ano H, et al. Detection of Babesia species from infected dog blood by polymerase chain reaction. J Vet Med Sci. 2001;63:111.
Birkenheuer AJ, et al. Babesia gibsoni infections in dogs from North Carolina. J Am Anim Hosp Assoc. 1999;35:125.
Birkenheuer AJ, et al. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian genotype) and B. canis DNA in canine blood samples. J Clin Microbiol. 2003;41:4172.
Birkenheuer AJ, et al. Serosurvey of anti-Babesia antibodies in stray dogs and American pit bull terriers and American Staffordshire terriers from North Carolina. J Am Anim Hosp Assoc. 2003;39:551.
Birkenheuer AJ, et al. Detection and molecular characterization of a novel large Babesia species in a dog. Vet Parasitol. 2004;124:151.
Birkenheuer AJ, et al. Efficacy of combined atovaquone and azithromycin for therapy of chronic Babesia gibsoni (Asian genotype) infections in dogs. J Vet Int Med. 2004;18:494.
Birkenheuer AJ, et al. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003). J Am Vet Med Assoc. 2005;227:942.
Boozer AL, Macintire DK. Canine babesiosis. Vet Clin North Am Small Anim Pract. 2003;33:885.
Breitschwerdt EB, et al. Babesiosis in the greyhound. J Am Vet Med Assoc. 1983;182:978.
Garcia AT. Piroplasm infection in dog in northern Spain. Vet Parasitol. 2006;138:97.
Jefferies R, et al. Babesia gibsoni: Detection during experimental infections and after combined atovaquone and azithromycin therapy. Exp Parasitol. 2007;117:15.
Kocan AA, et al. A genotypically unique Babesia gibsoni-like parasite recovered from a dog in Oklahoma. J Parasitol. 2001;87:437.
Kordick SK, et al. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631.
Lobetti RG, et al. Renal involvement in dogs with babesiosis. J S Afr Vet Assoc. 2001;72:23.
Mcintire DK, et al. Babesia gibsoni infection among dogs in the southeastern United States. J Am Vet Med Assoc. 2002;220:325.
Meinkoth JH, et al. Clinical and hematologic effects of experimental infection of dogs with recently identified Babesia gibsoni-like isolates from Oklahoma. J Am Vet Med Assoc. 2002;220:185.
Schetters TP, et al. Different Babesia canis isolates, different diseases. Parasitology. 1997;115:485.
Stegeman JR, et al. Transfusion-associated Babesia gibsoni infection in a dog. J Am Vet Med Assoc. 2003;222:959.
Tuttle AD, et al. Concurrent bartonellosis and babesiosis in a dog with persistent thrombocytopenia. J Am Vet Med Assoc. 2003;223:1306.
Wlosniewski A, et al. Asymptomatic carriers of Babesia canis in an enzootic area. Comp Immunol Microbiol Infect Dis. 1997;20:75.
Wardrop KJ, et al. Canine and feline blood donor screening for infectious disease. J Vet Intern Med. 2005;19:135-142.
Wozniak EJ, et al. Clinical, anatomic, and immunopathologic characterization of Babesia gibsoni infection in the domestic dog (Canis familiaris). J Parasitol. 1997;83:692.
Wulansari R, et al. Clindamycin in the treatment of Babesia gibsoni infections in dogs. J Am Anim Hosp Assoc. 2003;39:558.
Zahler M, et al. Characteristic genotypes discriminate between Babesia canis isolates of differing vector specificity and pathogenicity to dogs. Parasitol Res. 1998;84:544.
Zahler M, et al. “Babesia gibsoni” of dogs from North America and Asia belong to different species. Parasitology. 2000;120:365.
Zahler M, et al. Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol. 2000;89:241.
Birkenheuer AJ, et al. Cytauxzoon felis infection in cats in the mid-Atlantic states: 34 cases (1998-2004). J Am Vet Med Assoc. 2006;228:568.
Birkenheuer AJ, et al. Development and evaluation of a PCR assay for the detection of Cytauxzoon felis DNA in feline blood samples. Vet Parasitol. 2006;137:144.
Greene CE, et al. Administration of diminazene aceturate or imidocarb dipropionate for treatment of cytauxzoonosis in cats. J Am Vet Med Assoc. 1999;215:497.
Haber MD, et al. The detection of Cytauxzoon felis in apparently healthy free-roaming cats in the USA. Vet Parasitol. 2007;146:316.
Hoover JP, et al. Cytauxzoonosis in cats: eight cases (1985–1992). J Am Vet Med Assoc. 1994;205:455.
Jackson CB, Fisher T. Fatal cytauxzoonosis in a Kentucky cat (Felis domesticus). Vet Parasitol. 2006;139:192.
Kier AB, et al. Experimental transmission of Cytauxzoon felis from bobcats (Lynx rufus) to domestic cats (Felis domesticus). Am J Vet Res. 1982;43:97.
Meier HT, et al. Feline cytauxzoonosis: a case report and literature review. J Am Anim Hosp Assoc. 2000;36:493.
Meinkoth J, et al. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J Vet Intern Med. 2000;14:521.
Meinkoth JH, Kocan AA. Feline cytauxzoonosis. Vet Clin North Am Small Anim Pract. 2005;35:89.
Walker DB, et al. Survival of a domestic cat with naturally acquired cytauxzoonosis. J Am Vet Med Assoc. 1995;206:1363.
Baneth G, et al. Hepatozoon spp. parasitemia in a domestic cat. Fel Pract. 1995;23:10.
Baneth G, et al. Antibody response to Hepatozoon canis in experimentally infected dogs. Vet Parasitol. 1998;74:299.
Baneth G, et al. Hepatozoon species infection in domestic cats: a retrospective study. Vet Parasitol. 1998;79:123.
Baneth G, et al. Genetic and antigenic evidence supports the separation of Hepatozoon canis and Hepatozoon americanum at the species level. J Clin Microbiol. 2000;38:1298.
Ewing GO. Granulomatous cholangiohepatitis in a cat due to a protozoan resembling Hepatozoon canis. Fel Pract. 1977;7:37.
Ewing SA, et al. Transmission of Hepatozoon americanum (Apicomplexa: Adeleorina) by ixodids (Acari: Ixodidae). J Med Entomol. 2002;39:631.
Ewing SA, et al. Persistence of Hepatozoon americanum (Apicomplexa: Adeleorina) in a naturally infected dog. J Parasitol. 2003;89:611.
Macintire DK, et al. Treatment of dogs infected with Hepatozoon americanum: 53 cases (1989–1998). J Am Vet Med Assoc. 2001;218:77.
Mathew JS, et al. Experimental transmission of Hepatozoon americanum to dogs by the Gulf Coast tick, Amblyomma maculatum. Vet Parasitol. 1998;80:1.
Panciera RJ, et al. Skeletal lesions of canine hepatozoonosis caused by Hepatozoon americanum. Vet Pathol. 2000;37:225.
Panciera RJ, Ewing SA. American canine hepatozoonosis. Anim Health Res Rev. 2003;4:27.
Vincent-Johnson NA. American canine hepatozoonosis. Vet Clin North Am Small Anim Pract. 2003;33:905.
Vincent-Johnson NA, et al. A new Hepatozoon species from dogs: description of the causative agent of canine hepatozoonosis in North America. J Parasitol. 1997;83:1165.
Cavaliero T, et al. Clinical, serologic, and parasitologic follow-up after long-term allopurinol therapy of dogs naturally infected with Leishmania infantum. J Vet Intern Med. 1999;13:330.
Cortadella O. Initial and long-term efficacy of a lipid emulsion of amphotericin B desoxycholate in the management of canine leishmaniasis. J Vet Intern Med. 2003;17:808.
Craig TM, et al. Dermal leishmaniasis in a Texas cat. Am J Trop Med Hyg. 1986;35:1100.
Dantas-Torres F. Leishmune vaccine: the newest tool for prevention and control of canine visceral leishmaniosis and its potential as a transmission-blocking vaccine. Vet Parasitol. 2006;141:1.
de Freitas E, et al. Transmission of Leishmania infantum via blood transfusion in dogs: potential for infection and importance of clinical factors. Vet Parasitol. 2006;137:159.
Denerolle P, et al. Combination allopurinol and antimony treatment versus antimony alone and allopurinol alone in the treatment of canine leishmaniasis (96 cases). J Vet Intern Med. 1999;13:413.
Duprey ZH, et al. Canine visceral leishmaniasis, United States and Canada, 2000-2003. Emerg Infect Dis. 2006;12:440.
Eddlestone SM. Visceral leishmaniasis in a dog from Maryland. J Am Vet Med Assoc. 2000;217:1686.
Font A, et al. Monoclonal gammopathy in a dog with visceral leishmaniasis. J Vet Intern Med. 1994;8:233.
Francino O, et al. Advantages of real-time PCR assay for diagnosis and monitoring of canine leishmaniosis. Vet Parasitol. 2006;137:214.
Gaskin AA, et al. Visceral leishmaniasis in a New York foxhound kennel. J Vet Intern Med. 2002;16:34.
Grosjean NL, et al. Seroprevalence of antibodies against Leishmania spp among dogs in the United States. J Am Vet Med Assoc. 2003;222:603.
Kirkpatrick CE, et al. Leishmania chagasi and L. donovani: experimental infections in domestic cats. Exp Parasitol. 1984;58:125.
Manna L, et al. Real-time PCR assay in Leishmania-infected dogs treated with meglumine antimoniate and allopurinol. Vet J. Jun 4, 2007. [Epub ahead of print]
Oliva G, et al. Activity of liposomal amphotericin B (AmBisone) in dogs naturally infected with Leishmania infantum. J Antimicrob Chemother. 1995;36:1013.
Otranto D, et al. Efficacy of a combination of 10% imidacloprid/50% permethrin for the prevention of leishmaniasis in kennelled dogs in an endemic area. Vet Parasitol. 2007;144:270.
Pena MT, et al. Ocular and periocular manifestations of leishmaniasis in dogs: 105 cases (1993–1998). Vet Ophthalmol. 2000;3:35.
Plevraki K, et al. Effects of allopurinol treatment on the progression of chronic nephritis in Canine leishmaniosis (Leishmania infantum). J Vet Intern Med. 2006;20:228.
Reale S, et al. Detection of Leishmania infantum in dogs by PCR with lymph node aspirates and blood. J Clin Microbiol. 1999;37:2931.
Rosypal AC, et al. Emergence of zoonotic canine leishmaniasis in the United States: isolation and immunohistochemical detection of Leishmania infantum from foxhounds from Virginia. J Eukaryot Microbiol. 2003;50(Suppl):691.
Smith BE, et al. Antinuclear antibodies can be detected in dog sera reactive to Bartonella vinsonii subsp. berkhoffii, Ehrlichia canis, or Leishmania infantum antigens. J Vet Intern Med. 2004;18:47.
Vouldoukis I, et al. Canine visceral leishmaniasis: comparison of in vitro leishmanicidal activity of marbofloxacin, meglumine antimoniate and sodium stibogluconate. Vet Parasitol. 2006;135:137.
Barber TS, et al. Clinical aspects of 27 cases of neosporosis in dogs. Vet Rec. 1996;139:439.
Basso W, et al. First isolation of Neospora caninum from the feces of a naturally infected dog. J Parasitol. 2001;87:612.
Bresciani KD, et al. Antibodies to Neospora caninum and Toxoplasma gondii in domestic cats from Brazil. Parasitol Res. 2007;100:281.
Cuddon P, et al. Neospora caninum infection in English Springer spaniel littermates: diagnostic evaluation and organism isolation. J Vet Intern Med. 1992;6:325.
Dijkstra T, et al. Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine placenta but not after ingestion of colostrum spiked with Neospora caninum tachyzoites. Int J Parasitol. 2001;31:747.
Dubey JP, et al. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J Am Vet Med Assoc. 1988;193:1259.
Dubey JP, et al. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc. 1988;192:1269.
Dubey JP, et al. Neosporosis in cats. Vet Pathol. 1990;27:335.
Dubey JP, et al. Repeated transplacental transmission of Neospora caninum in dogs. J Am Vet Med Assoc. 1990;197:857.
Dubey JP, et al. High prevalence of antibodies to Neospora caninum in white-tailed deer (Odocoileus virginianus). Int J Parasitol. 1999;29:1709.
Dubey JP, et al. Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev. 2007;20:323.
Dubey JP, et al. Neosporosis in Beagle dogs: Clinical signs, diagnosis, treatment, isolation and genetic characterization of Neospora caninum. Vet Parasitol. Sep 21, 2007. [Epub ahead of print]
Gondim LF, et al. Transmission of Neospora caninum between wild and domestic animals. J Parasitol. 2004;90:1361.
Greig B, et al. Neospora caninum pneumonia in an adult dog. J Am Vet Med Assoc. 1995;206:1000.
Hill DE, et al. Specific detection of Neospora caninum oocysts in fecal samples from experimentally-infected dogs using the polymerase chain reaction. J Parasitol. 2001;87:395.
Holmberg TA, et al. Neospora caninum associated with septic peritonitis in an adult dog. Vet Clin Pathol. 2006;35:235.
Lindsay DS, et al. Neospora caninum and the potential for parasite transmission. Comp Contin Educ Pract Vet. 1999;21:317.
Lindsay DS, et al. Canine neosporosis. J Vet Parasitol. 2000;14:1.
McAllister MM, et al. Dogs are definitive hosts of Neospora caninum. Int J Parasitol. 1998;28:1473.
McGarry JW, et al. Protracted shedding of oocysts of Neospora caninum by a naturally infected foxhound. J Parasitol. 2003;89:628.
Meseck EK, et al. Use of a multiplex polymerase chain reaction to rapidly differentiate Neospora caninum from Toxoplasma gondii in an adult dog with necrotizing myocarditis and myocardial infarct. J Vet Diagn Invest. 2005;17:565.
Ordeix L, et al. Cutaneous neosporosis during treatment of pemphigus foliaceus in a dog. J Am Anim Hosp Assoc. 2002;38:415.
Petersen E, et al. Neospora caninum infection and repeated abortions in humans. Emerg Infect Dis. 1999;5:278.
Reichel MP, et al. Neosporosis and hammondiosis in dogs. J Small Anim Pract. 2007;48:308.
Ruehlmann D, et al. Canine neosporosis: a case report and literature review. J Am Anim Hosp Assoc. 1995;31:174.
Schatzerg SJ, et al. Use of a multiplex polymerase chain reaction assay in the antemortem diagnosis of toxoplasmosis and neosporosis in the central nervous system of cats and dogs. Am J Vet Res. 2003;64:1507.
Silva DA, et al. Evaluation of serological tests for the diagnosis of Neospora caninum infection in dogs: optimization of cut off titers and inhibition studies of cross-reactivity with Toxoplasma gondii. Vet Parasitol. 2007;143:234.
Spencer JA, et al. Seroprevalence of Neospora caninum and Toxoplasma gondii in captive and free-ranging nondomestic felids in the United States. J Zoo Wildl Med. 2003;34:246.
Tranas J, et al. Serological evidence of human infection with the protozoan Neospora caninum. Clin Diagn Lab Immunol. 1999;6:765.
Wouda W, et al. Seroepidemiological evidence for a relationship between Neospora caninum in dogs and cattle. Int J Parasitol. 1999;29:1677.
Angulo FJ, et al. Caring for pets of immunocompromised persons. J Am Vet Med Assoc. 1994;205:1711.
Baril L, et al. Risk factors for Toxoplasma infection in pregnancy: a case-control study in France. Scand J Infect Dis. 1999;31:305.
Barrs VR, et al. Antemortem diagnosis and treatment of toxoplasmosis in two cats on cyclosporin therapy. Aust Vet J. 2006;84:30.
Bernstein L, et al. Acute toxoplasmosis following renal transplantation in three cats and a dog. J Am Vet Med Assoc. 1999;215:1123.
Brownlee L, et al. Diagnosis of naturally occurring toxoplasmosis by bronchoalveolar lavage in a cat. J Am Anim Hosp Assoc. 2001;37:251.
Burney DP, et al. Detection of Toxoplasma gondii parasitemia in experimentally inoculated cats. J Parasitol. 1999;5:947.
da Silva AV, et al. Genotyping of Toxoplasma gondii strains isolated from dogs with neurological signs. Vet Parasitol. 2005;127:23.
Davidson MG, et al. Feline immunodeficiency virus predisposes cats to acute generalized toxoplasmosis. Am J Pathol. 1993;143:1486.
Dubey JP. Duration of immunity to shedding Toxoplasma gondii oocysts by cats. J Parasitol. 1995;81:410.
Dubey JP. Comparative infectivity of oocysts and bradyzoites of Toxoplasma gondii for intermediate (mice) and definitive (cats) hosts. Vet Parasitol. 2006;140:69.
Dubey JP, et al. Fatal toxoplasmosis in dogs. J Am Anim Hosp Assoc. 1989;25:659.
Dubey JP, et al. Histologically confirmed clinical toxoplasmosis in cats: 100 cases (1952–1990). J Am Vet Med Assoc. 1993;203:1556.
Dubey JP, et al. Neonatal toxoplasmosis in littermate cats. J Am Vet Med Assoc. 1993;203:1546.
Dubey JP, et al. Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J Parasitol. 2005;91:1082.
Dubey JP, et al. Clinical Sarcocystis neurona, Sarcocystis canis, Toxoplasma gondii, and Neospora caninum infections in dogs. Vet Parasitol. 2006;137:36.
Dubey JP, Lappin MR. Toxoplasmosis and neosporosis. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. St. Louis: Saunders/Elsevier; 2006:754.
Falzone C, et al. Toxoplasma gondii brain granuloma in a cat: diagnosis using cytology from an intraoperative sample and sequential magnetic resonance imaging. J Small Anim Pract. Sep 3, 2007. [Epub ahead of print]
Hass JA, et al. Neurological manifestations of toxoplasmosis: a literature review and case summary. J Am Anim Hosp Assoc. 1989;25:253.
Hawkins EC, et al. Cytologic identification of Toxoplasma gondii in bronchoalveolar lavage fluid of experimentally infected cats. J Am Vet Med Assoc. 1997;210:648.
Lappin MR. Feline toxoplasmosis: interpretation of diagnostic test results. Semin Vet Med Surg. 1996;11:154.
Lappin MR, et al. The effect of glucocorticoid administration on oocyst shedding, serology, and cell-mediated immune responses of cats with recent or chronic toxoplasmosis. J Am Anim Hosp Assoc. 1992;27:625.
Lappin MR, et al. Polymerase chain reaction for the detection of Toxoplasma gondii in aqueous humor of cats. Am J Vet Res. 1996;57:1589.
Lappin MR, et al. Primary and secondary Toxoplasma gondii infection in normal and feline immunodeficiency virus-infected cats. J Parasitol. 1996;82:733.
Lindsay DS, et al. Mechanical transmission of Toxoplasma gondii oocysts by dogs. Vet Parasitol. 1997;73:27.
McConnell JF, et al. Eosinophilic fibrosing gastritis and toxoplasmosis in a cat. J Fel Med Surg. 2007;9:82.
Park CH, et al. Cutaneous toxoplasmosis in a female Japanese cat. Vet Pathol. 2007;44:683.
Pearce J, et al. Management of bilateral uveitis in a Toxoplasma gondii-seropositive cat with histopathologic evidence of fungal panuveitis. Vet Ophthalmol. 2007;10:216.
Pfohl JC, Dewey CW. Intracranial Toxoplasma gondii granuloma in a cat. J Fel Med Surg. 2005;7:369.
Powell CC, Lappin MR. Clinical ocular toxoplasmosis in neonatal kittens. Vet Ophthalmol. 2001;4:87.
Simpson KE, et al. Suspected toxoplasma-associated myocarditis in a cat. J Fel Med Surg. 2005;7:203.
Vollaire MR, et al. Seroprevalence of Toxoplasma gondii antibodies in clinically ill cats in the United States. Am J Vet Res. 2005;66:874.
Vyas A, et al. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci USA. 2007;104:6442.
Wallace MR, et al. Cats and toxoplasmosis risk in HIV-infected adults. JAMA. 1993;269:76.
Webb JA, et al. Cutaneous manifestations of disseminated toxoplasmosis in an immunosuppressed dog. J Am Anim Hosp Assoc. 2005;41:198.
Baer S, et al. Trypanosomiasis and laryngeal paralysis in a dog. J Am Vet Med Assoc. 1986;188:1307.
Barr SC, et al. Chronic dilatative myocarditis caused by Trypanosoma cruzi in two dogs. J Am Vet Med Assoc. 1989;195:1237.
Barr SC, et al. Trypanosoma cruzi infection in Walker Hounds from Virginia. Am J Vet Res. 1995;56:1037.
Berger SL, et al. Neurologic manifestations of trypanosomiasis in a dog. J Am Vet Med Assoc. 1991;198:132.
Bradley KK, et al. Prevalence of American trypanosomiasis (Chagas disease) among dogs in Oklahoma. J Am Vet Med Assoc. 2000;217:1853.
Fox JC, et al. Trypanosoma cruzi infection in a dog from Oklahoma. J Am Vet Med Assoc. 1986;189:1583.
Gobbi P, et al. Allopurinol is effective to modify the evolution of Trypanosoma cruzi infection in mice. Parasitol Res. 2007;101:1459.
Meurs KM, et al. Chronic Trypanosoma cruzi infection in dogs: 11 cases (1987–1996). J Am Vet Med Assoc. 1998;213:497.
Nabity MB, et al. An atypical case of Trypanosoma cruzi infection in a young English Mastiff. Vet Parasitol. 2006;140:356.
Reithinger R, et al. Chagas disease control: deltamethrin-treated collars reduce Triatoma infestans feeding success on dogs. Trans R Soc Trop Med Hyg. 2005;99:502.
Shadomy SV, et al. Combined use of enzyme-linked immunosorbent assay and flow cytometry to detect antibodies to Trypanosoma cruzi in domestic canines in Texas. Clin Diagn Lab Immunol. 2004;11:313.
Snider TG. Myocarditis caused by Trypanosoma cruzi in a native Louisiana dog. J Am Vet Med Assoc. 1980;177:247.