CHAPTER 92 Laboratory Diagnosis of Infectious Diseases
Clinical syndromes induced by infectious agents are common in small animal practice. The combination of signalment, history, and physical examination findings are used to develop a list of differential diagnoses ranking the most likely infectious agents involved. For example, young, unvaccinated cats with conjunctivitis generally are infected by herpesvirus type 1, Chlamydophila felis, or Mycoplasma felis; if a dendritic ulcer is present, herpesvirus type 1 is most likely. Results of a complete blood count, serum biochemical panel, urinalysis, radiographs, or ultrasonography can also suggest infectious diseases. For example, a dog with polyuria, polydipsia, neutrophilic leukocytosis, azotemia, pyuria, and an irregularly marginated kidney on radiographic examination likely has pyelonephritis. After making a tentative diagnosis, the clinician then must determine whether to “test or treat.” Empiric treatment is often satisfactory in simple, first-time infections of dogs or cats without life-threatening disease (see Chapter 93). However, having a definitive diagnosis is usually preferred so that treatment, prevention, prognosis, and zoonotic issues can be addressed optimally.
Documenting that the infectious agent is still present is the best way to make a definitive diagnosis. However, with some infectious agents, organism demonstration techniques have low sensitivity, are expensive, are invasive, are not adequately validated, or require specialized equipment. Antibody detection is commonly used to aid in the diagnosis of specific infectious diseases in these situations. Antibody detection is generally inferior to organism demonstration for three reasons: (1) antibodies can persist long after an infectious disease has resolved, (2) positive antibody test results do not confirm clinical disease induced by the infectious agent, and (3) in peracute infections, results of serum antibody tests can be negative if the humoral immune responses have not had time to develop. This chapter discusses the common organism demonstration and antibody detection techniques used in small animal practice.
DEMONSTRATION OF THE ORGANISM
FECAL EXAMINATION
Examination of feces can be used to help diagnose parasitic diseases of the gastrointestinal (Table 92-1; see Chapter 29) and respiratory tracts (Table 92-2; see Chapter 20). The techniques used most frequently include direct and saline smear, stained smear, fecal flotation, and Baermann technique; each procedure can easily be performed in a small animal practice.
Direct Smear
Fresh, liquid feces or feces that contain large quantities of mucus should be microscopically examined immediately for the presence of protozoal trophozoites, including those of Giardia spp. (small-bowel diarrhea), Tritrichomonas foetus (large-bowel diarrhea), and Pentatrichomonas hominis (large-bowel diarrhea). A direct saline smear can be made to potentiate observation of these motile organisms. A 2 mm × 2 mm × 2 mm quantity of fresh feces is mixed thoroughly with 1 drop of 0.9% NaCl or water. The surface of the feces or mucus coating the feces should be used because the trophozoites are most common in these areas. After application of a coverslip, the smear is evaluated for motile organisms by examining it under × 100 magnification.
Stained Smear
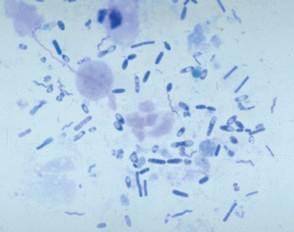
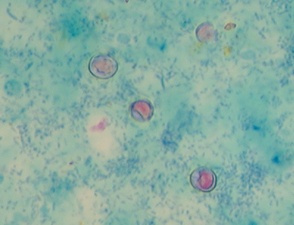
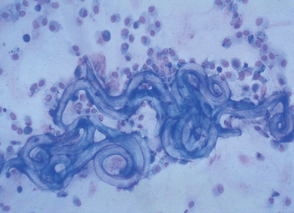
A thin smear of feces should be made from all dogs and cats with diarrhea. Material should be collected by rectal swab, if possible, to increase the chances of finding white blood cells. A cotton swab is gently introduced 3 to 4 cm through the anus into the terminal rectum, directed to the wall of the rectum, and gently rotated several times. Placing 1 drop of 0.9% NaCl on the cotton swab will facilitate passage through the anus and not adversely affect cell morphology. The cotton swab is rolled on a microscope slide gently multiple times to give areas with varying smear thickness (Fig. 92-1). After air drying, the slide can be stained. White blood cells and bacteria morphologically consistent with Campylobacter spp. (spirochetes) or Clostridium perfringens (spore-formingrods; Fig. 92-2) can be observed after staining with Diff-Quik (Medion GmbH, Dudingen, Switzerland) or Wright’s or Giemsa stains (see Cytology section). Histoplasma capsulatum or Prototheca may be observed in the cytoplasm of mononuclear cells. Methylene blue in acetate buffer (pH 3.6) stains trophozoites of the enteric protozoans. Iodine and acid methyl green stains are also used for the demonstration of protozoans. Modified acid-fast staining of a thin fecal smear can be performed in dogs and cats with diarrhea to aid in the diagnosis of cryptosporidiosis. Cryptosporidium spp. are the only enteric organisms of approximately 4 to 6 μm in diameter that will stain pink to red with acid-fast stain (Fig. 92-3). Presence of neutrophils on rectal cytology can suggest inflammation induced by Salmonella spp., Campylobacter spp., or Clostridium perfringens; fecal culture is indicated in these cases, particularly if the history supports these differentials.
Fecal Flotation
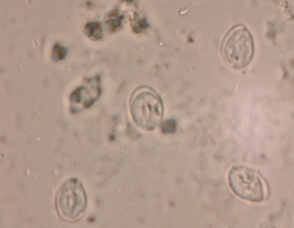
Cysts, oocysts, and eggs in feces can be concentrated to increase the sensitivity of detection. A variety of techniques are available for use in veterinary clinics. Centrifugation techniques are more sensitive than passive flotation techniques. Most eggs, oocysts, and cysts are easily identified after centrifugation in zinc sulfate solution (Box 92-1) or Sheather’s sugar solution. These procedures are particularly superior to passive flotation techniques for the demonstration of protozoan cysts (particularly Giardia spp.; Fig. 92-4). Fecal sedimentation recovers most cysts and ova but also contains debris.
 BOX 92-1 Zinc Sulfate Centrifugation Procedure
BOX 92-1 Zinc Sulfate Centrifugation Procedure
* Add 330 g zinc sulfate to 670 mL distilled water
Baermann Technique
This technique is used to concentrate motile larvae from feces. Some respiratory parasites are passed as larvated eggs but release larvae shortly after being passed in feces. Eggs or larvae from respiratory parasites can also be detected by cytologic evaluation of airway washings (Fig. 92-5).
Preservation of Feces
Feces should be refrigerated, not frozen, until assayed. If present, refrigerated Toxoplasma gondii oocysts will not likely sporulate and become infectious. In addition, refrigerated feces have less overgrowth of yeast, leading to fewer false-positive results. If a fecal sample is to be sent to a diagnostic laboratory for further analysis and will not be evaluated within 48 hours, it should be preserved. Polyvinyl alcohol, merthiolate-iodine-formalin, and 10% formalin preservation can be used. Ten percent formalin is commonly used because of its routine availability; the clinician should add 1 part feces to 9 parts formalin and mix well.
CYTOLOGY
Cytologic evaluation of exudates, bone marrow aspiration, blood smears, synovial fluid, gastric brushings, duodenal secretions, urine, prostatic washings, airway washings, fecal smears, tissue imprints, and aspiration biopsies is an inexpensive and extremely valuable tool for the documentation of infectious agents (Table 92-3). Cytologic demonstration of some infectious agents constitutes a definitive diagnosis. Morphologic appearance and Gram stain of bacteria aids in the selection of empiric antibiotics while waiting for results of culture and antimicrobial susceptibility testing (see Chapter 93).
 TABLE 92-3 Characteristic Cytologic Morphology of Small Animal Bacterial and Rickettsial Agents
TABLE 92-3 Characteristic Cytologic Morphology of Small Animal Bacterial and Rickettsial Agents
| AGENT | MORPHOLOGIC CHARACTERISTICS |
|---|---|
| Bacteria | |
| Actinomyces spp. | Gram-positive, acid-fast–negative filamentous rod within sulfur granules |
| Anaerobes | Usually occur in mixed morphologic groups |
| Bacteroides fragilis | Thin, filamentous, gram-negative rods |
| Campylobacter spp. | Seagull-shaped spirochete in feces |
| Chlamydophila felis | Large, cytoplasmic inclusions in conjunctival cells or neutrophils |
| Clostridium spp. | Large, gram-positive rods |
| Clostridium perfringens | Large, spore-forming rods in feces |
| Hemoplasmas* | Rod or ring shaped on the surface of RBCs |
| Helicobacter spp. | Tightly coiled spirochetes in gastric or duodenal brushings |
| Mycobacterium spp. | Intracytoplasmic acid-fast rods in macrophages or neutrophils |
| Nocardia spp. | Gram-positive, acid-fast–positive filamentous rod within sulfur granules |
| Leptospira spp. | Spirochetes in urine; darkfield microscopy required |
| Yersinia pestis | Bipolar rods in cervical lymph nodes or airway fluids |
| Rickettsia | |
| Ehrlichia canis | Clusters of gram-negative bacteria (morulae) in mononuclear cells |
| Ehrlichia ewingii | Clusters of gram-negative bacteria (morulae) in neutrophils |
| Anaplasma phagocytophilum | Clusters of gram-negative bacteria (morulae) in neutrophils and eosinophils |
| Anaplasma platys | Clusters of gram-negative bacteria (morulae) in platelets |
| Neorickettsia risticii | Clusters of gram-negative bacteria (morulae) in mononuclear cells |
* Previously known as Haemobartonella felis and H. canis. RBCs, Red blood cells.
For demonstration of most infectious agents, thin smears are preferred. Blood can be prepared as follows: 1 drop of blood approximately the size of a match head is placed at one end of a clean microscope slide. The short edge of another slide (i.e., spreader slide) is placed against the slide at a 30-degree angle and pulled back until the blood and the spreader slide make contact. After the blood spreads across the width of spreader slide, the slide is smoothly and quickly pushed away from the blood across the length of the slide (“push” smears). For materials other than blood, the spreader slide is laid gently on top of the material; the slides are then smoothly and rapidly pulled apart on parallel planes (“pull” smears). Cells in airway washings, prostatic washings, urine, aqueous humor, and cerebrospinal fluid (CSF) should be pelleted by centrifugation at 2000 g for 5 minutes before staining. For CSF the clinician should add 1 drop of 22% albumin or normal canine serum before centrifugation to help cells adhere to the slides. Multiple slides should always be made, if possible. After being placed on the microscope slide, the material is air dried at room temperature, fixed if indicated by the procedure used, and stained. Slides that are not stained immediately should be fixed by dipping in 100% methanol and air dried.
Cytologic specimens can be stained with routine stains; immunocytochemical techniques for certain pathogens are available (see Immunologic Techniques, p. 1287). Stains routinely used for the diagnosis of infectious diseases in small animal practice include Wright’s-Giemsa stain, Diff-Quik, Gram stain, and acid-fast stain. Immunocytochemical techniques (e.g., fluorescent antibody staining of bone marrow cells for feline leukemia virus) are only performed in refer ence or research laboratories (see Immunologic Techniques, p. 1287). The laboratory should be contacted for specific specimen handling information.
Bacterial Diseases
If bacterial disease is suspected, materials are collected aseptically and handled initially for culture (see Culture Techniques, p. 1287). After slides are prepared for cytologic evaluation, one is generally stained initially with Wright’s-Giemsa or Diff-Quik stain. If bacteria are noted Gram stain of another slide is performed to differentiate gram-positive and gram-negative agents. This information can be used to aid in the empiric selection of antibiotics (see Chapter 93). If filamentous, gram-positive rods are noted, acid-fast staining can help differentiate Actinomyces (not acid fast) from Nocardia (generally acid fast). If macrophages or neutrophils are detected, acid-fast staining is indicated to assess for Mycobacterium spp. within the cytoplasm. Bacteria can be present in small numbers, so failure to document organisms cytologically does not totally exclude the diagnosis. Bacterial culture of all samples with increased numbers of neutrophils or macrophages should always be considered. Some organisms such as Mycoplasma are rarely documented cytologically, whereas other organisms require special stains for optimal visualization. For some bacteria culture has never been successful. For example, the hemoplasmas of dogs and cats (previously called Haemobartonella felis and H. canis) can be detected on the surface of red blood cells (RBCs) but have never been successfully cultured. Until the advent of PCR (see p. 1288), documentation of infection was based on cytology; Wright’s-Giemsa stain is the best stain to use in practice for these organisms. The duration of parasitemia is short lived, and the organism commonly leaves the surface of the RBC if the blood is placed into ethylenediamine tetraacetic acid (EDTA), making it difficult to document the presence of the organism. Making thin blood smears immediately with blood that has not been placed into anticoagulant, or collecting blood into a heparinized syringe may help determine canine or feline hemoplasmas.
Rickettsial Diseases
Ehrlichia spp., Anaplasma spp., and Neorickettsia risticii (atypical ehrlichiosis) are occasionally found within the cytoplasm of cells in the peripheral blood, lymph node aspirates, bone marrow aspirates, or synovial fluid (see Chapter 96). Morulae of these genera can be found in different cell types (see Table 92-3). Wright’s-Giemsa stain is superior to Wright’s or Diff-Quik stain for the demonstration of morulae. Rickettsia rickettsii in endothelial cells lining vessels can be documented by immunofluorescent antibody staining (see Immunologic Techniques, p. 1287).
Fungal Diseases
Arthrospores and conidia of dermatophytes can be identified cytologically. Hairs plucked from the periphery of a lesion are covered with 10% to 20% potassium hydroxide on a microscope slide to clear debris. The slide is then heated, but not boiled, and examined for dermatophytes. All cats with chronic, draining skin lesions should have imprints of the lesions made and stained with Wright’s-Giemsa stain followed by microscopic examination for the characteristic round, oval, or cigar-shaped yeast phase of Sporothrix schenckii within the cytoplasm of mononuclear cells (see Chapter 98). Periodic acid–Schiff stain is superior to Wright’s-Giemsa stain for the demonstration of fungi. The cytologic appearance of the systemic fungi is presented in Table 98-1.
Cutaneous Parasitic Diseases
Cheyletiella spp., Demodex spp., Sarcoptes scabiei, Notoedres cati, and Otodectes cynotis are the most common small animal cutaneous parasites. Definitive diagnosis is based on cytologic demonstration of the organisms. Cheyletiella is demonstrated by pressing a piece of transparent tape against areas with crusts, placing the tape on a microscope slide, and examining it microscopically. Demodex spp. are most commonly detected in deep skin scrapings and follicular exudates; Cheyletiella spp., Sarcoptes scabiei, and Notoedres cati are detected in wide, more superficial scrapings. Otodectes cynotis or its eggs are detected in ceruminous exudates from the ear canals.
Systemic Protozoal Diseases
The most common systemic protozoal diseases and the cytologic appearance and location of these agents are summarized in Table 92-4. Cytologic demonstration of these agents leads to a presumptive or definitive diagnosis of the disease. Wright’s-Giemsa or Giemsa staining of thin blood films should be used to demonstrate Leishmania spp., Trypanosoma cruzi, Babesia spp., Hepatozoon americanum, and Cytauxzoon felis. Collection of blood from an ear margin vessel may increase the chances of demonstrating the protozoans found in blood, particularly Babesia spp. and Cytauxzoon felis. Toxoplasma gondii and Neospora caninum cause similar syndromes in dogs, but their tachyzoites are difficult to distinguish morphologically; immunocytochemical staining or PCR is required to differentiate these agents. These protozoans can also be distinguished by evaluating for seroconversion because antibodies are specific to each agent. With the exception of T. gondii and N. caninum, systemic protozoans are rare or regionally defined in the United States. See Chapter 99 for further discussion of these agents.
 TABLE 92-4 Characteristic Cytologic Morphology of Small Animal Systemic Protozoal Agents
TABLE 92-4 Characteristic Cytologic Morphology of Small Animal Systemic Protozoal Agents
| AGENT | MORPHOLOGIC CHARACTERISTICS |
|---|---|
| Babesia canis | Paired piroplasms (2.4 × 5.0 μm) in circulating RBCs |
| Babesia gibsoni | Single piroplasms (1.0 × 3.2 μm) in circulating RBCs |
| Cytauxzoon felis | Piroplasms (1.0 × 1.5 μm “signet ring” form; 1.0 × 2.0 μm oval form; 1.0 μm round form) in circulating RBCs; macrophages or monocytes of lymph node aspirates, splenic aspirates, or bone marrow |
| Hepatozoon canis and H. americanum | Gamonts in circulating neutrophils and monocytes |
| Leishmania spp. | Ovoid to round amastigotes (2.5-5.0 μm × 1.5-2.0 μm) in macrophages found on imprints of exudative skin lesions, lymph node aspirates, or bone marrow aspirates |
| Neospora caninum | Free or intracellular (macrophages or monocytes) tachyzoites (5-7 μm × 1-5 μm) in CSF, airway washings, or imprints of cutaneous lesions |
| Toxoplasma gondii | Free or intracellular (macrophages or monocytes) tachyzoites (6 × 2 μm) in pleural effusions, peritoneal effusions, or airway washings |
| Trypanosoma cruzi | Flagellated trypomastigotes (one flagellum; 15-20 μm long) free in whole blood, lymph node aspirates, and peritoneal fluid |
RBCs, Red blood cells; CSF, cerebrospinal fluid.
Viral Diseases
Rarely, viral inclusion bodies are detected cytologically after staining with Wright’s-Giemsa. Distemper virus infection causes inclusions in circulating lymphocytes, neutrophils, and erythrocytes of some dogs. Rarely, feline infectious peritonitis virus results in intracytoplasmic inclusions in circulating neutrophils. Feline herpesvirus 1 (FHV-1) transiently results in intranuclear inclusion bodies in epithelial cells.
TISSUE TECHNIQUES
Tissues collected from animals with suspected infectious diseases can be evaluated by several different techniques. Tissue samples should be aseptically placed in appropriate transport media for culture procedures or inoculated into laboratory animals, if indicated, before further handling.
Gently blotting the cut edge of the tissue on a paper towel to remove excess blood and then lightly touching the tissue multiple times to a microscope slide make tissue impressions for cytologic examination. Tissue specimens can then be frozen, placed into 10% buffered formalin solution, or placed into glutaraldehyde-containing solutions. Frozen specimens are generally superior for immunohistochemical staining and PCR. Routine histopathologic evaluation is performed on formalin-fixed tissues. Special stains can be used to maximize the identification of some infectious agents. The clinician should alert the histopathology laboratory to the infectious agents most suspected to allow for appropriate stain selection. Glutaraldehyde-containing fixatives are superior to other fixatives for electron microscopic examination of tissues; this technique can be more sensitive than other procedures for demonstration of viral particles.
CULTURE TECHNIQUES
Bacteria, fungi, viruses, and some protozoans can be cultured. In general, a positive culture can be used to establish a definitive diagnosis. Bacterial culture can be combined with antimicrobial susceptibility testing to determine optimal drug therapy. Successful culture depends on collecting the optimal materials without contamination, transporting the materials to the laboratory as quickly as possible in the most appropriate medium to minimize organism death or overgrowth of nonpathogens, and using the most appropriate culture materials.
Culture results of body systems with normal bacterial and fungal flora, including the skin, ears, mouth, nasal cavity, trachea, feces, and vagina, are the most difficult to interpret. Finding positive culture results and inflammatory cells cytologically suggests the organism is inducing disease. Culture of a single agent, particularly if the organism is relatively resistant to antimicrobials, is more consistent with a disease-inducing infection than if multiple, antibiotic-susceptible bacteria are cultured. Materials for routine aerobic bacterial culture can be placed on sterile swabs if the swabs remain moist and are placed on appropriate culture media within 3 hours of collection. If a delay of greater than 3 hours is expected, swabs containing transport medium should be used. These swabs should be refrigerated or frozen to inhibit bacterial growth if cultures are not to be started within 4 hours; some bacteria will grow more rapidly than others, potentially masking fastidious organisms. Most aerobes will survive at 4° C (routine refrigeration temperature) in tissue or on media-containing swabs for 48 hours. Solid-phase transport media that will support the growth of most aerobes, anaerobes, Mycoplasma spp., and fungi for several days if refrigerated are also routinely available. Routine aerobic culture is generally successful on fluid samples (e.g., urine, airway washings) stored at 20° C for 1 to 2 hours, 4° C for 24 hours, or 4° C for 72 hours if placed in transport medium.
Anaerobes can be successfully cultured from fluid collected aseptically into a syringe and the needle covered with a rubber stopper if the material is to be placed on culture media within 10 minutes of collection. Because of time limitations, transport media is generally required for samples from animals with suspected anaerobic infections. These media will support the growth of most anaerobes for 48 hours if stored at 4° C.
Samples for blood culture should be collected aseptically from a large vein after surgical preparation of the skin. In general, three 5-mL samples are collected over a 24-hour period in stable patients or at 1- to 3-hour intervals in septic patients. Unclotted whole blood is placed directly into transport media that will support the growth of aerobic and anaerobic bacteria, and it is incubated at 20° C for 24 hours. Culture for Bartonella spp. from the blood of dogs or cats is generally performed on a 1.5-mL whole blood sample collected aseptically and placed in an EDTA-containing tube (see Chapter 95).
Culture of feces for Salmonella spp., Campylobacter spp., and Clostridium perfringens is occasionally indicated in small animal practice. Approximately 2 to 3 g of fresh feces should be submitted to the laboratory immediately for optimal results; however, Salmonella and Campylobacter are usually viable in refrigerated fecal specimens for 3 to 7 days. To increase the likelihood of achieving positive culture results, a transport medium should be used if a delay is expected. The laboratory should be notified of the suspected pathogen so that appropriate culture media can be used.
Mycoplasma and Ureaplasma cultures are most commonly performed on airway washings, synovial fluid, exudates from chronic draining tracts in cats, urine from animals with chronic urinary tract disease, and the vagina of animals with genital tract disease. Samples should be transported to the laboratory in Amies medium or modified Stuart bacterial transport medium. Mycoplasma spp. culture should be specifically requested.
Mycobacterium spp. grow very slowly, and culture is often limited by overgrowth of other bacteria. Special medium is required; therefore the laboratory should be specifically instructed to culture for Mycobacterium spp. Tissue samples or exudates from animals with suspected Mycobacterium spp. infection should be refrigerated immediately after collection and transported to the laboratory as soon as possible. Exudates should be placed in transport media.
Cutaneous fungal agents can be cultured in the small animal office by using routinely available culture media. Materials from dogs or cats with suspected systemic fungal infection can be transported to the laboratory as described for bacteria, and the laboratory can be told specifically that fungal culture is needed. The yeast phase of the systemic fungi occurs in vivo and is not zoonotic; the mycelial phase of Blastomyces, Coccidioides, and Histoplasma grows in culture and will infect human beings. Thus in-house culture for these agents is not recommended.
Viral agents can be isolated from tissues or secretions at some laboratories. Contact the laboratory before submitting samples. Samples should be collected aseptically as for bacteria, placed in transport media, and immediately refrigerated to inhibit bacterial growth. The samples should be transported to the laboratory on cold packs but not frozen.
IMMUNOLOGIC TECHNIQUES
Infectious agents or their antigens can be detected in body fluids, feces, cells, or tissues by using immunologic tech niques. In general, polyclonal or monoclonal antibodies against the agent in question are used in a variety of different test methods, including direct fluorescent antibody assay with cells or tissue, agglutination assays, and enzyme-linked immunosorbent assay (ELISA). Sensitivities and specificities vary by test but are generally high for most assays. Positive results with these tests generally prove infection; this is in contrast to antibody detection procedures, which only document exposure to an infectious agent. Contact the laboratory for details concerning specimen transport before collection.
Commercially available assays are available for the detection of antigens of Dirofilaria immitis, Cryptococcus neoformans, Blastomyces dermatitidis, and FeLV in serum or plasma. The Cryptococcus neoformans latex agglutination procedure can also be performed on aqueous humor, vitreous humor, and CSF.
Parvovirus, Cryptosporidium spp., and Giardia spp. antigen detection procedures are available for use with feces. Parvovirus assays detect both canine and feline parvovirus antigen and may be affected transiently by administration of modified-live vaccines. Most Giardia antigen tests marketed for use with human feces and the test labeled for use with dog or cat feces detect the Giardia assemblages that infect dogs or cats. Samples are occasionally antigen positive but cyst negative on fecal flotation. In this situation whether the antigen test is falsely positive or the fecal flotation is falsely negative. None of the currently available C. parvum antigen tests marketed for use with human feces consistently detects C. felis or C. canis and should therefore not be used with feces from dogs and cats.
Immunocytochemistry and immunohistochemistry techniques are widely available for the documentation of a variety of infectious diseases. These procedures are particularly valuable for the detection of viral diseases, detection of agents present in small numbers, and for differentiation among agents with similar morphologic features. In general, these techniques are more sensitive and specific than histopathologic techniques and are comparable to culture. For example, focal feline infectious peritonitis granulomatous disease can be documented by immunohistochemical staining (see Chapter 97).
POLYMERASE CHAIN REACTION
PCR amplifies small quantities of DNA to detectable levels (Fig. 92-6). With a reverse transcriptase step, RNA is converted to DNA; therefore the technique can also be used to detect RNA (RT-PCR). In general PCR is much more sensitive than cytologic or histopathologic techniques and is comparable to culture and laboratory animal inoculation. PCR assays are of great benefit for documentation of infections, particularly if the organism in question is difficult to culture (e.g., Ehrlichia spp.) or cannot be cultured (e.g., hemoplasmas). Specificity can be quite high depending on the primers used in the reaction. For example, primers can be designed to detect one bacterial genus but not others. Primers can also be designed to identify only one species. For example, a PCRassay can be developed to detect all Ehrlichia spp. or just one species, such as E. canis.
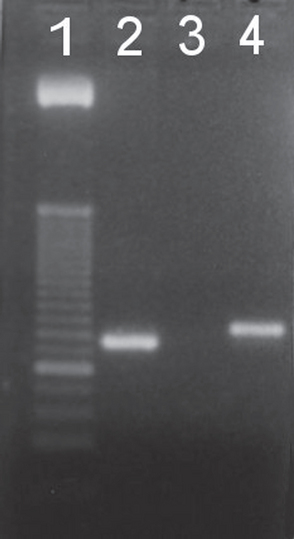
FIG 92-6 Photograph of a PCR assay for hemoplasmas showing the two different band sizes that help differentiate species: Mycoplasma haemofelis (Lane 2) and Candidatus M. haemominutum (Lane 4). Lane 1 is a base pair ladder and Lane 3 is a negative sample. In this assay Candidatus M. turicensis is included in the M. haemofelis amplicon.
Because of the inherent sensitivity of the reaction, PCR can give false-positive results if sample contamination occurs during collection or at the laboratory performing the procedure. False-negative results can occur if the sample is handled inappropriately; this is of particular importance for detection of RNA viruses by RT-PCR. Results may also be affected by treatment. Another potential problem is that no standardization exists among commercial laboratories offering PCR techniques; in addition, no external quality control exists (see Chapter 97).
Although PCR assays can be one of the most sensitive for documentation of infections, positive test results do not always prove that the infection is causing clinical illness. For example, because the technique detects DNA of both live and dead organisms, positive test results may be achieved even if the infection has been controlled. When the organism being tested for commonly infects the background population of healthy pets, interpretation of results for a single animal can be difficult. For example, FHV-1 commonly infects cats and is commonly carried by healthy cats. Thus although PCR is the most sensitive way to document infection by FHV-1, the positive predictive value for disease of a FHV-1 PCR result is actually quite low. In one study more positive FHV-1 PCR results were detected in the healthy control group than in the group with conjunctivitis (Burgesser et al., 1999). In addition, the currently available PCR assays for FHV-1 also amplify modified-live vaccine strains, so a positive result does not even indicate presence of a pathogenic strain. Real-time PCR can be used to determine the amount of microbial DNA in a sample. The DNA load may correlate to the presence of disease for some agents. Because of these findings, small animal practitioners must carefully assess the predictive values of currently available PCR assays and the expertise and reliability of the laboratory that will be performing the assays. New PCR assays are being developed almost daily. See specific chapters for a discussion of the use of PCR for the detection of the agents.
ANIMAL INOCULATION
Animal inoculation can be used to identify some infectious diseases. For example, oocysts of Toxoplasma gondii cannot be distinguished morphologically from those of Hammondia hammondi or Besnoitia darlingi; only T. gondii is infectious for human beings. T. gondii can be differentiated from the other coccidians by inoculation of sporulated oocysts into mice and monitoring for T. gondii–specific antibody production. However, because live animals are required, animal inoculation is rarely used in small animal practice.
ELECTRON MICROSCOPY
Electron microscopy is a highly sensitive procedure for organism identification in body fluids and tissues. Glutaraldehyde-containing fixatives are used most commonly. One of the most clinically relevant uses of electron microscopy is for the detection of viral particles in feces of animals with gastrointestinal signs of diseases. Approximately 1 to 3 g of feces without fixative should be transported to the laboratory (e.g., Diagnostic Laboratory, Colorado State University, College of Veterinary Medicine and Biomedical Sciences, Fort Collins) by overnight mail on cold packs.
ANTIBODY DETECTION
SERUM
A variety of different methods exists for detecting serum antibodies against infectious agents; complement fixation, hemagglutination inhibition, serum neutralization, agglutination assays, agar gel immunodiffusion, indirect fluorescent antibody assay (IFA), ELISA, and Western blot immunoassay are commonly used methods. Complement fixation, hemagglutination inhibition, serum neutralization, and agglutination assays generally detect all antibody classes in a serum sample. Western blot immunoassay, IFA, and ELISA can be adapted to detect specific immunoglobulin (Ig) M, IgG, or IgA responses.
Comparison of IgM, IgA, and IgG antibody responses against an infectious agent can be used to attempt to prove recent or active infection. In general, IgM is the first antibody produced after antigenic exposure (Fig. 92-7). Antibody class shift to IgG occurs in days to weeks. Serum and mucosal IgA immune responses have also been studied for some infectious agents, including T. gondii, feline coronaviruses, and Helicobacter felis.
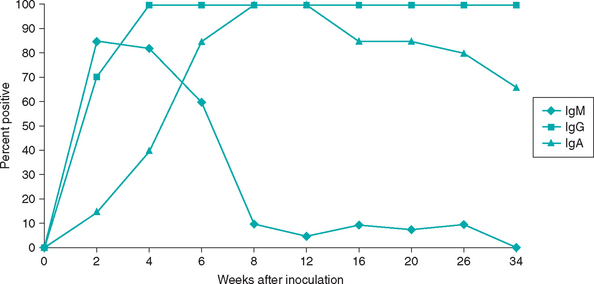
FIG 92-7 Serum Toxoplasma gondii IgM, IgG, and IgA immune responses after experimental inoculation in cats.
Timing of antibody testing is important. In general, serum antibody tests in puppies and kittens cannot be interpreted as specific responses until at least 8 to 12 weeks of age because of the presence of antibodies from the dam passed to the puppy or kitten in the colostrum. Most infectious agents can induce disease within 3 to 10 days after initial exposure; with many assays serum IgG antibodies are usually not detected until 1 to 2 weeks after initial exposure. On the basis of these facts, falsely negative serum antibody tests during acute disease can be common in small animal practice. If specific serum antibody testing is initially negative in an animal with acute disease, repeat antibody testing should be performed in 2 to 3 weeks to assess for seroconversion. Documentation of increasing antibody titers is consistent with recent or active infection. Assessment of both the acute and convalescent sera in the same assay on the same day is preferable to avoid interassay variation.
Sensitivity is the ability of an assay to detect a positive sample; specificity is the ability of an assay to detect a negative sample. Sensitivity and specificity vary with each assay. Positive predictive value is the ability of a test result to predict presence of disease; negative predictive value is the ability of a test result to predict absence of disease. Many of the infectious agents encountered in small animal practice infect a large percentage of the population, resulting in serum antibody production. However, they only induce disease in a small number of animals in the infected group. Examples include coronaviruses, canine distemper virus, T. gondii, Bartonella spp., and Borrelia burgdorferi. For these examples, even though assays with good sensitivity and specificity for the detection of serum antibodies are available, the predictive value of a positive test for presence of disease is extremely low. This is because antibodies are commonly detected in nondiseased animals. Diagnostic utility of some serologic tests is also limited because of the presence of antibodies induced by vaccination. Examples include feline coronaviruses, some B. burgdorferi assays, FHV-1, parvoviruses, FIV calicivirus, and canine distemper virus.
The clinician should interpret positive results in serum antibody tests only as evidence of present or prior infection by the agent in question. Recent or active infection is suggested by the presence of IgM, an increasing antibody titer over 2 to 3 weeks, or seroconversion (negative antibody result on the first test and positive antibody result on convalescent testing). However, detection of recent infection based on antibody testing does not always prove disease because of the agent in question. Conversely, failure to document recent or active infection based on serologic testing does not exclude a diagnosis of clinical disease. For example, many cats with toxoplasmosis develop clinical signs of disease after serum antibody titers have reached their plateau. The magnitude of antibody titer does not always correlate with active or clinical disease. For example, many cats with clinical toxoplasmosis have IgM and IgG titers that are at the low end of the titer scale; conversely, many healthy cats have IgG titers greater than 1 : 16,384 years after infection with T. gondii. The clinical diagnosis of an infectious disease usually includes the combination of the following
BODY FLUIDS
Some infectious agents induce disease of the eyes or central nervous system (CNS). Documentation of agent-specific antibodies in aqueous humor, vitreous humor, or CSF can be used to support the diagnosis of infection of these tissues. Quantification of ocular and CSF antibodies is difficult to interpret if serum antibodies and inflammatory disease are present; serum antibodies leak into ocular fluids and CSF in the face of inflammation. Detection of local production of antibodies within the eye or CNS has been used to aid in the diagnosis of canine distemper virus infection, feline toxoplasmosis, and feline bartonellosis (see Chapters 95, 97, and 99). The following is a method to prove local antibody production by the eye or CNS:
A ratio greater than 1 suggests that the antibody in the aqueous humor or CSF was produced locally. This formula has been used extensively in the evaluation of cats with uveitis. Approximately 60% of cats with uveitis in the United States have T. gondii–specific IgM, IgA, or IgG values greater than 1 (see Chapter 99). The technique was also used to help prove that FHV-1 and Bartonella henselae are causes of uveitis in cats.
Burgesser KM, et al. Comparison of PCR, virus isolation, and indirect fluorescent antibody staining in the detection of naturally occurring feline herpesvirus infections. J Vet Diagn Invest. 1999;11:122.
Dryden MW, et al. Accurate diagnosis of Giardia spp and proper fecal examination procedures. Vet Therapeutics. 2006;7:4.
Jensen WA, et al. Prevalence of Haemobartonella felis infection in cats. Am J Vet Res. 2001;62:604.
Lappin MR, et al. Enzyme-linked immunosorbent assays for the detection of Toxoplasma gondii-specific antibodies and antigens in the aqueous humor of cats. J Am Vet Med Assoc. 1992;201:1010.
Lappin MR, et al. Polymerase chain reaction for the detection of Toxoplasma gondii in aqueous humor of cats. Am J Vet Res. 1996;57:1589.
Lappin MR, et al. Laboratory diagnosis of protozoal infections. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 2. Philadelphia: WB Saunders; 1998:437.
Lappin MR. Microbiology and infectious disease. In: Willard MD, et al, editors. Small animal clinical diagnosis by laboratory methods. ed 3. Philadelphia: WB Saunders; 1999:288.
Lappin MR, et al. Bartonella spp. antibodies and DNA in aqueous humor of cats. Fel Med Surg. 2000;2:61.
Lappin MR, et al. Use of serologic tests to predict resistance to feline herpesvirus 1, feline calicivirus, and feline parvovirus infection in cats. J Am Vet Med Assoc. 2002;220:38.