CHAPTER 2 Basic structure, function and control of the lower urinary tract
INTRODUCTION
The urinary tract consists of two distinct and mutually dependent components:
These provide a highly sophisticated system of conduits and a reservoir that converts the continuous involuntary production of urine by the kidneys into the intermittent, consciously controlled voiding of urine (micturition at a convenient time and place).
A thorough understanding of the structure, function and control of the lower urinary tract is vital for the accurate interpretation of urodynamic investigations.
THE KIDNEYS AND URETERS
Both kidneys continuously produce greater than 0.5 ml of urine per kg of body weight per hour (i.e. >35 ml per hour in a 70 kg man) when functioning properly and adequately hydrated. This urine empties into the kidney’s collecting systems which drain via the ureters.
The ureters function as low-pressure distensible conduits with intrinsic peristalsis, which transport urine from the kidneys to the bladder. The urine drains into the bladder at the vesico-ureteric junction (VUJ) at the termination of each ureter. Each junction if correctly functioning only allows the one way flow of urine and contains a mechanism to prevent retrograde transmission of urine back into the ureters from the bladder. This serves to protect the upper tract from the high pressures encountered within the bladder during voiding and to prevent infection entering the upper tracts.
THE URINARY BLADDER
The bladder is a hollow, muscular organ. It has two main functions:
Histologically the bladder is composed of three distinct layers:
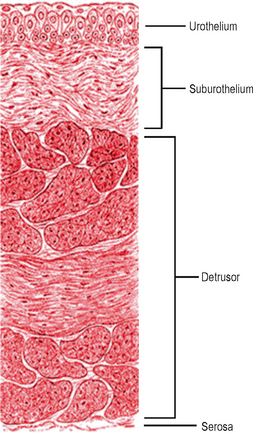
Figure 2.1 Structure of the bladder wall. The bladder wall is composed of four distinct layers and includes highly metabolically active urothelial and suburothelial layers.
The base of the bladder extends circumferentially from the ureteric orifices. This region contains the trigone which is a small triangular muscular area between the two ureteric orifices and the bladder neck. The trigone contains a complex plexus of nerves. Above the ureteric orifices is the main body of the bladder.
THE URETHRA AND SPHINCTERIC MECHANISMS
The urethra has two main functions:
A further role for the urethra which seems likely but remains hypothetical is to provide afferent feedback which may have important implications in influencing bladder function. The innermost mucosal layer in both sexes is organized in longitudinal folds and during the storage phase when the urethra is ‘closed’ this appears in a stellate configuration on cross section. Such a configuration allows significant distensibility, which is necessary during urethral ‘opening’.
The submucosal layer contains a vascular plexus which may be involved in improving the seal of a ‘closed urethra’ by transmitting the tension of the urethral muscle to the mucosal folds.
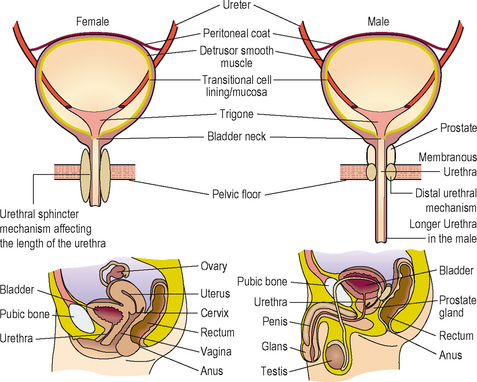
Apart from the obvious anatomical differences there are important differences in the configuration of the sphincter mechanisms between males and females (Figure 2.2).
Table 2.1 contrasts the longer urethra, a prostate and two powerful sphincter mechanisms in the male compared to the single weaker intrinsic sphincter mechanism with a weaker bladder neck and also a shorter urethra in the female.
Table 2.1 Comparison of male and female sphincteric mechanisms – showing why females are much more likely to develop an incompetent urethral mechanism and be prone to urinary incontinence from intrinsic sphincter deficiency (ISD).
| Comparison of male and female sphincteric mechanisms | ||
|---|---|---|
| Male | Female | |
| Proximal bladder neck mechanism | Powerful | Weak |
| Distal urethral mechanism/urethal sphincter mechanism (in the female) | Powerful | Prone to the effect of exogenous influences such as pelvic floor weakness and damage or denervation consequent upon childbirth |
| Prostate | Further increases bladder outlet resistance | Not present |
| Urethra | Long | Short (∼3.5 cm) |
Male sphincteric mechanisms
In the male there are two important sphincteric mechanisms:
The proximal sphincter in the male bladder neck provides a powerful mechanism in both maintaining urinary continence and also preventing retrograde ejaculation of semen during sexual activity. In patients with a damaged distal urethral sphincter (e.g. a pelvic fracture-associated urethral disruption) continence can be maintained solely by the proximal bladder neck mechanism. Ultrastructurally it consists of a powerful inner layer of muscle bundles arranged in a circular orientation.
The distal sphincteric mechanism is also extremely important, as evidenced by its ability to maintain continence even when the proximal bladder neck mechanism has been rendered totally incompetent by surgical bladder neck incision or a prostatectomy. It is confined to the 3–5 mm thickness of the wall of the membranous urethra from the level of the verumontanum down to the distal aspect of the membranous urethra. It is composed mainly of extrinsic striated muscle which is capable of the sustained contraction necessary for continence and to a lesser degree by intrinsic smooth muscle.
Prostate gland
The prostate is made up of smooth muscle and glandular tissue, with the proportion of smooth muscle being increased in benign prostatic hyperplasia (BPH). The prostatic smooth muscle is controlled by the sympathetic nervous system, which acts by releasing noradrenaline onto α1a-adrenoceptors located on the smooth muscle cells; the resulting contraction increases the bladder outlet resistance and further aids continence in the male.
Female sphincteric mechanisms
Females are much more likely to suffer from urinary incontinence due to sphincteric deficiency than males, due to the much less powerful sphincteric mechanisms. The bladder neck is a far weaker structure than the male bladder neck and is often incompetent, even in nulliparous young women. The bladder neck is poorly defined with the muscle fibres having a mainly longitudinal orientation.
Urinary continence is usually reliant upon the integrity of the urethral sphincteric mechanism, which like the male distal mechanism is composed of a longitudinal intrinsic urethral smooth muscle and a larger extrinsic striated muscle component. This sphincter extends throughout the proximal two-thirds of the urethra, being most developed in the middle one-third of the urethra. Damage to the sphincter or it’s innervation (in particular the pudendal nerve) by obstetric trauma reduces the effectiveness of this mechanism and predisposes to stress urinary incontinence.
Pelvic floor muscles
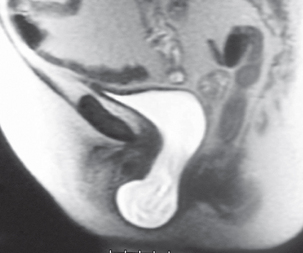
In females the pelvic floor muscles also have an important role in maintaining continence. The pelvic floor is composed primarily of the levator ani muscle group, the endo-pelvic fascia and the supporting ligaments. The pelvic organs are maintained in the correct position by this pelvic floor. These tissues form a supporting ‘hammock’ beneath the urethra and during increases in intra-abdominal pressure (such as coughing, sneezing) the urethra is compressed against this hammock, thereby keeping the urethra closed and the patient continent (Figure 2.3).
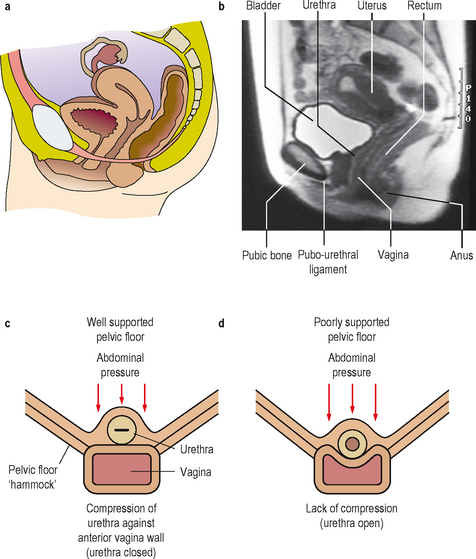
Figure 2.3 Midline sagittal illustration of the female pelvis (a) with corresponding MRI scan appearances (b). Loss of pelvic floor (hammock) support (c), causing a loss of transmission/compression of intra-abdominal pressure to the urethra.
Failure of this mechanism causes descent (prolapse) (Figure 2.4) and also hyper-mobility of the bladder neck and is an important cause of stress urinary incontinence (see Chapter 5).
FUNCTION OF THE LOWER URINARY TRACT
The function of the lower urinary tract can be split into two distinct phases:
For the majority of the time (greater than 99%) the lower urinary tract will be in the storage phase, whilst less than 1% of time is spent voiding.
Storage phase
During the storage phase the bladder is filled with urine from the ureters. The bladder needs to accommodate the increase in volume without an appreciable rise in bladder (intra-vesical) pressure. This receptive relaxation property is called the ‘compliance’ of the bladder. Factors that contribute to compliance are:
During the storage phase the urethra and sphincteric mechanisms should be closed, thereby maintaining a high outlet resistance and continence.
Voiding phase
During the voiding phase the reverse activity to that seen during the storage phase must occur. The bladder must cease relaxing and instead contract to expel the urine and the urethra and sphincteric mechanisms must ‘open’ to decrease the outlet resistance and allow passage of urine. Voiding should be efficient and there should be minimal or no urine remaining in the bladder at the end of the voiding phase.
Return to storage phase
At the end of voiding the proximal urethra is closed in a retrograde fashion, thus milking back the urine into the bladder. This ‘milkback’ is seen during contrast studies of the lower urinary tract when the patient is asked to stop voiding. Following this the bladder returns to a state of relaxation.
NEURONAL CONTROL OF THE LOWER URINARY TRACT
Control of the lower urinary tract is executed via a complex series of peripheral and central neuronal pathways. These pathways:
Motor (efferent) control
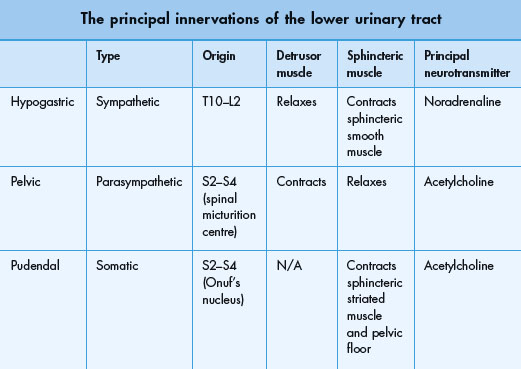
The peripheral innervation of the lower urinary tract is principally from three groups of nerves (Table 2.2 and Figure 2.5):
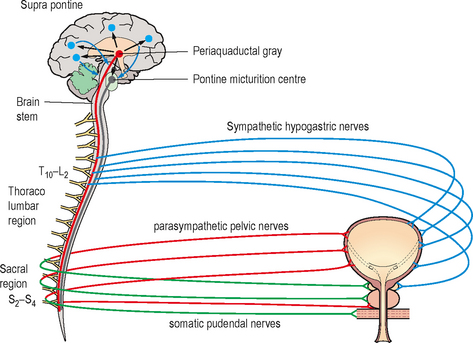
Figure 2.5 Neural control of the lower urinary tract. Showing the somatic, sympathetic and parasympathetic peripheral nerves; the pontine micturition centre, the periaqueductal gray and the suprapontine areas involved in control of storage and voiding of urine. There are extensive interactions at all levels (not shown).
Sensory (afferent) control
Afferent signalling from the lower urinary tract also occurs along the hypogastric, pelvic and pudendal nerves. These nerves transmit information regarding the fullness of the bladder as well as the presence of any noxious (chemical or cold) stimuli.
Afferent signalling is involved in:
In certain neurogenic or inflammatory conditions there is up-regulation of some of these afferent nerves, causing bladder pain and it is thought that type C afferent nerve fibres are implicated in the involuntary stimulation of detrusor contractions (detrusor overactivity).
Involuntary storage reflexes
A number of involuntary reflexes exist, which are mainly located in the lumbo-sacral spinal cord. With increasing bladder fullness these reflexes will increase sympathetic activity and inhibit parasympathetic activity and also activate the pudendal (somatic) neurones. These reflexes therefore enhance storage by relaxing the bladder and maintain continence by increasing both intrinsic and extrinsic sphincter tone (guarding reflex).
Voiding reflexes are not confined to the spinal cord and there are a number of supra-spinal reflexes involved in co-ordinating lower urinary tract activity that have been discovered in experimentally produced high spinal cord transections.
Desire to void
After infancy, voluntary control of voiding is achieved, thus allowing voiding to be initiated only in appropriate circumstances. To achieve this, information regarding bladder fullness must be sent to the brain, and when appropriate to void the brain must override the peripheral storage reflexes and ‘switch’ the lower urinary tract into the voiding phase.
Once a threshold level of fullness has been achieved (which will depend upon circumstances and vary between individuals) there will be increasing afferent activity emanating from sensory neurones in the suburothelial plexus associated with the bladder wall. Parasympathetic afferents in the pelvic nerve will communicate this activity via the spinal cord to the periaqueductal gray (PAG) area in the midbrain. At the PAG the bladder filling information is processed and from here the signals are sent to the pontine micturition centre (PMC) in the brainstem and the suprapontine areas of the brain.
The suprapontine areas of the brain comprises the frontal cortex, the hypothalamus, the para-central lobule, the limbic system and the cingulate gyrus. These are important in the conscious and unconscious control of the PMC. They have a role in delaying micturition, inhibiting premature detrusor contractions and in initiating voiding at an appropriate time.
Voluntary control of voiding
The PMC is an essential control centre in co-ordinating the micturition process and is itself under the control of the suprapontine area.
If the bladder is sensed to be full but it is inappropriate to void then the PMC will send descending signals to inhibit parasympathetic activity, increase sympathetic activity and increase the pudendal somatic activity to contract the urethral sphincter mechanism and pelvic floor muscles. These mechanisms voluntarily ‘tighten up’ the bladder outlet and so maintain continence until an appropriate time and place is found to void.
If the bladder is sensed to be full and it is appropriate to void then the PMC will ‘switch’ the lower urinary tract into the voiding phase by sending descending signals to increase parasympathetic activity and inhibit sympathetic and somatic activity.
Once voiding is initiated, secondary reflexes in the urethra, activated by urine flow also further facilitate bladder emptying.
Neuronal interactions
The account of the neuronal control of the lower urinary tract given above is a simplification; there are extensive interactions between the neuronal populations at all levels in both the peripheral, spinal and higher central areas. These complex neuronal interactions have led to much controversy regarding the motor control of the lower urinary tract and also more recently regarding the sensory feedback. In particular the role of the sub-urothelial layer in afferent feedback involving a variety of neurotransmitters is currently the focus of much research. In recent years it has been recognised that stretch of the urothelial mucosa can lead to the nonneuronal release of a number of neurotransmitters such as acetylcholine, nitric oxide and ATP. In addition, an important group of cells, so called ‘interstitial cells’ have also been identified and these undoubtedly have an important role in the coordination of lower urinary tract function. This remains an evolving area with continuing research into the mechanisms underpinning lower urinary tract function.