CHAPTER 102 Diagnostic Testing for Autoimmune Disease
CLINICAL DIAGNOSTIC APPROACH
The diagnostic approach to a dog or cat with suspected immune-mediated disease depends on the clinical presentation and organ(s) involved. A complete history including questions regarding environmental or drug exposures, previous medical history, exposure to infectious agents, and vaccination history should be obtained. A thorough physical examination should also be performed. The next step is to define the extent of the problem and rule out other more common causes of the clinical signs. A typical minimal database includes a complete blood count, serum biochemical profile, and urinalysis. Because many immune-mediated diseases are characterized by fever and leukocytosis, ruling out infectious agents as the primary cause of the clinical signs is important before pursuing other less common causes. The diagnostic evaluation for immune-mediated disease is similar to that for fever of unknown origin. Bacterial culture of the urine, blood, or both—testing for common viral pathogens such as feline leukemia virus, feline immunodeficiency virus, and feline infectious peritonitis—and diagnostic imaging (thoracic and abdominal radiographs, abdominal ultrasound) are important. Investigation for vector-borne diseases such as ehrlichiosis, anaplasmosis, borreliosis, and leishmaniasis as well as more fastidious organisms such as mycoplasma and L-forms, is usually only considered once more common bacterial and viral infections have been excluded because these tests are more expensive and the results are often not immediately available. The specific infectious agents tested for depend on whether the patient is a dog or cat as well as the disease presentation and geographic location because many infectious diseases have regional distributions.
If infection is ruled out or considered unlikely, further diagnostic evaluation should focus on organs identified as potentially involved according to the physical examination and results of the minimal database and diagnostic imaging. Organ-specific diagnostic tests may include evaluation of joint or cerebrospinal fluid, quantification of urine protein excretion, and biopsy of affected organs. (These tests are discussed in more detail in the sections on specific diseases.)
Specific tests of immune dysfunction are indicated once infectious and neoplastic diseases have been excluded and when the organ system(s) of interest has been identified. For example, in a dog with a regenerative anemia the clinician should consider doing a direct antiglobulin (Coombs) test, whereas in a dog with polyarthritis a test for rheumatoid factor would be indicated. Immune panels that include a selection of tests with different indications are rarely necessary and may result in excessive testing and results that are difficult to interpret. For example, a positive Coombs test has little relevance in a dog that is not anemic.
SPECIFIC DIAGNOSTIC TESTS
COOMBS TEST (DIRECT ANTIGLOBULIN TEST)
The direct Coombs test, or direct antiglobulin test (DAT), detects the presence of antibody and/or complement bound to patient red blood cell (RBC) membranes. The test is used for diagnosis of immune-mediated hemolytic anemia (IMHA) and for diagnosis of hemolytic transfusion reactions. The DAT uses antidog or anticat antiglobulin antibody produced in a different species (usually goats or rabbits); the reagents are species specific. The DAT is best performed on ethylenediamine tetraacetic acid (EDTA) anticoagulated blood at body temperature (37° C). Most frequently a combined Coombs reagent containing goat anticanine immunoglobulin (Ig) G, IgM, and complement component C3 is used. Addition of the Coombs reagent to washed patient RBCs results in agglutination if more than approximately 100 IgG antibody or C3 molecules are bound to the RBCs. Because the end point of the test is agglutination, the test cannot be interpreted if spontaneous agglutination persists after washing the RBCs. Results of the DAT may be reported in various forms depending on the laboratory: positive or negative, 1+ to 4+ agglutination, or as the lowest dilution of the reagent that results in agglutination. Modifications of the DAT that may improve diagnostic performance include use of monospecific antisera (usually IgG, IgM, and C3), and using more dilutions of the reagents than typically performed. The pattern of the antibody binding (IgG vs. IgM, vs. C3) can be used to increase specificity of the DAT because some patterns of binding tend to be more specific for IMHA than others. For example, in dogs with IMHA the most common pattern of binding is IgG and C3, whereas C3 alone is most commonly seen in dogs with nonhemolytic disorders and underlying inflammatory or neoplastic diseases. Using more dilutions of reagent can potentially improve the sensitivity of the DAT because it allows detection of the prozone effect in which a lack of reactivity is observed with high concentrations of antibody. Another modification of the DAT involves performing the test at 4° C to identify cold-acting agglutinins. This test should only be used in animals with clinical signs of cold agglutinin disease (e.g., ear or tail tip necrosis) because nonspecific RBC agglutination occurs commonly at 4° C in many normal dogs. In some dogs with IMHA that have spontaneous agglutination, agglutination will resolve after washing of the RBCs. In this scenario a DAT may still be indicated because resolution of a previously positive DAT may be useful for disease monitoring. Both false-positive and false-negative results may occur with the DAT (Box 102-1). For this reason the DAT should be interpreted in the light of other clinical and clinicopathologic information. Recognizing that a positive Coombs test does not distinguish primary from secondary IMHA is also important (see Chapter 104). Other more sensitive techniques such as enzyme-linked antiglobulin tests, flow cytometric techniques, and column agglutination assays have also be used to detect the presence of antibody on RBCs; however, these tests are not yet widely available in commercial laboratories.
 BOX 102-1 Causes of False-Positive and False-Negative Results for the Direct Antiglobulin (Coombs Test)
BOX 102-1 Causes of False-Positive and False-Negative Results for the Direct Antiglobulin (Coombs Test)
| FALSE-POSITIVE RESULT | FALSE-NEGATIVE RESULT |
|---|---|
The indirect antiglobulin test is used to detect antibody in patient serum that is capable of binding to RBCs collected from a different animal. This test is both less sensitive and less specific than the direct test and is rarely used clinically except when screening blood donor serum for anti–dog erythrocyte antigen antibodies or as part of some cross-matching procedures.
SLIDE AGGLUTINATION TEST
The slide agglutination test is used to detect the presence of spontaneous agglutination of RBCs. Spontaneous agglutination (autoagglutination) is a three-dimensional clustering of RBCs that occurs from cross-linking of RBC surface-associated antibodies. Autoagglutination occurs as a result of the presence of either high titer IgG or IgM on the RBC membrane. Agglutination must be distinguished from rouleaux formation (stacking of RBCs that occurs most often in the presence of high globulin concentrations). To evaluate for the presence of agglutination, 1 drop of saline should be added to 5 to 10 drops of blood and mixed. The RBC suspension is then evaluated both by macroscopic and microscopic examination at a temperature as close to 37° C as possible. The temperature is important because clinically insignificant cold-acting agglutinins are common in normal dogs. In most laboratories spontaneous autoagglutination that persists after saline dilution is considered diagnostic for IMHA. In other laboratories only RBC agglutination that persists after three washings of the RBCs is considered diagnostic for IMHA.
ANTIPLATELET ANTIBODIES
Detection of platelet surface–associated antibody (direct antibody) or serum platelet bindable antibody (indirect antibody) may be useful in evaluation of dogs and cats with suspected immune-mediated thrombocytopenia. Tests for antiplatelet antibody are most commonly performed by using flow cytometric techniques. Detection of platelet surface–associated IgG is more sensitive than detection of serum platelet–bindable antibodies, presumably because the majority of antiplatelet antibody is bound to platelets rather than free in the circulation. The direct assay has a sensitivity of greater than 90% in dogs with confirmed idiopathic thrombocytopenic purpura (ITP). Because of the high sensitivity of the direct assay, a negative result for platelet surface–associated antibody makes a diagnosis of ITP unlikely. Detection of antiplatelet antibodies by either the direct or indirect technique implies an immune-mediated pathogenesis for thrombocytopenia but is not specific for primary immune-mediated thrombocytopenia. Many infectious and neoplastic diseases as well as drug exposure may cause thrombocytopenia by immune-mediated mechanisms; therefore blood samples from such patients may be positive for platelet-associated antibody. A flow cytometric assay for platelet surface–associated antibody for both dogs and cats is currently available at Kansas State University. The test requires 2 mL of EDTA blood and currently costs $60 plus shipping. Blood samples should be shipped overnight on ice.
MEGAKARYOCYTE DIRECT IMMUNOFLUORESCENCE
Antibodies directed against megakaryocytic cells in the bone marrow may be detected by direct immunofluorescence (see below for more details on immunofluorescent testing). Variable sensitivity (30% to 80%) for diagnosis of ITP has been reported. This test is also offered at Kansas State University and costs $40. A bone marrow aspirate is required, and slides should be air dried before being sent to the testing laboratory.
ANTINUCLEAR ANTIBODY TEST
Measurement of antinuclear antibody (ANA) is useful in the evaluation of dogs and cats with suspected systemic lupus erythematosus (SLE). SLE should be suspected in patients with evidence of an immune-mediated process affecting a minimum of two organ systems (see Chapter 104). Antinuclear antibodies are heterogenous antibodies directed against nuclear antigens. They are typically detected by immunofluorescent staining of frozen sections of rat liver or tissue culture monolayers of human epithelial cell lines. Results are reported as a titer that is the highest dilution of patient serum that causes definitive immunofluorescent nuclear staining. Various patterns of nuclear staining (diffuse, speckled, peripheral, and nucleolar) can be identified, but the clinical significance of the various staining patterns is still under investigation in dogs and cats. Measurement of ANA antibodies is sensitive for diagnosis of SLE in dogs and cats, although ANA-negative cases of SLE do occur. In one study of 75 dogs with SLE, 100% had a positive ANA titer (Fournel et al., 1992). In most cases the ANA titer was greater than 1:256 and the magnitude of the titer correlated with disease severity. Other studies have demonstrated lower sensitivity of the ANA for diagnosing SLE. The variability in diagnostic sensitivity probably arises from differences in stringency in the diagnostic criteria for confirming a diagnosis of SLE and variability among laboratories in assay sensitivity and specificity. Many normal animals have low positive titers for ANA, so a cut-off for a significant positive titer should be established for each individual laboratory. The cut-off titer varies depending on the substrate and techniques used by the laboratory. Low positive ANA titers may also occur after exposure to certain drugs and in animals with chronic inflammatory or neoplastic diseases. ANAs are detected in 10% to 20% of dogs with seroreactivity to Bartonella vinsonii, Ehrlichia canis, and Leishmaniasis infantum. Dogs with seroreactivity to multiple pathogens are more likely to be ANA positive. Chronic or high-dose corticosteroid treatment may decrease the ANA titer.
LUPUS ERYTHEMATOSUS TEST
The lupus erythematosus (LE) test is a highly specific test for SLE but is rarely used clinically because it lacks sensitivity and the ANA test is more sensitive and less time consuming. LE cells are neutrophils that contain phagocytosed nuclear material. The test is performed in vitro. Blood collected from the patient is allowed to clot and is damaged to release free nuclei. If ANA is present it binds to nuclear material and the resulting complex is phagocytosed by neutrophils and can be identified as an LE cell by visual inspection. LE cells may also rarely be identified in vivo in blood, bone marrow, or joint fluid and, when present, are highly suggestive of SLE. The LE cell test is more sensitive to the effects of steroids than is the ANA titer. The test has been reported to be positive in the blood of 30% to 90% of dogs with SLE but may also be positive in other immune or neoplastic disorders.
RHEUMATOID FACTOR
Rheumatoid factor (RF) is antibody directed against an individual’s own IgG. The antibody is directed against sites on the Fc portion of immunoglobulin molecules that become exposed only after antibody binds to antigen. The test is used as one of the diagnostic criteria for rheumatoid arthritis; however, the utility of the test is limited by a lack of sensitivity and specificity. The most common technique for detection of RF is the Rose-Waaler, test which uses sheep RBCs sensitized to rabbit IgG. If RF is present in patient serum, agglutination occurs. The test is performed on refrigerated serum. Samples should not be frozen because RF activity may be destroyed. Only 40% to 75% of dogs with rheumatoid arthritis are positive for RF, so a negative titer does not rule out the disease. In addition, any disease with longstanding immune complex formation may eventually cause RF, so a positive titer should not be the sole criterion for a diagnosis of rheumatoid arthritis.
IMMUNOFLUORESCENCE AND IMMUNOHISTOCHEMISTRY
In many type II and type III immune-mediated diseases the presence of antibody in fixed tissues (e.g., kidney, skin) can be detected by immunofluorescent or immunoperoxidase techniques. Numerous variations on these methods exist, but in general, sections of tissue are labeled with a primary antibody (e.g., rabbit antidog IgG) and then a secondary antibody is added (e.g., antirabbit IgG), which has been conjugated to either fluorescein or the enzyme peroxidase. If antibodies are present in the tissue sample, apple green fluorescence is seen under ultraviolet light with immunofluorescent staining. In the case of immunoperoxidase peroxide, when a substrate is added in the presence of hydrogen peroxide, deposition of a brown color can be visualized with the light microscope. Tissue samples for immunofluorescent testing should be collected in Michel’s medium. Routinely fixed tissue can be used for immunohistochemistry. Common uses for immunofluorescent staining include evaluation of renal biopsies in dogs with suspected glomerulonephritis, detection of antibodies directed against megakaryocytic cells in the bone marrow, and evaluation of skin biopsies from patients with suspected autoimmune skin disease.
AUTOIMMUNE PANELS
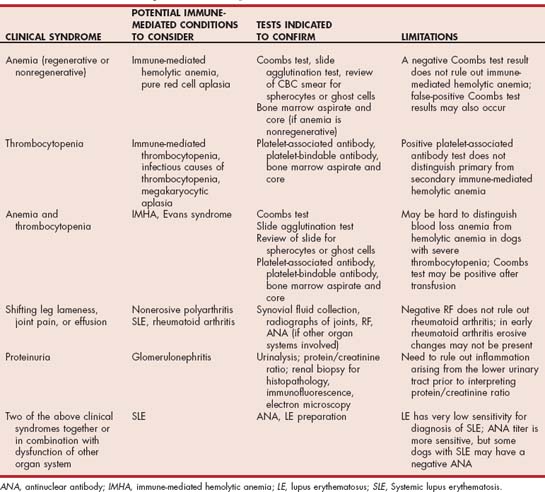
Many laboratories offer an immune panel that typically includes a complete blood count and platelet count, Coombs test, ANA, and RF. It would be unusual for all these tests to be appropriate in an individual patient (Table 102-1). In addition to the cost of running such a panel, the significance of a positive test may be difficult to determine in patients in which the test was initially not indicated. For these reasons the clinician is encourage to pick individual tests rather than automatically choosing an autoimmune panel in a dog or cat with suspected autoimmune or immune-mediated disease.
Fournel C, et al. Canine systemic lupus erythematosus I: a study of 75 cases. Lupus. 1992;1:133.
Lewis DC, et al. Canine idiopathic thrombocytopenia. J Vet Intern Med. 1996;10:207.
Smee NM, et al. Measurement of serum antinuclear antibody titer in dogs with and without systemic lupus erythematosus: 120 cases (1997-2005). J Am Vet Med Assoc. 2007;230:1180.
Smith BE, et al. Antinuclear antibodies can be detected in dog sera reactive to Bartonella vinsonii subsp., berkhoffii, Ehrlichia canis, or Leishmania infantum antigens. J Vet Intern Med. 2004;18:47.
Wardrop KJ. The Coombs’ test in veterinary medicine: past, present, and future. Vet Clin Pathol. 2005;34:325.