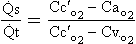THE PULMONARY CIRCULATION
BRINGING BLOOD AND GAS TOGETHER
The functions of the pulmonary circulation
So far, we have considered how the respiratory system transports gas between the atmosphere and the alveoli. In this chapter we will consider how blood and gas are brought together so that gas exchange can take place between them. This is a complex process: in order for all blood to undergo gas exchange, virtually the entire cardiac output is directed through the lungs, and brought close to gas within the alveoli. Furthermore, it is very important that ventilation (gas flow) and perfusion (blood flow) to a given area of the lung are matched. There is no point in directing air to areas of the lung that have little blood flow, and conversely there is no point in perfusing an area of lung with little or no ventilation. This matching of ventilation and perfusion is vital to maintaining adequate oxygenation and carbon dioxide removal from the blood.
In this chapter we will consider the anatomy of the pulmonary circulation, and compare it to the systemic circulation. We will then consider the factors that influence the flow of blood to different regions of the lungs. Finally, we will consider the very important issue of ventilation/perfusion matching: the way in which air and blood are brought together in order to achieve optimal gas exchange of oxygen and carbon dioxide.
The anatomy of the pulmonary circulation
In humans the circulation behaves in many ways as if it consisted of two parts. In the systemic circulation the left ventricle pumps oxygenated blood through the organs and tissues of the body, where oxygen is removed and carbon dioxide added before the blood returns to the right atrium. In the pulmonary circulation the right ventricle pumps deoxygenated blood through the lungs, where oxygen is added and carbon dioxide is removed (Fig. 7.1). Of course, the two parts of the circulation work in series to form a single circuit of blood, but there are a number of differences between the pulmonary and systemic circulations which reflect their different functions.
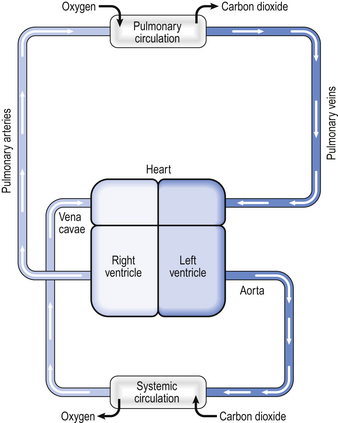
Fig. 7.1 Diagrammatic representation of the circulation. In humans the circulation behaves as if it consisted of two parts, the systemic circulation and the pulmonary circulation. The blood flow through the two parts is the same. In both circulations arteries carry blood away from the heart and veins carry blood to the heart. For this reason, the pulmonary artery carries deoxygenated blood and the pulmonary vein carries oxygenated blood.
First, virtually the entire cardiac output is directed through the pulmonary circulation. This means that, at any one time, there is as much blood flowing through the lungs as through all the other organs and tissues in the body put together.
Second, the purpose of the pulmonary circulation is to bring blood and air into very close contact in order to allow gas exchange to take place. This requires a very thin separating membrane and for this reason the pressure in the pulmonary circulation needs to be very low compared to the systemic circulation. If the pressure in the pulmonary circulation were higher, it could cause fluid to leak from the pulmonary capillaries into alveoli. In fact, the pressure in the pulmonary artery is about 25/10 mmHg, compared to 120/70 mmHg in the systemic circulation. The pulmonary circulation is therefore a low-pressure, high-flow system, which implies it has a low resistance. The components of the pulmonary circulation differ from their systemic counterparts in a way that reflects this.
The right ventricle
Every minute, the same volume of blood – about 5 L at rest – flows through both the right ventricle and the left ventricle. However, the two ventricles look very different (Fig. 7.2). The left ventricle has a thick, muscular wall that takes up most of the cross-section of the heart. The right ventricle has a much thinner wall, about one-third of the thickness of the left, and to accommodate the muscle of the left ventricle the right almost seems to be ‘wrapped around’ the left. Why is there such a difference between the two?
Fig. 7.2 Cross-section of the heart. Note that the left ventricle has a thick wall, reflecting the high pressures it generates. At rest, the systolic pressure in the left ventricle is about 120 mmHg, but during exercise, for example, the pressure can be much higher. In contrast, the right ventricle has a much thinner wall. The systolic pressure in the right ventricle is only about 25 mmHg, and in health does not increase much above this, even during exercise.
The reason is that the left ventricle pumps blood into the systemic circulation, which has a high resistance and which operates at a relatively high pressure. If cardiac output increases, for example during exercise, this pressure can increase even more, which means that the left ventricle needs a thick, muscular wall to produce these high pressures. On the other hand, the right ventricle pumps blood into the pulmonary circulation, which has a very low resistance and which operates at a low pressure, always less than that in the systemic circulation. Furthermore, as we shall see later, if cardiac output increases the pulmonary artery pressure does not increase very much. For this reason, the right ventricle needs only a relatively thin muscular wall in comparison to the left.
Pulmonary blood vessels
Pulmonary blood vessels are very different from their systemic counterparts. The larger vessels have much thinner walls, reflecting the lower blood pressure they need to withstand: for example, the thickness of the wall of the pulmonary artery is only about one-third of the thickness of the aorta.
As well as having thinner walls, the pulmonary vessels are much more distensible than systemic arteries, which is important in keeping pulmonary blood pressure low during systole and in the face of increases in cardiac output. In circumstances such as exercise, cardiac output can increase from its normal 5 L per minute to as much as 25 L per minute. In order to keep pulmonary blood pressure low, the pulmonary circulation is able to reduce its resistance to even lower values than normal. It does so by two mechanisms, illustrated in Figure 7.3.
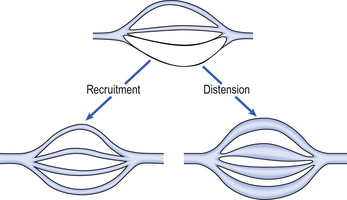
Fig. 7.3 Recruitment and distension of pulmonary blood vessels. If cardiac output increases the resistance of the pulmonary circulation decreases, meaning that the pulmonary artery pressure remains relatively low. This occurs because some pulmonary vessels increase in diameter (distension, lower right of diagram) and because some vessels which were virtually closed begin to open and blood can flow along them (recruitment, lower left of diagram).
1. Pulmonary blood vessels are able to dilate or distend. A small increase in the diameter of a vessel decreases the resistance of that vessel substantially (Poiseuille’s Law, p. 156).
2. During normal conditions, some blood vessels in the lungs are closed. During periods of high cardiac output these vessels open up and blood is able to flow through them. The increase in number of blood vessels able to carry blood is called recruitment. However, it is likely that very few vessels are completely collapsed under normal conditions, and distension is probably much more important than recruitment in reducing vascular resistance.
The anatomical position of the capillaries and their function in gas exchange means that they are different from their systemic counterparts. The density of the capillary network in the alveolar walls is extremely high so that efficient gas exchange can take place. In fact, there are so few cells between the capillaries in the alveolar walls that the alveolar circulation behaves almost like a film of blood flowing around the alveoli. At rest, blood flows through an alveolar capillary in about 0.8 seconds, which is about three times longer than the time needed for oxygenation of mixed venous blood.
There is very little space between the blood in the pulmonary capillaries and the air in the alveoli. In fact, for the most part, the only cells separating blood from air are the endothelial cells of the pulmonary capillary and the epithelial type I cells of the alveolar wall (Fig. 2.9). This is clearly very important in allowing the most efficient transfer of gases between the alveoli and the blood (see Chapter 6).
However, because the pulmonary capillaries are very thin-walled and lie in the alveolar walls they can be readily influenced by changes in the gas pressure within the alveoli. Increased alveolar gas pressure can compress the capillaries, with a consequent increase in capillary resistance and reduction in blood flow, which can affect the distribution of blood flow within the lungs (see below). This is very different from the conditions affecting systemic capillaries, which are supported by surrounding tissues.
In contrast to the situation in the systemic circulation, the pressure in the pulmonary veins exerts a considerable influence over the pressure in the pulmonary arteries. This is another consequence of the low pressures in the pulmonary circulation.
In the systemic circulation, the pressure at the precapillary sphincters, which regulate the blood flow through the capillary beds, is about 90 mmHg, very much higher than the pressure at the venous end of the capillary beds. The difference in pressure between the arterial and venous ends of a capillary bed is called the driving pressure and flow is dependent on this. Because the venous pressure in the systemic circulation is so much less than the arterial pressure, changes in venous pressure do not make large changes to the driving pressure.
However, in the pulmonary circulation the difference between pulmonary arterial and venous pressures is much less, and a relatively small change in venous pressure can make a considerable change to the driving pressure. To keep a constant driving pressure the pulmonary artery pressure has to increase. In certain pathological situations this can eventually lead to failure of the right ventricle (cor pulmonale; see below).
The bronchial circulation
Not all the blood flowing into the lungs does so via the pulmonary artery – a small volume of arterial blood flows through the bronchial circulation (Fig. 7.4). The bronchial circulation supplies blood to the airways and to the lung parenchyma, although it is not essential to their survival; during a lung transplant the bronchial circulation is not reconnected, and this apparently does not produce any serious ill effects. Blood flowing through the bronchial circulation does not pass through the alveolar capillaries and therefore does not take part in gas exchange.
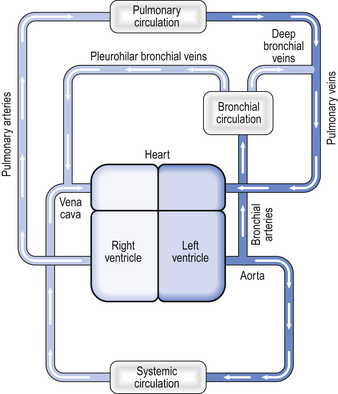
Fig. 7.4 The bronchial circulation. The bronchial arteries arise from the aorta and therefore carry oxygenated blood. The blood in the bronchial circulation supplies tissues within the lung but does not take part in gas exchange, and the blood in the bronchial veins is therefore deoxygenated. Some of the blood from the bronchial circulation drains via the pleurohilar bronchial veins into the azygous vein or the vena cava, whereas blood draining the deep bronchial veins drains into the pulmonary veins.
The bronchial arteries arise from the aorta. Bronchial vessels supply blood to the lower trachea, the bronchi, and to the smaller airways as far as the respiratory bronchioles. Blood from the proximal part of the bronchial circulation around the bronchi drains via the pleurohilar bronchial veins into the azygous vein and into the superior vena cava. This blood is effectively part of the systemic circulation in that it flows from the aorta to the vena cava.
However, the blood from the more distal parts of the bronchial circulation drains via the deep bronchial veins into the pulmonary circulation. This blood therefore forms a shunt (see below). In other words, the blood arises from the aorta, but instead of draining into the right-hand side of the circulation, the partly deoxygenated blood of the deep bronchial veins drains into the oxygenated blood that has passed through the alveolar capillaries. The resulting mixture of blood therefore has an oxygen content less than that of the original pulmonary artery blood. The significance of this will be discussed later in the chapter.
Matching ventilation and perfusion
In an ideal pair of lungs all the alveoli would be supplied with equal volumes of air of uniform gas composition during inspiration. Also, all the alveoli would be ideally supplied with the same flow of mixed venous blood. The ventilation and perfusion of all parts of these ideal lungs would therefore be optimally matched, and optimal gas exchange between the blood and the alveoli would take place.
However, in real lungs this is not the case. Per unit of lung volume, ventilation and perfusion both tend to be greater at the bases of the lungs compared to the apices. Nevertheless, for most of the lung tissue the two tend to be fairly optimally matched. This means that the ratio of ventilation to blood flow, the ventilation/perfusion ratio, or 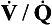 ratio, varies by a relatively small amount throughout the lungs.
ratio, varies by a relatively small amount throughout the lungs.
In order to see how ventilation/perfusion matching takes place we will first look at the way in which the blood flow is distributed throughout the lungs, then we will see how this is matched with ventilation before looking at how regional variations in the 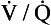 ratio in the lungs affects arterial blood gases.
ratio in the lungs affects arterial blood gases.
Distribution of blood flow through the lungs
As we have just seen, the distribution of blood to different regions of the lungs is not uniform but varies considerably. Furthermore, the blood flow to different parts of the lungs tends to be directed towards maintaining as normal a 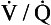 ratio as possible.
ratio as possible.
In the systemic circulation, blood flow through organs is almost entirely determined by high-resistance arterioles that regulate the blood flow through capillary beds. The arterioles in the pulmonary circulation do not have a high resistance and play only a small role in determining the blood flow to different parts of the lungs. The distribution of blood flow through the lungs is influenced instead by a number of different factors, including gravity, alveolar gas pressure, hypoxic pulmonary vasoconstriction and, to a lesser extent, the nervous control of blood vessel resistance.
Gravity
The diastolic blood pressure in the systemic circulation is about 80 mmHg, which is enough pressure to raise a column of water by a height of over a metre. In other words, there is more than enough pressure to carry blood from the heart up to the head. However, in the pulmonary circulation the diastolic blood pressure is about 12 mmHg, enough pressure to raise a column of water about 15 cm. In other words, there is only just enough pressure to pump blood from the right ventricle up to the lung apices. On the other hand, at the lung bases the blood pressure in the pulmonary circulation is equal to the pressure generated by the right ventricle plus the hydrostatic pressure of a column of blood extending up to the heart. Because the pressure generated by the right ventricle is not very high, this extra hydrostatic pressure makes a very significant difference. Thus there is a very considerable difference in arterial blood pressure between the bases and the apices of the lungs owing to gravity. In other words, gravity tends to direct blood towards the lung bases.
The regional flow of blood within the lungs can be demonstrated by dissolving a radioactive gas (usually Xenon-133 (133Xe)) in saline and then injecting this into the right side of the heart via an intravenous catheter. During the injection the subject holds his breath, and some of the radioactive xenon leaves the blood and enters the alveoli. By measuring the level of radioactivity from outside the body it is possible to estimate the blood flow to different regions of the lungs (Fig. 7.6).
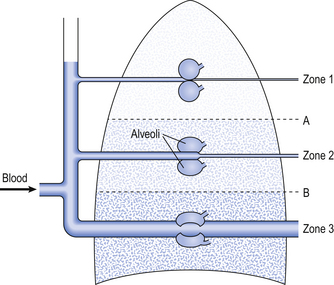
Fig. 7.6 The effect of blood pressure and alveolar pressure on regional blood flow through the lungs. In zone 1, the air pressure in the alveoli is greater than the pressure within the alveolar capillaries and therefore no blood flow takes place. If such a zone exists in healthy individuals, it is very small and situated at the apices of the lungs. In zone 2, the average alveolar gas pressure is lower than the pressure of blood entering the alveolar capillaries but higher than the pressure of blood leaving the capillaries. In zone 2, blood flow varies with changes in alveolar gas pressure. In zone 3, the blood pressure throughout the alveolar capillaries is higher than the alveolar gas pressure, and blood flow through the pulmonary capillaries is independent of alveolar gas pressure. In healthy individuals it is likely that most of the alveoli lie within zone 3.
Although the difference in regional blood flow between the apices and bases of the lungs is thought to be due largely to the effect of gravity, it is nevertheless the case that the gradient remains in subjects who are in the supine position. Furthermore, the anatomy of the branching pulmonary vessels results in greater variation between different parts of the same level in the lung than between mean flow in adjacent levels.
Gravity is one factor influencing regional differences in blood flow within the lungs. However, other factors include the air pressure in the alveoli around the pulmonary capillaries, and hypoxic pulmonary vasoconstriction.
Pressure around the blood vessels
As we have seen, the pulmonary capillaries are influenced by the air pressure in the alveoli that surround them. If the blood pressure within a capillary is less than the pressure of the gas in the alveoli adjacent to it, the pressure in the alveoli will compress the capillary, limiting the blood flow through it. The pressure in the alveoli remains close to atmospheric during quiet breathing, but may become significantly positive during artificial ventilation or during heavy breathing. In these circumstances, alveolar gas pressure may have a significant effect on the distribution of pulmonary blood flow.
These two factors combine to influence the distribution of blood flow within the lungs. Three zones have been described related to the blood pressure at the beginning and end of a capillary and the gas pressure within the adjacent alveoli (Fig. 7.6):
• Zone 1. In this zone, the pressure in the alveoli is always greater than the pressure in the capillaries. This means that in zone 1 there is no blood flow. It is unlikely that such a zone exists in healthy individuals, but if it does so it occupies a very small volume of the apices of the lungs, where the pulmonary blood pressure is lowest.
• Zone 2. In this zone, the alveolar gas pressure is greater than the blood pressure at the venous end of the capillary but less than the blood pressure at the arterial end of the capillary. In zone 2, the flow through the capillaries is influenced by changes in blood pressure and changes in alveolar gas pressure. If alveolar gas pressure increases, then flow through the capillaries in zone 2 will be reduced.
• Zone 3. In zone 3, the blood pressure throughout the capillaries, from the arterial end through to the venous end, is greater than the gas pressure in the surrounding alveoli. Therefore, in this zone, flow through the capillaries is influenced only by the difference in blood pressure along the capillary and is not influenced at all by changes in alveolar pressure. It is likely that in healthy humans, the majority of the volume of the lungs lies within zone 3, and blood flow is independent of alveolar gas pressure.
This ‘three zone’ model emphasizes the role of gravity in directing blood flow in the lungs although recent evidence suggests that gravity is not as important a factor in determining regional differences in blood flow as was once thought. In particular, blood flow to the very lowest parts of the lung bases appears to be less than this model predicts and so the difference between the bases and the apices of the lungs in blood flow per unit lung volume is relatively modest.
Furthermore, at any given height from the base, there is a wide variation in blood flow to different lung regions. It is thought likely that this variation is due to the fact that when a blood vessel divides, the two vessels formed are not necessarily the same size. This effect leads to a random variation in blood flow between lung regions at the same height from the lung base. This effect is probably at least as important as gravity in producing variation in regional perfusion of the lungs.
Hypoxic pulmonary vasoconstriction
Arterioles in the lungs differ considerably from their systemic counterparts in their response to hypoxia. Systemic arterioles vasodilate in response to hypoxia. This tends to increase the blood flow to hypoxic tissues and therefore tends to increase oxygen delivery where it is required.
However, arterioles in the lungs vasoconstrict in response to hypoxia: this effect is called hypoxic pulmonary vasoconstriction. Hypoxic pulmonary vasoconstriction is important in maintaining ventilation/perfusion matching and tends to direct blood away from underventilated parts of the lungs. Underventilated regions of the lungs have a low oxygen concentration, and therefore blood leaving them tends to be relatively deoxygenated. Hypoxic pulmonary vasoconstriction in these areas directs blood towards better-ventilated parts of the lungs where there is a higher oxygen concentration. In other words, hypoxic pulmonary vasoconstriction tends to promote an optimum 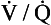 ratio for the lungs as a whole by increasing the
ratio for the lungs as a whole by increasing the 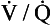 ratio in areas of the lungs where it is lower than normal.
ratio in areas of the lungs where it is lower than normal.
In its role in optimizing the 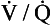 ratio, the vasoconstrictor response of the blood vessels to low oxygen concentrations is analogous to the bronchodilator response of bronchi to high levels of carbon dioxide (see Chapter 4). In addition, hypoxic pulmonary vasoconstriction plays an important role in limiting blood flow through the non-ventilated and therefore hypoxic lungs of the fetus. After the baby is delivered and takes its first breath, the oxygen concentration in its lungs increases, the hypoxic pulmonary vasoconstriction reduces and blood flow to the lungs is increased.
ratio, the vasoconstrictor response of the blood vessels to low oxygen concentrations is analogous to the bronchodilator response of bronchi to high levels of carbon dioxide (see Chapter 4). In addition, hypoxic pulmonary vasoconstriction plays an important role in limiting blood flow through the non-ventilated and therefore hypoxic lungs of the fetus. After the baby is delivered and takes its first breath, the oxygen concentration in its lungs increases, the hypoxic pulmonary vasoconstriction reduces and blood flow to the lungs is increased.
The mechanism behind hypoxic pulmonary vasoconstriction is not fully understood. What is known is that hypoxia causes membrane depolarization of the smooth muscle cells in the pulmonary blood vessels (Fig. 7.7). This is followed by contraction of the smooth muscles in the blood vessel wall. It is thought that the depolarization of the smooth muscle cells is mediated, at least in part, by a membrane potassium channel called the Kv channel. The conductance of Kv increases in response to hypoxia, resulting in membrane depolarization, although it is not certain whether the channel itself is sensitive to hypoxia or whether it responds to another detector of hypoxia.
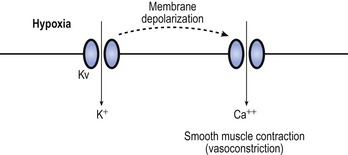
Fig. 7.7 Hypoxic pulmonary vasoconstriction. Pulmonary blood vessels constrict in response to hypoxia. The potassium channel Kv, which lies in the vascular smooth muscle membrane, opens in response to hypoxia, leading to membrane depolarization. This results in the opening of a calcium channel which allows extracellular calcium to enter the cell. This in turn triggers muscle contraction.
The membrane depolarization opens voltage-sensitive calcium channels that allow calcium to enter the cell. A rise in intracellular calcium concentration triggers smooth muscle contraction, resulting in vasoconstriction. Mediators such as endothelin (a vasoconstrictor) and nitric oxide (a vasodilator) released from the overlying endothelial cells also influence hypoxic pulmonary vasoconstriction, although they are not necessary for it to take place.
Although hypoxic pulmonary vasoconstriction is usually beneficial in maintaining ventilation/perfusion matching, it can cause problems in patients with lung conditions such as chronic obstructive pulmonary disease that result in long-term hypoxia. This chronic hypoxia affects all parts of the lungs and results in a widespread hypoxic pulmonary vasoconstriction. This means that the resistance of the pulmonary circulation as a whole is much higher than normal, and the pulmonary blood pressure increases. The right ventricle is not well suited to producing a high blood pressure and under these circumstances can start to fail. The patient may become increasingly breathless on exertion, as fluid tends to seep into the alveoli as cardiac output increases. Furthermore, the patient may notice oedema around their ankles. Failure of the right ventricle means that the pressure in the systemic veins increases. This sort of heart failure due to lung disease is called cor pulmonale.
Innervation of the pulmonary vessels
Fibres of the sympathetic and parasympathetic nervous system innervate the pulmonary vessels and may cause vasoconstriction or vasodilatation (Fig. 7.8). Sympathetic fibres release noradrenaline (norepinephrine), which acts predominantly on α1 adrenoceptors on the smooth muscle of arteries and arterioles to produce vasoconstriction. Noradrenaline (norepinephrine) may also act on α2 receptors to produce vasodilatation, but it is generally accepted that the overall effect of the sympathetic nervous system on the pulmonary vessels is vasoconstriction.
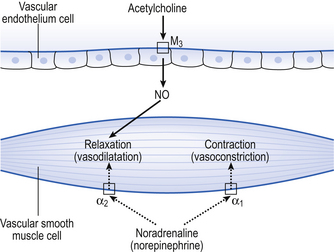
Fig. 7.8 Action of the sympathetic and parasympathetic nervous systems on pulmonary vessels. Noradrenaline (norepinephrine) released from sympathetic nerve fibres acts on smooth muscle α1 receptors to produce vasoconstriction and on α2 receptors to cause vasodilatation. Overall, the effect of the sympathetic nervous system is to cause vasoconstriction. Acetylcholine acts on the vascular endothelium, causing the release of nitric oxide that causes vasodilatation of the underlying vascular smooth muscle.
The parasympathetic nervous system produces most of its effects on the pulmonary blood vessels by the release of acetylcholine. The acetylcholine is thought to act on muscarinic M3 receptors on the endothelium of the blood vessels, rather than on the smooth muscle cells themselves. It is thought that stimulation of the M3 receptors by acetylcholine results in the release of nitric oxide (NO) from the vascular endothelial cells. Nitric oxide is a small gaseous molecule that is very rapidly broken down and is a very important mediator throughout many organ systems in the body. The NO released from the endothelium in response to acetylcholine causes pulmonary vascular smooth muscle cells to relax, and therefore produces vasodilatation.
Like the bronchial smooth muscle, the pulmonary vascular smooth muscle also has a non-adrenergic, non-cholinergic parasympathetic innervation. There are many possible neurotransmitters in this system. It seems to produce vasodilatation, and may well act via NO. However, its importance in humans is not fully understood.
The sympathetic and parasympathetic innervation of the pulmonary blood vessels is probably not very important in maintaining ventilation/perfusion matching in humans. During a lung transplant the sympathetic and parasympathetic nerves cannot be reconnected, and the lack of innervation does not appear to be a serious problem to patients who have undergone this operation.
Regional differences in ventilation in the lungs
As we have seen, blood flow to different regions of the lung is not uniform but tends to increase towards the bases of the lungs, as well as varying quite widely at a particular height from the lung base.
Fortunately, ventilation is also directed towards the bases. This happens essentially because the lungs ‘sag’. The weight of the tissue making up the lungs tends to cause the alveoli and airways at the bases to collapse, while at the same time the airways and alveoli at the apices tend to be ‘pulled’ open by the lung tissue below them. This means that at the beginning of a breath, the alveoli at the apices of the lungs are nearer to being ‘full’ than the alveoli at the bases, and cannot take much ‘fresh’ air, and therefore ventilation tends to increase towards the bases. However, this difference in ventilation between the apices and the bases of the lungs becomes less pronounced (and may even reverse) with high airway gas flows. There is also variability in the distribution of gas flow to different lung regions at a given height from the lung base, similar to the variability that is seen in blood flow. This is probably due to random differences in the diameters of bronchi formed at the bifurcation of the airways, comparable to the same random variation that occurs in regional perfusion of the lungs.
We have seen that ventilation and perfusion, for a given volume of lung tissue, both increase towards the bases of the lungs. The rate of increase in perfusion is greater than the increase in ventilation, meaning that there is an increase in 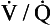 ratio from the apices to the bases of the lungs.
ratio from the apices to the bases of the lungs.
We have also seen that at any given height above the base, there is a wide variation in regional ventilation and regional perfusion. Despite this, the range of 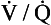 ratios found in the lung is relatively narrow. Figure 7.9 shows both ventilation and perfusion plotted against
ratios found in the lung is relatively narrow. Figure 7.9 shows both ventilation and perfusion plotted against 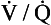 ratio on a logarithmic scale. As you can see, most of the ventilation (open circles) and most of the perfusion (closed circles) take place in lung regions that encompass a relatively narrow range of
ratio on a logarithmic scale. As you can see, most of the ventilation (open circles) and most of the perfusion (closed circles) take place in lung regions that encompass a relatively narrow range of 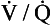 ratios. In other words, regions of the lung with relatively low ventilation tend to have relatively low perfusion.
ratios. In other words, regions of the lung with relatively low ventilation tend to have relatively low perfusion.
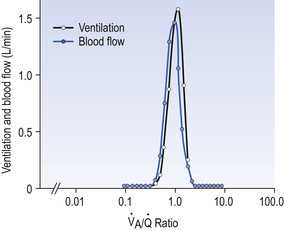
Fig. 7.9 Distribution of ventilation (O) and perfusion (•). Ventilation and perfusion are both plotted against 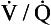 ratio on a logarithmic scale. Most ventilation and perfusion takes place in lung regions having a relatively narrow range of
ratio on a logarithmic scale. Most ventilation and perfusion takes place in lung regions having a relatively narrow range of 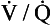 ratio.
ratio.
How ventilation and perfusion are so closely matched is not fully understood. Matching is partly due to the fact that both ventilation and perfusion increase towards the lung bases and hypoxic pulmonary vasoconstriction plays a small part. Ignoring these effects, it nevertheless appears that compliant regions of the lung that are relatively well ventilated also seem to have lower vascular resistances and are therefore better perfused. It has been speculated that this relationship between lung compliance and vascular resistance in a given region is one that is formed in the developing lung.
Although fairly well matched there is nevertheless a range of 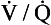 ratios found in normal lungs. In diseased lungs, this variation is very much increased. What effect does variability in
ratios found in normal lungs. In diseased lungs, this variation is very much increased. What effect does variability in 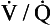 ratios have on gas exchange?
ratios have on gas exchange?
Ventilation/perfusion matching and its effect on blood O2 and CO2 content
It is useful to think of ventilation and perfusion matching as representing the mixing together of air and mixed venous blood (although obviously blood and gas cannot be physically mixed in the body). If the 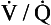 ratio is high, the mixture contains more air than blood and therefore has a relatively high Po2, and a relatively low Pco2. If, however, the
ratio is high, the mixture contains more air than blood and therefore has a relatively high Po2, and a relatively low Pco2. If, however, the 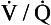 ratio is low, the mixture contains a higher concentration of blood and therefore has a relatively low Po2 and a relatively high Pco2. In fact, the
ratio is low, the mixture contains a higher concentration of blood and therefore has a relatively low Po2 and a relatively high Pco2. In fact, the 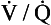 ratio can vary between zero and infinity. A
ratio can vary between zero and infinity. A 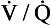 ratio of zero means that there is perfusion but no ventilation: in other words, an area of the lungs where shunting is taking place and the gas concentrations are similar to mixed venous blood. A
ratio of zero means that there is perfusion but no ventilation: in other words, an area of the lungs where shunting is taking place and the gas concentrations are similar to mixed venous blood. A 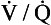 ratio of infinity means that there is ventilation but no perfusion, in other words an area of the lungs that is dead space, where the gas concentrations are similar to those of air. Each value of the
ratio of infinity means that there is ventilation but no perfusion, in other words an area of the lungs that is dead space, where the gas concentrations are similar to those of air. Each value of the 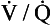 ratio is therefore associated with a particular Po2 and Pco2.
ratio is therefore associated with a particular Po2 and Pco2.
If the transfer of oxygen and carbon dioxide were based on a simple mixing effect then it would be straightforward to calculate the composition of gas in an alveolus in the same way we could calculate the composition of a beaker of saline (i.e. blood) to which various known amounts of water (i.e. alveolar gas) were added. Adding a small amount of water to the saline would result in a mixture with a composition close to that of the saline. Adding a large volume of water to the saline would result in a mixture with a composition similar to that of water. Figure 7.10 shows how the composition of the saline/water mixture varies with the proportions of each making up the mixture. If saline represents the composition of mixed venous blood (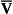 ) and water represents the composition of air (I), then there would be a straight line joining point
) and water represents the composition of air (I), then there would be a straight line joining point 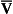 (zero
(zero 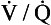 ratio, dead space) and I (infinite
ratio, dead space) and I (infinite 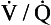 ratio, shunt). This straight line would represent the gas tensions at different parts of the lungs with ratios that lie between these two extremes.
ratio, shunt). This straight line would represent the gas tensions at different parts of the lungs with ratios that lie between these two extremes.
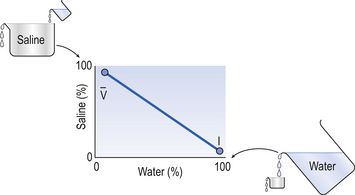
Fig. 7.10 An analogy of under- and overventilated regions of the lung. The exchange of gases is simplified as the displacement of saline by water. Saline represents mixed venous blood (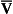 ) and pure water represents alveolar air. Point
) and pure water represents alveolar air. Point 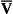 represents the composition of blood in poorly ventilated regions of the lung, not enough water being added to wash the salt away. Point I represents regions with ventilation in excess. Nearly all the salt is washed away and the composition of I approaches that of pure water.
represents the composition of blood in poorly ventilated regions of the lung, not enough water being added to wash the salt away. Point I represents regions with ventilation in excess. Nearly all the salt is washed away and the composition of I approaches that of pure water.
However, in this region gas exchange in the lungs is not a simple exchange of one molecule of carbon dioxide for one molecule of oxygen, and the line joining the two extremes of 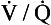 ratio is, in fact, curved, mainly because the oxygen–haemoglobin dissociation curve is sigmoid in shape. In a region of lung with a high
ratio is, in fact, curved, mainly because the oxygen–haemoglobin dissociation curve is sigmoid in shape. In a region of lung with a high 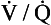 ratio more CO2 is removed from the blood but the blood does not take up much more oxygen. This is because at the higher Pao2, that is found here the haemoglobin is already 97% saturated and can therefore carry very little extra oxygen. Similarly, in an area of lung with a low
ratio more CO2 is removed from the blood but the blood does not take up much more oxygen. This is because at the higher Pao2, that is found here the haemoglobin is already 97% saturated and can therefore carry very little extra oxygen. Similarly, in an area of lung with a low 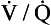 ratio, relatively more oxygen is taken up by the blood because at the lower Pao2 here, haemoglobin is at the middle, steep part of the oxygen–haemoglobin dissociation curve. However, relatively little extra carbon dioxide is released from the blood because the carbon dioxide dissociation curve is straighter than the oxygen–haemoglobin dissociation curve.
ratio, relatively more oxygen is taken up by the blood because at the lower Pao2 here, haemoglobin is at the middle, steep part of the oxygen–haemoglobin dissociation curve. However, relatively little extra carbon dioxide is released from the blood because the carbon dioxide dissociation curve is straighter than the oxygen–haemoglobin dissociation curve.
In other words, the ratio of carbon dioxide output to oxygen uptake (usually called the respiratory exchange ratio, R) varies throughout the lungs. The value of R for the lungs as a whole is about 0.8, varying according to what food is being metabolized. (The fact that this number is similar to the 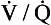 ratio for the lungs is coincidental.) In regions near the lung apices with a high
ratio for the lungs is coincidental.) In regions near the lung apices with a high 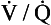 ratio the value of R is high (up to 2.0), whereas in regions near the bases with a low
ratio the value of R is high (up to 2.0), whereas in regions near the bases with a low  ratio the value of R is low (as low as 0.5).
ratio the value of R is low (as low as 0.5).
The relationship between Po2 and Pco2 in regions of the lungs with different 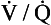 ratios is usually expressed in a diagram such as Figure 7.11C. This is called the oxygen–carbon dioxide diagram, or O2–CO2 diagram. The values of Po2 and Pco2 at point ‘
ratios is usually expressed in a diagram such as Figure 7.11C. This is called the oxygen–carbon dioxide diagram, or O2–CO2 diagram. The values of Po2 and Pco2 at point ‘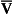 ’ are those of mixed venous blood (perfusion, no ventilation,
’ are those of mixed venous blood (perfusion, no ventilation,  ratio of zero). The values of Po2 and Pco2 at I are those of moist air (ventilation, no perfusion,
ratio of zero). The values of Po2 and Pco2 at I are those of moist air (ventilation, no perfusion, 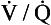 ratio of infinity). The
ratio of infinity). The 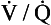 ratio can be any value between these two extremes, and each value is associated with a particular respiratory exchange ratio as well as a particular Po2 and Pco2.
ratio can be any value between these two extremes, and each value is associated with a particular respiratory exchange ratio as well as a particular Po2 and Pco2.
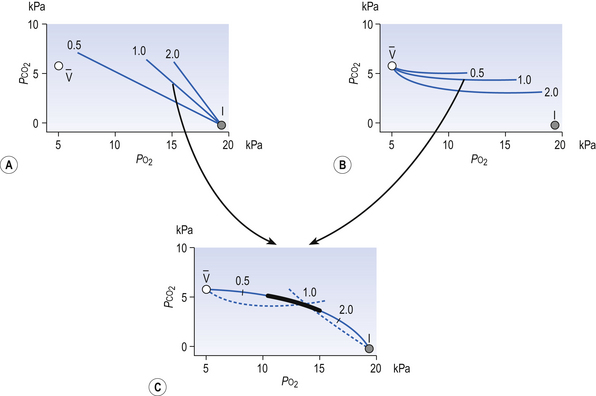
Fig. 7.11 The respiratory exchange ratio (R) for the gases in alveoli and the blood associated with them indicates how Pco2 and Po2 change in the alveolar gas and blood. Combination of the blood and gas lines enables us to determine the partial pressures of CO2 and O2 at any combination of ventilation and perfusion. Points 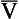 and I correspond to mixed venous blood and moist inspired air, respectively. The theoretical effects of different gas exchange ratios (R) are shown in (A), on the composition of the alveolar gas, and (B) on the blood gas tensions in the blood (in reality it is not possible for gas exchange to take place in either the alveoli or blood independently of each other). (C) shows the combined alveolar–blood gas tension relationships for different values of R. The gas R line for R = 1.0 and the blood R line for R = 1.0 have been drawn in interrupted lines. Because the gas tensions must be the same in the alveoli and the blood, the point where the gas and blood R lines intersect represents the blood gas tensions that are actually seen in the alveolar gas and the alveolar capillary blood. Plotting points for all values of R gives the O2–CO2 diagram for the lungs. The normal range of
and I correspond to mixed venous blood and moist inspired air, respectively. The theoretical effects of different gas exchange ratios (R) are shown in (A), on the composition of the alveolar gas, and (B) on the blood gas tensions in the blood (in reality it is not possible for gas exchange to take place in either the alveoli or blood independently of each other). (C) shows the combined alveolar–blood gas tension relationships for different values of R. The gas R line for R = 1.0 and the blood R line for R = 1.0 have been drawn in interrupted lines. Because the gas tensions must be the same in the alveoli and the blood, the point where the gas and blood R lines intersect represents the blood gas tensions that are actually seen in the alveolar gas and the alveolar capillary blood. Plotting points for all values of R gives the O2–CO2 diagram for the lungs. The normal range of 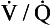 ratios is shown by the heavy line.
ratios is shown by the heavy line.
It is also possible to draw separate hypothetical O2–CO2 diagrams for the composition of alveolar gas and for the gas tensions in the blood, although of course in reality neither arterial gas nor pulmonary capillary blood exists in isolation. Figure 11A shows the theoretical effect of different gas exchange ratios on the composition of alveolar gas. If R = 1.0, each molecule of O2 removed from an alveolus is replaced by one molecule of CO2. The Po2 and Pco2 change according to line 1.0. If R = 0.5, two molecules of O2 are replaced by one of CO2 and the gas tensions follow the 0.5 line; similarly, a line for R = 2.0 can be drawn. No line passes through the point, as this would represent a value of R that lies outside the range of R in normal healthy lungs. Figure 7.11B represents the theoretical effects of different gas exchanges on the gas tensions in the blood. In this diagram, the relationships between Po2 and Pco2 are not straight lines, because the oxygen and carbon dioxide dissociation curves are not straight lines.
As we have said, the alveoli and the pulmonary capillaries do not act in isolation. Clearly, each molecule of CO2 lost from the blood is gained by the alveolus, and each molecule of O2 gained by the blood must have come from the alveolus. The alveolar gas and pulmonary capillary blood must have the same value for R. This means that for each value of R in a particular lung region, the values of Po2 and Pco2 must lie on both the alveolar gas R line and the blood R line, i.e. they must lie where the two lines intersect, as in Figure 7.11. Plotting points for every possible value of R leads to the O2–CO2 diagram for the lungs shown in Figure 7.11C. Notice how the line joining all the values of Po2 and Pco2 is curved. On the diagram, the heavy line represents the normal ranges of Po2, Pco2, 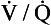 ratio and R that are found in healthy individuals.
ratio and R that are found in healthy individuals.
As a result of regional differences in the 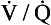 ratio and R, the alveoli at the apices of the lungs have a higher Pao2 and a lower Paco2 than those at the bases of the lungs. Does this matter? After all, the blood from different regions of the lungs is mixed together in the left atrium. Surely the low Pao2 and high Paco2 in blood leaving regions of the lung with a low
ratio and R, the alveoli at the apices of the lungs have a higher Pao2 and a lower Paco2 than those at the bases of the lungs. Does this matter? After all, the blood from different regions of the lungs is mixed together in the left atrium. Surely the low Pao2 and high Paco2 in blood leaving regions of the lung with a low 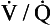 ratio will be ‘balanced’ by the high Pao2 and low Paco2 in blood leaving regions of the lungs with a high
ratio will be ‘balanced’ by the high Pao2 and low Paco2 in blood leaving regions of the lungs with a high 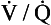 ratio?
ratio?
In the case of carbon dioxide this is roughly true: blood leaving regions of the lungs with a high 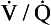 ratio has a low Paco2 and low CO2 content, and mixes with blood leaving regions of the lungs with a low
ratio has a low Paco2 and low CO2 content, and mixes with blood leaving regions of the lungs with a low 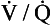 ratio, a high, Paco2 and high CO2 content. The resulting mixture has a Paco2 and CO2 content, which is ‘normal’.
ratio, a high, Paco2 and high CO2 content. The resulting mixture has a Paco2 and CO2 content, which is ‘normal’.
Unfortunately, this is not the case for oxygen, for two reasons:
1. The blood flow to the apices of the lungs is very much less than the blood flow to the bases, as we have already seen. The blood leaving the apices of the lungs has a higher Pao2 than that leaving the bases; however, there is very much more blood leaving the bases of the lungs.
2. Although blood from the apices of the lungs has a higher Pao2 this does not translate into a higher blood oxygen content. The haemoglobin leaving regions of the lungs with a higher Po2 is 97% saturated with oxygen and can therefore carry very little extra oxygen compared with regions of the lungs with a normal Po2. In other words, the content of oxygen in blood leaving regions of the lungs with a high Po2 is very similar to that of blood leaving regions of the lungs with a ‘normal’ Po2. Clearly, then, it cannot ‘compensate’ for the low oxygen content of blood leaving regions of the lungs with a low Po2.
To summarize these two points: if there is a wide range of 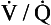 ratios, blood in the left atrium will tend to have a reduced Pao2 (because the volume of blood with a low Pao2 from the bases of the lungs is greater than the volume of blood with a high Pao2 from the apices) and will also have a reduced oxygen content (the blood from the apices has a high PaO2, but its oxygen content is only very slightly higher than normal because of the shape of the oxygen–haemoglobin dissociation curve). It is therefore very important that there is a relatively narrow range of
ratios, blood in the left atrium will tend to have a reduced Pao2 (because the volume of blood with a low Pao2 from the bases of the lungs is greater than the volume of blood with a high Pao2 from the apices) and will also have a reduced oxygen content (the blood from the apices has a high PaO2, but its oxygen content is only very slightly higher than normal because of the shape of the oxygen–haemoglobin dissociation curve). It is therefore very important that there is a relatively narrow range of 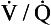 ratios within the lungs; in other words, ventilation and perfusion matching should be as good as possible. Poor ventilation/perfusion matching leads to deoxygenation. In healthy individuals, the degree of ventilation/perfusion matching is so good that arterial blood carries only 2% less oxygen than blood, leaving a theoretical ‘ideal’ set of lungs that have perfect ventilation/perfusion matching.
ratios within the lungs; in other words, ventilation and perfusion matching should be as good as possible. Poor ventilation/perfusion matching leads to deoxygenation. In healthy individuals, the degree of ventilation/perfusion matching is so good that arterial blood carries only 2% less oxygen than blood, leaving a theoretical ‘ideal’ set of lungs that have perfect ventilation/perfusion matching.
Although much of the difference in oxygen content between arterial blood and that of blood leaving an ‘ideal’ pair of lungs is due to ventilation/perfusion mismatching, there is a second source of relatively deoxygenated blood. This blood makes up part of the anatomical shunt. Anatomical shunt is deoxygenated blood that drains into the left (arterial) side of the circulation, and therefore is another reason why arterial blood does not contain as much oxygen as hypothetical blood from an ‘ideal’ pair of lungs.
Shunt
The content of oxygen in the systemic arteries is not as high as would be predicted from the alveolar gas equation. This is due partly to shunting (i.e. relatively deoxygenated blood being added to oxygenated blood leaving well-ventilated alveoli) and partly to ventilation mismatching.
Shunted blood comes from two main sources. The blood draining the bronchial circulation via the deep bronchial veins has already been mentioned. The second source of shunted blood is the thebesian veins in the myocardium. Most of the blood drains from the myocardium into the cardiac veins and then into the cardiac sinus. The cardiac sinus is essentially a large vein that drains into the right atrium. Deoxygenated blood draining through the cardiac vein system therefore does not form a shunt because it drains into the right-hand side of the circulation. The thebesian veins are small vessels that drain blood from the myocardium into the cavity of the underlying atrium or ventricle. Blood draining through the thebesian veins on the left-hand side of the heart therefore forms a shunt. Although the flow of blood through the left-sided thebesian veins is not great, the oxygen content of the blood is very low.
The amount of shunting and ventilation/perfusion mismatching may be expressed in a number of ways. One way is to calculate the difference in oxygen tension between the alveoli and the systemic arteries. The oxygen tension in the alveoli is calculated using the alveolar gas equation, and the oxygen tension in the systemic arteries is measured directly from a blood sample. In normal subjects the alveolar–arterial difference (A–a difference) is usually less than 5 kPa.
Another way of expressing the amount of shunting and ventilation/perfusion mismatching that is taking place is to calculate the venous admixture. This is defined as the amount of mixed venous blood that would need to be added to blood leaving a well-perfused and well-ventilated area of lung in order to produce the arterial oxygen concentration that is actually seen in the systemic arteries. The venous admixture is usually expressed as a proportion of the total cardiac output (shunt fraction). The venous admixture is a theoretical volume of blood, as the blood that actually flows through the physiological shunts has an oxygen content that may be higher (deep bronchial veins) or lower (thebesian veins) than that of mixed venous blood. The venous admixture may nevertheless be calculated using the shunt equation:
where 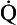 s is the venous admixture flow,
s is the venous admixture flow, 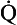 t is the total blood flow,
t is the total blood flow, 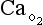 is the oxygen content of arterial blood, C
is the oxygen content of arterial blood, C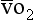 is the oxygen content of mixed venous blood and
is the oxygen content of mixed venous blood and 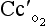 is the oxygen content of the capillary blood draining an alveolus with an ideal ventilation/perfusion ratio.
is the oxygen content of the capillary blood draining an alveolus with an ideal ventilation/perfusion ratio.
The oxygen content of blood can be calculated knowing the partial pressure of oxygen and the haemoglobin concentration, using the oxygen dissociation curve. Mixed venous blood needs to be obtained from a catheter placed in the pulmonary artery. How can the oxygen content of capillary blood draining an ‘ideal’ alveolus be measured? Of course it cannot, but it can be inferred by calculating the Pao2 in an ‘ideal’ alveolus by solving the alveolar gas equation (see p. 69) and then calculating the oxygen content of blood at equilibrium with such an alveolus, as above.
The shunt fraction in normal individuals is usually less than 5% of the total cardiac output. Most of the shunt is made due to ventilation/perfusion mismatching, rather than true shunting of blood from the deep bronchial and thebesian veins.
A salutary tale
A student illicitly eating peanuts in the library is surprised by the approach of the librarian. He inhales a nut, which completely blocks his right main bronchus. Leaping about in an attempt to expel the offending fruit our hero merely succeeds in dislodging a large clot which has formed in a leg vein due to the hours of immobility he has spent at his studies (this is a fairy story). If the clot obstructs his right pulmonary artery all will probably be well; although his right lung will be functionally non-existent, his left lung will be normal and sufficient for him to survive. If the clot lodges in the left pulmonary artery the consequences of this extreme case of ventilation/perfusion mismatch will be grave. Despite the fact that his total lung ventilation and total lung perfusion may be sufficient for survival, both lungs will be functionally useless, but each for a different reason: one will have no ventilation and be a shunt and one will have no perfusion and be dead space. This story may help you understand the importance of matching ventilation and perfusion and a law-abiding lifestyle.
Further reading
Fishman, A. P., 1988. Normal pulmonary circulation. In: Pulmonary Diseases and Disorders, Vol. 2. McGraw-Hill, New York.
Glazier, J. B., Hughes, J. M. B., Maloney, J. E., West, J. B. Measurements of capillary dimensions and blood volume in rapidly frozen lungs. J. Appl. Physiol.. 1969; 26:65–76.
Hughes, J. M. B. Distribution of pulmonary blood flow. In Crystal R. G., West J. B., Barnes P. J., Weibel E. R., eds. : The Lung: Scientific Foundations, second ed, New York: Raven Press, 1997.
Weir E. K., Reeves J. T., eds. Pulmonary Vascular Physiology and Pathophysiology. New York: Marcel Dekker, 1989.
West, J. B., Wagner, P. D. Ventilation–perfusion relationships. In Crystal R. G., West J. B., Barnes P. J., Weibel E. R., eds. : The Lung: Scientific Foundations, second ed, New York: Raven Press, 1997.
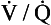 .
.