GAS EXCHANGE BETWEEN AIR AND BLOOD
DIFFUSION
The path from air to tissue
The lungs are so good at maintaining O2 levels in the blood that it was until quite recently thought to be an active process, with the body actively extracting O2 from the air. It is now known that the process is purely passive diffusion down concentration gradients. Diffusion is so important to our obtaining O2 that the ability of the lungs to allow diffusion to happen is frequently measured, and is sometimes called the lung’s diffusing capacity. This capacity is reduced in many diseases, including fibrosis, asbestosis, sarcoidosis and pneumonia.
In Chapter 5 we described the way in which ventilation of different regions of the lung results in different compositions of gas in the alveoli. In understanding the next step in the journey from air to blood or blood to air it is important to differentiate clearly between the amount of a gas present in a mixture and the fraction or concentration of that gas, frequently expressed as partial pressure (P). This concept is important because it is only the difference in partial pressure that drives diffusion from one place to another. To use an analogy: the pressure across the enormous lock gates holding the Atlantic Ocean out of a dock in which the water is 10 cm lower than the ocean is the same as that across the walls of a child’s paddling pool containing water 10 cm deep. The amounts of water either side (or gas in the case of the lung) having nothing to do with the motive force (driving pressure): the difference in depth of water (or difference in partial pressure of gas) provides the motive force.
The partial pressure of a gas depends on its concentration, and so the partial pressure gradient across a membrane depends on the difference in concentration on either side. It is diffusion alone that transports gas between air and blood at the lungs, and between blood and cells at the tissues, and the circulation of blood effectively links the two sites. In the case of O2 (Fig. 6.1) we can see that diffusion at the lungs is sufficient to reduce to virtually zero any difference in partial pressure (concentration) between alveolar air and pulmonary blood, whereas at the tissues a large difference in partial pressure between arterial blood and the mitochondria ensures a vigorous flow into these organelles of oxidative metabolism.
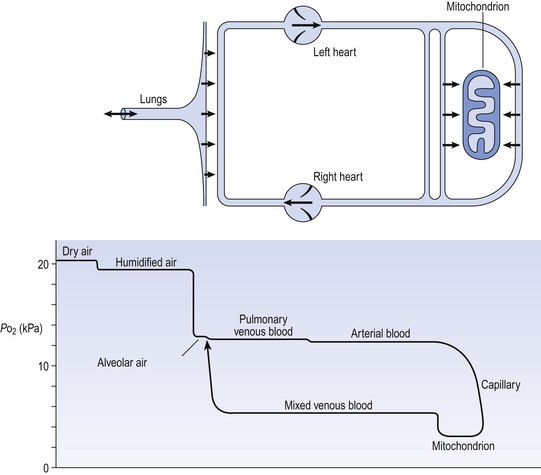
Fig. 6.1 The path of oxygen. The physical path is shown as a diagram above. The partial pressure of O2 in air, blood and tissue fluid at the different points on its journey is shown below.
The single step of O2 from air to blood in Figure 6.1 is in fact a series of steps through components which are in series and which will be considered in detail below. The important thing to remember is that they are in series (i.e. one after another), like the segments of a hosepipe made up of a number of bits joined together. Constricting one segment reduces flow in all.
Lung disease and diffusion
The lungs have such an enormous capacity for diffusion that there is some doubt in many diseases (e.g. emphysema) as to what part the impairment of diffusion plays in producing the characteristic hypoxia of the disease. In many cases a diffusional component can only be demonstrated when the patient is exercising. The destruction of the architecture of the respiratory regions in emphysema (Fig. 4.16) must impair diffusion, but the effect on distribution of ventilation and blood flow must be at least as important. Other diseases (e.g. interstitial pulmonary fibrosis) which produce profound thickening of the respiratory membranes reduce the lungs’ capacity for diffusion to one-sixth of normal, and it is difficult to assume that this does not affect their function.
Fick’s Law of Diffusion
The movement of gas into and out the blood is described by Fick’s Law (Appendix, p. 155), which describes the rate of diffusion of a gas across a membrane as follows:
where A is the area of the membrane available for diffusion. S is the solubility of the gas in the membrane, ΔC is the concentration gradient – brought about by the differences in concentration (or partial pressure) either side of the membrane – t is the thickness of the membrane and MW is the molecular weight of the gas (Fig. 6.2).
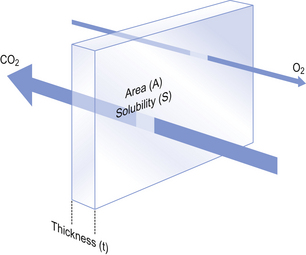
Fig. 6.2 Diffusion through a membrane. It is obvious that gas will diffuse more quickly if the area is great, the thickness is small and the concentration difference of the gas, which is highly soluble in the membrane, is high.
The area available for diffusion is the area of the pulmonary capillaries that is in contact with alveolar air. This area can be changed by diseases which destroy alveolar septa, and by changes during exercise, when capillaries that were closed at rest open up and those already open become distended.
The solubility of a gas determines the rate of diffusion of a gas in solution. The gases we are particularly interested in are CO2 and O2. Carbon dioxide is 23 times more soluble in tissue fluid than is O2 but has a higher molecular weight; it will therefore diffuse from blood to air 20 times more readily for the same partial pressure gradient than will O2 moving in the opposite direction. However, equilibrium for CO2 is established at about the same rate as for O2 because:
1. the reaction releasing CO2 from blood is relatively slow
2. the concentration gradient driving CO2 from blood to alveolar air is only 0.8 kPa, whereas that driving O2 in the opposite direction is 8 kPa.
Although the process of diffusion is a physical one, chemical reactions exert their influence on this process in the lungs. In particular, the rate at which O2 can be taken up or released by haemoglobin in the red blood corpuscles (RBC) acts as a rate-limiting step, reducing its overall rate of transfer.
Although there are situations when the lung can be so damaged as to restrict the diffusion of CO2 it is usually O2 that is first affected by diffusional difficulties. The rate of removal of CO2 is governed primarily by alveolar ventilation (chapter 5; p. 62 and p.83 of this chapter).
In Figure 6.1 there are three places where diffusion is the major transport mechanism and where this transport can effectively be impeded by failure of diffusion:
As this is a textbook on the respiratory system we will concentrate on the first two. The anatomical sites of these two sources of impedance to diffusion are shown in Figure 6.3.
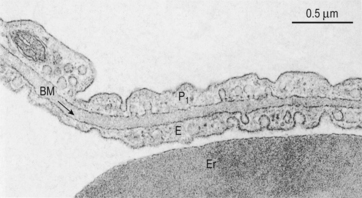
Fig. 6.3 The site of diffusion in the lungs. The distance involved in diffusion within the alveolar space is much greater than the distance within the lung tissue. However, diffusion within the alveolar space is in air and therefore much more rapid than in the tissue. The diffusional barrier between blood and alveolar air consists of attenuated cytoplasm of a Type 1 pneumocyte P1, a common basement membrane BM, cytoplasm of a capillary endothelial cell E. Er is an erythrocyte in a capillary. Source: Stevens et al., 2002.
Within the alveoli
In healthy lungs the average diameter of the alveoli is about 200 μm. Any differences in the concentrations of the respiratory gases at different points within the alveolus will be obliterated by diffusion over these distances in less than 10 ms. It might be imagined, however, that in emphysema (where the air spaces are enlarged) increased distances for diffusion slow O2 transport to unacceptable levels. Although it is true that these changes in geometry must have an effect, destruction of the alveolar septa, reducing surface area, together with ventilation/perfusion mismatching, exerts a malign influence in this disease well before diffusion problems need be considered.
Air to RBC
This step from air to blood or blood to air has a number of components of its own:
• Length of time blood is in the capillaries
• Crossing the alveolar/capillary membrane
Time in the capillaries
Although blood flow through the lungs is highly pulsatile (p. 87) it is legitimate when considering diffusion to consider blood flowing smoothly and taking on average 0.8 s to transit the pulmonary capillary. The way concentration and hence the partial pressure of O2 in the blood changes during transit along the capillary is shown in Figure 6.4.
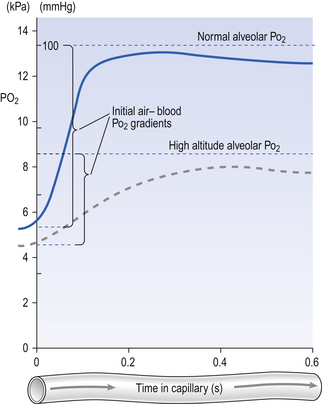
Fig. 6.4 Partial pressure of O2 in blood during its transit through a pulmonary capillary. This example shows the mean transit time for a population which has a high degree of variation. The broken line represents reduced partial pressure of oxygen (as at altitude).
Consideration of this figure reveals a number of potential complications which can arise because:
• There are great differences in transit time through different regions of the lung. Transit times less than 0.2 s do not allow sufficient time for equilibration, and capillaries with these low transit times pass deoxygenated blood to the pulmonary veins. This is functionally equivalent to a spread of 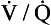 (ventilation/perfusion) ratios, and this is ‘a bad thing’ (see Chapter 7).
(ventilation/perfusion) ratios, and this is ‘a bad thing’ (see Chapter 7).
• During heavy exercise cardiac output increases and all transit times are reduced.
• At altitude the partial pressure of ambient O2 driving O2 into the blood is reduced. This effect is shown as a dotted line in Figure 6.4. Clearly, exercise at altitude is the worst of all possible combinations, except for disease, and these effects account for much of the limitation of mountaineering achievement.
Crossing the alveolar membrane
The lining epithelium and basement membrane of the alveoli and the endothelium and basement membrane of the pulmonary capillaries are each about 0.2 μm thick on one side of the capillary and somewhat thicker on the other supporting side (Fig. 6.3). The capillaries occupy most of the alveolar wall, generally being less than one capillary diameter apart, and with each capillary passing through up to three alveoli. It might be expected that diseases such as pulmonary fibrosis, sarcoidosis, asbestosis and pulmonary oedema, which affect the alveolar/capillary membrane, would interfere with diffusion. Remarkably, however, these problems seem to affect diffusion less than might be expected, because they are usually found on the inactive, supporting, side of the pulmonary capillary, leaving the functional side relatively undamaged. Their major effects are to reduce the surface area for diffusion by gross destruction of tissue and increased ventilation/perfusion mismatching.
Diffusion in blood plasma
Red blood corpuscles are about the same diameter as pulmonary capillaries: in fact, they have to be distorted to squeeze through many capillaries. This means there is very little plasma between the corpuscle and the capillary wall for gas to diffuse through. Also, the ‘doughnut’ shape of the corpuscles means that most of the haemoglobin within is close to the wall, again shortening the distance for diffusion. The ‘kneading’ of the corpuscles as they squeeze through the capillaries probably causes mass movements of haemoglobin within the corpuscle, which also aids diffusion.
Reaction with haemoglobin
As the vast majority of O2 in the blood is carried by haemoglobin, the rate at which they combine is an important limiting step in O2 diffusion into the blood.
Because the transfer of O2 into the blood is profoundly affected by such factors as reaction rates with haemoglobin and transit time through pulmonary capillaries, which have nothing to do with the physical process of diffusion, it has been suggested that ‘diffusing capacity’ is an inappropriate term to describe the process. In England, where both the language and the technique for measuring this transfer were developed, the term transfer factor is generally preferred.
Measuring transfer factor
It is important, clinically and scientifically, to be able to measure transfer factor (diffusing capacity). The physiology on which the technique of measuring transfer factor is based will repay study here.
Transfer factor is primarily to do with the rate at which the lungs allow O2 to diffuse into the blood. We wish to measure this – or at least to compare this ability in people we know are normal with those suspected of having lung disease.
Frequently when we say we are measuring something we are in fact comparing it with a known standard. (When we carry out the simple act of measuring someone’s height we are in fact comparing their length with ‘the distance travelled by light in a vacuum in 1/29 792 458 seconds’, which is the physicists’ definition of the metre, but any other standard length would do. We could use the ‘hand’ (102 mm), normally used to measure the height of horses, so long as we define what we are using. Applying this principle to transfer factor means we do not have to actually measure the flow of O2 into the body to compare transfer factor between people: we can measure the flux of another, more convenient, gas so long as it is influenced by the same things as those that influence O2 transfer. This is fortunate because, as we will see, O2 presents difficulties.)
How do we choose a gas to measure transfer factor?
We therefore need to know the rate at which our chosen gas is taken up and the pressure which is driving it into the body.
If the blood in the pulmonary capillaries is very quickly saturated with the gas we are using it cannot take up any more, and this ‘puts a stop’ to the movement of the gas into the body. In this case, the rate at which blood flows through the lungs, and not diffusion, determines the rate of transfer and we are measuring pulmonary blood flow not transfer factor. Nitrous oxide behaves in this way and is used to measure pulmonary blood flow, but it is useless for measuring transfer factor (Fig. 6.6A).
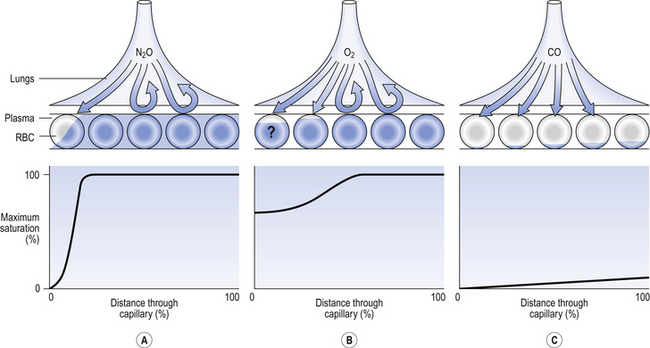
Fig. 6.6 Measuring transfer factor. Nitrous oxide immediately saturates pulmonary capillary blood and so prevents any further transfer. Its uptake is therefore limited by pulmonary blood flow, not transfer factor. Oxygen partial pressure in the pulmonary capillary is impossible to measure accurately along the capillary, and so driving pressure into the blood cannot be calculated. Carbon monoxide is so tightly bound to haemoglobin in the blood that it exerts no partial pressure there. Driving partial pressure is the partial pressure in the inhaled air.
As we are interested in the transfer of O2 why not use O2? We can certainly measure the rate at which O2 is taken up by a subject, but the problem comes with having to measure the ‘driving partial pressure (air-to-blood)’. Blood enters the lung capillaries already carrying some O2. Venous blood, which contains variable amounts of O2 is beginning to be oxygenated in the pulmonary arterioles even before it reaches the capillaries, and so it is very difficult to know the exact driving pressure between alveolar air and this variable capillary blood. Also, except at low inhaled Po2 blood is almost totally saturated with O2 when it leaves the lungs, producing the same difficulties as with nitrous oxide, where uptake is governed by blood flow rather than transfer factor (Fig. 6.6B).
The gas that turns out to be most suitable for measuring transfer factor is the poisonous gas carbon monoxide (CO). This is poisonous because it competes with O2 for binding sites on haemoglobin, and binds 250 times more strongly than O2, thereby denying O2 access to the haemoglobin – the victim of CO poisoning therefore dies of O2 lack. (For this reason only very low concentrations of CO are used in measuring transfer factor.) Because it binds so strongly CO is ‘locked up’ in the haemoglobin and to all intents and purposes disappears from the blood. In other words, its partial pressure in the blood is zero. Knowing the rate at which CO disappears from a gas mixture the subject holds in his lungs for 10 seconds, and the partial pressures of CO in that mixture before and after inhalation, transfer factor can be calculated (Fig. 6.6C).
Treating diffusion difficulties
Bearing in mind that a large part of the problems of patients who might be suspected of having diffusional difficulties may be due to ventilation or 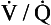 abnormalities, the rational treatment of patients whose lung diffusion problems lead to hypoxaemia depends on understanding the properties of the lung membrane illustrated in Figure 6.2. In these patients diffusion may have been reduced by thickening of the membrane (fibrosis) or loss of surface area (emphysema). These conditions are usually irreversible, and so we are left with only the option of increasing the partial pressure driving O2 into the blood. Fortunately this is very effective, and increasing the inspired fraction of O2 from the normal 21% in room air to 30% doubles the O2 flux into the blood. In appropriate cases of progressive lung disease O2 supplied via a nasal cannula or mask can much improve the quality of life of the unfortunate patient who requires O2 to relieve his breathlessness at rest. These patients, perhaps realizing how precarious their existence is, were once thought to become dependent on O2 administration. This dependency is usually psychological and is best avoided by giving the gas only to patients who obtain relief, and then only when needed and at the minimum effective dose.
abnormalities, the rational treatment of patients whose lung diffusion problems lead to hypoxaemia depends on understanding the properties of the lung membrane illustrated in Figure 6.2. In these patients diffusion may have been reduced by thickening of the membrane (fibrosis) or loss of surface area (emphysema). These conditions are usually irreversible, and so we are left with only the option of increasing the partial pressure driving O2 into the blood. Fortunately this is very effective, and increasing the inspired fraction of O2 from the normal 21% in room air to 30% doubles the O2 flux into the blood. In appropriate cases of progressive lung disease O2 supplied via a nasal cannula or mask can much improve the quality of life of the unfortunate patient who requires O2 to relieve his breathlessness at rest. These patients, perhaps realizing how precarious their existence is, were once thought to become dependent on O2 administration. This dependency is usually psychological and is best avoided by giving the gas only to patients who obtain relief, and then only when needed and at the minimum effective dose.
Carbon dioxide and other gases
Gases such as nitrous oxide, helium or carbon monoxide may be administered experimentally to those unfortunate enough to fall into the hands of respiratory physiologists. Vapours of volatile anaesthetics such as halothane or ether are administered therapeutically.
The major determinant of how readily these substances diffuse across the lung membrane is their solubility in water. Ether is almost 600 times more soluble in water than O2, and diffuses into the blood almost 400 times more readily. Nitrogen, on the other hand, is about half as soluble and diffuses at half the rate.
Carbon dioxide is a special case. We have seen that it is 24 times more soluble than O2 and diffuses 20 times as readily. Returning to the series nature of transfer of gases, we know that rate of diffusion depends on the rate of supply of CO2, which in turn depends on the chemical reactions that release CO2 from carbamino compounds and bicarbonate in the blood. The only time hypercapnia occurs other than as a result of hypoventilation is when the enzyme that accelerates the equilibrium of bicarbonate and CO2 in the blood (carbonic anhydrase; see p. 109) is inhibited. So, the rate of CO2 removal from the body is determined primarily by alveolar ventilation.
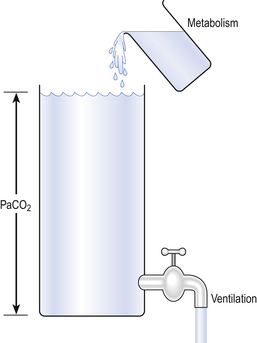
Carbon dioxide diffuses so readily that its arterial partial pressure is almost entirely the result of a balance between CO2 production by metabolism and CO2 removal by ventilation (Fig. 6.7).
Further reading
Forster, R. E., Crandall, E. D. Pulmonary gas exchange. Ann. Rev. Physiol.. 1976; 38:69.
Krogh, M. The diffusion of gases through the lungs of man. J. Physiol. (Lond). 1914–1915; 49:271–300.
Roughton, F. J. W., Forster, R. E. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J. Appl. Physiol.. 1957; 11:290–302.
Scheid, P., Piiper, J. Diffusion. In Crystal R. G., West J. B., Barnes P. J., Weibel E. R., eds. : The Lung: Scientific Foundations, second ed., New York: Raven Press, 1997.
Stevens, A., Lowe, J. S., Young, B. Wheater’s Basic Histopathology, fourth ed. Edinburgh: Churchill Livingstone; 2002.
West, J. B. Ventilation Blood Flow and Gas Exchange, fifth ed. Oxford: Blackwell Science; 1990.