CHAPTER 103 Treatment of Primary Immune-Mediated Diseases
PRINCIPLES OF TREATMENT OF IMMUNE-MEDIATED DISEASES
When treating an animal with immune-mediated disease, any underlying disease must also be treated. Although concurrent immunosuppressive therapy is typically necessary, effective treatment of underlying disease (if possible) may minimize the length of immunosuppressive therapy. The aim of treatment is to control the immune-mediated process while minimizing the adverse effects of the drugs used. In many situations short-term adverse effects must be tolerated to put the immune-mediated disease into remission. In the long term, however, medications must be tapered to minimize adverse effects. If this is not possible or if the initial drug chosen elicits a poor response, alternate or additional therapy should be considered. Monitoring the disease process before each dose reduction is critical and should be individualized depending on the underlying disease process. For example, in immune-mediated hemolytic anemia (IMHA), monitoring the complete blood count (CBC) and reticulocyte count (plus the Coombs test if it was initially positive) is adequate, whereas in dogs with immune-mediated polyarthritis repeated joint taps for synovial fluid analysis are recommended.
Supportive care and aggressive monitoring for complications caused by the immunosuppressive drugs is also critical. Detection and treatment of complications of therapy can improve long-term outcome and minimize adverse sequelae. For example, patients receiving glucocorticoids should be carefully monitored for evidence of gastrointestinal ulceration, and animals receiving azathioprine should be monitored for hepatotoxicity and bone marrow suppression. In addition, supportive care is also needed while waiting for the full effects of immunosuppressive therapy. For example, dogs with IMHA and immune-mediated thrombocytopenia (ITP) may require several transfusions before immunosuppressive treatment adequately controls the immunemediated destruction of red blood cells (RBCs) or platelets. Other forms of supportive care that may be necessary include care of the skin in animals that are recumbent, nutritional support, monitoring for and treatment of infection, and prevention of gastrointestinal ulceration.
OVERVIEW OF IMMUNOSUPPRESSIVE THERAPY
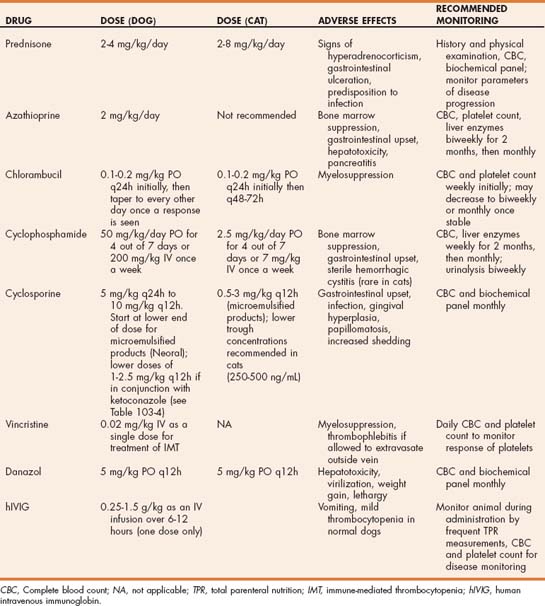
The first line of treatment for the majority of immunemediated diseases is treatment with glucocorticoids (Table 103-1). The reasons for using glucocorticoids as the first line of therapy include rapid onset of action, low risk of toxicity, and low cost. Even in patients with concurrent conditions such as diabetes mellitis, in which long-term glucocorticoid treatment is relatively contraindicated, glucocorticoids should still be used initially with a plan to transition to other drugs (such as azathioprine) that are less likely to complicate management of the concurrent disease. In some immune-mediated diseases additional immunosuppressive drugs should be added at the start of treatment. These are diseases in which a positive response to glucocorticoids alone is unlikely. Examples include canine Evans syndrome, canine IMHA with multiple poor prognostic indicators (intravascular hemolysis, agglutination that persists after washing the RBCs, high bilirubin concentration), systemic lupus erythematosus, rheumatoid arthritis, and the polyarthritis syndrome of Akitas. In most other immune-mediated diseases, the response to glucocorticoids should be assessed before adding other immunosuppressive drugs. If response to glucocorticoids is inadequate or the adverse effects of glucocorticoids are unacceptable, azathioprine is the next drug that is most commonly added to the treatment protocol in the dog, and chlorambucil should be considered in the cat. Cyclophosphamide and cyclosporine are typically considered third-line drugs, although some exceptions are discussed in the sections on the individual immune-mediated diseases (see Chapter 104). For example, cyclosporine is used as a first-line drug in treatment of perianal fistulas in dogs, and cyclophosphamide is used as a second-line drug in cats with red cell aplasia. If immune-mediated disease has an underlying infectious cause, more caution should be used before adding an additional immunosuppressive drug. When adding a third-line drug, in most circumstances it should replace the second-line drug. Treatment with more than one additional immunosuppressive drug at the same time (e.g., azathioprine and cyclosporine together) is usually unnecessary and is likely to cause much more severe immunosuppression and predisposition to infection.
 TABLE 103-1 First-, Second-, and Third-Line Drugs Commonly Used in the Management of Immune-Mediated Disease of the Dog and Cat
TABLE 103-1 First-, Second-, and Third-Line Drugs Commonly Used in the Management of Immune-Mediated Disease of the Dog and Cat
| DOG | CAT | |
|---|---|---|
| Initial treatment | Prednisone | Prednisolone |
| Second line | Azathioprine | Chlorambucil |
| Third line | Cyclophosphamide or cyclosporine | Cyclophosphamide or cyclosporine |
GLUCOCORTICOIDS
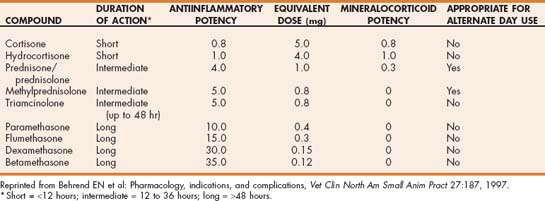
Glucocorticoids (corticosteroids with primarily glucocorticoid activity) are the mainstay of treatment of most immune-mediated diseases because they are effective, rapid acting, and cheap. Several different glucocorticoid drugs vary according to duration, potency, and route of administration. Glucocorticoids are characterized by their biologic half-life as measured by duration of suppression of the hypothalamic pituitary adrenocortical axis (Table 103-2). Short-acting glucocorticoids such as hydrocortisone and cortisone have a biologic half-life of less than 12 hours. Intermediate-acting steroids such as prednisone, prednisolone, methylprednisolone, and triamcinolone have a biologic half-life of 12 to 36 hours, and betamethasone, dexamethasone, flumethasone, and paramethasone have a biologic half-life of 48 hours or longer. The duration of effect of a glucocorticoid preparation is also influenced by the chemical form of the steroid. Parenteral glucocorticoid preparations are either esters or free steroid alcohols. Highly soluble esters (e.g., dexamethasone sodium phosphate, prednisolone sodium succinate) and solutions of free steroid alcohols in polyethylene glycol (dexamethasone, flumethasone) have a duration of action similar to the biologic half-life, but long-acting suspensions of insoluble steroid esters (e.g., methylprednisolone acetate suspension, triamcinolone acetamide suspension) are absorbed slowly from the injection site and do not achieve high plasma concentrations. The slow absorption also dramatically prolongs the duration of effect. Oral preparations are usually composed of the free steroid alcohol; because absorption from the gastrointestinal tract is quite rapid, the duration of effect is similar to the biologic half-life. The antiinflammatory effects of corticosteroids correlate with their glucocorticoid activity, and undesirable adverse effects such as sodium retention and edema formation are associated with mineralocorticoid activity. Synthetic steroids that possess higher glucocorticoid and lower mineralocorticoid activity than cortisol include prednisone, which has four times the potency of cortisone but 0.3 times the mineralocorticoid activity; dexamethasone, which has eight times the potency with no mineralocorticoid activity; and triamcinolone, which also has no mineralocorticoid activity.
In most patients with immune-mediated disease the ideal route of glucocorticoid administration is oral; however, in animals that are vomiting or have diseases that interfere with swallowing or gastrointestinal absorption, intravenous administration of either prednisolone or dexamethasone may be necessary. The use of long-acting parenteral drugs for treatment of immune-mediated disease is not recommended because of the failure to achieve high plasma concentrations and the long duration of effect.
The actions of corticosteroids that make them useful drugs in the treatment of various immune-mediated diseases are shown in Box 103-1. The early effects of corticosteroids are believed to result from a rapid decrease in the phagocytic activity of splenic and hepatic macrophages, whereas the long-term effects result from suppression of cell-mediated immunity. How much suppression of antibody production occurs in steroid-resistant species such as the dog and cat is controversial, but effects on B lymphocytes likely occur from suppression of T-helper cells that are required for full antibody response to an antigen.
For treatment of most immune-mediated diseases, an intermediate-acting corticosteroid such as prednisone is the treatment of choice. Prednisone is converted in the liver to prednisolone. The two drugs have historically been considered clinically identical except in the presence of hepatic failure; however, some evidence now suggests that cats do not convert prednisone to prednisolone as well as other species, and thus prednisolone may be a better choice than prednisone in cats. The starting dose for prednisone in dogs is 2 to 4 mg/kg/day usually given in two divided doses. Cats are more resistant to the effects of glucocorticoids than are dogs. In cats doses of 2 to 8 mg/kg/day of prednisolone or 4 mg/week per cat of dexamethasone are recommended. For immunosuppressive therapy with other glucocorticoids, the dose is based on the drug’s comparative potency to prednisone. For example, the dose of dexamethasone should be approximately eight times less than the dose of prednisone for an equivalent effect. Other than this difference in potency, no evidence currently suggests that dexamethasone is more effective than prednisone or prednisolone in the treatment of immune-mediated disease. The most common reason for choosing dexamethasone rather than prednisone is for parenteral administration in patients that are vomiting or cannot tolerate oral medication.
Although glucocorticoids are extremely useful in the management of immune-mediated disease, long-term adverse effects may be debilitating to the animal and intolerable to the owner. Common adverse effects include polyuria, polydipsia, panting, weakness, dermatologic changes, predisposition to infection, gastrointestinal ulceration (at higher doses) and muscle atrophy (Fig. 103-1). Insulin resistance and hyperglycemia as well as a steroid-induced hepatopathy may also occur. Individual patients vary in their tolerance of the side effects of glucocorticoid therapy, with larger dogs often being particularly sensitive. Cats seem to be much more resistant to the side effects of glucocorticoids than are dogs.

FIG 103-1 Severe temporal muscle atrophy in a 7-year-old castrated male Weimaraner treated with immunosuppressive doses of prednisone for immune-mediated disease.
Strategies to minimize the adverse effects of glucocorticoid therapy include using the lowest dose that will control the disease of interest, using shorter acting rather than longer acting steroids, and switching to alternate-day therapy as soon as possible. To maximize the likelihood of a good response to treatment, start with high doses initially and then slowly taper the dose rather than start with a more conservative dose and increase the dose if required. Tapering of the dose should be based on an objective measure of response to treatment (e.g., hematocrit or joint fluid analysis), and tapering of the dose should be done slowly to minimize the chance of disease relapse. As a general rule the dose should not be tapered faster than 50% per month. Remission may be harder to achieve a second time if the disease is allowed to relapse because of premature tapering of the dose. If clinical signs of glucocorticoid treatment are intolerable, other immunosuppressive drugs should be added to the treatment protocol so that the dose of glucocorticoids can be tapered more rapidly and ultimately discontinued.
AZATHIOPRINE
Azathioprine (Imuran) is a thiopurine antimetabolite that is a sulfur analog of adenine. After absorption, azathioprine is converted into 6-mercaptopurine and then into a number of thiopurine antimetabolites within the liver. The active cytotoxic metabolites of azathioprine are the 6-thioguanine nucleotides, which compete with purines in the synthesis of nucleic acids. This results in formation of nonfunctional nucleic acid strands. DNA and RNA synthesis is inhibited, leading to decreased proliferation of rapidly dividing cells. In hepatic insufficiency the immunosuppressive effects of azathioprine are diminished, and concurrent administration of allopurinol results in increased concentration of active metabolites. Azathioprine has a preferential effect on Tlymphocyte function with inhibition of cell-mediated immunity and T-lymphocyte–dependent antibody synthesis. Numbers of circulating monocytes are also decreased. Some confusion exists in the veterinary literature about the length of time required for azathioprine to have a clinical effect. The experimental data are sparse, but in one study azathioprine inhibited blastogenic response of canine lymphocytes to mitogens after 7 days of treatment, although serum immunoglobulin concentrations were unchanged. Clinical experience, however, suggests that the full effects of azathioprine treatment may not occur until 4 to 8 weeks after initiation of treatment.
Azathioprine is commonly used as a second-line drug in a variety of immune-mediated diseases, including immune-mediated hemolytic anemia, immune-mediated thrombocytopenia, immune-mediated polyarthritis, inflammatory bowel disease, and systemic lupus erythematosus (SLE) (see Chapter 104 for the specific indications for each of these diseases). Azathioprine at the typical starting dose of 2 mg/kg PO q24h is well tolerated in dogs. Adverse effects are uncommon but bone marrow suppression, gastrointestinal upset, pancreatitis, and hepatotoxicity have been reported. A small percentage of canine patients experience life-threatening myelosuppression, characterized by neutropenia, thrombocytopenia, and sometimes anemia, when treated with azathioprine. Lower doses of azathioprine (50 mg/m2 q24h or 1 mg/kg PO q24h) should be considered in dogs that show evidence of myelosuppression at the usual 2 mg/kg dose. Attempts to predict which patients are likely to have these reactions by measuring thiopurine methyltransferase activity have not been rewarding. Bone marrow suppression usually occurs within 1 to 4 months after initiation of therapy and is typically reversible within 7 to 14 days after discontinuation of therapy. Because of the potential for myelosuppression and hepatotoxicity, dogs receiving azathioprine should have a CBC and hepatic enzymes measured every 1 to 2 weeks for the first month of treatment and then every 1 to 3 months. In dogs azathioprine is typically initially used in conjunction with immunosuppressive doses of prednisone. If a positive response is observed to combined therapy, the prednisone dose should be tapered over a period of 2 to 4 months. During this time daily azathioprine should be continued at the same dose (if adverse effects are not seen). If complete discontinuation of prednisone is possible without disease relapse, then the dose of azathioprine can be gradually decreased. This is usually accomplished by initially changing the dose schedule to every other day and then to every third day before complete cessation of treatment. In patients for whom prior relapse of immune-mediated disease has already occurred, the clinician may choose to continue life-long low-dose azathioprine treatment (2 mg/kg every other day). Of note, bone marrow suppression has been reported as long as 12 months after starting azathioprine treatment, so monitoring of CBC and hepatic enzymes should be continued for the duration of treatment. Azathioprine is not recommended for use in cats because severe neutropenia and thrombocytopenia have been reported to occur even at reduced doses. Chlorambucil is a better choice for adjunctive immunosuppression in cats with immune mediated disease.
CYCLOPHOSPHAMIDE
Cyclophosphamide (Cytoxan) is an alkylating agent that decreases cell division of both B and T lymphocytes. Alkylating agents form covalent bonds with organic compounds, specifically nucleic acids, with resultant cross-linking of DNA, inhibition of DNA synthesis, and death in rapidly dividing cells. Cyclophosphamide affects both the cell-mediated and the humoral immune responses, but the effects on the humoral system are more pronounced. Cyclophosphamide requires hepatic transformation to its active metabolites (nornitrogen mustard, phosphoramide mustard, and acrolein). Cyclophosphamide is used to treat a range of immune-mediated diseases, but it is less commonly used than azathioprine because of the higher risk of adverse effects. In the past cyclophosphamide was a commonly used drug for adjunctive treatment of dogs with IMHA; however, recent studies suggest that other drugs such as azathioprine and cyclosporine are better choices in this disease. Adverse effects of cyclophosphamide include bone marrow suppression, gastrointestinal upset, poor hair growth, alopecia, and sterile hemorrhagic cystitis from the toxic effects on the bladder of the metabolite acrolein. Sterile hemorrhagic cystitis is most commonly reported in dogs treated with cyclophosphamide for 2 months or longer and is rare in cats. Cyclophosphamide is typically dosed in dogs either at 50 mg/m2 daily for 4 days each week or as a single intravenous dose of 200 mg/m2 once a week. The latter dose schedule tends to cause more profound bone marrow suppression. Lower doses are recommended in cats (Table 103-3).
CHLORAMBUCIL
Chlorambucil (Leukeran) is an alkylating agent that is most commonly used as an alternative to azathioprine in cats with immune-mediated disease. Chlorambucil is a prodrug metabolized to the active metabolite phenylacetic acid mustard. It can also be used as an alternate immunosuppressive drug in dogs that do not tolerate the more commonly used cytotoxic drugs. The usual starting dose for treatment of immune-mediated diseases in both dogs and cats is 0.1 to 0.2 mg/kg PO q24h or 20 to 40 mg/m2 PO q2wk (see Table 103-3). Adverse effects include bone marrow suppression, gastrointestinal upset, and predisposition to infection.
CYCLOSPORINE
Cyclosporine, a potent immunomodulating agent, is a cyclic polypeptide extracted from fungi. The major mode of action is by inhibition of the initial activation phase of CD4 T lymphocytes. Cyclosporine blocks the transcription of genes encoding several cytokines, in particular interleukin-2 (IL-2). This prevents the activation and proliferation of T lymphocytes and the secondary synthesis of other cytokines. Cyclosporine does not affect the humoral immune system; therefore treatment with cyclosporine should not influence response to vaccination. Cyclosporine is the treatment of choice for perianal fistulas in dogs and is as effective as glucocorticoids in the management of atopic dermatitis. Cyclosporine has also been used to treat other refractory immune-mediated diseases in dogs and cats, such as immune-mediated hemolytic anemia, inflammatory bowel disease, myasthenia gravis, granulomatous meningoencephalomyelitis, pure red cell aplasia, and a variety of immune-mediated dermatologic diseases.
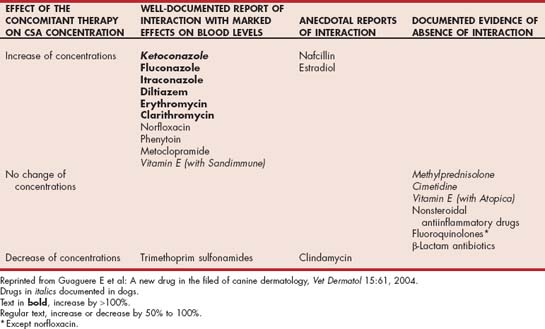
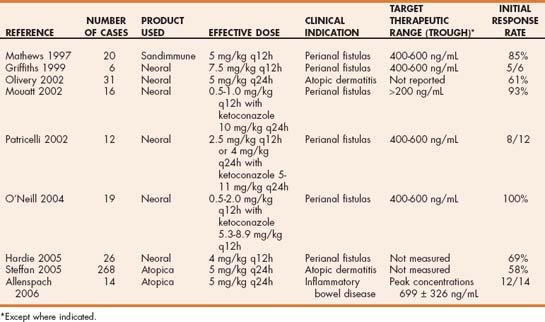
Cyclosporine is available as a vegetable oil formulation (Sandimmune, Sandoz, Holzkirchen, Germany) or as a microemulsion in gelatin capsules (Atopica, Novartis Animal Health, Basel, Switzerland; Neoral, Sandoz). Bioavailability of the microemulsion is higher than that of the oil-based product, and drug absorption is less variable. Because food intake delays drug absorption and increases variability of absorption, the microemulsion form of cyclosporine should be given 2 hours before or after feeding. Doses of cyclosporine depend on the product used and the disease being treated but range from 5 mg/kg q24h to 10 mg/kg PO q12h (Tables 103-3 and 103-4). Lower doses are typically necessary when the microemulsion product is used. Measurement of blood cyclosporine concentration for dose individualization is recommended; however, clear-cut guidelines for appropriate therapeutic concentrations are lacking. In addition, considerable variability exists between commercial assays for cyclosporine, so following the guidelines of individual laboratories regarding the therapeutic range is important. Blood cyclosporine levels measured with high-performance liquid chromatography techniques are typically lower than those measured with other commercial techniques (fluorescent polarization immunoassay, radioimmunoassay) because these techniques also detect some cyclosporine metabolites. Trough concentrations of 400 to 600 ng/mL (depending on the assay used) are considered to be in the therapeutic range, but positive clinical responses for some disorders may be observed at lower concentrations.
 TABLE 103-4 Selected Studies of Dosing Recommendations and Therapeutic Monitoring for Dogs Treated with Cyclosporine
TABLE 103-4 Selected Studies of Dosing Recommendations and Therapeutic Monitoring for Dogs Treated with Cyclosporine
Numerous interactions between cyclosporine and other drugs occur because of shared metabolic pathways involving the cytochrome P450 enzyme system. Therapeutic monitoring is especially important in animals receiving concurrent therapy with such drugs (Table 103-5). In dogs treated with cyclosporine (2 to 5 mg/kg PO q24h), concurrent ketoconazole administration (5 to 15 mg/kg q24h) can be used to decrease the required dose of cyclosporine, with considerable resultant cost savings. This strategy has primarily been used in dogs with perianal fistulas and dogs undergoing organ transplantation; however, it could also be considered in other diseases for which cyclosporine is indicated, although the effectiveness is unproven. Therapeutic monitoring of the cyclosporine concentration is important when using this strategy.
Adverse effects of cyclosporine include gastrointestinal disturbance, predisposition to infection, gingival hyperplasia, papillomatosis, and increased shedding of the haircoat. A dermatosis from atypical staphylococcal infection (psoriasiform lichenoid–like dermatosis) has also been reported in dogs treated with cyclosporine. Affected dogs improved after antibiotic therapy and a decreased dose of cyclosporine. At the doses used to treat atopic dermatitis (5 mg/kg PO q24h), no difference in prevalence of bacterial infection was demonstrated between dogs treated with prednisone and those treated with cyclosporine. The risk of infection is increased in dogs treated with higher doses of cyclosporine, such as those used to prevent transplant rejection (20 mg/kg PO q24h) and when cyclosporine is combined with other immunosuppressive drugs such as prednisone and azathioprine.
VINCRISTINE
Vincristine is an alkaloid derived from the periwinkle plant used as an antineoplastic and immunosuppressive agent. Vincristine binds to the microtubular structural protein tubulin, which is abundant within platelets. At low doses the drug causes a transient increase in circulating platelet numbers; at higher doses it can cause myelosuppression and thrombocytopenia. Proposed mechanisms for increased platelet numbers in normal dogs include stimulation of thrombopoiesis by circulating thrombopoietic factors (perhaps by concealing platelets from the thrombopoietic regulatory system) or by inducing acute fragmentation of mature megakaryocytes. In immune-mediated thrombocytopenia, in which stimulation of thrombopoiesis is already maximal, the mechanisms for increased platelet numbers are most likely increased platelet release from the bone marrow and impaired platelet destruction from inhibition of phagocytosis, or interference with antibody binding to platelets. Decreased antibody synthesis seems less likely considering the short time course for the increase in platelet count. Disruption of structure and function of platelets has been reported after exposure to vincristine in vitro and in vivo in dogs with lymphoma; however, the clinical significance of this finding is unclear.
The major indication for vincristine in treatment of immune-mediated disease is as an adjunctive therapy for ITP. Vincristine is administered at 0.02 mg/kg IV as a single dose in addition to treatment with glucocorticoids. Vincristine-treated dogs with ITP have a more rapid increase in platelet number and shorter duration of hospitalization than dogs treated with prednisone alone. The advantages of vincristine are that it is readily available and inexpensive. Although bone marrow suppression may occur at higher doses, this has not been reported at the low single dose used for treatment of immune-mediated thrombocytopenia. Care should be taken during intravenous administration because the drug is highly caustic if allowed to extravasate outside the vein.
DANAZOL
Danazol (Danocrine) is an attenuated synthetic androgen that has been used as an immunomodulating drug in dogs. In theory androgens suppress the immune response, and in people with immune-mediated hemolytic anemia and immune-mediated thrombocytopenia danazol has been found to decrease the amount of immunoglobulin and complement on the surface of RBCs and platelets. Isolated case reports have also suggested a beneficial effect in dogs with IMHA and thrombocytopenia, but confounding effects of other drugs occurred in both reports. In a double-blind study of danazol treatment in dogs with IMHA also treated with prednisone and azathioprine, no beneficial effect of danazol could be demonstrated. Side effects of danazol in dogs are uncommon but include hepatotoxicity, virilization (of females), weight gain, and lethargy. The recommended dose for danazol is 5 mg/kg q12h; however, no good evidence currently exists to support the use of this drug for treatment of immune-mediated diseases in dogs and cats.
HUMAN INTRAVENOUS IMMUNOGLOBULIN
Human intravenous immunoglobulin (hIVIG) is a preparation of polyspecific immunoglobulin G (IgG) obtained from the plasma of a large number (more than 1000) of healthy human blood donors. hIVIG is available either as a solution or a lyophilized product, and a wide range of concentrations and vial sizes are available (5% to 10%, 1- to 12-g vials). Numerous commercial products are available and vary in price and availability (e.g., Gammagard S/D, Baxter Healthcare Corporation, Deerfield, Ill.; Gamimune N, Bayer Pharmaceuticals, Leverkusen, Germany). Human hIVIG is the treatment of choice for immune-mediated thrombocytopenic purpura and is also used for the treatment of a wide variety of other immune-mediated diseases in human beings. The mechanism(s) by which hIVIG modulates the immune system is unknown. In dogs the primary mechanism of hIVIG is hypothesized to be blockade of Fc receptors on mononuclear phagocytes, thereby inhibiting phagocytosis. Other potential mechanisms include decreased production of autoantibodies, possibly from effects of anti-idiotypic antibodies in hIVIG, functional modulation of T cells, decreased natural killer cell activity, blockade of complement-mediated cell damage, and modulation of the release and function of proinflammatory cytokines.
hIVIG has been used in veterinary medicine to treat immune-mediated hemolytic anemia, pure red cell aplasia, myelofibrosis, ITP, erythema multiforme, pemphigus foliaceus, and toxic epidermal necrolysis, although prospective studies evaluating the efficacy of hIVIG have yet to be performed in any of these diseases. Doses recommended for use in dogs range from 0.25 to 1.5 g/kg administered as an intravenous infusion over 6 to 12 hours. The potential limitation of treatment of dogs and cats with hIVIG is that administration of an infusion containing human protein could lead to sensitization and potential anaphylaxis if the treatment is repeated. However, no reports of anaphylactic reactions have yet been reported despite administration of the products at least twice (and in one case multiple times) in some dogs and cats. To date no clinically significant side effects have been reported in dogs or cats treated with hIVIG, although a high rate of thromboembolism was reported in one study of dogs with IMHA treated with hIVIG (Scott-Moncrieff et al., 1997). Whether this was related to the underlying disease or the treatment was not clear. Risk of thromboembolism is also a concern in people treated with hIVIG, especially in those with other risk factors for thromboembolism. Mild thrombocytopenia and occasional vomiting have been reported in normal dogs treated with hIVIG. The major limitation of hIVIG treatment is the expense, which has limited prospective studies of this mode of therapy in veterinary medicine. hIVIG is currently most commonly used as a rescue agent in dogs with immune-mediated diseases that are not responding to conventional immunosuppressive agents. Because of the rapid but short-acting effect of hIVIG on phagocytosis, the most logical use is as a bridge to suppress phagocytosis in diseases such as IMHA and ITP while waiting for other immunosuppressive drugs to become effective; however, clinical studies are currently lacking.
PENTOXIFYLLINE
Pentoxifylline belongs to the methylxanthine drug class and is a derivative of theobromine. Despite this derivation the drug does not have cardiac or bronchodilatory effects. The major properties of the drug relate to its effects on the immune system and blood viscosity. By mechanisms that are unclear, pentoxifylline improves the deformability of RBCs. Pentoxifylline also has a number of immunomodulating effects, including inhibition of IL-1, IL-6, and tumor necrosis factor-α as well as inhibition of B- and T-cell activation. The pharmacokinetics of pentoxifylline have been described in the dog, and the current dose recommendation is 15 mg/kg PO q8h. In veterinary medicine pentoxifylline has primarily been used for the management of cutaneous immune-mediated diseases, including dermatomyositis, SLE, and various forms of vasculitis. Whether the drug might be beneficial in other immune-mediated diseases is still to be determined. Adverse side effects in dogs are uncommon but may include vomiting, diarrhea, bone marrow suppression, and flushing.
SPLENECTOMY
Splenectomy is an adjunctive therapy that has been recommended in the management of hematologic immune-mediated diseases such as IMHA and ITP. Splenectomy is theorized to decrease the number of phagocytic mononuclear cells available for phagocytosis of antibody-coated RBCs and platelets. It is typically recommended in dogs with IMHA and ITP resistant to medical therapy. In dogs with relapsing ITP good evidence supports the merits of splenectomy in the subset of dogs with ITP that relapses after tapering of prednisone and azathioprine therapy. The merits of splenectomy in dogs with IMHA are less clear. Some case reports suggest that some dogs have better control of disease after splenectomy, but in other cases no benefit occurred. One concern regarding splenectomy in dogs with IMHA is that the spleen is an important site of extramedullary hematopoiesis, so splenectomy decreases the regenerative response. In addition, most dogs with IMHA are not good candidates for a major surgical procedure such as splenectomy.
Allenspach K, et al. Pharmacokinetics and clinical efficacy of cyclosporine treatment of dogs with steroid refractory inflammatory bowel disease. J Vet Intern Med. 2006;20:239.
Beale KM, et al. Systemic toxicosis associated with azathioprine administration in domestic cats. Am J Vet Res. 1992;53:1236.
Beale KM. Azathioprine for treatment of immune-mediated diseases of dogs and cats. J Am Vet Med Assoc. 1988;192:1316.
Behrend E, et al. Pharmacology, indications, and complications. Vet Clin North Am Small Anim Pract. 1997;27:187.
Grau-Bassas ER, et al. Vincristine impairs platelet aggregation in dogs with lymphoma. J Vet Intern Med. 2000;14:81.
Griffiths LG, et al. Cyclosporine as the sole treatment for anal furunculosis: preliminary results. J Small Anim Pract. 1999;40:569-572.
Guaguere E, et al. A new drug in the filed of canine dermatology. Vet Dermatology. 2004;15:61.
Hardie RJ, et al. Cyclosporine treatment of anal furunculosis in 26 dogs. J Small Anim Pract. 2005;46:3-9.
Matthews KA, et al. Randomized controlled trial of cyclosporine for treatment of perianal fistulas in dogs. J Am Vet Med Assoc. 1997;211:1249-1253.
Miller E. The use of cytotoxic agents in the treatment of immune-mediated diseases of dogs and cats. Semin Vet Med Surg (Small Anim). 1997;12:144.
Mouatt JG, et al. Cyclosporine and ketoconazole interaction for treatment of perianal fistulas in the dog. Aust Vet J. 2002;80:207-211.
Ogilvie GK, et al. Short-term effect of cyclophosphamide and azathioprine on selected aspects of the canine blastogenic response. Vet Immunol Immunopath. 1988;18:119.
Olivry T, et al. Randomized controlled trial of the efficacy of cyclosporine in the treatment of atopic dermatitis in dogs. J Am Vet Med Assoc. 2002;221:370-377.
O’Neill T, et al. Efficacy of combined cyclosporine A and ketoconazole treatment of anal furunculosis. J Small Anim Pract. 2004;45:238.
Patricelli AJ, et al. Cyclosporine and ketoconazole for the treatment of perianal fistulas in dogs. J Am Vet Med Assoc. 2002;220:1009.
Rinkardt NE, et al. Azathioprine induced bone marrow toxicity in four dogs. Can Vet J. 1996;37:612.
Rodriguez DB, et al. Relationship between red blood cell thiopurine methyltransferase activity and myelotoxicity in dogs receiving azathioprine. J Vet Intern Med. 2004;18:339.
Scott-Moncrieff JC, et al. Human intravenous immunoglobulin therapy. Semin Vet Med Surg (Small Anim). 1997;12:178.
 BOX 103-1
BOX 103-1