CHAPTER 104 Common Immune-Mediated Diseases
IMMUNE-MEDIATED HEMOLYTIC ANEMIA
Etiology
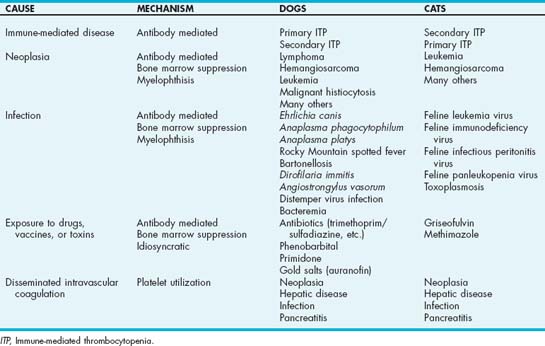
Immune-mediated hemolytic anemia (IMHA) is a clinical syndrome in which anemia results from the accelerated destruction of red blood cells (RBCs) by immune-mediated mechanisms (see Chapter 83). IMHA is the most common cause of hemolytic anemia in dogs but is much less common in cats. In primary IMHA (true autoimmune hemolytic anemia) antibodies are directed against RBC membrane antigens. These antigens have not been well characterized in the dog or cat, but antibodies directed against spectrin, band 3, and the family of erythrocyte membrane glycoproteins, known as glycophorins, have been identified. True autoimmune hemolytic anemia may also be a manifestation of systemic lupus erythematosus (SLE). In secondary IMHA an underlying disease is identified as a precipitating factor for the immune-mediated hemolytic process. Examples of causes of secondary IMHA include infection and neoplastic diseases (Box 104-1). Secondary IMHA may also occur after exposure to certain drugs, venoms, and possibly vaccines. Most studies in dogs suggest that primary autoimmune hemolytic anemia is more common than the secondary form, although the frequency of identification of a secondary cause likely depends in part on how thoroughly the clinician searches for it. Secondary IMHA is more common than primary IMHA in cats.
 BOX 104-1 Infectious Diseases Implicated as Causing IMHA in Dogs and Cats
BOX 104-1 Infectious Diseases Implicated as Causing IMHA in Dogs and Cats
IMHA, Immune-mediated hemolytic anemia.
The most common antibody classes identified on the RBC in both dogs and cats with IMHA are immunoglobulin (Ig) G and IgM, with IgA being least common. Complement is usually also present on the RBC. In secondary IMHA antibodies may be directed against antigens that adsorb to the RBC membrane or against a microbial antigen combined with a self-determinant, with the RBCs destroyed as an “innocent bystanders.” Alternatively, previously hidden membrane antigens may be exposed by membrane damage from microbes or toxins, or microbial and drug antigens may be cross-reactive with self-determinants. Lastly, nonspecific activation of lymphocytes can result in formation of self-reacting lymphocytes in any chronic inflammatory process.
Recent vaccination has been implicated in the pathogenesis of IMHA. The occurrence of IMHA within 2 to 4 weeks of vaccination has been a clinical observation of concern to many owners and veterinarians. In one study of 58 dogs with IMHA, 26% of dogs had been vaccinated within 4 weeks of developing IMHA compared with a control group presenting for other disorders in which no increase in frequency of vaccination in the previous 4 weeks was observed (Duval et al., 1996). Mortality rates between the dogs that had been recently vaccinated and those that had not were not significantly different. In a later study that compared 72 dogs with IMHA to a control group, a temporal association between vaccination and development of IMHA was not identified (Carr et al., 2002). The importance of vaccination in the etiology of IMHA remains unclear.
IMHA clearly has a genetic predisposition, with the disease recognized more frequently in certain breeds (Box 104-2). The Cocker Spaniel appears to be the breed at greatest risk, accounting for as many as one third of cases. The presence of dog erythrocyte antigen 7 is associated with a protective effect in Cocker Spaniels (Miller et al., 2004). Female dogs and neutered dogs are overrepresented, suggesting a possible hormonal influence.
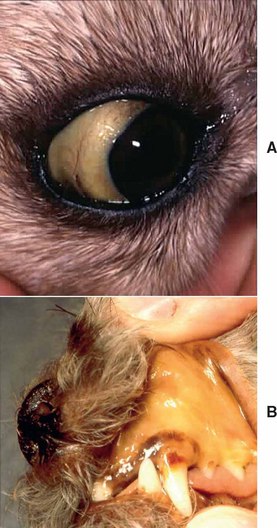
In IMHA the presence of antibody and/or complement on the RBC ultimately results in intravascular or extravascular hemolysis (see Chapter 83). Extravascular hemolysis is more common than intravascular hemolysis, is typically a less-acute process, and is commonly accompanied by spherocytes and hyperbilirubinemia (Figs. 104-1 and 104-2). Although hyperbilirubinemia is a common feature of IMHA, it does not occur in all cases and lack of hyperbilirubinemia does not rule out IMHA. Little clinical significance can be attributed to the relative proportions of conjugated and unconjugated bilirubin on the biochemical panel. Factors that determine the presence and severity of hyperbilirubinemia include the rate of hemolysis as well as hepatic function. In dogs with IMHA, hepatic function may be compromised by hypoxia and hepatic necrosis. In one study of 34 dogs that died of IMHA, 53% had moderate to severe centrilobular hepatic necrosis at necropsy (McManus et al., 2001).
Clinical Features
Dogs with primary IMHA are typically young to middle-aged adults, with a reported age range of 1 to 13 years and amedian age of 6 years. Females and neutered dogs of both sexes appear predisposed compared with sexually intact male dogs, and several breeds are overrepresented (see Box 104-2). Cats with primary IMHA tend to be younger than dogs, with a median age of 2 years. Males are slightly overrepresented, with no influence of neuter status (Kohn et al., 2006). Common clinical signs of IMHA are listed in Box 104-3. The duration of clinical signs before presentation to the veterinary hospital is typically short in both dogs and cats, with a median of 4 days. Seasonal increases in diagnosis of IMHA have been reported, although the findings are not consistent among studies. The majority of reports suggest an increased frequency of IMHA during the warmer months of the year.
 BOX 104-3 Historic and Physical Examination Findings in Dogs and Cats with IMHA
BOX 104-3 Historic and Physical Examination Findings in Dogs and Cats with IMHA
IMHA, Immune-mediated hemolytic anemia.
| DOGS | CATS |
|---|---|
| History | |
| Lethargy | Lethargy |
| Anorexia | Anorexia |
| Pallor | Pallor |
| Icterus | Icterus |
| Vomiting | Vomiting |
| Collapse | Pica |
| Weakness | |
| Physical Examination (Additional Findings) | |
| Systolic heart murmur | Systolic heart murmur |
| Pyrexia | Pyrexia |
| Tachycardia | Hypothermia |
| Tachypnea | Lymphadenomegaly |
| Pallor | Pallor |
| Icterus | Icterus |
| Splenomegaly | |
| Hepatomegaly | |
| Abdominal pain | |
Diagnosis
Diagnosis of IMHA relies on identifying abnormalities consistent with hemolytic anemia on a complete blood count (CBC), serum biochemistry panel, and urinalysis (Box 104-4) followed by identification of antibodies directed against the RBC membrane. Further diagnostic testing is then directed at establishing whether a secondary underlying cause for IMHA can be identified.
 BOX 104-4 Abnormalities on the CBC and Serum Chemistry Profile in Dogs with IMHA
BOX 104-4 Abnormalities on the CBC and Serum Chemistry Profile in Dogs with IMHA
CBC, Complete blood cell count; IMHA, immune-mediated hemolytic anemia.
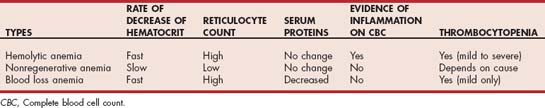
The first requirement for making a diagnosis of IMHA is the presence of anemia. The anemia is typically moderate to marked (median hematocrit of 13%) and is usually regenerative, although in approximately 30% of dogs and more than 50% of cats the anemia is nonregenerative. Reasons for nonregenerative anemia in IMHA include an acute onset and presentation before the bone marrow has had time to respond (typically takes 3 to 5 days for maximal regenerative response) and the presence of antibodies directed against bone marrow precursors. In the latter situation, reticulocytes are destroyed before they enter the peripheral circulation. In the absence of a regenerative response, a rapid fall in the hematocrit with little change in the serum total protein or albumin concentration should be considered suspicious for hemolysis. In anemia caused by decreased RBC production from the bone marrow, the hematocrit should not decrease by more than approximately 1% per day, whereas in blood loss anemia the drop in the hematocrit is usually accompanied by a concurrent decrease in the total protein or albumin (Table 104-1).
Most dogs with IMHA also have an inflammatory leukogram, often with a shift toward immature cells; thrombocytopenia; and abnormalities of the coagulation system, including prolongation of both the activated partial thromboplastin time (aPTT) and prothrombin time, elevations in d-dimer and fibrinogen degradation products, decreased antithrombin, and increased fibrinogen. Reasons for thrombocytopenia include the presence of antibodies directed against platelets as well as RBCs (Evans syndrome), disseminated intravascular coagulation, or sequestration in the spleen.
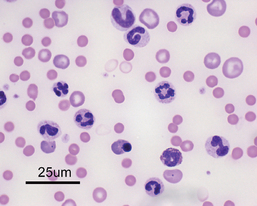
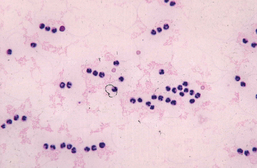
Identification of autoagglutination or spherocytosis (2+ or more) on a blood smear is considered definitive evidence of antibody-mediated RBC hemolysis (Fig. 104-3). Autoagglutination is detected by macroscopic or microscopic examination of the blood smear and is generally considered diagnostic for IMHA. Agglutination must be distinguished from rouleaux formation (see Chapter 83).
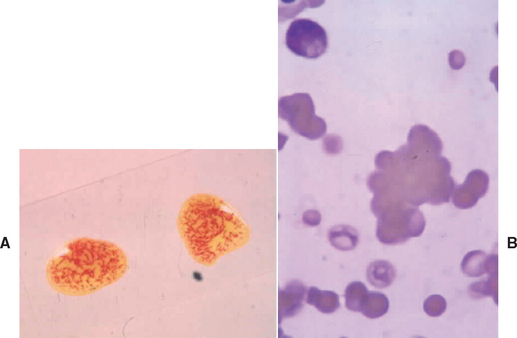
FIG 104-3 Blood smear showing gross (A) and microscopic (B) agglutination. Note the three-dimensional clustering of red blood cells on the microscopic view.
Spherocytes are formed by partial removal of antibody-coated RBC membranes by macrophages (see Fig. 104-2). This results in a loss of the normal discoid shape, decreased size, and loss of central pallor. Spherocytes are more rigid and less deformable than normal RBCs and are removed when they pass through the spleen. Spherocytes are readily identified in the dog but difficult to recognize in cats because of the lack of significant central pallor in their normal RBCs. Spherocytes are considered a hallmark morphologic change in IMHA, and when present in sufficient numbers (2+ or greater) may be regarded as diagnostic for IMHA in dogs. Of note, low numbers of spherocytes (1+) may be observed on a blood smear when damage to the RBC is nonimmune (e.g., zinc toxicosis, hypophosphatemia, oxidative damage, rickettsial diseases, neoplasia, microangiopathic anemia). Techniques for quantitation of spherocyte numbers are typically semiquantitative (Table 104-2). In retrospective studies approximately 90% of dogs with IMHA have spherocytes present on the blood smear; however, low numbers may be present in dogs with per-acute hemolysis. Ghost cells are remnant membranes of RBCs that have undergone intravascular lysis. Lysis can be induced by immune- or non–immune-mediated mechanisms, so ghost cells are not diagnostic for IMHA.
 TABLE 104-2 Semiquantitative Scoring System for Numbers of Spherocytes on a Slide
TABLE 104-2 Semiquantitative Scoring System for Numbers of Spherocytes on a Slide
| APPROXIMATE NUMBER OF SPHEROCYTES PER × 1000 FIELD | ASSIGNED SCORE |
|---|---|
| 1-10 | 1 + |
| 11-50 | 2+ |
| 51-150 | 3+ |
The direct Coombs test with polyvalent antisera is the most commonly used diagnostic test for IMHA when autoagglutination or spherocytosis is not present; however, this test is neither particularly sensitive nor specific for confirming a diagnosis of IMHA. A positive Coombs test indicates that antibody, complement, or both are on the surface of the RBC but does not mean that the antibody is directed specifically against the RBC membrane or that the antibody is causing hemolysis. Approximately 60% to 80% of canine patients with IMHA have a positive Coombs test. Conversely, a positive Coombs test can occur in a variety of other inflammatory diseases causing false-positive results (see Chapter 102).
A search for secondary causes of IMHA should always be undertaken in a dog or cat with IMHA because the underlying disease may influence management strategy and prognosis. Potential secondary causes of IMHA are listed in Table 104-3. The diagnostic approach to ruling out secondary IMHA includes a thorough history of drug, vaccine, and toxin exposure; detailed physical examination, including rectal, ophthalmologic, and neurologic examinations; tests for specific infectious diseases; investigation into causes of chronic antigenic stimulation; and a search for evidence of neoplasia. Diagnostic tests to consider in addition to a CBC, biochemical panel, and urinalysis include urine culture, abdominal and thoracic radiographs, abdominal ultrasound, bone marrow cytology or histopathology (or both if the anemia is nonregenerative), and appropriate titers for infectious diseases.
 TABLE 104-3 Secondary Causes of IMHA in Dogs and Cats
TABLE 104-3 Secondary Causes of IMHA in Dogs and Cats
| EXAMPLES | DIAGNOSTIC TESTS INDICATED | |
|---|---|---|
| Neoplasia | ||
| Infection (see Box 104-1) | ||
| Chronic inflammation | ||
| Exposure to drugs vaccines or toxins | Antibiotics (sulfonamides, β-lactam antibiotics) | Detailed history |
IMHA, Immune-mediated hemolytic anemia; IFA, immunofluorescent antibody; PCR, polymerase chain reaction.
Results of bone marrow evaluation in dogs with nonregenerative primary IMHA typically reveal erythroid hyperplasia with a low mycloid/erythroid (M/E) ratio, although maturation arrest at the rubricyte or metarubricyte stage may also be observed. Some dogs initially suspected to have IMHA based on the presence of spherocytosis or a positive Coombs test have pure red cell aplasia. Myelofibrosis can be detected on bone marrow core biopsy in many dogs with nonregenerative IMHA. In dogs with myelofibrosis, collection of adequate bone marrow elements by aspiration cytology is difficult. Myelofibrosis is likely a secondary response to bone marrow injury and may resolve in dogs that respond to treatment.
In dogs without the classic morphologic changes of immune-mediated hemolysis (regenerative anemia, autoagglutination, spherocytes), confirming a diagnosis of IMHA may be challenging. A positive direct Coombs test should be interpreted cautiously in such cases because false-positive results may occur. The logical approach is to rule out other causes of anemia (see Chapter 83) and use the Coombs test and other indications of hemolysis as supporting evidence of IMHA if no other cause of anemia is identified.
Treatment
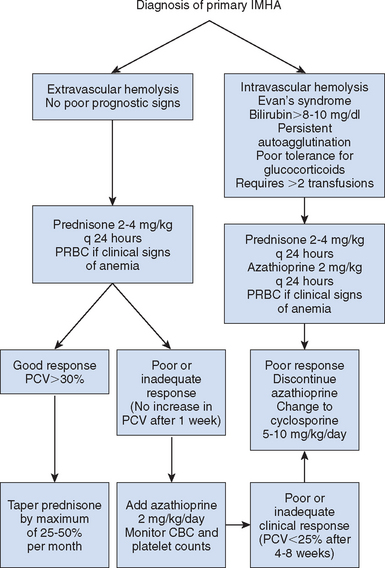
Choosing an appropriate treatment regimen for dogs with IMHA is a frustrating task for the clinician (Fig. 104-4). Lack of prospective studies of treatment efficacy, the poor prognosis associated with the disease, and the high cost of treatment and supportive care are some reasons for this frustration. In addition, serious complications such as pulmonary thromboembolism and disseminated intravascular coagulation are relatively common occurrences but hard to predict in individual patients. Because of the lack of prospective studies of treatment efficacy, recommendations for approach to treatment in dogs with IMHA are based primarily on clinical experience rather than objective data.
When planning the management of a dog with IMHA, the goals of treatment should include prevention of RBC hemolysis, alleviation of tissue hypoxia by blood transfusion, prevention of thromboembolism, and provision of supportive care.
Prevention of hemolysis
Immunosuppressive drugs are the key for prevention of RBC hemolysis in dogs with IMHA. The mechanism of action and adverse effects associated with the use of various immunosuppressive drugs recommended for use in dogs and cats with autoimmune disorders are discussed in Chapter 103.
High doses of glucocorticoids are the first line of treatment for controlling RBC hemolysis in dogs with IMHA. In dogs that can tolerate oral medication, prednisone at a dose of 1 to 2 mg/kg PO q12h is the corticosteroid of choice. The higher end of the dose range is recommended as a starting dose except in large breed dogs (more than 30 kg). Most dogs that will respond to prednisone show some improvement within the first 7 days of treatment, but the full therapeutic effect may not be evident until 2 to 4 weeks after initiation of treatment. Once the hematocrit increases above 30%, the dose may be decreased to 1 mg/kg q12h. Subsequently the dose is tapered by a maximal rate of 25% to 50% per month over a 3- to 6-month period depending on the hematocrit and severity of side-effects. If after 6 months the prednisone dose is tapered to a low every-other-day dose and the disease is in remission, discontinuation of medication should be attempted. A CBC and reticulocyte count should be performed before and 2 weeks after any change in immunosuppressive therapy. Indications of resolution of the hemolytic process in addition to improvement in the anemia include a negative Coombs test (if it was initially positive), resolution of autoagglutination, resolution of spherocytosis, normalization of the reticulocyte count, and improvement in the leukogram with resolution of inflammation.
Most cats with IMHA respond to prednisone alone and rarely have problems with the adverse effects of glucocorticoids. In the occasional cat that needs an additional immunosuppressive drug to treat IMHA, treatment with chlorambucil, cyclophosphamide, or cyclosporine should be considered. Not enough published information exists on which to base a recommendation of one drug over another. Azathioprine is not recommended in cats because of the risk of unacceptable side effects (see Chapter 103).
Some dogs with IMHA do not respond to glucocorticoid treatment alone, or the dose of prednisone cannot be tapered enough for adequate resolution of adverse effects of glucocorticoids. In those cases an additional cytotoxic drug should be added to the treatment regimen. One common clinical dilemma is whether all dogs with IMHA should be treated with an additional immunosuppressive drug early in the course of treatment, or whether waiting and identifying which dogs are likely to benefit is more appropriate. The advantage of starting another immunosuppressive drug early is that no time is lost waiting to identify which patients will respond to glucocorticoid treatment alone. The disadvantages include the risk of adverse effects and the lack of evidence of benefit in all cases. In studies at Purdue University approximately 20% of dogs with IMHA are ultimately treated with another immunosuppressive drug in addition to prednisone. Use of more than one additional immunosuppressive drug at any one time is not recommended because of the potential for severe immunosuppression and resultant susceptibility to infection.
The choice for additional immunosuppression varies among clinicians. Viable options include azathioprine, cyclophosphamide, and cyclosporine. In our hospital azathioprine is added early in the course of treatment in dogs that do not respond within 5 to 7 days of initiating glucocorticoid treatment and in dogs that require more than two transfusions of blood or a hemoglobin-based oxygen carrier. Azathioprine is also used in dogs known to have a poor tolerance of the side-effects of glucocorticoids (e.g., large-breed dogs) and in those with other poor prognostic indicators (e.g., intravascular hemolysis, serum bilirubin level greater than 8 to 10 mg/dL, persistent autoagglutination, Evans syndrome). The recommended starting dose for azathioprine in dogs is 2 mg/kg q24h. Once control of IMHA is attained, azathioprine should be continued at the same dosage while the dose of prednisone is tapered. Azathioprine is then tapered slowly once the prednisone has been discontinued. If a relapse occurs, life-long prednisone, azathioprine, or both are recommended at the lowest dose that controls RBC hemolysis. CBC and hepatic enzymes should be monitored biweekly initially, then every 1 to 2 months in dogs treated with azathioprine.
Historically, cyclophosphamide has been recommended for treatment of dogs with severe acute IMHA. However, evidence is mounting that addition of cyclophosphamide does not improve outcome and that its use may be associated with a poorer prognosis in dogs with IMHA. Cyclophosphamide is usually reserved for dogs that do not tolerate oral drugs because of persistent vomiting or gastrointestinal disease (cyclophosphamide can be administered intravenously; see Table 103-3) or because of expense (cyclosporine).
Cyclosporine is currently the preferred immunosuppressive drug for dogs that do not respond to prednisone and azathioprine. The cost of cyclosporine is a major deterrent to its use, and its potent immunosuppressive effects mandate frequent monitoring of the patient for infections. Interestingly, in a prospective study of 38 dogs with IMHA, no difference in survival was found between dogs treated with prednisone alone and those treated with prednisone and cyclosporine; however, most of the deaths occurred early before the effects of cyclosporine had likely reached maximal effect (Husbands et al., 2004). Cyclosporine appears to be relatively safe in dogs with IMHA, and clinical experience suggests that it is useful and effective in the treatment of dogs with IMHA that do not respond to prednisone or azathioprine. (For dosing and monitoring recommendations for cyclosporine, see Tables 103-3 and 103-4.)
Human intravenous immunoglobulin (hIVIG) has had beneficial effects in dogs with IMHA that are refractory to other therapy. Administration of hIVIG may be most useful early in the treatment of acute severe IMHA to control acute hemolysis while waiting for other immunosuppressive drugs to become effective. Cost is a deterrent to using hIVIG, and multiple treatments are not currently recommended because of the potential for sensitization to this human product, although dogs have been treated twice with no obvious deleterious effects.
Blood transfusion
Most dogs and cats with acute, severe IMHA need oxygen-carrying support while waiting for the anemia to improve. Oxygen supplementation alone is of limited benefit. The need for blood transfusion depends on the severity of anemia, the rapidity of onset and chronicity of the anemia, and the presence and severity of concurrent disease such as pulmonary thromboembolism and gastrointestinal blood loss. No specific hematocrit level is necessary as a transfusion trigger; rather, each patient should be considered individually. In general, transfusion should be considered when the dog has problems with tachycardia, tachypnea, anorexia, lethargy, or weakness while at rest. Most dogs with acute IMHA and a hematocrit level less than 15% have some degree of tissue hypoxia and will benefit from a blood transfusion regardless of how the dog appears to be doing clinically. Severe tissue hypoxia likely exacerbates the complications of IMHA, such as hepatic necrosis, disseminated intravascular coagulation, and thromboembolism.
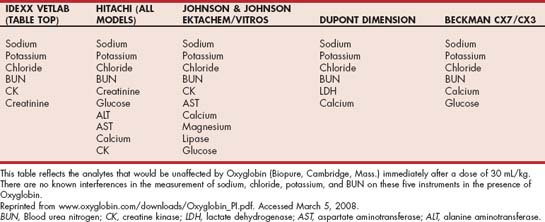
Options for providing oxygen-carrying support include transfusion of packed RBCs (pRBCs) or a hemoglobin-based oxygen carrier (HBOC) such as Oxyglobin (Biopure, Cambridge, Mass.). Transfusion of whole blood is acceptable but less ideal because the plasma component is not necessary and may increase the risk of transfusion reactions. Disadvantages of HBOC include the short duration of effect (74 to 82 hours at the 30-mL/kg dose) and the discoloration of serum caused by the transfused hemoglobin, which interferes with many analytes on the biochemical profile (see Table 104-4 for a list of valid analytes). In dogs with hematocrit levels less than 8%, HBOC alone will not provide adequate oxygen-carrying support, and additional support with pRBCs or whole blood is recommended. (See Chapter 83 for more information about blood transfusions and HBOCs.)
Prevention of thromboembolism
Thromboembolic events (TEs) are a common complication and important cause of death in dogs with IMHA. TEs have been documented at necropsy in 29% to 80% of dogs with IMHA. Intravenous catheter placement and identification of certain laboratory abnormalities, such as thrombocytopenia, hyperbilirubinemia, leukocytosis, and hypoalbuminemia, are associated with an increased risk of TE in dogs with IMHA. The pathogenesis of thrombus formation is unknown, and effective regimens for prophylaxis have not been established. Treatment options currently used for prevention of thromboembolic complications include heparin, low-molecular-weight heparin, aspirin, or a combination of these modalities. The recommended starting dose for heparin in patients with IMHA is 200 to 300 U/kg q6h, and the dose is adjusted by measuring anti-Xa activity (0.35 to 0.7 U/mL) or, less ideally, monitoring the aPTT with the aim to prolong aPTT by 25% to 50% of baseline. (For a discussion of the use of low-molecular-weight heparin, see Chapter 12.) Low-dose aspirin (0.5 mg/kg q24h) has also been used to prevent thromboembolic complications in dogs with IMHA. Weinkle et al (2005) reported that dogs treated with a protocol that included prednisone, azathioprine, and low-dose aspirin had the longest survival times. (See Chapter 12 for more information on treatment and prevention of thromboembolism.)
Supportive care
Aggressive supportive care is critical to a good outcome in dogs with IMHA. Identification and treatment of underlying disease, detection of complications associated with immunosuppressive drug therapy, and good nursing care positively influence outcome. In addition to transfusion, fluid therapy should be administered in dogs with evidence of dehydration to improve tissue perfusion. In dehydrated dogs fluid therapy will decrease the measured hematocrit, but this does not change the total RBC mass. Fluid therapy should not be withheld because of fear of exacerbating anemia. In reality, fluid therapy reveals the true severity of the anemia.
Careful investigation and treatment of underlying disease in dogs with IMHA are important. Immunosuppressive therapy is usually still necessary in dogs with secondary IMHA. However, the duration of immunosuppression may be shorter if an underlying cause can be identified and treated. If an infectious disease is identified, addition of cytotoxic drugs (e.g., azathioprine, cyclophosphamide, chlorambucil) should be avoided.
Complications of immunosuppressive drug therapy include bone marrow suppression, infection, gastrointestinal ulceration, and iatrogenic hyperadrenocorticism. Gastrointestinal hemorrhage can contribute to anemia in dogs with IMHA, either from the gastrointestinal effects of high doses of glucocorticoids or concurrent thrombocytopenia, vasculitis, ischemia, or other concurrent disease. Recognition of occult gastrointestinal hemorrhage is important because the resulting anemia may be confused with a failure to respond to treatment for IMHA (see Chapter 83). Drugs used for treatment of gastrointestinal hemorrhage include gastrointestinal protectants such as sucralfate and H2 blockers (e.g., famotidine).
Prognosis
Reported mortality rates of dogs with primary IMHA range from 26% to 70%, with thromboembolism being the cause of death in at least 30% to 60% of cases. Other common causes of death include infection, disseminated intravascular coagulation, and failure to control anemia. Factors that clinically appear to confer a good prognosis in dogs with IMHA include a rapid response to treatment with glucocorticoids, ability to maintain the packed cell volume at greater than 25% to 30% with glucocorticoids alone, and identification of a treatable secondary cause. The prognosis is more guarded in dogs that require multiple drugs to control the disease and those with persistent autoagglutination, an elevated bilirubin concentration, marked thrombocytopenia, and severe leukocytosis. If a major TE does occur in a dog with IMHA, particularly if blood supply to a major organ is disrupted, the long-term prognosis is typically very poor. Contrary to popular opinion the prognosis in Cocker Spaniels with IMHA does not differ from that of other breeds. In approximately 60% of dogs with IMHA, medications can ultimately be discontinued after a slow tapering of the dose. The remaining dogs require long-term immunosuppressive therapy.
PURE RED CELL APLASIA
Pure red cell aplasia (PRCA) is a rare disorder characterized by severe, nonregenerative anemia with marked depletion or absence of erythroid precursors in the bone marrow. In some cases evidence of concurrent peripheral RBC hemolysis is present, based on the presence of spherocytes and a positive antiglobulin test. Other cell lines are usually normal. The erythroid aplasia in PRCA is in contrast to the nonregenerative form of IMHA, in which there is erythroid hyperplasia or sometimes maturation arrest of the erythroid maturation sequence. PRCA is likely one end of the spectrum of IMHA, with acute peripheral hemolysis at the other end of this spectrum (Table 104-5). The affinity of circulating antibody for different erythroid precursors likely influences the level at which damage occurs in the bone marrow. As with IMHA, both primary and secondary forms of PRCA are recognized. Secondary causes of PRCA include treatment with recombinant human erythropoietin and parvovirus infection in dogs. Infection with feline leukemia virus subtype C is a cause of PRCA in cats.
Dogs with PRCA have a similar signalment and present with similar clinical signs as dogs with IMHA. Cats with primary PRCA are typically younger than dogs, with an age range of 8 months to 3 years. Dogs and cats with PRCA have severe, nonregenerative anemia; the platelet count and leukogram are typically normal. In contrast to IMHA the biochemical panel and urinalysis are also usually unremarkable, with no evidence of peripheral hemolysis. Low numbers of spherocytes are sometimes present in dogs with PRCA. The Coombs test is usually negative.
Diagnosis of PRCA is made by evaluation of a bone marrow aspirate and bone marrow core biopsy. In dogs with PRCA, erythroid precursors are rare or absent and the M/E ratio is quite high (more than 99 : 1). In contrast to dogs with nonregenerative IMHA, severe myelofibrosis is rare.
Treatment of PRCA is similar to IMHA. Most dogs with PRCA respond to prednisone alone. Azathioprine or cyclophosphamide may be necessary for a complete response in some dogs or may be added to allow tapering of the prednisone dose in dogs with unacceptable side effects of corticosteroid therapy. The time taken to achieve complete remission (2 to 6 months) is longer in dogs with PRCA compared with IMHA, and it is sometimes difficult to judge whether a particular protocol is failing or whether inadequate time has been allowed for the bone marrow to respond to treatment and begin to produce and release RBCs into the circulation. Sequential bone marrow evaluations should ideally be used to determine when to change the treatment protocol. A repeat bone marrow aspirate should be considered after 2 months of treatment if no improvement in the anemia is observed. Repeated transfusion of pRBCs or whole blood is necessary while waiting for a response to treatment. Dogs with PRCA do not typically have evidence of systemic inflammation and are not at increased risk of TEs, so anticoagulant treatment is not indicated. The prognosis for PRCA in dogs is better than for IMHA, with mortality rates being less than 20%. The major cause of death is euthanasia because of the high cost of supportive care. Response to treatment and mortality rates in cats with PRCA appears to be similar to dogs, although cats respond to treatment more quickly (1.5 to 5 weeks). See Chapter 83 for additional information on PRCA.
IMMUNE-MEDIATED THROMBOCYTOPENIA
Classification/Etiology
Immune-mediated thrombocytopenia (idiopathic thrombocytopenic purpura [ITP]) is a clinical syndrome in which thrombocytopenia results from antibody-mediated accelerated destruction of platelets. Immune-mediated thrombocytopenia is diagnosed in approximately 3% to 18% of cases of thrombocytopenia and is the most common cause of severe thrombocytopenia in dogs (Table 104-6). Immune-mediated thrombocytopenia is classified as primary or secondary. In primary thrombocytopenia (true autoimmune thrombocytopenia) antibodies are directed against platelet antigens, presumably because of an underlying defect in immune regulation. Antibodies directed against platelet membrane glycoproteins IIb and IIIa have been identified as target antigens in dogs, although others may be important as well. Primary ITP is a common cause of thrombocytopenia in dogs but is rare in cats. Environmental factors suspected to precipitate ITP in some cases include stress, changes in environmental temperature, hormonal changes, vaccination, and surgery.
In secondary ITP antibody-mediated platelet destruction occurs as a result of an underlying inflammatory or neoplastic disease. Causes of secondary immune-mediated thrombocytopenia in dogs and cats are listed in Table 104-6. Immune-mediated thrombocytopenia may also be a component of SLE and may occur in conjunction with IMHA (Evans syndrome).
Clinical Features
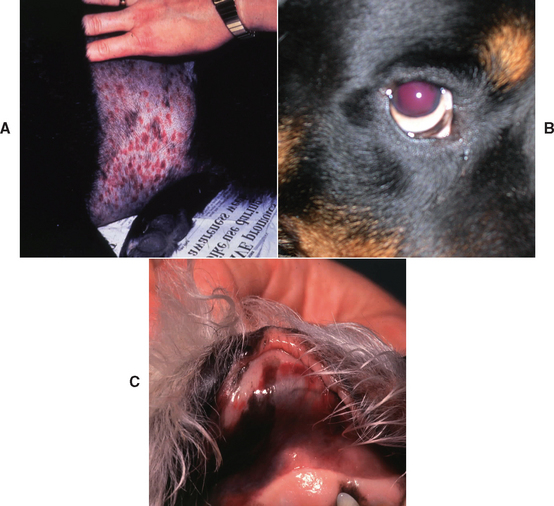
Dogs with primary ITP range in age from 8 months to 15 years, with a median age of 6 years. Females are affected twice as often as males, and although any breed can be affected the Cocker Spaniel, Poodle (all varieties), German Shepherd dog, and Old English Sheepdog are overrepresented. Common findings include sudden onset of petechial and ecchymotic hemorrhages in the skin and mucous membranes, epistaxis, hematochezia, hematemesis, easy bruising, lethargy, weakness, and anorexia. Additional findings on physical examination may include melena, hematuria, hyphema, retinal hemorrhage, and pale mucous membranes (Fig. 104-5). Neurologic signs and blindness may occur from bleeding into the CNS and eye, respectively. Because rapid-onset, life-threatening hemorrhage is rare in dogs with ITP, anemia is usually initially mild and slowly progressive unless IMHA is concurrent. As affected dogs become moderately to severely anemic, lethargy, exercise intolerance, tachypnea, tachycardia, and a heart murmur may be evident. In some dogs with ITP clinical signs of hemorrhage are not present and thrombocytopenia is an incidental finding on bloodwork performed for another reason. The platelets present in dogs with ITP are often larger and may be hemostatically more competent, which may explain why not all dogs with severe ITP bleed spontaneously. However, platelet dysfunction (impaired aggregation) has been documented in normal canine platelets after incubation with serum from dogs with ITP, suggesting that antibodies or other factors in the serum impair platelet function in some dogs with ITP. Certain breeds, such as the Cavalier King Charles Spaniel and the Greyhound, are known to have lower platelet counts than other dogs and do not appear to have increased risk of bleeding.
Diagnosis
Because immune-mediated thrombocytopenia can occur in association with many other disorders (see Table 104-6 and Chapter 87), a diagnosis of primary ITP can only be made by ruling out other causes of thrombocytopenia. Dogs with ITP usually have severe thrombocytopenia (less than 50,000 platelets per μL), and platelet fragments (microthrombocytosis) may be present on the blood smear. Platelet fragments are reported to be a specific but insensitive indication of ITP. Platelet fragments may be present as a result of immune injury or because larger platelets are preferentially removed from circulation. The presence of enlarged platelets on the blood smear supports the presence of increased bone marrow production of platelets, but this is not specific for a regenerative response because bone marrow injury may also cause enlarged platelets.
Diagnosis of ITP is confirmed by ruling out other cause of severe thrombocytopenia (see Table 104-6 and Chapter 87). Spurious thrombocytopenia from platelet clumping, other technical problems, and breed-related thrombocytopenia should be considered in dogs that do not have clinical signs of bleeding. In dogs with thrombocytopenia, examination of a bone marrow aspirate should be performed early in the diagnostic workup to rule out disorders such as myelophthisis, neoplasia, megakaryocytic aplasia, and aplastic anemia (see Chapter 87). Bone marrow aspiration and biopsy can be safely performed even in severely thrombocytopenic dogs because hemorrhage can be controlled with local pressure. In most dogs with ITP normal to increased numbers of megakaryocytes are present on a bone marrow aspirate. Decreased numbers of megakaryocytes in the bone marrow have been associated with a poorer prognosis in dogs with ITP. Megakaryocytic aplasia is a rare disorder in which aplasia of the megakaryocytic cell line results in severe thrombocytopenia. This disease may be a primary immune-mediated disease or occur secondary to infections such as Ehrlichia canis and Borrelia burgdorferi. Immune-mediated megakaryocytic aplasia has a poor prognosis unless it is caused by underlying infection.
The presence of a positive assay for platelet-bound antibody (see Chapter 102) is highly sensitive but not specific for a diagnosis of ITP. A diagnosis of ITP is unlikely if the test result is negative. A positive test result is not specific for ITP because immune-mediated mechanisms are responsible for many causes of thrombocytopenia in dogs, including Babesia canis, Dirofilaria immitis, E. canis, myelodysplasia, SLE, drug reactions to trimethoprim sulfadiazine, and various forms of neoplasia.
Treatment
Immunosuppression
Immunosuppressive drugs are the key to treating ITP. High doses of corticosteroids block macrophage-mediated destruction of platelets and are the first line of treatment in dogs with ITP. Prednisone at a dose of 1 to 2 mg/kg q12h is the corticosteroid of choice. Treatment with one dose of vincristine (0.02 mg/kg IV) should also be considered early in the course of treatment for dogs with severe ITP (platelet count less than 15,000/μL). Dogs treated with vincristine have a more rapid increase in platelet count and shortened duration of hospitalization compared with untreated dogs. Most dogs with ITP have a rapid response to prednisone or prednisone combined with vincristine, and in most cases the platelet count increases to more than 50,000 per μL within 7 days of treatment. Once the platelet count is in the reference range, the dose of prednisone can be slowly tapered. Because of the risk of relapse the dose should not be tapered more rapidly than 25% to 50% per month over a 3- to 6-month period. If after 6 months the prednisone dose has been tapered to a low every-other-day dose and the disease is in remission, discontinuation of medication should be attempted.
Azathioprine therapy should be considered in dogs that do not have an adequate response to prednisone alone (platelet count less than 100,00 per μL) or in whom the dose of prednisone cannot be tapered low enough to manage the adverse effects of glucocorticoids. The dose of azathioprine is 2 mg/kg q24h. If azathioprine is tolerated the dose should be continued while the dose of prednisone is tapered. Azathioprine is tapered slowly once prednisone has been discontinued. If a relapse occurs, life-long prednisone and/or azathioprine should be continued at the lowest dose that maintains the platelet count within the reference range. A platelet count should be performed before and 2 weeks after any change in immunosuppressive therapy. In some dogs with ITP maintaining the platelet count within the reference range is difficult without severe glucocorticoid side effects. In these dogs maintaining the platelet count greater than 100,000 per μL is acceptable because this level of thrombocytopenia is usually not associated with increased risk of bleeding. Other drugs that can be considered in dogs with refractory ITP include danazol, cyclophosphamide, cyclosporine, and hIVIG (see Chapter 103). None of these drugs has been extensively evaluated in dogs with ITP, but they may be useful in treatment of refractory cases. Splenectomy may also be indicated in dogs with ITP that have chronic relapses after tapering prednisone and azathioprine therapy (see Chapter 103).
Supportive care
Supportive care for dogs with ITP is critical to a positive outcome. Cage rest and exercise restriction to prevent trauma, eliminating all except absolutely necessary diagnostic procedures, and minimizing other invasive procedures will decrease risk of hemorrhage. A balance between appropriate monitoring and minimizing venipuncture is important. Careful monitoring for clinically significant changes that could be from new hemorrhage, especially involving the nervous system or eye, should be performed frequently. Blood transfusions should be administered to actively bleeding patients and those with clinically significant anemia. The only blood products that provide clinically significant platelet activity are fresh whole blood, platelet-rich plasma, and platelet concentrate. Fresh whole blood often provides enough platelets to stop an episode of clinical bleeding, although an increase in the platelet count is not expected. The beneficial effect of a fresh whole blood trans fusion typically lasts approximately 48 hours. Blood typing of the donor and cross-matching of the recipient should be performed as described in Chapter 83. Platelet-rich plasma or platelet concentrate are the ideal products for administration to actively bleeding patients before they become anemic. However, availability and cost limit their use in most hospitals. Administration of gastric protectants such as H2 blockers (e.g., famotidine), or proton pump inhibitors (e.g., omeprazole) and sucralfate may help prevent adverse effects of glucocorticoid treatment on the gastrointestinal tract, especially in dogs with gastrointestinal bleeding.
Treatment of Evans syndrome (concurrent IMHA and ITP) is managed as described for IMHA. However, azathioprine should be administered in addition to glucocorticoids. One dose of vincristine should be considered if the thrombocytopenia is severe (platelet count less than 15,000/μL). Whole blood transfusion rather than pRBCs should be administered in dogs with Evans syndrome that are actively bleeding. Dogs with Evans syndrome should not be treated with heparin because of the risk of hemorrhage.
Prognosis
The prognosis for dogs with ITP is good to guarded, with a mortality rate of approximately 30%. Most dogs respond to medical treatment, although relapse is common, occurring in as many as 50% of dogs. Dogs with megakaryocytic hypoplasia have a more guarded prognosis. The prognosis for dogs with concurrent IMHA and ITP is poor, with a reported mortality rate of 80% or higher. See Chapter 87 for more information on this topic.
IMMUNE-MEDIATED NEUTROPENIA
Etiology
Autoimmune causes of neutropenia are rare in dogs and cats, accounting for approximately 0.4% of cases of neutropenia (see Chapter 85). In immune-mediated neutropenia (also called idiopathic neutropenia or steroid-responsive neutropenia), serum antineutrophil IgG antibodies can be detected by flow cytometry in the serum (Weiss, 2007). Antibody and complement directed against myeloid cells within the bone marrow have also been identified. In most cases of suspected immune-mediated neutropenia, the diagnosis is one of exclusion because commercial testing for antineutrophil antibodies is not readily available. As with other immune-mediated disorders, immune-mediated neutropenia may be a primary disorder or occur secondary to drug therapy, neoplasia, or other immune-mediated disorder (Table 104-7). The majority of canine cases reported in the literature have been primary. Only one case of suspected immune-mediated neutropenia in a cat has been reported.
 TABLE 104-7 Causes of Severe Neutropenia in Dogs and Cats
TABLE 104-7 Causes of Severe Neutropenia in Dogs and Cats
| ETIOLOGY | EXAMPLE |
|---|---|
| Infection | Parvovirus, ehrlichiosis, bacterial sepsis |
| Drug associated | Chemotherapeutic agents, cytotoxic drugs, vincristine, estrogens, trimethoprim/sulfadiazine, phenobarbital |
| Bone marrow suppression | Aplastic anemia, Ehrlichia canis infection, myelodysplasia, myeloid hypoplasia, leukemia |
| Immune mediated | Primary immune-mediated neutropenia |
Clinical Features
In a retrospective report of 11 dogs with suspected immune-mediated neutropenia, a variety of breeds were represented and eight of 11 cases were female (Brown et al., 2006). Affected dogs were typically young, with a median age of 4 years. Clinical signs included fever, lameness, anorexia, and lethargy and the duration of clinical signs ranged from 3 to 180 days. Common abnormalities detected on CBC, serum biochemistry panel, and urinalysis included severe neutropenia (median 110 cells/μL), mild anemia, hyperglobulinemia, and increased alkaline phosphatase level. Further evaluation of affected dogs with bacterial culture, infectious disease serology, and imaging did not reveal a cause for the neutropenia. Bone marrow cytology and histopathology revealed myeloid hyperplasia in the majority of affected dogs and myeloid hypoplasia in two dogs. All dogs had resolution of neutropenia 1 to 18 days after initiation of treatment with glucocorticoids.
Diagnosis and Treatment
A clinical diagnosis of immune-mediated neutropenia is made by exclusion of other causes of neutropenia and by rapid response to treatment with glucocorticoids at an initial dose of 2 to 4 mg/kg/day of prednisone. Gradual withdrawal of corticosteroid therapy is possible without relapse in most dogs; however, some dogs require long-term immunosuppression. Routine monitoring is important to detect recurrence of neutropenia and monitor for infection. See Chapter 85 for more information on this topic.
IDIOPATHIC APLASTIC ANEMIA
Aplastic anemia (aplastic pancytopenia) is characterized by cytopenia of all three marrow-derived cell lines and a hypocellular/acellular bone marrow, with the marrow elements replaced by adipose tissue. Reported causes of aplastic anemia in dogs and cats include infectious agents (Ehrlichia spp, parvovirus, sepsis, feline leukemia virus, feline immunodeficiency virus) hormonal (estrogens), drug associated, radiation associated, and idiopathic. By definition the cause of idiopathic aplastic anemia is unknown; however, evidence in humans suggests that it may be immune mediated. Although an immune-mediated cause has not been established for idiopathic aplastic anemia in dogs and cats, trial therapy with prednisone, cyclosporine, or both may be considered once other causes of aplastic anemia, most notably infectious agents, have been ruled out. An immune-mediated cause for idiopathic anemia is currently difficult to prove but should be suspected in cases that respond to immunosuppressive therapy. The prognosis for idiopathic aplastic anemia is generally guarded to poor. See Chapter 86 for more information on this topic.
POLYARTHRITIS
Etiology
Immune-mediated polyarthritis is defined as chronic synovial inflammation in two or more joints, failure to isolate an organism from the joint fluid, and a positive response to immunosuppressive therapy. Immune-mediated polyarthritis is primarily a type III immune complex hypersensitivity disorder (see Chapter 101) in which immune complexes are deposited in the synovial membrane, initiating local inflammation and release of proteolytic enzymes and cytokines, with resultant cartilage degeneration. In rheumatoid arthritis type IV hypersensitivity may also be present with perivascular infiltration of mononuclear cells into the synovial membrane (see Chapter 101). Immune-mediated polyarthritis may be classified as primary or secondary. In secondary polyarthritis immune complex deposition in the joints is secondary to an underlying inflammatory or neoplastic disease. Infectious agents are an important cause of secondary polyarthritis. Chronic bacterial infections may cause secondary or reactive polyarthritis, and Anaplasma spp., Ehrlichia spp., and Borrelia burgdorferi also cause polyarthritis, although they cannot usually be visualized in or cultured from affected joints. Administration of live calicivirus vaccine also causes transient polyarthritis in cats.
In primary immune-mediated polyarthritis no underlying cause of polyarthritis can be identified. This form of polyarthritis is believed to be attributable to an underlying immune system dysfunction or imbalance (true autoimmunity; see Chapter 101). The most commonly recognized forms of polyarthritis in the dog and cat are idiopathic nonerosive polyarthritis, reactive nonerosive polyarthritis secondary to underlying inflammatory disease (gastrointestinal disease, chronic inflammation, neoplasia, or infection), rheumatoid arthritis, and proliferative polyarthritis (Table 104-8). A number of breed-specific syndromes are recognized in dogs. A nonerosive polyarthritis is also a prominent feature of SLE. See Chapter 74 for a more detailed discussion of the various forms of polyarthritis.
 TABLE 104-8 Forms of Polyarthritis Recognized in Dogs and Cats
TABLE 104-8 Forms of Polyarthritis Recognized in Dogs and Cats
| SYNDROME | CLINICAL MANIFESTATIONS | BREED PREDISPOSITION |
|---|---|---|
| Idiopathic nonerosive | Small distal joints | Large-breed dogs, rarely cats |
| Secondary nonerosive | Similar to idiopathic but clinical signs of underlying disease also present | Any breed |
| Breed-specific idiopathic nonerosive | Similar to idiopathic but more severe and often concurrent meningeal inflammation | Akita, Weimaraner, Newfoundlands |
| Familial Sharpei fever | Recurrent fever, soft tissue swelling around affected joints, predisposition to systemic amyloidosis | Sharpei |
| Lymphoplasmacytic synovitis | No sign of systemic illness, cranial cruciate rupture, lymphocytes and plasma cells in synovial fluid | Rottweiler, Labrador Retrievers, Newfoundlands, Staffordshire Terriers |
| SLE | Multisystemic immune disease | German Shepherd dogs, rarely cats |
| Rheumatoid arthritis | Initially similar to nonerosive form but progresses to joint crepitus, laxity, luxation, and deformity of affected joints (carpi, hocks, phalanges) | Small and toy breeds |
| Erosive polyarthritis of greyhounds | Erosive changes in phalanges, carpi, hocks, elbow, stifles; lymphoplasmacytic inflammation in synovial fluid | Young Greyhounds |
| Feline chronic progressive polyarthritis | Erosive or proliferative changes in multiple joints | Young male cats infected with FeFSV or feline leukemia virus |
SLE, Systemic lupus erythematosus; FeFSV, feline synctium-forming virus.
Clinical Features
The clinical hallmark of immune-mediated polyarthritis is the presence of nonseptic inflammation within the synovial membrane of two or more joints. Consequently the diagnosis is made by analysis of synovial fluid collected from joints suspected to be affected. Common clinical signs are listed in Box 104-5. In some cases neurologic disease is initially suspected because the animal is unable to ambulate; however, the neurologic examination in dogs with polyarthritis is normal. Many dogs and cats with polyarthritis have clinical signs of systemic illness, including fever, anorexia, and lethargy. In some cases joint pain and swelling may be mild or not clinically detected and fever is the only clinical sign. Polyarthritis is one of the most common causes of unexplained fever in dogs. Joint pain from polyarthritis may also cause cervical pain, and concurrent meningeal inflammation has been reported in dogs with polyarthritis (Webb et al., 2002). Polyarthritis should therefore be considered in any dog or cat presenting with cervical pain without neurologic deficits. Cats with polyarthritis may appear to have generalized hyperesthesia and resist handling. Cats may also present for decreased activity, and the owners often note that the animal has become withdrawn, often hiding in inaccessible locations. In the less-common erosive forms of polyarthritis, affected joints may become distorted or collapsed as the disease progresses, resulting in a severe gait abnormality. These changes are typically irreversible.
Diagnosis
Diagnosis of immune-mediated polyarthritis is made by documentation of inflammation within the synovial fluid, synovial membrane, or both (Fig. 104-6). Synovial fluid for cytologic evaluation and culture should be collected from at least three and preferably four joints. Synovial fluid should be collected from the more distal joints (carpus, tarsus, stifle) because these are the most commonly affected. The approach to joint fluid collection is discussed in Chapter 73. Joint fluid may be grossly turbid, with decreased viscosity and increased volume. Cytologic evaluation reveals neutrophilic inflammation with no evidence of sepsis. Fluid should always be collected for culture and sensitivity to rule out an occult infection (especially likely if the animal has been previously treated with antibiotics). Once inflammation within multiple joints has been documented, the next step is to identify the type of polyarthritis (see Table 104-8) and whether it is from a primary autoimmune disease or secondary to underlying inflammation, infection, or neoplasia. Diagnostic tests should include a CBC, biochemistry profile, urinalysis, urine culture, thoracic radiographs, abdominal ultrasound, and infectious disease titers or SNAP test (E. canis, A. phagocytophilum, B. burgdorferi) (SNAP test, IDEXX, Westbrook, Maine). In some cases blood cultures may also be indicated. In dogs with suspected rheumatoid arthritis, a rheumatoid factor test should be performed (see Chapter 102). In dogs and cats with evidence of multiple organ involvement, an antinuclear antibody (ANA) titer is indicated to investigate for SLE (see Chapter 102).
Treatment
Treatment of secondary immune-mediated polyarthritis depends on identification of an underlying cause. Polyarthritis usually resolves with appropriate treatment and use of antiinflammatory doses of glucocorticoids or nonsteroidal antiinflammatory drugs. In dogs with primary (autoimmune) polyarthritis, immunosuppressive dosages of glucocorticoids are the initial treatment of choice (2 to 4 mg/kg/day). Additional immunosuppressive treatment is necessary in dogs that do not respond to corticosteroids alone or that relapse as glucocorticoids are withdrawn. Drugs that are useful include azathioprine, cyclophosphamide, and cyclosporine. Azathioprine is typically the first drug added to the treatment regimen. More aggressive immunosuppression is often necessary in polyarthritis from SLE, in polyarthritis seen in Akitas, and in rheumatoid arthritis.
Response to treatment should be monitored by assessment of clinical signs and cytologic changes within the joint fluid. Joint fluid should be cytologically normal before tapering immunosuppressive therapy. Failure to establish cytologic remission in addition to clinical remission may result in disease relapse or progressive injury to the joints that ultimately results in degenerative joint disease. Approximately 80% of dogs with idiopathic nonerosive polyarthritis treated with prednisone alone respond well to initial treatment, and half of these dogs can be weaned off therapy after 3 to 4 months. The prognosis for idiopathic nonerosive polyarthritis is good, with a mortality/euthanasia rate of less than 20%. Relapses are common, however, and some dogs require life-long therapy. The prognosis for other forms of immune-mediated polyarthritis varies with the different forms of the disease. See Chapters 73 and 74 for more information on this topic.
SYSTEMIC LUPUS ERYTHEMATOSUS
Etiology
SLE is a multisystemic immune disorder in which antibodies to specific tissue proteins (type II hypersensitivity) and immune complex deposition (type III hypersensitivity) result in immune-mediated damage to multiple organs. Type IV mechanisms (delayed hypersensitivity) may also contribute to tissue damage. The underlying cause of SLE is still poorly understood, but an increased CD4/CD8 ratio, increased expression of a T-cell activation marker, and marked lymphopenia have been reported in dogs with active disease. These findings suggest that T-suppressor cells may be defective in dogs with SLE. The disease is heritable although not by simple autosomal mechanisms. Breeds that are predisposed include the German Shepherd dog, Shetland Sheepdog, Collie, Beagle, and Poodle. Several colonies of dogs with a high predisposition toward SLE have been established, and an association with certain MHC (DLA) types exists. Other risk factors likely include environmental factors and exposure to certain infectious agents and drugs.
Clinical Features
The disease is uncommon in dogs and rare in cats. In dogs SLE most commonly occurs in middle-aged dogs (age range, 1 to 11 years), and there is no sex predisposition. Because any organ system may be affected in SLE, a wide range of clinical signs is possible. The most common signs are fever (100%), lameness or joint swelling from nonerosive polyarthritis (91%), dermatologic manifestations (60%), and signs of renal failure such as weight loss, vomiting, polyuria, and polydipsia. Proteinuria from glomerulonephritis is detected in 65% of patients. The dermatologic lesions often involve areas of skin exposed to sunlight, with photosensitization being common. The dermatologic manifestations are highly variable, with alopecia, erythema, ulceration, crusting, or hyperkeratosis common. Mucocutaneous lesions may also occur. Other clinical manifestations may include hemolytic anemia, PRCA, thrombocytopenia, leukopenia, myositis, pleuropericarditis, and central nervous system dysfunction. A similar spectrum of disease manifestations has been reported in cats with SLE. SLE typically has a relapsing and remitting course, and different organ systems may be involved with subsequent relapses. For example, a dog initially presenting with clinical signs predominantly relating to the neuromuscular system (polyarthritis or myositis) may later relapse with signs of IMHA or ITP.
Diagnosis
A diagnosis of SLE should be suspected when evidence of involvement of more than one organ system is present in a dog or cat with immune-mediated disease. Because of the large number of organ systems that may be involved, the diagnostic testing required varies widely from patient to patient. Diagnostic tests that should be performed in all dogs and cats with suspected SLE include a CBC, serum biochemical profile, urinalysis, quantitation of urine protein, collection of synovial fluid for cytology and culture, and fundic examination. Additional tests that may be indicated include thoracic and abdominal radiographs (investigating fever), abdominal ultrasonography (investigating renal dysfunction), infectious disease titers (investigating fever, thrombocytopenia, hemolytic or nonregenerative anemia, proteinuria, or polyarthritis), Coombs test (in presence of hemolytic anemia), bone marrow aspirate and core (in cases of cytopenia), and skin or kidney biopsy if dermatologic or renal lesions are present. The extent of diagnostic testing for infectious disease will depend on the species and geographic location. For example, testing for feline leukemia virus, feline immunodeficiency virus, and feline infectious peritonitis should be considered in any cat with suspected SLE. In dogs in Europe, testing for leishmaniasis should be strongly considered because this disease can mimic SLE.
Numerous criteria for the diagnosis of SLE in dogs have been extrapolated from the literature in humans. The most commonly accepted and clinically applicable criteria are shown in Table 104-9. Measurement of serum ANA titers is a relatively sensitive test to confirm the diagnosis of SLE, although the sensitivity reported in the literature ranges from 50% to 100% (see Chapter 102). The variability in diagnostic sensitivity probably arises from variation in the diagnostic criteria for confirming the diagnosis as well as variations in the populations of dogs tested. When used in dogs that have appropriate clinical criteria for SLE, the ANA test is an excellent test; however, false-positive results can occur in dogs and cats with other inflammatory or infectious disorders or neoplasia. ANAs are detected in 10% to 20% of dogs with seroreactivity to Bartonella vinsonii, Ehrlichia canis, and Leishmania infantum. Dogs with seroreactivity to multiple pathogens are more likely to be ANA positive. A recent study of 120 dogs in which an ANA titer was measured emphasized the importance of appropriate patient selection for testing (Smee et al., 2007). In this study measurement of an ANA titer was not a useful diagnostic test in dogs without any major clinical or clinicopathologic abnormalities suggestive of SLE. Only one of 47 dogs tested that did not have any major signs of SLE had immune-mediated disease, and this dog was seronegative for ANA. Ten (21%) of 47 dogs were seropositive for ANA. Conversely, 13 of 16 dogs with two major signs compatible with SLE had immune-mediated disease, and ANA was positive in 10 of these dogs. These results emphasize that the positive predictive value of a diagnostic test is lower in a population of animals in which the disease prevalence is low.
The LE test is rarely used clinically for diagnosis of SLE because of very low sensitivity. A number of other antibody tests have been investigated in groups of dogs with SLE, including antinative DNA antibodies, antiextractable nuclear antigen antibodies, and antihistone antibodies. None of these tests has been extensively evaluated in dogs, and none is currently commercially available.
Treatment
Immunosuppressive therapy for SLE begins with high doses of corticosteroids (1 to 2 mg/kg q12h). The dose is then tapered if disease remission is achieved. Addition of other cytotoxic drugs (e.g., azathioprine, cyclophosphamide, cyclosporine) is usually necessary to induce or maintain remission. Little information is available on the efficacy of drug protocols for treating SLE. One study reported a protocol of prednisone (0.5 to 1.0 mg/kg q12h) combined with levamisole (2 to 5 mg/kg [maximum 150 mg per patient] every other day; Chabanne et al., 1999b). The prednisone is tapered over a 1- to 2-month period and the levamisole continued for 4 months. In cases that relapse, levamisole is administered for a further 4 months. This protocol was effective in inducing remission in 25 of 33 dogs with SLE. The prognosis for dogs with SLE is guarded to poor. Relapse is common regardless of the drug protocol used, and long-term and often life-long immunosuppressive therapy is necessary to control the disease. Relapses may involve different organ systems and clinical signs than at initial presentation (e.g., hemolytic anemia initially and polyarthritis at relapse).
GLOMERULONEPHRITIS
Etiology
Acquired glomerulonephritis (GN) is more common in dogs than cats and results from the presence of immune complexes within the glomerular capillary walls. Immune complexes may be circulating antigen-antibody complexes that are deposited or trapped in the glomerulus or may form in situ when circulating antibodies react with either endogenous glomerular antigens or nonglomerular antigens within the glomerular capillary wall. Soluble circulating immune complexes formed in the presence of mild antigen excess, or when both antigen and antibody are present in approximately equal quantities, may be deposited along capillary walls resulting in a granular pattern observed on immunofluorescent or immunoperoxidase staining. Infectious and inflammatory diseases are common identifiable causes for deposition of immune complexes within the glomerulus (Box 104-6). Unfortunately in the majority of cases of GN, an underlying cause is not identified. When immune complexes form in situ, a smooth linear pattern is observed with immunofluorescent or immunoperoxidase staining. Causes of in situ deposition of immune complexes may be either true autoimmune disease when antibodies are directed against the basement membrane of the glomerular capillaries (not yet documented as a spontaneous disease in dogs and cats) or when antigen becomes localized in the glomerular capillary wall. For example, in dogs with heartworm disease, soluble Dirofilaria immitis antigens have been shown to adhere to the glomerular capillary wall by a carbohydrate-glycoprotein interaction.
 BOX 104-6 Infectious and Inflammatory Diseases Implicated in Pathogenesis of GN in Dogs
BOX 104-6 Infectious and Inflammatory Diseases Implicated in Pathogenesis of GN in Dogs
GN, Glomerulonephritis.
Whatever the cause of immune complex deposition, the consequences are similar (see Chapter 43) and ultimately lead to severe proteinuria, systemic hypertension, renal failure, and predisposition to thromboembolism.
Clinical Features
The hallmark of GN is proteinuria, which is readily detected on routine urinalysis. In many cases proteinuria is initially identified as an incidental finding and the animal may have no obvious clinical signs or only subtle abnormalities (e.g., weight loss, lethargy, decreased appetite). In other cases animals present with clinical signs of renal failure (e.g., anorexia, weight loss, vomiting, polyuria, polydipsia), and proteinuria is identified in the course of the evaluation. In nephrotic syndrome, which is defined as the presence of proteinuria, hypoalbuminemia, hypercholesterolemia, and either edema or ascites, the clinical signs are more severe and often rapidly progressive. Other clinical signs in dogs with glomerulonephritis may relate to the presence of hypertension or hypercoagulability. Hypertension may result in retinal changes and blindness, whereas TEs may occur as a result of the hypercoagulable state.
Diagnosis
A diagnosis of protein-losing nephropathy is made by documentation of persistent proteinuria that cannot be explained by inflammation of the lower urinary tract or blood contamination of the urine. Initial dipstick estimates of urine protein should be evaluated in the light of the urine sediment and specific gravity of the urine. The severity of protein loss should then be quantitated by measurement of a protein/creatinine ratio, preferably on a urine sample with no inflammation or hematuria. A protein/creatinine ratio greater than 0.5 is abnormal; most dogs and cats with protein-losing nephropathy have a ratio greater that 2.0. Once persistent proteinuria has been documented, further testing is necessary to determine whether evidence of tubular dysfunction also exists and to investigate for the presence of underlying infectious or inflammatory diseases implicated as causes of GN. Diagnostic tests that should be performed include a CBC, serum biochemical profile, urinalysis and urine culture, blood pressure, and radiographs of the thorax and abdomen. Ultrasonography of the kidneys is useful to investigate for evidence of pyelonephritis, nephroliths, or other underlying renal disease, but it rarely detects changes associated with glomerulonephritis. An occult heartworm test should be performed and serum titers submitted for the infectious diseases discussed in Box 104-6. Testing for hyperadrenocorticism should be considered in dogs if the appropriate signalment and clinical signs are present. Renal biopsy should be considered if an underlying cause for the proteinuria cannot be identified. Tissue samples should be submitted for routine histopathology, electron microscopy, and immunopathology. Goals of renal biopsy should be to confirm the underlying disease process (specific type of GN, hereditary nephritis, glomerulosclerosis, amyloidosis), determine severity of the disease and, if possible, determine a prognosis as well as guide specific therapy.
Treatment
Therapy for immune-mediated glomerulonephritis should be directed at treating the underlying disease (if identified), decreasing protein loss in the urine, decreasing the likelihood of thromboembolism, and initiating appropriate dietary therapy and supportive care. Angiotensin converting enzyme inhibitors (ACEI) (e.g., enalapril 0.25-0.5 mg/kg q12-24h) are currently the most effective treatment for proteinuria. Anticoagulation is recommended to decrease the likelihood of thromboembolism in dogs with GN, especially in those with documented antithrombin deficiency (less than 70%). Low-dose aspirin (0.5 mg/kg q24h) may be beneficial for its anticoagulant effects and for decreasing the glomerular response to immune complexes. Other supportive measures include control of hypertension (if not controlled by ACEI alone); dietary sodium restriction; a low-protein, high-quality protein diet with n-3 fatty acid supplementation; and control of ascites and edema if present. Therapy for overt renal failure may also be necessary. See Chapter 44 for further details on general management of renal failure.
In theory, immunosuppression should be useful in idiopathic immune-mediated GN; however, no studies have documented beneficial responses to immunosuppressive therapy in dogs with GN, and the use of corticosteroids may exacerbate rather than ameliorate proteinuria. Immunosuppressive therapy is indicated when glomerulonephritis occurs as part of an immune-mediated disease known to respond to corticosteroids, such as SLE. Other indications for immunosuppressive treatment are currently poorly defined.
Careful monitoring of response to therapy with monthly measurement of protein/creatine ratios, blood urea nitrogen, creatine, and blood pressure is important to assess adequacy of therapy. Prognosis for GN varies depending on the severity of disease, underlying histopathology, and response to treatment. In general, the prognosis is guarded in animals that initially present with concurrent azotemia. The outcome is best in dogs with reversible causes of immune complex deposition and those that respond to diet and ACEI to control proteinuria. See Chapter 43 for more information on this topic.
ACQUIRED MYASTHENIA GRAVIS
Myasthenia gravis (MG) is a disorder of neuromuscular transmission resulting from deficiency or dysfunction of the nicotinic acetylcholine receptor (AChR) on the postsynaptic membrane. Acquired myasthenia gravis is an autoimmune disease in which antibodies directed against the AChR interfere with the interaction between acetylcholine and its receptor. Antibodies also cross-link AChR and cause receptor internalization. Complement-mediated damage to the postsynaptic membrane also contributes to neuromuscular blockade. As with other immune-mediated diseases, MG may be a primary autoimmune disorder or occur in association with other disorders, such as thymoma, and other neoplasms. Hypothyroidism and hypoadrenocorticism, which are also immune-mediated disorders, may also occur in association with MG. A breed predisposition exists for MG in dogs, with the Akita, various terrier breeds, and German Short-Haired Pointer being at increased risk. Abyssinian and Somali cats also have an increased risk of MG compared with other breeds.
The most common clinical presentation of MG is generalized weakness (60% of cases), either with or without concurrent megaesophagus. In focal MG, in which signs of generalized weakness are absent, the most common clinical sign is regurgitation because of megaesophagus, but dysphagia, voice change, and cranial nerve dysfunction may also occur. An acute fulminating form of MG is characterized by severe weakness, sometimes with loss of spinal reflexes and usually in conjunction with megaesophagus and aspiration pneumonia. In cats, the two most common clinical presentations are generalized weakness without megaesophagus and generalized weakness associated with a cranial mediastinal mass.
Definitive diagnosis of MG is by measurement of serum autoantibodies against AChR by immunoprecipitation radioimmunoassay. The assay is sensitive and specific and false-positive results are rare. Seronegative MG occurs in only 2% of dogs with MG. Canine and feline specific assay systems should be used. Immunosuppressive doses of corticosteroids lower the antibody concentration and can interfere with testing. Because antibodies are not the cause of congenital MG, results of antibody testing will be negative. Other useful tests in diagnosis of MG include evaluation of the response of clinical signs to a short-acting anticholinesterase drug (edrophonium chloride [Tensilon]) and electrodiagnostic testing. Once a diagnosis of MG has been confirmed, additional testing is necessary to investigate for the presence of other underlying disorders that may lead to secondary MG or occur concurrently.
The first line of treatment for MG is oral or injectable anticholinesterase inhibitors such as neostigmine or pyridostigmine (Table 104-10). These drugs act by prolonging the action of acetylcholine at the neuromuscular junction. Immunosuppression with glucocorticoids should be considered in patients that do not respond well to anticholinesterase inhibitors alone. The advantages of the immunosuppressive effects of glucocorticoids in MG are often outweighed by adverse effects such as worsening of muscle weakness and muscle atrophy. Corticosteroids may be problematic in animals with aspiration pneumonia, diabetes mellitus, and gastrointestinal ulceration, and if corticosteroids are necessary for MG care should be used to avoid excessive doses. Therapeutic approaches include starting glucocorticoids at the low end of the immunosuppressive range (prednisone 1 mg/kg q12h) or starting glucocorticoids at an even lower dose (prednisone 0.5 mg/kg PO every other day) and slowly increasing the dose after 2 weeks if a satisfactory response is not seen. Other immunosuppressive drugs that have been used for adjunctive management of MG include azathioprine and cyclosporine. Drug regimens and doses used in the routine management of MG are given in Table 104-10.
 TABLE 104-10 Drug Regimens and Doses Used for Routine Management of MG in Dogs and Cats
TABLE 104-10 Drug Regimens and Doses Used for Routine Management of MG in Dogs and Cats
| DRUG | DOGS | CATS |
|---|---|---|
| Pyridostigmine | 0.5-3.0 mg/kg PO q8-12h | 0.25-3.0 mg/kg PO q8-12h (start at low end of dose) |
| Neostigmine (use to bypass gastrointestinal tract in presence of severe regurgitation) | 0.04 mg/kg IM q6h | 0.04 mg/kg IM q6h |
| Prednisone | 0.5 mg/kg PO q48h to 1.0 mg/kg q12h | 0.5 mg/kg PO q48h to 1.0 mg/kg q12h |
| Azathioprine | 2 mg/kg PO q24h | Do not use in cats |
| Cyclosporine | 5 mg/kg PO q24h to 10 mg/kg PO q12h (see Chapter 103) | 0.5-3 mg/kg PO q12h (microemulsified) |
MG, Myasthenia gravis.
Spontaneous remission of acquired MG is common in dogs. Clinical remission is accompanied by a decrease of the AChR antibody titer into the reference range. Repeated measurement of the AChR titer is a useful guide for identifying when clinical remission is occurring and when adjustments to therapy may be indicated. The majority of dogs that do not go into remission have underlying neoplasia. See Chapter 71 for more information on this topic.
IMMUNE-MEDIATED MYOSITIS
MASTICATORY MYOSITIS
Masticatory myositis is a focal myositis affecting the muscles of mastication (temporalis, masseter, digastricus). Masticatory muscles contain a unique muscle fiber type (type 2M) that differs histopathologically, immunologically, and bio chemically from fiber types in limb musculature. Antibodies directed against this unique muscle fiber type are present in more than 80% of dogs with masticatory myositis.
Masticatory myositis is the most common form of myositis that occurs in dogs. It has not been reported in cats. Young large-breed dogs are overrepresented, and there is no breed or gender predisposition. Clinical signs include inability to open the mouth (trismus), swelling and/or pain of the masticatory muscles, and severe muscle atrophy. In some dogs an acute phase is recognized in which muscle swelling and pain predominate. If untreated this acute phase progresses to a chronic phase characterized by severe muscle atrophy and trismus. In many affected dogs the acute phase is not recognized and the first clinical signs that are recognized are severe muscle atrophy and inability to open the jaws. In severe cases the jaws can only be separated by a few centimeters, and the affected animal is unable to eat or drink. Less severely affected dogs may be able to use the tongue to lick up fluids or liquidized food. Other clinical signs include fever, depression, weight loss, dysphagia, dysphonia, and exophthalmus from swelling of the pterygoid muscles.
Diagnosis of masticatory myositis is made based on the characteristic clinical signs, and presence of antibodies against type 2M fibers. This test is positive in greater than 80% of cases and has a specificity approaching 100%. Muscle biopsy is useful to determine the degree of fibrosis and likelihood of return to normal function with treatment and to confirm the diagnosis in dogs in which the antibody test is negative. Multifocal infiltration with lymphocytes, histiocytes, and macrophages, with or without eosinophils, is found on histopathology. Moderate to severe muscle fiber atrophy, fibrosis, and sometimes complete loss of muscle fibers with replacement by connective tissue may be present. Other adjunctive tests that may be useful include measurement of creatinine kinase, which is increased in some but not all dogs with masticatory myositis, and electrodiagnostic testing, which allows identification of the most severely affected muscles. Typical electrodiagnostic findings include presence of fibrillation potentials and positive sharp waves.
Treatment of masticatory myositis relies on the use of immunosuppressive doses of corticosteroids (prednisone 2-4 mg/kg PO q24h). Under no circumstances should force be used to open the jaws because fracture or luxation of the temporomandibular joint may result. Once resolution of clinical signs is achieved with corticosteroids, the dose should then be slowly tapered over several months. Disease activity and progression should be monitored by clinical signs (especially range of motion) and measurement of creatinine kinase (if elevated at presentation). Long-term treatment with prednisone or an additional immunosuppressive drug such as azathioprine is required in dogs that relapse when prednisone is tapered. Tapering of prednisone too quickly increases the chance of relapse. The goal of therapy is a return to normal muscle function and a normal quality of life. In many cases, especially in the presence of severe fibrotic changes, muscle atrophy persists and is exacerbated by glucocorticoid therapy. Prognosis for return to function is good in most cases. See Chapter 72 for more information on this topic.
POLYMYOSITIS
Polymyositis is characterized by multifocal or diffuse infiltration of skeletal muscle by lymphocytic cells with negative serology for infectious disease. Although most cases are primary autoimmune, paraneoplastic immune-mediated myositis may be associated with malignancies such as lymphoma (particularly in Boxers), bronchogenic carcinoma, myeloid leukemia, and tonsillar carcinomas in dogs. The specific inciting antigen is not known, although the mechanism of injury is believed to be mediated by cytotoxic T cells (type IV delayed-type hypersensitivity).
Polymyositis is uncommon in dogs and rare in cats. The disease is most common in young large-breed dogs, and Boxers and Newfoundlands are overrepresented. Clinical signs include generalized weakness that worsens with exercise and a characteristic stiff gait. Cervical ventriflexion may occur, especially in cats. Most animals show pain on palpation of affected muscles, particularly the proximal muscle groups. Dysphagia, generalized muscle atrophy, dysphonia, and fever may also be present. Megaesophagus has been reported in 15% of cases. Some dogs with polymyositis also have signs of masticatory myositis, and these dogs are positive for antibodies against type 2M fibers. Polymyositis may also occur in SLE and in canine polyarthritis/myositis syndrome.
Diagnosis of polymyositis is based on characteristic clinical signs, presence of an elevated creatinine kinase level (more commonly increased in polymyositis than in masticatory myositis), electrophysiologic testing abnormalities consistent with myositis, and muscle biopsy. Muscle biopsy is very important in dogs with polymyositis to rule out infectious causes of myositis (Box 104-7). Muscle biopsies have similar changes to those described for dogs with masticatory myositis; however, the presence of eosinophils in dogs with polymyositis increases the index of suspicion for an infectious cause. Polymyositis may be a preneoplastic syndrome, especially in Boxers, so a complete evaluation for neoplasia should be performed in Boxers with polymyositis.
Treatment of polymyositis is similar to treatment of masticatory myositis (see previous page). Prognosis for return to function is good in most cases. See Chapter 72 for more information on this topic.
DERMATOMYOSITIS
Dermatomyositis is an uncommon immune-mediated disorder affecting the skin, skeletal muscle, and vasculature of Collies and Shetland Sheepdogs. The disorder has an autosomal-dominant pattern of inheritance, and the pathogenesis is suspected to be immune complex deposition, although the target antigen is not known.
In dermatomyositis cutaneous lesions develop between 2 and 4 months of age, with signs of myositis developing later. The temporalis muscle is most commonly affected and clinical signs include dysphagia and muscle atrophy. More severe signs may include megaesophagus and generalized polymyositis with diffuse muscle atrophy, especially of the distal appendicular muscles. Diagnosis of dermatomyositis is based on the classic signalment (age, breed, presence of cutaneous signs). The creatinine kinase level is usually only minimally increased. Definitive diagnosis is based on skin and muscle biopsy.
Treatment of dermatomyositis relies on symptomatic care of cutaneous lesions and immunosuppression. The protocol for corticosteroid therapy is similar to that used for polymyositis, but prolonged therapy is needed and relapses are common. Additional recommendations include avoidance of exposure to sunlight, neutering of sexually intact dogs, and vitamin E supplementation. Pentoxifylline has also been shown to be of some benefit in affected dogs (see Chapter 103). The prognosis depends on severity, being good for mild cases and poor for severely affected dogs. See Chapter 72 for more information on dermatomyositis.
Brown CD, et al. Evaluation of clinicopathologic features, response to treatment, and risk factors associated with idiopathic neutropenia in dogs: 11 cases (1990-2002). J Am Vet Med Assoc. 2006;229:87.
Carr AP, et al. Prognostic factors for mortality and thromboembolism in canine immune-mediated hemolytic anemia: a retrospective study of 72 dogs. J Vet Intern Med. 2002;16:504.
Chabanne L, et al. Canine systemic lupus erythematosus: part I, clinical and biologic aspects. Compendium. 1999;21:135. (small animal/exotics)
Chabanne L, et al. Canine systemic lupus erythematosus: part II, diagnosis and treatment. Compendium. 1999;21:402. (small animal/exotics)
Clements DN, et al. Type I immune-mediated polyarthritis in dogs: 39 cases (1997-2002). J Am Vet Med Assoc. 2004;224:1323.
Duval DJ, et al. Vaccine associated immune-mediated hemolytic anemia in the dog. J Vet Intern Med. 1996;10:290.
Evans J, et al. Canine inflammatory myopathies: a clinicopathologic review of 200 cases. J Vet Intern Med. 2004;18:679.
Gilmour MA, et al. Masticatory myopathy in the dog: a retrospective study of 18 cases. J Am Anim Hosp Assoc. 1992;28:300.
Grauer GF. Canine glomerulonephritis: new thoughts on proteinuria and treatment. J Small Anim Pract. 2005;46:469.
Husbands B, et al. Prednisone and cyclosporine versus prednisone alone for treatment of canine immune mediated hemolytic anemia (IMHA). J Vet Int Med. 2004;18:389.
Jans HE, et al. Therapy of immune-mediated thrombocytopenia: a retrospective study of 15 dogs. J Vet Intern Med. 1990;4:4.
Jordan HL, et al. Thrombocytopenia in cats: a retrospective study of 41 cases. J Vet Intern Med. 1993;7:261.
King LG, et al. Acute fulminating myasthenia in five dogs. J Am Vet Med Assoc. 1998;212:830.
Kohn B, et al. Primary immune-mediated hemolytic anemia in 19 cats: diagnosis, therapy, and outcome (1998-2004). J Vet Intern Med. 2006;20:159.
Lachowicz JL, et al. Acquired amegakaryocytic thrombocytopenia—four cases and a literature review. J Small Anim Pract. 2004;45:507.
Lewis DC, et al. Canine idiopathic thrombocytopenia. J Vet Intern Med. 1996;10:207.
Marks SL, Henry CJ. CVT update: diagnosis and treatment of systemic lupus erythematosus. In: Bonagura JD, editor. Kirk’s current veterinary therapy XIII: Small animal practice. ed 13. Philadelphia: WB Saunders; 2000:514-516.
McManus PM, et al. Correlation between leukocytosis and necropsy findings in dogs with immune-mediated hemolytic anemia: 34 cases (1994–1999). J Am Vet Med Assoc. 2001;218:1308.
McManus PM, et al. Immune-mediated neutropenia in 2 dogs. J Vet Intern Med. 1999;13:372.
Miller SA, et al. Case control study of blood type, breed, sex, and bacteremia in dogs with immune-mediated hemolytic anemia. J Am Vet Med Assoc. 2004;224:232.
Podell M. Inflammatory myopathies. Vet Clin North Am Small Anim Pract. 2002;32:147.
Rondeau MP, et al. Suppurative non-septic polyarthropathy in dogs. J Vet Intern Med. 2005;19:654.
Rozanski EA, et al. Comparison of platelet count recovery with use of vincristine and prednisone or prednisone alone for treatment for severe immune-mediated thrombocytopenia in dogs. J Am Vet Med Assoc. 2002;220:477.
Scott-Moncrieff JC, et al. Hemostatic abnormalities in dogs with primary immune-mediated hemolytic anemia. J Am Anim Hosp Assoc. 2001;37:220.
Shelton GD. Myasthenia gravis and disorders of neuromuscular transmission. Vet Clin North Am Small Anim Pract. 2002;32:189.
Shelton GD, et al. Risk factors for acquired myasthenia gravis in cats: 105 cases (1986–1998). J Am Vet Med Assoc. 2000;216:55.
Shelton DG, et al. Risk factors for acquired myasthenia gravis in dogs: 1,154 cases (1991–1995). J Am Vet Med Assoc. 1997;211:11428.
Smee NM, et al. Measurement of serum antinuclear antibody titer in dogs with and without systemic lupus erythematosus: 120 cases (1997-2005). J Am Vet Med Assoc. 2007;230:1180.
Smith BE, et al. Antinuclear antibodies can be detected in dog sera reactive to Bartonella vinsonii subsp. Berkhoffii, Ehrlichia canis, or Leishmania infantum antigens. J Vet Intern Med. 2004;18:47.
Stokol T, et al. Idiopathic pure red cell aplasia and nonregenerative immune-mediated anemia in dogs: 43 cases (1998–1999). J Am Vet Med Assoc. 2000;216:1429.
Stokol T, et al. Pure red cell aplasia in cats: 9 cases (1989–1997). J Am Vet Med Assoc. 1999;214:75.
Webb AA, et al. Steroid responsive meningitis-arteritis in dogs with noninfectious nonerosive idiopathic immune-mediated polyarthritis. J Vet Intern Med. 2002;16:269.
Weinkle TK, et al. Evaluation of prognostic factors, survival rates, and treatment protocols for immune-mediated hemolytic anemia in dogs: 151 cases (1993-2002). J Am Vet Med Assoc. 2005;226:1869.
Weiss DJ. Primary pure red cell aplasia in dogs: 13 cases (1996-2000). J Am Vet Med Assoc. 2002;221:93.
Weiss DJ. Evaluation of antineutrophil IgG antibodies in persistently neutropenic dogs. J Vet Intern Med. 2007;21:440.