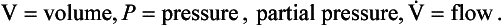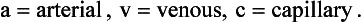INTRODUCTION
Introduction
The aim of this book is to provide an understanding of the respiratory system: its structure, function, and the diseases and conditions that may affect it. In attempting to do this we are adopting the philosophy of the new curriculum in medicine, which involves bringing to bear on a particular topic all the sciences relevant to that topic. To include in one book all that a student should know about the anatomy, histology, physiology, pharmacology and medicine of the respiratory system would result in a gigantic and intimidating tome. Equally unsatisfactorily, all these subjects could be treated superficially. We have adopted the policy of basing an understanding of the respiratory system on a full description of its physiology and anatomy, with specific topics of particular clinical importance being expanded upon in terms of clinical sciences.
For students to learn effectively, the material they must master should be broken down into manageable portions with a coherent theme: these are the chapters of this book, with each theme being based on a particular function of the respiratory system.
Students must also know what is expected of them, and each chapter is preceded by a list of aims and objective – things you should be able to do when you have mastered the material of that chapter. To provide experience of that bane of student life, examinations, each chapter contains questions of the type you might be asked at an undergraduate level.
What is respiration?
That depends on the context in which you use the word. Biochemists use it to describe the energy-producing chemical processes that take place in tissues, cells or even parts of cells. In this book we will use the physiologist’s definition, which is ‘An interchange of gases between an organism and its environment’. To all intents and purposes, for human beings this means ‘breathing’ (Latin, spiro, ‘I breathe’). The movement of air into and out of the lungs, which most people call breathing, is called by physiologists ventilation. Breathing is brought about by specific structures of the body, including (but not exclusively) the lungs. A description of these structures at a macroscopic (anatomical) and microscopic (histological) level helps us to understand the processes of the respiratory system and the disruption of these processes and structures (pathology) that brings about disease.
The part of our environment involved in this ‘interchange of gases’ mentioned above is of course the air around us, and our need for air must have been obvious to even our most distant ancestors. This need is recorded in some very ancient writings. For example, Anaximenes of Miletus (c. 570 bc) observed that air or pneuma (Greek, ‘breath’) was essential to life.
What was not clear to the ancients was what the air was used for. Aristotle, drawing on theories dating from the 5th century bc which noted the rapid and repeated movements of the heart, relegated the function of the lungs to a sort of radiator, and stated with his usual authority:
…as the heart might easily be raised to too high a temperature by hurtful irritation (by its rapid movements) the genii placed the lungs in its neighbourhood, which adhere to it and fill the cavity of the thorax, in order that air vessels might moderate the great heat.
Galen (130–199 ad), probably more by an accident of metaphor rather than on any scientific evidence, came close to describing the true nature of respiration when he compared it to a lamp burning in a gourd:
When an animal inspires it is, I think, similar to a perforated gourd, but when respiration is prevented at the appropriate place on the trachea, you may compare it to a gourd unperforated and everywhere closed.
If Galen had had the benefit of modern gas analysing facilities he would have found even closer parallels between breathing and burning, with oxygen (O2) being consumed and carbon dioxide (CO2) being produced in both cases.
The ‘bottom line’ of an account of the complicated process of respiration begins with a flow of
These two flows are the first and final results of the complex metabolism of the body, and this book describes the respiratory system that facilitates these flows.
The need for respiration
One definition of the success of a species of organism, in evolutionary terms, is how well it can maintain constant the composition of the fluid surrounding its individual cells (its internal environment) despite changes in its external environment (surroundings getting dryer, colder, warmer etc.). This process is called homeostasis and requires energy. Most of the energy generated by our tissues is the result of oxidation of food substrates, and this is the reason we need a flow of OXYGEN IN. Neophytes in physiology often emphasize the role of the respiratory system in providing this oxygen, and certainly an uninterrupted supply is important, particularly for the nervous system, but of more immediate importance is the removal of CO2. The word oxygen means ‘acid producer’ (Greek, oxy; acid; gen, to produce), and the major product of our oxidative metabolism is the acid gas CO2. The accumulation of CO2 would result in acidification of the body fluids. The importance of removing this CO2 can be demonstrated by rebreathing from a plastic bag for a few minutes. The unpleasant sensation that forces you to stop this rather dangerous experiment is due to over stimulation of the reflex that controls breathing to get rid of this gas. You will see later (Chapter 8) that CO2 produces its acidic effect by reacting with water to form carbonic acid.
Ventilation of the lungs would not fulfil the needs of the cells of our bodies if the results of this ventilation did not diffuse into the blood, which is then carried close to the cells of the body by the circulation.
Diffusion in respiration and the circulation
The flows of O2 and CO2 into and out of the body take place as a result of one very basic physical phenomenon: diffusion, which results in the movement of molecules in liquids and gases from regions of high concentration to regions of lower concentration. Because they are small, microscopic organisms such as the humble amoeba in its pond can rely on this phenomenon alone to carry O2 to and remove CO2 from its single cell. Multicellular creatures are too large to rely on diffusion alone: the distances gases would have to diffuse are too great, and the movement of gas therefore too slow to maintain life.
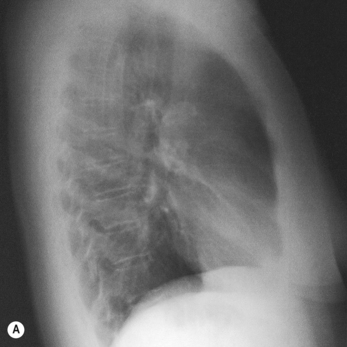
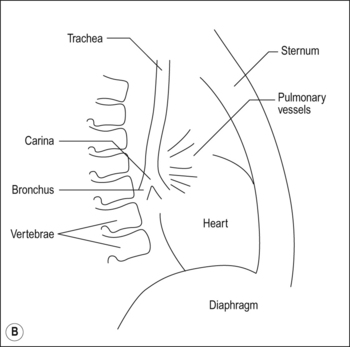
Although in human beings the same passive mechanism of diffusion alone supplies and removes these gases from our bodies (there is no active chemical transport), the phenomenon of diffusion is maximized by complicated respiratory and circulatory systems which accomplish what the pond water does for the amoebae in providing a supply of and a sink for these gases. The lungs promote diffusion by having an enormous surface area, which is very thin, through which diffusion can take place easily. A surface of over 90 m2 is enclosed in a lung volume of less than 10 L. This functional 90 m2 is often reduced in disease, by thickening of the membrane, excess fluid in the lungs, or by a reduction in the supply of air or blood. The circulation of the blood forms the transport link between the diffusion site of the lungs and the diffusion site of the capillaries within the tissues. The distances involved in this link are enormous in molecular terms, and diffusion would be totally useless to transport gas over the metre or so between the lungs and distal tissues of our bodies. This transport is accomplished in seconds by the circulation (Fig. 1.1).
Timing in the circulation and respiration
The processes of breathing and the beating of the heart are both cyclic events. One involves the inhalation of air and then its exhalation; the other involves filling of the heart with blood and then its ejection into the circulation. The time courses of these two cycles are very different: at rest you may take 12 breaths in the minute the heart beats 60 times, ejecting 5 L of blood through the lungs (see Fig. 1.2).
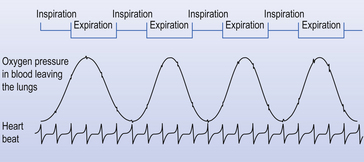
Fig. 1.2 Timing. Because the heart beats so much faster than the respiratory cycle there is effectively a continuous flow of blood through the lungs during inspiration and expiration. Expiration is like breath-holding – no fresh air is added, and the composition of blood leaving the lungs changes accordingly.
The composition of air in the lungs changes as a result of two effects: during inspiration it is altered by the addition of fresh air to that already in the lungs and the effects of exchange with the blood passing through the lungs. Expiration in this context is the equivalent of breath-holding, because no new air is added: the only effect is due to exchange of gas in the lungs with that in the blood. These changes in the composition of the air in the lungs are picked up by the blood flowing in the pulmonary circulation, which therefore shows cyclic changes in its composition that coincide with the breathing cycle.
Basic science of respiration
All these changes, just like all the events in respiration, are properly described in terms of the basic sciences physics and chemistry. These are generally not the favourite subjects of students of the basic medical sciences. We have therefore included at the end of the book a short section (Appendix) on the most relevant parts of physics and chemistry that a student should understand in order to understand respiration. That section is not obligatory to students confident with these subjects. The Appendix, which is intended to aid not torment, will repay scrutiny by students who have any doubts about their grasp of basic science. That this basic science is integral to understanding normal respiration and diseased states is illustrated when we take an overview of human respiration and point out where the phenomena we describe apply (Fig. 1.3).
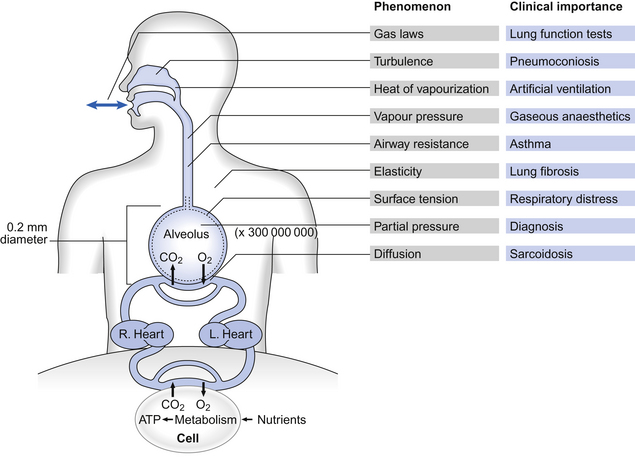
Fig. 1.3 An overview of respiration, showing the physical phenomena that make it up and the clinical situations where understanding these phenomena is important.
Taking examples from this list of phenomena we see that solids are elastic, and this elasticity of our respiratory system determines part of our work of breathing. Liquids exert a vapour pressure, a property important in humidifying the air we breathe and in the administration of gaseous anaesthetics. Gases exert a partial pressure, the understanding of which is essential to the monitoring of how well the lungs are working. The volume of a mass of gas is described by laws relating it to temperature and pressure, and the resistance to flow of gases is related to the dimensions of the tube in which it is flowing.
These examples of the importance of the basic sciences in understanding the respiratory system do not mean that a great deal, or a great depth, of knowledge is required. The Appendix contains all that is required to understand the contents of this book. However, there is a vocabulary that is specific to the respiratory system, and it is probably helpful for you to be introduced to it here.
Respiratory symbols – the language of the respiratory system
Respiratory physiology and medicine contain some intimidating symbols which lead students to fear that some unpleasant mathematical exercises are immanent. Not so: the symbols used in respiratory physiology are assigned logically and make the description of processes and the identification of where measurements were made very much easier than using words.
Primary units are given in capital letters (see Table 1.1):
Table 1.1
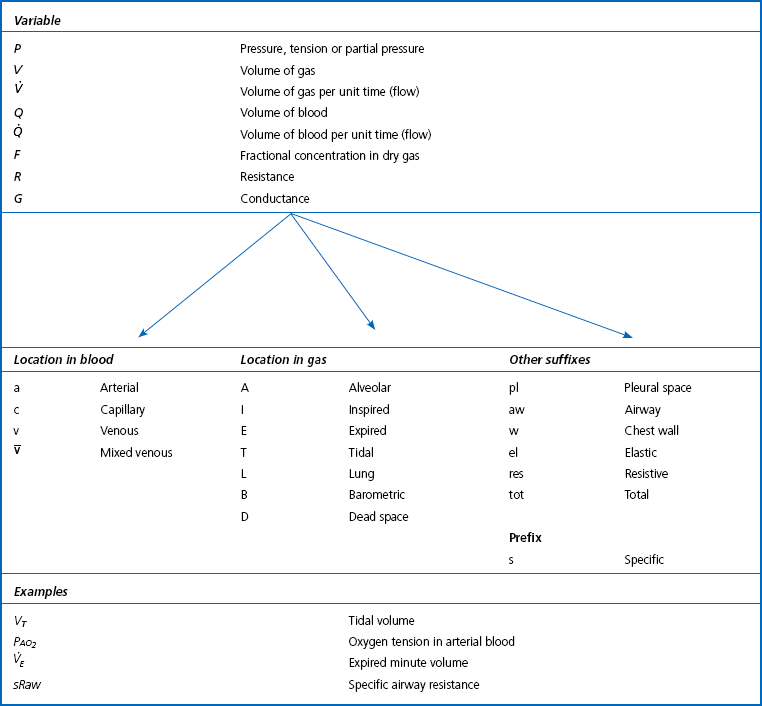
Note: Sometimes S is used for saturation and C for content. These are not used here because of confusion with chemical names (e.g. SO2, CO2).
Locations in the gas phase are also given capital letters but smaller than the primary units:
Locations in blood are identified by lower-case letters:
The primary symbol is written first, followed by the qualifying symbol at a lower level.
Drugs
Drugs are chemicals which change the natural functions of the body. Most prescribed drugs have therapeutic properties. Just as Fig. 1.3 demonstrates where specific physical phenomena have particular importance in the respiratory system, Table 1.2 gives examples of conditions where specific types of drugs are used therapeutically in treatment of specific conditions or for specific procedures.
Table 1.2
Drugs and the respiratory system
| Drug name | Type | Condition treated |
| Oxymetazoline | α-agonist | Nasal congestion |
| Atropine | Muscarinic cholinergic antagonist | Excess mucus secretion |
| Prednisolone | Corticosteroid | Allergic rhinitis |
| Chlorpheniramine | Antihistamine | Rhinorrhea |
| Succinylcholine | Neuromuscular blocking | Facilitate tracheal intubation |
| Dextromethorphan | Synthetic narcotic analgesic | Non-productive cough (suppression) |
| Salbutamol (Isoproterenol, USA) | β2 agonist (bronchodilator) | Asthma |
| Cromoglicate | Inflammatory-cell stabilizer | Asthma |
| Beclometasone | Anti-inflammatory corticosteroid | Asthma |
| Azathioprine | Cytotoxic immunosuppressant | Diffuse connective tissue |
| Aminopenicillin etc. | Antibiotic | Pneumonia and other infections |
| Amphotericin B | Antifungal | Fungal infections |
This list is, of course, far from exhaustive. The examples are chosen to demonstrate that several approaches may be used to treat a specific disease (e.g. asthma). It also demonstrates that the UK and the USA are ‘two nations separated by a common tongue’ (Salbutamol, UK = Isoproterenol, USA). This dichotomy extends to the units of measurement used in Europe and the USA and this sometimes causes problems.
CGS and SI units
The centimetre, gram, second system (CGS) of measurement, which has been in use in Europe since the French Revolution, is being displaced by Système International (SI), based on the kilogram, metre and second. The CGS system still receives considerable use in North America.
The SI unit of force is the newton and the unit of volume the cubic metre (m3); as this is rather large, the cubic decimetre (dm3), which is equivalent to a litre, is frequently used.
The unit of pressure in the SI system is the newton per square metre – the pascal (Pa). This is too small for practical use and so the kilopascal (kPa) is used with some surprisingly convenient results. 1 kPa = 7.5 mm Hg or 10 cm of water; equally usefully, barometric pressure at sea level is close to 100 kPa, which makes the arithmetic of calculating partial pressures easier.
In the SI system, concentration is measured in moles per litre, where a mole is 6.02 × 1023 molecules of the substance in solution. Measurement of blood pressure is still widely expressed in mm Hg, probably because it is usually measured using a mercury manometer.