Principles of Management and Prevention of Odontogenic Infections
MICROBIOLOGY OF ODONTOGENIC INFECTIONS
NATURAL HISTORY OF PROGRESSION OF ODONTOGENIC INFECTIONS
PRINCIPLES OF THERAPY OF ODONTOGENIC INFECTIONS
Principle 1: Determine Severity of Infection
Principle 2: Evaluate State of Patient’s Host Defense Mechanisms
Principle 3: Determine Whether Patient Should Be Treated by General Dentist or Oral and Maxillofacial Surgeon
Principle 4: Treat Infection Surgically
Principle 5: Support Patient Medically
Principle 6: Choose and Prescribe Appropriate Antibiotic
Determine the Need for Antibiotic Administration
Use Empirical Therapy Routinely
Use the Narrowest-Spectrum Antibiotic
Use the Antibiotic with the Lowest Incidence of Toxicity and Side Effects
Use a Bactericidal Antibiotic, If Possible
PRINCIPLES OF PREVENTION OF INFECTION
One of the most difficult problems to manage in dentistry is an odontogenic infection. Odontogenic infections arise from the teeth and have a characteristic flora. Caries, periodontal disease, and pulpitis are the initiating infections that can spread beyond the teeth to the alveolar process and the deeper tissues of the face, oral cavity, head, and neck. These infections may range from low-grade, well-localized infections that require only minimal treatment to severe, life-threatening deep fascial space infections. Although the overwhelming majority of odontogenic infections are readily managed by minor surgical procedures and supportive medical therapy that includes antibiotic administration, the practitioner must constantly bear in mind that these infections occasionally become severe and life-threatening in a short time.
This chapter is divided into several sections. The first section discusses the typical microbiologic organisms involved in odontogenic infections. Appropriate therapy of odontogenic infections depends on a clear understanding of the causative bacteria. The second section discusses the natural history of odontogenic infections. When infections occur, they may erode through bone and into the overlying soft tissue. Knowledge of the usual pathway of infection from the teeth and surrounding tissues through the bone and into the overlying soft tissue planes is essential when planning appropriate therapy. The third section of this chapter deals with the principles of management of odontogenic infections. A series of principles are discussed, with consideration of the microbiology and typical pathway of infection. The chapter concludes with a section on prophylaxis against infection. The prophylaxis of wound infection and of metastatic infection is discussed.
MICROBIOLOGY OF ODONTOGENIC INFECTIONS
The bacteria that cause infection are most commonly part of the indigenous bacteria that normally live on or in the host. Odontogenic infections are no exception because the bacteria that cause odontogenic infections are part of the normal oral flora: those that comprise the bacteria of plaque, those found on the mucosal surfaces, and those found in the gingival sulcus. These bacteria are primarily aerobic gram-positive cocci, anaerobic gram-positive cocci, and anaerobic gram-negative rods. These bacteria cause a variety of common diseases, such as dental caries, gingivitis, and periodontitis. When these bacteria gain access to deeper underlying tissues, as through a necrotic dental pulp or through a deep periodontal pocket, they cause odontogenic infections. As the infection progresses more deeply, different members of the infecting flora can find better growth conditions and begin to outnumber the previously dominant species.
Many carefully performed microbiologic studies of odontogenic infections have demonstrated the microbiologic composition of these infections. Several important factors must be noted. First, almost all odontogenic infections are caused by multiple bacteria. The polymicrobial nature of these infections makes it important that the clinician understand the variety of bacteria that are likely to cause the infection. In most odontogenic infections, the laboratory can identify an average of five species of bacteria. For as many as eight different species to be identified in a given infection is not unusual. On rare occasions a single species may be isolated. New molecular methods, which identify the infecting species by their genetic makeup, have allowed scientists to identify greater numbers and a whole new range of species not previously associated with these infections, including unculturable pathogens.
A second important factor is the oxygen tolerance of the bacteria causing odontogenic infections. Because the mouth flora is a combination of aerobic and anaerobic bacteria, it is not surprising to find that most odontogenic infections have anaerobic and aerobic bacteria. Infections caused by aerobic bacteria alone account for 6% of all odontogenic infections. Anaerobic bacteria alone are found in 44% of odontogenic infections. Infections caused by mixed anaerobic and aerobic bacteria compose 50% of all odontogenic infections (Table 15-1).
TABLE 15-1
Role of Anaerobic Bacteria in Odontogenic Infections
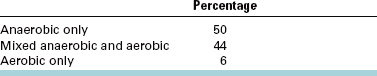
Brook I, Frazier EH, Gher ME: Aerobic and anaerobic microbiology of periapical abscess, Oral Microbiol Immunol 6:123-125, 1991.
The predominant aerobic bacteria in odontogenic infections (found in about 65% of cases) are the Streptococcus milleri group, which consists of three members of the S. viridans group of bacteria: S. anginosus, S. intermedius, and S. constellatus. These facultative organisms, which can grow in the presence and the absence of oxygen, may initiate the process of spreading into the deeper tissues (Table 15-2). Miscellaneous bacteria contribute 5% or less of the aerobic species found in these infections. Rarely found bacteria include staphylococci, group D Streptococcus organisms, other streptococci, Neisseria spp., Corynebacterium spp., and Haemophilus spp.
TABLE 15-2
Major Pathogens in Odontogenic Infections
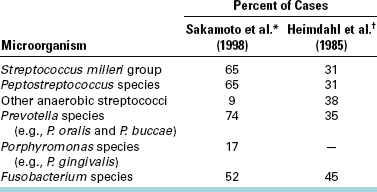
*Sakamoto H, Kato H, Sato T, Sasaki J: Semiquantitative bacteriology of closed odontogenic abscesses, Bull Tokyo Dent Coll 39:103-107, 1998.
†Heimdahl A, Von Konow L, Satoh T et al: Clinical appearance of orofacial infections of odontogenic origin in relation to microbiological findings, J Clin Microbiol 22:299, 1985.
The anaerobic bacteria found in odontogenic infections include an even greater variety of species (Table 15-2). Two main groups, however, predominate. The anaerobic gram-positive cocci are found in about 65% of cases. These cocci are anaerobic Streptococcus and Peptostreptococcus. Oral gram-negative anaerobic rods are cultured in about three quarters of the infections. The Prevotella and Porphyromonas spp. are found in about 75% of these, and Fusobacterium organisms are present in more than 50%.
Of the anaerobic bacteria, several gram-positive cocci (i.e., anaerobic Streptococcus and Peptostreptococcus spp.) and gram-negative rods (i.e., Prevotella and Fusobacterium spp.) play a more important pathogenic role. The anaerobic gram-negative cocci and the anaerobic gram-positive rods appear to have little or no role in the cause of odontogenic infections; instead, they appear to be opportunistic organisms.
The method by which mixed aerobic and anaerobic bacteria cause infections is known with some certainty. After initial inoculation into the deeper tissues, the facultative S. milleri group organisms can synthesize hyaluronidase, which allows the infecting organisms to spread through connective tissues, initiating a cellulitis type of infection. Metabolic by-products from the streptococci then create a favorable environment for the growth of anaerobes: the release of essential nutrients, lowered pH in the tissues, and consumption of local oxygen supplies. The anaerobic bacteria are then able to grow, and as the local oxidation-reduction potential is lowered further, the anaerobic bacteria predominate and cause liquefaction necrosis of tissues by their synthesis of collagenases. As collagen is broken down and invading white blood cells necrose and lyse, microabscesses form and may coalesce into a clinically recognizable abscess. In the abscess stage, the anaerobic bacteria predominate and may eventually become the only organisms found in culture. Early infections appearing initially as a cellulitis may be characterized as aerobic streptococcal infections, and late, chronic abscesses may be characterized as anaerobic infections.
Clinically, this progression of the infecting flora from aerobic to anaerobic seems to correlate with the type of swelling that can be found in the infected region. Thus, odontogenic infections seem to pass through four stages. In the first 3 days of symptoms, a soft, mildly tender, doughy swelling represents the inoculation stage, in which the invading streptococci are just beginning to colonize the host. After 3 to 5 days, the swelling becomes hard, red, and acutely tender as the infecting mixed flora stimulates the intense inflammatory response of the cellulitis stage. At 5 to 7 days after the onset of swelling, the anaerobes begin to predominate, causing a liquefied abscess in the center of the swollen area. This is the abscess stage. Finally, when the abscess drains spontaneously through skin or mucosa or it is surgically drained, the resolution stage begins as the immune system destroys the infecting bacteria and the processes of healing and repair ensue. The clinical and microbiologic characteristics of edema, cellulitis, and abscess are summarized and compared in Table 15-3.
NATURAL HISTORY OF PROGRESSION OF ODONTOGENIC INFECTIONS
Odontogenic infections have two major origins: (1) periapical, as a result of pulpal necrosis and subsequent bacterial invasion into the periapical tissue, and (2) periodontal, as a result of a deep periodontal pocket that allows inoculation of bacteria into the underlying soft tissues. Of these two, the periapical origin is the most common in odontogenic infections.
Necrosis of the dental pulp as a result of deep caries allows a pathway for bacteria to enter the periapical tissues. Once this tissue has become inoculated with bacteria and an active infection is established, the infection spreads equally in all directions but preferentially along the lines of least resistance. The infection spreads through the cancellous bone until it encounters a cortical plate. If this cortical plate is thin, the infection erodes through the bone and enters the surrounding soft tissues. Treatment of the necrotic pulp by standard endodontic therapy or extraction of the tooth should resolve the infection. Antibiotics alone may arrest, but do not cure, the infection because the infection is likely to recur when antibiotic therapy has ended without treatment of the underlying dental cause. Thus the primary treatment of pulpal infections is endodontic therapy or tooth extraction, as opposed to antibiotics.
When the infection erodes through the cortical plate of the alveolar process, it spreads into predictable anatomic locations. The location of the infection arising from a specific tooth is determined by the following two major factors: (1) the thickness of the bone overlying the apex of the tooth and (2) the relationship of the site of perforation of bone to muscle attachments of the maxilla and mandible.
Figure 15-1 demonstrates how infections perforate through bone into the overlying soft tissue. In Figure 15-1, A, the labial bone overlying the apex of the tooth is thin compared with the bone on the palatal aspect of the tooth. Therefore, as the infectious process spreads, it goes into the labial soft tissues. In Figure 15-1, B, the tooth is severely proclined, which results in thicker labial bone and a relatively thinner palatal bone. In this situation, as the infection spreads through the bone into the soft tissue, the infection is seen as a palatal abscess.
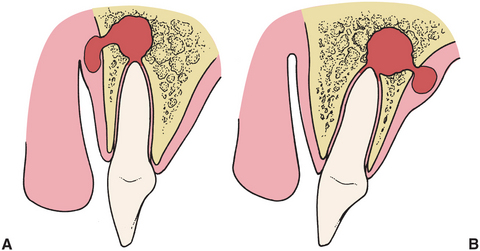
FIGURE 15-1 When infection erodes through bone, it will enter soft tissue through thinnest bone. A, Tooth apex is near thin labial bone, so infection erodes labially. B, Right apex is near palatal aspect, so palatal bone will be perforated.
Once the infection has eroded through the bone, the precise location of the soft tissue infection is determined by the position of the perforation relative to the muscle attachments. In Figure 15-2, A, the infection has eroded through to the facial aspect of the tooth and inferior to the attachment of the buccinator muscle, which results in an infection that appears as a vestibular abscess. In Figure 15-2, B, the infection has eroded through the bone superior to the attachment of the buccinator muscle and is expressed as an infection of the buccal space because the buccinator muscle separates the buccal and vestibular spaces.
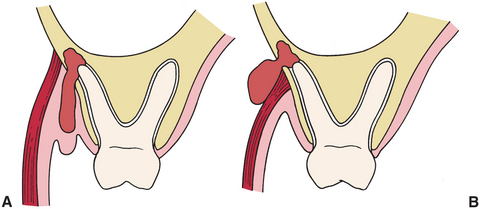
FIGURE 15-2 Relationship of point of bone perforation to muscle attachment determines fascial space involved. A, When tooth apex is lower than muscle attachment, vestibular abscess results. B, If apex is higher than muscle attachment, the adjacent fascial space is involved.
Infections from most maxillary teeth erode through the facial cortical plate. These infections also erode through the bone below the attachment of the muscles that attach to the maxilla, which means that most maxillary dental abscesses appear initially as vestibular abscesses. Occasionally, a palatal abscess arises from the apex of a severely inclined lateral incisor or the palatal root of a maxillary first molar or premolar (Fig. 15-3). More commonly, the maxillary molars cause infections that erode through the bone superior to the insertion of the buccinator muscle, which results in a buccal space infection. Likewise, on occasion a long maxillary canine root allows infection to erode through the bone superior to the insertion of the levator anguli oris muscle and causes an infraorbital (canine) space infection.
In the mandible, infections of the incisors, canines, and premolars usually erode through the facial cortical plate superior to the attachment of the muscles of the lower lip, resulting in vestibular abscesses. Mandibular molar teeth infections erode through the lingual cortical bone more frequently than with the anterior teeth. First molar infections may drain buccally or lingually. Infections of the second molar can perforate buccally or lingually (but usually lingually), and third molar infections almost always erode through the lingual cortical plate. The mylohyoid muscle determines whether infections that drain lingually go superior to that muscle into the sublingual space or below it into the submandibular space.
The most common odontogenic deep fascial space infection is a vestibular space abscess (Fig. 15-4). Occasionally, patients do not seek treatment for these infections, and the process ruptures spontaneously and drains, resulting in resolution or chronicity of the infection. The infection recurs if the site of spontaneous drainage closes. Sometimes the abscess establishes a chronic sinus tract that drains to the oral cavity or to the skin (Fig. 15-5). As long as the sinus tract continues to drain, the patient experiences no pain. Antibiotic administration usually stops the drainage of infected material temporarily, but when the antibiotic course is over, the drainage recurs. Definitive treatment of a chronic sinus tract requires treatment of the original causative problem, which is usually a necrotic pulp. In such a case the necessary surgery is endodontic therapy or extraction of the infected tooth.

FIGURE 15-4 Vestibular abscess arising from maxillary incisor. Overlying mucosa is thin because pus is near surface. (From Flynn TR: Anatomy of oral and maxillofacial infections. In Topazian RG, Goldberg MH, Hupp JR, editors: Oral and maxillofacial infections, ed 4, Philadelphia, 2002, WB Saunders.)
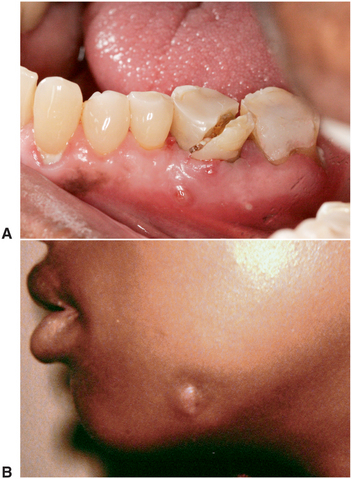
FIGURE 15-5 Chronic drainage sinus tracts that result from low-grade infections may drain intraorally (A) or extraorally (B). (A courtesy Sasha B. Ross, DMD. B from Flynn TR, Topazian RG: Infections of the oral cavity. In Waite D, editor: Textbook of practical oral and maxillofacial surgery, Philadelphia, 1987, Lea & Febiger.) Lea & Febiger
PRINCIPLES OF THERAPY OF ODONTOGENIC INFECTIONS
This section discusses the management of the odontogenic infection. A series of principles are discussed that are useful in treating patients who come to the dentist with infections related to the teeth and gingiva. The clinician must keep in mind the information in the preceding two sections of this chapter to understand these principles. By following these principles in stepwise fashion, the clinician may not always achieve the expected result, but he or she will certainly have met the standard of care. The first three principles are perhaps the most important in determining the outcome, yet they can be accomplished by the experienced practitioner within the first few minutes of the initial patient encounter.
Principle 1: Determine Severity of Infection
Most odontogenic infections are mild and require only minor surgical therapy. When the patient comes for treatment, the initial goal is to assess the severity of the infection. This determination is based on a complete history of the current infectious illness and a physical examination.
Complete History
The history of the patient’s infection follows the same general guidelines as any history. The initial purpose is to find out the patient’s chief complaint. Typical chief complaints of patients with infections are “I have a toothache,” “My jaw is swollen,” or “I have a gum boil in my mouth.” The complaint should be recorded in the patient’s own words.
The next step in taking of the history is determining how long the infection has been present. First, the dentist should inquire as to time of onset of the infection. How long ago did the patient first have symptoms of pain, swelling, or drainage, which indicated the beginning of the infection? The course of the infection is then discussed. Have the symptoms of the infection been constant, have they waxed and waned, or has the patient steadily grown worse since the symptoms were first noted? Finally, the practitioner should determine the rapidity of progress of the infection. Has the infection process progressed rapidly over a few hours, or has it gradually increased in severity over several days to a week?
The next step is eliciting the patient’s symptoms. Infections are actually a severe inflammatory response, and the cardinal signs of inflammation are clinically easy to discern. These signs and symptoms are the Latin terms dolor (pain), tumor (swelling), calor (warmth), rubor (erythema, redness), and functio laesa (loss of function).
The most common complaint is pain. The patient should be asked where the pain actually started and how the pain has spread since it was first noted. The second sign is tumor (swelling). Swelling is a physical finding that is sometimes subtle and not obvious to the practitioner, although it is obvious to the patient. It is important that the dentist ask the patient to describe any area of swelling. The third characteristic of infection is calor (warmth). The patient should be asked whether the area has felt warm to the touch. Redness of the overlying area is the next characteristic to be evaluated. The patient should be asked if there has been or currently is any change in color, especially redness, over the area of the infection. Functio laesa should then be checked. When inquiring about this characteristic, the dentist should ask about trismus (difficulty in opening the mouth widely) and difficulty in chewing, swallowing (dysphagia), or breathing (dyspnea).
Finally, the dentist should ask how the patient feels in general. Patients who feel fatigued, feverish, weak, and sick are said to have malaise. Malaise usually indicates a generalized reaction to a moderate to severe infection (Fig. 15-6).
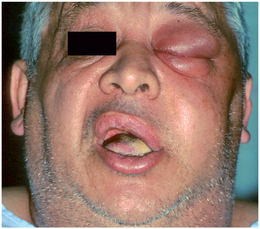
FIGURE 15-6 Patient with severe infection and elevated temperature, pulse rate, and respiratory rate. The patient feels sick and tired; he has a “toxic appearance.”(From Flynn TR: Atlas Oral Maxillofac Surg Clin North Am 8:79, 2000.)
In the next step the dentist inquires about treatment. The dentist should ask about previous professional treatment and self-treatment. Many patients doctor themselves with leftover antibiotics, hot soaks, and a variety of other home or herbal remedies. Occasionally, a dentist sees a patient who received treatment in an emergency room 2 or 3 days earlier and was referred to a dentist by the emergency room physician. The patient may have neglected to follow that advice until the infection became severe. Sometimes, the patient did not take the prescribed antibiotic because he or she could not afford to purchase it.
The patient’s complete medical history should be obtained in the usual manner by interview or by self-administered questionnaire with verbal follow-up of any positive findings.
Physical Examination
The first step in the physical examination is to obtain the patient’s vital signs, including temperature, blood pressure, pulse rate, and respiratory rate. The need for evaluation of temperature is obvious. Patients who have systemic involvement of infection have elevated temperatures. Patients with severe infections have temperatures elevated to 101° F or higher (greater than 38.3° C).
The patient’s pulse rate increases as the patient’s temperature increases. Pulse rates of up to 100 beats/min are not uncommon in patients with infections. If pulse rates increase to greater than 100 beats/min, the patient may have a severe infection and should be treated more aggressively.
The vital sign that varies the least with infection is the patient’s blood pressure. Only if the patient has significant pain and anxiety will there be an elevation in systolic blood pressure. However, severe septic shock results in hypotension.
Finally, the patient’s respiratory rate should be closely observed. One of the major considerations in odontogenic infections is the potential for partial or complete upper airway obstruction as a result of extension of the infection into the deep fascial spaces of the neck. As respirations are monitored, the dentist should carefully check to ensure that the upper airway is clear and that breathing is without difficulty. The normal respiratory rate is 14 to 16 breaths per minute. Patients with mild to moderate infections may have elevated respiratory rates greater than 18 breaths/min.
Patients who have normal vital signs with only a mild temperature elevation usually have a mild infection that can be readily treated. Patients who have abnormal vital signs with elevation of temperature, pulse rate, and respiratory rate are more likely to have serious infection and require more intensive therapy and evaluation by an oral and maxillofacial surgeon.
Once vital signs have been taken, attention should be turned to physical examination of the patient. The initial portion of the physical examination should be inspection of the patient’s general appearance. Patients who have more than a minor, localized infection have an appearance of fatigue, feverishness, and malaise. This is a “toxic appearance” (Fig. 15-6).
The patient’s head and neck should be carefully examined for the cardinal signs of infection, and the patient should be inspected for any evidence of swelling and overlying erythema. The patient should be asked to open the mouth widely, swallow, and take deep breaths so that the dentist can check for trismus, dysphagia, or dyspnea. These are ominous signs of a severe infection, and the patient should be referred immediately to an oral and maxillofacial surgeon or emergency room. A recent study of severe odontogenic infections requiring hospitalization found trismus (maximum interincisal opening less than 20 mm) in 73% of cases, dysphagia in 78%, and dyspnea in 14%.
Areas of swelling must be examined by palpation. The dentist should gently touch the area of swelling to check for tenderness, amount of local warmth or heat, and the consistency of the swelling. The consistency of the swelling may vary from very soft and almost normal through a firmer, fleshy swelling (described as having a doughy feeling) to an even firmer or hard swelling (described as feeling indurated). An indurated swelling has similar firmness to a tightened muscle. Another characteristic consistency is fluctuant. Fluctuance is the feeling of a fluid-filled balloon. Fluctuant swelling almost always indicates an accumulation of pus in the center of an indurated area.
The dentist then performs an intraoral examination to try to find the specific cause of the infection. There may be severely carious teeth, an obvious periodontal abscess, severe periodontal disease, combinations of caries and periodontal disease, or an infected fracture of a tooth or the entire jaw. The dentist should look and feel for areas of gingival swelling and fluctuance and for localized vestibular swellings or draining sinus tracts.
The next step is to perform a radiographic examination. This usually consists of the indicated periapical radiographs. Occasionally, however, extraoral radiographs, such as a panoramic radiograph, may be necessary because of limited mouth opening or other extenuating circumstances.
After the physical examination, the practitioner should begin to have a sense of the stage of the presenting infection. Very soft, mildly tender, edematous swellings indicate the inoculation stage, whereas an indurated swelling indicates the cellulitis stage (Fig. 15-7), and central fluctuance indicates an abscess (Fig. 15-8). Soft tissue infections in the inoculation stage may be cured by removal of the odontogenic cause with or without supportive antibiotics, and infections in the cellulitis or abscess stages require removal of the dental cause of infection plus incision and drainage and antibiotics.
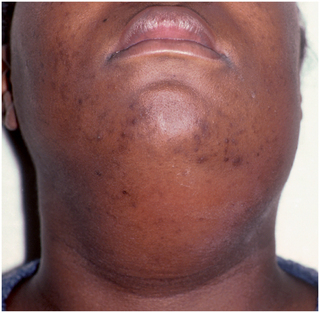
FIGURE 15-7 Cellulitis involving the submental and submandibular region. The cellulitis is indurated on palpation, and the patient is sick. (From Flynn TR: Atlas Oral Maxillofac Surg Clin North Am 8:79, 2000.)
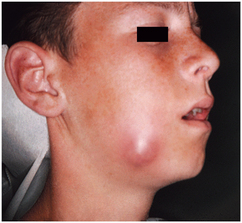
FIGURE 15-8 Well-localized abscess with fluctuance in the center and induration at its periphery. (Courtesy Richard G. Topazian, DDS.)
Distinctions between the inoculation, cellulitis, and abscess stages are typically in duration, pain, size, peripheral definition, and consistency on palpation, presence of purulence, infecting bacteria, and potential danger (Table 15-3). The duration of cellulitis is usually thought to be acute and is the most severe presentation of the infection. An abscess, however, is a sign of increasing host resistance to the infection. Cellulitis is usually described as more painful than an abscess, which may be the result of its acute onset and distention of tissues.
Edema, the hallmark of the inoculation stage, is typically diffuse and jellylike, with minimal tenderness to palpation. The size of a cellulitis is typically larger and more widespread than that of an abscess or edema. The periphery of a cellulitis is usually indistinct, with a diffuse border that makes it difficult to determine where the swelling begins or ends. The abscess usually has distinct and well-defined borders. Consistency to palpation is one of the primary distinctions among the stages of infection. When palpated, edema can be very soft or doughy; a severe cellulitis is almost always described as indurated or even as being “boardlike.” The severity of the cellulitis increases as its firmness to palpation increases. On palpation, an abscess feels fluctuant because it is a pus-filled cavity in the tissue. Thus an infection may appear innocuous in its early stages and extremely dangerous in its more advanced, indurated, rapidly spreading stages. A localized abscess is typically less dangerous, because it is more chronic and less aggressive.
The presence of pus usually indicates that the body has locally walled off the infection and that the local host resistance mechanisms are bringing the infection under control. In many clinical situations, the distinction between severe cellulitis and abscess may be difficult to make, especially if an abscess lies deeply within the soft tissue. In some patients an indurated cellulitis may have areas of abscess formation within it (see Chapter 16).
Severe infections occupying multiple deep fascial spaces may be in an early stage in one anatomic space, and in a more severe, rapidly progressive stage in another fascial space. A severe, deeply invading infection may pass through ever deeper anatomic spaces in a predictable fashion like a fire in a house, where there may be smoke in one room, intense heat in another room, and open flames near the source of the fire. The goal of therapy in such infections is to abort the spread of the infection in all involved anatomic spaces. These infections are discussed in detail in Chapter 16.
In summary, edema represents the earliest, inoculation stage of infection that is most easily treated. A cellulitis is an acute, painful infection with more swelling and diffuse borders. Cellulitis has a hard consistency on palpation and contains no pus. Cellulitis may be a rapidly spreading process in serious infections. An acute abscess is a more mature infection with more localized pain, less swelling, and well-circumscribed borders. The abscess is fluctuant on palpation because it is a pus-filled tissue cavity. A chronic abscess is usually slow growing and less serious than a cellulitis, especially if it has drained spontaneously to the external environment.
Principle 2: Evaluate State of Patient’s Host Defense Mechanisms
Part of the evaluation of the patient’s medical history is designed to estimate the patient’s ability to defend against infection. Several disease states and several types of drug use may compromise this ability. Compromised patients are more likely to have infections, and these infections often become serious more rapidly. Therefore, to manage their infections more effectively, it is important to be able to discern those patients who may have compromised host defenses.
Medical Conditions That Compromise Host Defenses
Delineation of those medical conditions that may result in decreased host defenses is important. These compromises allow more bacteria to enter the tissues or to be more active, or they prevent the humoral or cellular defenses from exerting their full effect. Several specific conditions may compromise patients’ defenses (Box 15-1).
Uncontrolled metabolic diseases—such as uncontrolled diabetes, end-stage renal disease with uremia, and severe alcoholism with malnutrition—result in decreased function of leukocytes, including decreased chemotaxis, phagocytosis, and bacterial killing. Of these metabolic diseases, poorly controlled type 1 (insulin-dependent) and type 2 (non–insulin-dependent) diabetes are the most common immunocompromising diseases, and worsening control of hyperglycemia correlates directly with lowered resistance to all types of infections.
The second major group of immunocompromising diseases is those that interfere with host defense mechanisms, such as leukemias, lymphomas, and many types of cancer. These diseases result in decreased white cell function and decreased antibody synthesis and production.
Human immunodeficiency virus (HIV) infection attacks the T lymphocytes, affecting resistance to viruses and other intracellular pathogens. Fortunately, odontogenic infections are caused largely by extracellular pathogens (bacteria). Therefore, HIV-seropositive individuals are able to combat odontogenic infections fairly well until acquired immunodeficiency syndrome has progressed into advanced stages, when the B lymphocytes are also severely impaired. Nonetheless, care for the HIV-seropositive patient with an odontogenic infection is usually more intensive than for the otherwise normal patient.
Pharmaceuticals That Compromise Host Defenses
Patients taking certain drugs are also immunologically compromised. Cancer chemotherapeutic agents can decrease circulating white cell counts to low levels, commonly less than 1000 cells/mL. When this occurs, patients are unable to defend themselves effectively against bacterial invasion. Patients receiving immunosuppressive therapy, usually for organ transplantation or autoimmune diseases, are compromised. The common drugs in these categories are cyclosporin, corticosteroids, and azathioprine (Imuran). These drugs decrease T and B lymphocyte function and immunoglobulin production. Thus, patients taking these medications are more likely to have severe infections. The immunosuppressive effects of some cancer chemotherapeutic agents can last for up to a year after the completion of therapy.
In summary, when evaluating a patient whose chief complaint may be an infection, the patient’s medical history should be carefully reviewed for the presence of diabetes, severe renal disease, alcoholism with malnutrition, leukemias and lymphomas, cancer chemotherapy, and immunosuppressive therapy of any kind. When the patient’s history includes any of these, the patient with an infection must be treated much more vigorously because the infection may spread more rapidly. Referral to an oral-maxillofacial surgeon for early and aggressive surgery to remove the cause and initiate more intensive parenteral antibiotic therapy must be considered.
Additionally, when a patient with a history of one of these problems is seen for routine oral surgical procedures, it may be necessary to provide the patient with prophylactic antibiotic therapy to decrease the risk of postoperative wound infection. Use of the guidelines and regimens for prevention of endocarditis published by the American Heart Association and American Dental Association is a practical way to manage this problem.
Principle 3: Determine Whether Patient Should Be Treated by General Dentist or Oral and Maxillofacial Surgeon
Most odontogenic infections seen by the dentist can be managed with the expectation of rapid resolution. Odontogenic infections, when treated with minor surgical procedures and antibiotics, if indicated, almost always respond rapidly. However, some odontogenic infections are potentially life-threatening and require aggressive medical and surgical management. In these special situations, early recognition of the potential severity is essential, and these patients should be referred to an oral-maxillofacial surgeon for definitive management. As the specialist with the best training and experience in the management of severe odontogenic infections, the oral and maxillofacial surgeon can optimize the outcomes and minimize the complications of these infections. For some patients, hospitalization is required, whereas others can be managed as outpatients.
When a patient with an odontogenic infection comes for treatment, the dentist must have a set of criteria by which to judge the seriousness of the infection (Box 15-2). If some or all of these criteria are met, immediate referral must be considered.
Three main criteria indicate immediate referral to a hospital emergency room because of an impending threat to the airway. The first is a history of a rapidly progressing infection. This means that the infection began 1 or 2 days before the interview and is growing rapidly worse, with increasing swelling, pain, and other associated signs and symptoms. This type of odontogenic infection may cause swelling in deep fascial spaces of the neck, which can compress and deviate the airway. The second criterion is difficulty in breathing (dyspnea). Patients who have severe swelling of the soft tissue of the upper airway as the result of infection may have difficulty maintaining a patent airway. In these situations the patient often will not lie down, has muffled or distorted speech, and is obviously distressed with breathing difficulties. This patient should be referred directly to an emergency room because immediate surgical attention may be necessary to maintain an intact airway. The third urgent criterion is difficulty in swallowing (dysphagia). Patients with acutely progressive deep fascial space infections may also have difficulty swallowing their saliva. This is an ominous sign because the inability to control one’s secretions frequently indicates a narrowing of the oropharynx and potential for acute airway obstruction. This patient should also be transported to the hospital emergency room because surgical intervention or intubation may be required for airway maintenance. Definitive treatment of the infection can follow once the airway is secure.
Several other criteria should indicate referral to an oral and maxillofacial surgeon. Patients who have involvement of extraoral fascial spaces, such as buccal space infections or submandibular space infections, may require extraoral surgical incision and drainage (I&D), as well as hospitalization. Next, although infection frequently causes an elevated temperature, a temperature higher than 101° F indicates a greater likelihood of severe infection, and this patient should be referred. Another important sign is trismus, which is the inability to open the mouth widely. In odontogenic infections, trismus results from the involvement of the muscles of mastication in the inflammatory process. Mild trismus can be defined as a maximum interincisal opening between 20 and 30 mm; moderate trismus is between 10 and 20 mm; and severe trismus is an interincisal opening of less than 10 mm.
Moderate or severe trismus may be an indication of the spread of the infection into the masticator space (surrounding the muscles of mastication) or worse, the lateral pharyngeal and/or retropharyngeal spaces surrounding the pharynx and trachea. In this situation, referral to a specialist is necessary for evaluation of upper airway patency. In addition, systemic involvement of an odontogenic infection is an indication for referral. Patients with systemic involvement have a typical toxic facial appearance: glazed eyes, open mouth, and a dehydrated, sick appearance. When this is seen, the patient is usually fatigued, has a substantial amount of pain, has an elevated temperature, and is dehydrated. Finally, if the patient has compromised host defenses, hospitalization is likely to be required. An oral and maxillofacial surgeon is qualified to admit the patient expeditiously to the hospital for definitive care.
In summary, within the first few minutes of the initial patient encounter, the aforementioned three principles allow the dentist to assess the severity of the infection, evaluate host defenses, and decide expeditiously on the best setting for the patient’s care. In doubtful situations, it is always best to err on the side of caution and refer the patient for a higher level of care. Appropriate decision making at this stage can prevent serious morbidity and the occasional mortality that still occurs because of odontogenic infections.
Principle 4: Treat Infection Surgically
The primary principle of management of odontogenic infections is to perform surgical drainage and to remove the cause of the infection. Surgical treatment may range from something as simple as an endodontic access opening and extirpation of the necrotic tooth pulp to treatment as complex as the wide incision of soft tissue in the submandibular and neck regions for a severe infection or even open drainage of the mediastinum.
The primary goal in surgical management of infection is to remove the cause of the infection, which is most commonly a necrotic pulp or deep periodontal pocket. A secondary goal is to provide drainage of accumulated pus and necrotic debris.
When a patient has a typical odontogenic infection, the most likely appearance is a carious tooth with a periapical radiolucency and a small vestibular abscess. With this presentation, the dentist has the following surgical options: endodontic treatment or extraction, with or without I&D. If the tooth is not to be extracted, it should be opened and its pulp removed, which results in elimination of the cause and limited drainage through the apical foramen of the tooth. If the tooth cannot be salvaged or is not restorable, it should be extracted as soon as possible.
Extraction provides removal of the cause of the infection and drainage of the accumulated periapical pus and debris. In addition to the endodontic procedure or extraction of the tooth, an I&D procedure may be required for an infection that has spread beyond the periapical region. Incision of the abscess or cellulitis allows removal of the accumulated pus and bacteria from the underlying tissue. Evacuation of the abscess cavity dramatically decreases the load of bacteria and necrotic debris. Evacuation also reduces the hydrostatic pressure in the region by decompressing the tissues, which improves the local blood supply and increases the delivery of host defenses to the infected area. I&D of a cellulitis serves to abort the spread of the infection into deeper anatomic spaces. The I&D procedure includes the insertion of a drain to prevent premature closure of the mucosal incision, which could allow the abscess cavity to reform. It is important to remember that the surgical goal is to achieve adequate drainage. If endodontic opening of the tooth does not provide adequate drainage of the abscess, it is essential to perform an I&D.
The technique for I&D of a vestibular abscess or cellulitis is straightforward (Fig. 15-9). The preferred site for intraoral incision is directly over the site of maximum swelling and inflammation. However, it is important to avoid incising across a frenum or the path of the mental nerve in the lower premolar region. When I&D procedures are performed extraorally, a more complex set of criteria must be met when selecting a site for the incision. Once the area of incision has been selected, a method of pain control must be used. Regional nerve block anesthesia is preferred when it can be achieved by injecting in an area away from the site of the incision. Alternatively, infiltration of local anesthetic solution into and around the area to be drained can be performed. Once the local anesthetic needle has been used in an infected site, however, it should not be reused in an uninfected area.
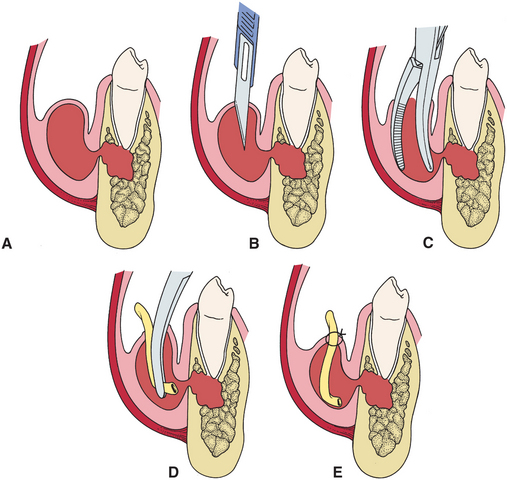
FIGURE 15-9 A, Periapical infection of lower premolar extends through buccal plate and creates sizable vestibular abscess. B, Abscess is incised with No. 11 blade. C, Beaks of hemostat are inserted through incision and opened so that beaks spread to break up any loculations of pus that may exist in abscessed tissue. D, Small drain is inserted to depths of abscess cavity with hemostat. E, Drain is sutured into place with single black silk suture.
Before the actual incision of the abscess is performed, one must consider obtaining a specimen for culture and sensitivity (C&S) testing. If the decision is made to perform a culture, culture is carried out as the initial portion of the surgery (Box 15-3). After the site of surgery has been anesthetized, the surface mucosa is disinfected with a solution such as povidone-iodine (Betadine) and dried with sterile gauze. A large-gauge needle, usually 18 gauge, is used for specimen collection. A small syringe, usually 3 mL, is adequate. The needle is then inserted into the abscess or cellulitis, and 1 or 2 mL of pus or tissue fluid is aspirated. The specimen may contain only tissue fluid and blood instead of pus, yet it still most often provides sufficient bacteria for an accurate culture. The specimen is then inoculated directly into aerobic and anaerobic culturettes, which are sterile tubes containing a swab and bacterial transport medium. Culturettes and specimen bottles that are appropriate for aerobes and anaerobes are also available. All culturettes and specimen bottles have a limited shelf-life, so the expiration date should be checked before use. Care must be taken to keep the anaerobic culture tube upright while open to prevent the escape of the carbon dioxide that is needed to maintain the tube’s anaerobic environment. As discussed before, anaerobic bacteria are almost always present in odontogenic infections, and therefore care must be taken to provide the laboratory the best opportunity to find them. The surgeon should request a Gram stain, aerobic and anaerobic cultures, and antibiotic sensitivity testing in writing.
Once the culture specimen is obtained, an incision is made with a scalpel blade just through the mucosa and submucosa into the abscess cavity (Fig. 15-9). The incision should be short, usually no more than 1 cm in length. Once the incision is completed, a closed curved hemostat is inserted through the incision into the abscess cavity. The hemostat is then opened in several directions to break up any small loculations or cavities of pus that have not been opened by the initial incision. Any pus or tissue fluid that drains out during this time should be aspirated into the suction and should not be allowed to drain into the patient’s mouth. If, however, an adequate specimen has not been obtained by aspiration, aerobic and anaerobic culturette swabs may be carefully introduced into the wound without contaminating them on the surface mucosa. Adequate specimens can be obtained in this manner even if obvious pus is not present in the wound.
Once all areas of the abscess cavity have been opened and all pus has been removed, a small drain is inserted to maintain the opening. The most commonly used drain for intraoral abscesses is a ¼-inch sterile Penrose drain. A frequently used substitute is a small strip of sterilized rubber dam or surgical glove material. A piece of drain of adequate length to reach the depth of the abscess cavity is prepared and inserted into the cavity using a hemostat. The drain is then sutured to one edge of the incision with a nonresorbable suture. The suture should be placed in viable tissue to prevent loss of the drain because it might tear through friable, nonvital tissue.
The drain should remain in place until all the drainage has stopped, usually 2 to 5 days. Removal is done by simply cutting the suture and slipping the drain from the wound.
Inoculation stage infections that initially appear as edema with soft, doughy, diffuse, mildly tender swelling do not typically require I&D. Surgical management of infections of this type is limited to removal of the necrotic pulp or removal of the involved tooth. Adjunctive antibiotic therapy may be used, according to the following indications.
It is critical to keep in mind that the primary method for treating odontogenic infections is to perform surgery to remove the source of the infection and drain anatomic spaces affected by indurated cellulitis or abscess. Whenever an abscess or cellulitis is diagnosed, the surgeon must drain it. Failure to do so will result in worsening of the infection and failure of the infection to resolve, even if antibiotics are given. Even if the tooth cannot be opened or extracted, an I&D should be done.
Some clinicians believe that I&D of a cellulitis may allow the infection to spread into deeper tissues by opening them up to infecting bacteria. The experience of others has shown that establishing drainage for a cellulitis serves to abort the spread of infection. In a prospective study of 37 patients hospitalized for severe odontogenic infection, approximately 25% of the cases had drainage in the cellulitis stage.* On multivariate analysis the stage of infection had no significant effect on complications or the length of hospital stay.
The algorithm presented in Figure 15-10 is a decision pathway for the management of uncomplicated odontogenic infections, which follows the principles described in this chapter. After deciding to treat the patient in the outpatient setting, the dentist should determine whether the infection is in the inoculation (edema) stage or if it has progressed to a cellulitis or abscess. In the inoculation stage the dental cause of the infection should be treated surgically. An antibiotic may also hasten resolution of the infection at this stage. If the infection has progressed to cellulitis or abscess, then an I&D and the appropriate dental therapy should be performed. Sometimes, a separate I&D is not necessary if the abscess cavity drains completely through an extraction socket. Antibiotic therapy should be used when complete abscess drainage cannot be achieved by extraction alone.
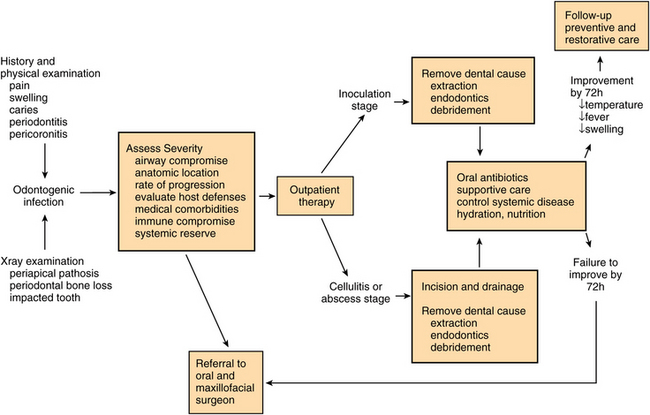
FIGURE 15-10 Management algorithm for odontogenic infections. (Adapted from Flynn TR: Deep fascial space infections. In Laskin DM, Abubaker AO, editors. Decision making in oral and maxillofacial surgery, Chicago, 2007, Quintessence.)
The criteria for referral to an oral and maxillofacial surgeon are listed in Box 15-2. In summary, when there is potential or actual airway compromise, infection spreading beyond the alveolar process, medical or immune system compromise, or signs of systemic involvement, immediate referral to an oral and maxillofacial surgeon or, in life-threatening cases, referral to a hospital emergency room is indicated.
Principle 5: Support Patient Medically
A patient’s systemic resistance to infection is perhaps the most important determinant of a good outcome. Host systemic resistance must be considered in three areas: immune system compromise, control of systemic diseases, and physiologic reserves.
Diseases that compromise the immune system are listed in Box 15-1. Odontogenic infections that occur in patients with immune system compromise should be treated by a specialist. Often, hospitalization and medical consultation are required. The treatment team selects therapies designed to enhance the immune response, combat the infection medically with bactericidal antibiotics, and optimize surgical management of the infection.
Many systemic diseases also reduce the ability of the patient to resist infection and to undergo treatment. In diabetes, for example, the control of blood sugar is directly correlated with resistance to infection. Host response to a significant infection increases the blood sugar levels and therefore the insulin requirements of a diabetic person. Moreover, cardiovascular diseases decrease the ability of the host to respond to the stress of infection and surgery. Therefore, optimizing control of hypertension, cardiac dysrhythmias, and atherosclerotic heart disease is an essential part of the comprehensive management of odontogenic infections. Medications may also affect the treatment of odontogenic infections. For example, the patient receiving anticoagulant therapy with warfarin (Coumadin) may need reversal of the anticoagulation before surgery can be safely performed. Patients with systemic conditions, especially of the immune, cardiovascular, respiratory, hematologic, and metabolic systems, often need sophisticated medical support from a team of specialists.
Even patients without medically compromising diseases may have reduced or altered physiologic reserves to draw upon as they combat an odontogenic infection. Children, for example, are particularly susceptible to dehydration and high fevers. Elderly patients, however, have a reduced ability to mount a fever, and yet they are susceptible to dehydration. Fever increases daily fluid requirements in the adult by about 800 mL per degree Fahrenheit per day, and daily caloric requirements by 3% to 5% per degree per day. However, temperatures up to 103° F may be beneficial in combating infections. Therefore, judicious control of highly elevated fever along with active hydration and nutritional support are important components of the management of odontogenic infections.
Because of pain and/or difficulty swallowing, patients frequently have not had adequate fluid intake, nutritional intake, or rest. During the immediate posttreatment period, patients should be encouraged to drink sufficient water or juice to require them to need to urinate regularly and to take high-calorie nutritional supplements. Patients should also be prescribed adequate analgesics for relief of pain so that they can rest. Patients should be given careful postoperative instructions and should be able to manage their self-care. The clinician is responsible to make sure patients are provided careful instructions about these important issues.
Principle 6: Choose and Prescribe Appropriate Antibiotic
Choosing the appropriate antibiotic for treating an odontogenic infection must be done carefully. When all factors are weighed, the clinician may decide that no antibiotic is necessary at all, whereas in other situations, broad-spectrum or even combination antibiotic therapy may be indicated. A variety of factors must be considered when choosing an antibiotic from the nearly 70 antibiotics currently available. Antibiotics must be viewed as a double-edged sword. Although appropriate use may result in dramatic resolution and cure of patients with infections, misuse of antibiotics provides little benefit to offset the associated risks and expense of antibiotic administration. Recent studies have shown that even the administration of oral penicillin promotes the growth of penicillin-resistant organisms in the oropharyngeal flora of the patient, the patient’s family, and even the patient’s co-workers or classmates. Therefore the following guidelines are recommended for consideration when choosing a specific antibiotic.
Determine the Need for Antibiotic Administration
A common misconception is that all infections, by definition, require antibiotic administration. This is not necessarily the case. In some situations, antibiotics are not useful and may be contraindicated. In making this determination, three factors must be considered: The first factor is the seriousness of the infection when the patient comes to the dentist. If the infection has caused swelling, has progressed rapidly, or is a diffuse cellulitis, the evidence supports the use of antibiotics in addition to surgical therapy. The second factor is whether adequate surgical treatment can be achieved. In many situations, extraction of the offending tooth may result in rapid resolution of the infection. Contrary to widely held opinion, extraction of a tooth in the presence of infection does not promote the spread of infection. Several studies have shown that removal of a tooth in the presence of infection hastens its resolution and minimizes the complications of the infection, such as time out of work, hospitalization, and the need for extraoral I&D. Therefore, prompt removal of the offending tooth (teeth) in the presence of infection is to be encouraged; a prior period of antibiotic therapy is not necessary. However, when the appropriate surgery cannot be immediately performed, antibiotics may be useful to retard the progression of infection. The third consideration is the state of the patient’s host defenses. A young, healthy patient may be able to mobilize host defenses and may not need antibiotic therapy for resolution of a minor infection. However, patients who have any type of decreased host resistance, such as those with severe metabolic disease or those receiving cancer chemotherapy, may require vigorous antibiotic therapy even for minor infections.
When these three factors are balanced, several definite indications for antibiotic use in dentistry become clear (Box 15-4). The first and most common indication is the presence of an acute-onset infection with diffuse swelling and moderate to severe pain. This infection is usually in the cellulitis stage; and with appropriate antibiotic therapy, I&D, and treatment of the offending tooth, rapid resolution is expected. The second indication is almost any type of infection in a patient who is medically compromised. Such patients who have infections of any severity should be considered candidates for antibiotic administration. The third indication for antibiotic therapy is the presence of an infection that has progressed to involvement of the deep fascial spaces. In these situations, the infection is aggressive enough to have spread beyond the alveolar process of the jaws, indicating that the host defenses are inadequate to contain the infection. The fourth indication is severe pericoronitis, with temperatures higher than 100° F, trismus, and swelling of the lateral aspect of the face, which occurs most commonly around impacted mandibular third molars. Finally, the patient who has osteomyelitis requires antibiotic therapy in addition to surgery to achieve resolution of the infection.
Based on the same three criteria, antibiotic therapy is not indicated and is even contraindicated in other situations (Box 15-5). The first is a minor, chronic, well-localized abscess for which extraction of the offending tooth results in complete evacuation of a periapical abscess, assuming that the patient’s host defenses are intact and that the patient has no other compromising conditions. An example of this is the patient without symptoms who may require the extraction of teeth with chronic periapical abscesses, a draining parulis, or severe periodontitis. A second, albeit similar, contraindication is a well-localized dentoalveolar abscess, with little or no facial swelling. In these situations, endodontic therapy can be performed or the tooth can be extracted along with I&D of the swelling on the alveolar process, which will result in rapid resolution in most patients. Third is a localized alveolar osteitis, or dry socket. Treatment of the dry socket is primarily palliative, and dry socket is not treated as an infection. Although bacterial pathogens may play a role in dry socket, the clinical problem of dry socket (alveolar osteitis) is self-limiting and appears to be due to premature fibrinolysis (loss of the blood clot). Fourth, patients who have mild pericoronitis with minor gingival edema and mild pain do not require antibiotics for resolution of their infection. Irrigation with hydrogen peroxide or chlorhexidine, plus extraction of the partially erupted tooth will result in resolution. Antibiotics should not prescribed simply because a patient demands them for a routine toothache or for dental extractions in a patient without immune system compromise.
In summary, antibiotics should be used when clear evidence exists of bacterial invasion into deeper tissues that is greater than the host defenses can overcome. Patients who have an impaired ability to defend themselves against infection and patients who have infections that are not immediately amenable to surgical treatment should be considered for antibiotic therapy. Antibiotics should not be used when no evidence of bacterial invasion of deeper tissues is found. Antibiotics do not hasten wound healing and do not provide any benefit for nonbacterial (e.g., viral) conditions. Patients who have inflammatory pulpitis have severe pain, but the pain results from the local inflammatory reaction within the pulp, not from bacterial infection spreading into deeper tissues. These patients should not routinely be given antibiotic therapy.
Use Empirical Therapy Routinely
Odontogenic infections are caused by a highly predictable group of bacteria. Additionally, the antibiotic sensitivity of these organisms is well known and consistent. As a result, the use of C&S testing is not necessary for routine odontogenic infections. The bacteria that cause odontogenic infections are overwhelmingly the facultative oral streptococci, anaerobic streptococci including peptostreptococci, and Prevotella and Fusobacterium species. Other species of bacteria may also be cultured from these infections, but they appear to be opportunistic rather than causative bacteria. Fortunately, the antibiotic susceptibility of the causative bacteria is fairly predictable. The orally administered antibiotics that are effective against odontogenic infections include penicillin, amoxicillin, clindamycin, azithromycin, metronidazole, and moxifloxacin (Box 15-6).
These antibiotics are effective against aerobic and facultative streptococci (except metronidazole) and oral anaerobes. Metronidazole is effective only against obligate anaerobic bacteria, yet the effectiveness of this antibiotic class in odontogenic infections has been shown in a prospective study. Several important variations can be found among these antibiotics. (See Appendix VI for detailed description of the various antibiotics.)
Because the microbiology and antibiotic sensitivity of the oral pathogens is well known, it is a reasonable therapeutic maneuver to use one of these antibiotics empirically; that is, to give the antibiotic with the assumption that an appropriate drug is being given. The drug of choice is usually penicillin. Alternative drugs for use in the penicillin-allergic patient are clindamycin and azithromycin. Metronidazole is useful only against anaerobic bacteria and should be reserved for a situation in which only anaerobic bacteria are suspected (or in combination with an antibiotic that has antiaerobic activity, such as penicillin).
Clearly, patients frequently fail to take the medication in the way in which it was prescribed. In fact, Socrates in 400 bc cautioned physicians to be aware that patients will lie about taking the medications prescribed.
Reliable data exist from many studies that demonstrate that patient compliance decreases with increasing number of pills per day. When it is necessary to take the prescription 1 time daily, patient compliance is approximately 80%. However, when it is necessary to take the pill 2 times daily, compliance decreases to 69%, and drops even further to 35% for 4 times daily. Therefore, if the clinician has a reasonable choice, antibiotics should be prescribed that can be given the fewest times daily to improve patient compliance.
For example, amoxicillin and clindamycin are usually given 3 times daily instead of 4 times daily (as is penicillin). Azithromycin is given twice a day, instead of 4 times daily (as is erythromycin). Moxifloxacin is given once daily. Thus when other important factors, such as antibacterial effectiveness, side effects, drug interactions, and cost are reasonably equal, a drug that can be given less frequently is preferable. As is discussed subsequently, however, there are significant differences among these antibiotics in their side effects, drug interactions, and cost.
Routine C&S testing is not cost-effective for the routine odontogenic infection. However, in some cases the dentist should seriously consider sending a specimen for C&S testing (Box 15-3). The first is the rapid onset of severe infection and its rapid spread. Delay in bacterial identification may have disastrous consequences in this situation. The second case is postoperative infection. If a patient had no signs of infection when the original surgery was done but returns 3 or 4 days later with an infection, the possibility of nonindigenous bacteria causing the infection is increased. Precise identification of the causative bacteria early on may facilitate the timely administration of the appropriate antibiotic and thus the resolution of the infection. The third case is an infection that is not resolving. In these situations the clinician should make every effort to obtain a specimen of pus or tissue fluid for C&S testing. The fourth case is a recurrent infection. When the initial infectious problem has resolved and there has been an infection-free period of 2 days to 2 weeks but a second infection occurs, the probability is high that the infection is caused by bacteria that are resistant to the previously used antibiotic. The fifth case is the patient who has compromised host defenses. Patients with immune system compromise have a propensity to harbor unusual pathogens that can be identified by C&S testing.
In the foreseeable future, conventional C&S testing may be replaced by molecular methods that are currently used in research. Bacteria can be identified even after their demise by their genetic material, using the polymerase chain reaction to amplify tiny amounts of bacterial DNA and RNA. Single-stranded nucleic acids from the unknown sample can be hybridized to single-stranded genes from known species, resulting in a positive identification of the infecting bacteria. These methods have identified the involvement of a large number of unculturable pathogens in odontogenic infections the presence of which was only suspected in the past. In the future, these methods may be able to detect antibiotic-resistance genes directly as well, resulting in prompt diagnosis of the infecting species and their antibiotic sensitivity patterns.
Use the Narrowest-Spectrum Antibiotic
When an antibiotic is administered to a patient, most susceptible bacteria are killed. If the antibiotic is a narrow-spectrum antibiotic, it kills bacteria of a narrow range. For example, penicillin will kill streptococci and oral anaerobic bacteria but will have little effect on the staphylococci of the skin and almost no effect on gastrointestinal tract bacteria. As a result, penicillin has little or no effect on the gastrointestinal tract and does not expose a multitude of other bacteria to the opportunity to develop resistance. By contrast, drugs such as amoxicillin-clavulanate (Augmentin) are broad-spectrum antibiotics, inhibiting not only the streptococci and oral anaerobes but also a variety of staphylococci and enteric gram-negative rods. Thus when this antibiotic is given, it has an effect on skin and gastrointestinal bacteria that may result in problems caused by alterations of host flora and overgrowth of resistant bacteria. In addition, broad-spectrum antibiotics provide a multitude of bacteria with the opportunity to develop resistance, which can be communicated by our patients to their families, co-workers, and entire communities.
The American Dental Association’s (ADA’s) Council on Scientific Affairs has issued guidelines, based on a review of the available scientific literature, which recommends that dentists use only narrow-spectrum antibiotics for simple infections. Narrow-spectrum and broad-spectrum antibiotics are listed in Box 15-7. Broad-spectrum antibiotics may be used for complex infections, which are not defined in the ADA advisory statement. Nonetheless, a simple odontogenic infection can be defined as one involving only the alveolar process or the oral vestibule, in its first course of treatment, and in an immunocompetent individual. A complex infection may be defined as one that has spread beyond the alveolar process and oral vestibule, with prior treatment failures, or in an immunocompromised patient. The characteristics of simple and complex odontogenic infections are differentiated in Box 15-8.
In summary, antibiotics that have narrow-spectrum activity against the causative organisms are just as effective as antibiotics that have broad-spectrum activity, without the problems of upsetting normal host microflora populations and increasing the chance of bacterial resistance.
Use the Antibiotic with the Lowest Incidence of Toxicity and Side Effects
Most antibiotics have a variety of toxicities and side effects that limit their usefulness. These range from mild to so severe that the antibiotic cannot be used in clinical practice. The older antibiotics usually used for odontogenic infections have a surprisingly low incidence of toxicity-related problems. The newer antibiotics, however, can have significant toxicities and drug interactions. Therefore, it is becoming increasingly important for the clinician to understand the toxicities, side effects, and drug interactions of the drugs he or she may prescribe.
Allergy is the major side effect of penicillin. Approximately 2% or 3% of the total population is allergic to penicillin. Patients who have allergic reactions to penicillin, as exhibited by hives, itching, or wheezing, should not be given penicillin again. Penicillin does not have other major side effects or toxicities in the normal dose range used by dentists.
Likewise, azithromycin and clindamycin have a low incidence of toxicity and side effects. Clindamycin may cause a severe diarrhea, called pseudomembranous colitis. Several other drugs, such as ampicillin and the oral cephalosporins, also cause this problem. However, with clindamycin and other antibiotics, this problem is usually confined to severely ill and debilitated patients and is rare in other patients. The elimination of much of the anaerobic gut flora allows the overgrowth of an antibiotic-resistant bacterium, Clostridium difficile. This bacterium produces toxins that injure the gut wall, which results in colitis. Patients who take clindamycin, amoxicillin, or cephalosporins should be warned of the possibility of profuse watery diarrhea and should be told to contact their prescribing dentist if it occurs.
Among the new members of the macrolide (erythromycin) family, azithromycin has the best combination of effectiveness, low toxicity, and infrequent drug interactions. Erythromycin is no longer considered effective against the oral pathogens, and it shares with clarithromycin a propensity to cause drug interactions involving the liver microsomal enzyme system.
Moxifloxacin is a new member of the fluoroquinolone class of antibiotics that has much better effectiveness against the oral pathogens than older members of this class. However, it has significant toxicities, including muscle weakness and mental clouding, and serious, potentially fatal drug interactions with many of the commonly used cardiovascular drugs. Moxifloxacin is also contraindicated in children and pregnant females. As a new antibiotic, moxifloxacin is expensive. Moxifloxacin should be reserved for use by specialists treating severe, recalcitrant infections for which no other effective drug is available.
The oral cephalosporins, such as cephalexin and cefadroxil, have lost much of their effectiveness in odontogenic infections. These antibiotics are no longer commonly used for odontogenic infections, even though they are associated with only mild toxicity problems. As with penicillin, the cephalosporins may cause allergic reactions. Cephalosporins should be given cautiously to patients with penicillin allergies because these patients may also be allergic to the cephalosporins. Patients who have experienced an anaphylactic type of reaction to penicillin should not be given a cephalosporin because of increased chance for that life-threatening event to recur.
The tetracyclines, like the cephalosporins, are no longer considered useful for odontogenic infections, except when they are used topically in very high local concentrations, such as when they are inserted into periodontal pockets. They have minor toxicities for most patients (i.e., the commonly encountered gastrointestinal problems of nausea, abdominal cramping, and diarrhea). Some patients may have a photosensitivity while they are taking this drug systemically and should be warned to stay out of the sun. Finally, tetracyclines may produce tooth discoloration if given to patients who are pregnant or who are in the tooth development stages of their lives (under 12 years of age). This discoloration is the result of chelation of the tetracycline to calcium, which results in incorporation of the tetracycline into developing teeth.
Metronidazole has mild toxicities, the most prominent being the typical gastrointestinal disturbances discussed previously. The drug may also produce a disulfiram effect; that is, the patient taking metronidazole who also drinks ethanol may experience sudden, violent abdominal cramping and vomiting.
Use a Bactericidal Antibiotic, If Possible
Antibiotics may kill bacteria (i.e., bactericidal antibiotics) or interfere with their reproduction (i.e., bacteriostatic antibiotics). Bactericidal antibiotics usually interfere with cell wall production in newly forming, growing bacteria. The resultant defective cell wall is not able to withstand the osmotic pressure differential between the cytoplasm and the environment, and the bacteria virtually explode. The antibiotic actually kills the bacteria, whereas the white blood cells, complement, and antibodies of the host play a less important role in fighting the bacteria.
Bacteriostatic antibiotics interfere with bacterial reproduction and growth. This slowing of bacterial reproduction allows the host defenses to move into the area of infection, phagocytize the existing bacteria, and kill them. Bacteriostatic antibiotics require reasonably intact host defenses. This type of antibiotic should be avoided in patients who have compromised host defense systems.
For patients with compromised host defenses, bactericidal antibiotics should be the drug of choice. For example, the bactericidal antibiotic penicillin would be preferred over the bacteriostatic antibiotic azithromycin in a patient who is receiving cancer chemotherapy.
Be Aware of the Cost of Antibiotics
Antibiotics vary widely in their cost to patients. Newer drugs tend to be more expensive, whereas older drugs, which are made by a variety of companies, tend to be less expensive. Drugs prescribed generically also tend to be less expensive than their brand-name counterparts. Generic prescriptions for newer drugs are not available. When other factors are equal, the clinician should prescribe the less expensive antibiotic. Table 15-4 provides a cost comparison among commonly used antibiotics.
TABLE 15-4
Cost Comparison of Orally Administered Antibiotics

Usual doses and intervals are for moderate infections and are not to be considered prescriptive. Penicillin cost ratio = Retail cost of antibiotic for 1 week/Retail cost of penicillin V for 1 week.
*Source: Gilbert DN, Moellering RC Jr, Eliopoulos GM, Sande MA: The Sanford guide to antimicrobial therapy 2006, ed 36, Sperryville, Va, 2006, Antimicrobial Therapy. Prices are wholesale cost per dose, generic when available.
†Retail cost/1 Week = Retail price charged for a 1-week prescription at a large pharmacy chain in the Boston region. Courtesy Gabriela Hoffens, CPHT.
‡Pharmacy cost per pill from Mosby’s drug consult 2006, St Louis, 2006, Mosby.
Summary
Antibiotics should be used to assist the dentist in treating patients with infections that are spreading beyond the alveolar processes of the jaws and to prevent endocarditis or infection of prosthetic-implanted devices arising from bacteremia induced by dental manipulations. Surgical treatment of the infection remains the primary method of treatment in most patients; antibiotic therapy plays an adjunctive role. Antibiotics are especially important in patients who have infections that are spreading beyond the alveolar process and in patients who have compromise of their host defense mechanisms. When antibiotic therapy is to be used a simple odontogenic infection, empiric antibiotic therapy with a narrow-spectrum antibiotic is recommended because the microbiology of odontogenic infections is well known and usually consistent from patient to patient. The antibiotic of choice for odontogenic infections is still penicillin. Penicillin has been shown to be as effective as other antibiotics in several prospective studies. Penicillin is bactericidal; has a narrow spectrum that includes streptococci and the oral anaerobes, which are responsible for odontogenic infections; has low toxicity; and is inexpensive.
Although about 25% of Prevotella strains are resistant to penicillin, when used in conjunction with adequate surgery, penicillin almost always results in cure. Amoxicillin and amoxicillin/clavulanate (Augmentin) are broad-spectrum penicillins that should be reserved for complex infections. Amoxicillin may be used for prophylaxis of endocarditis and late prosthetic joint infections as per the formal guidelines of the ADA in conjunction with the American Heart Association and the American Academy of Orthopaedic Surgeons. An alternative drug is azithromycin, which is a useful medication for patients who are allergic to penicillin. Clindamycin is also a useful alternative for patients with penicillin allergy or in special situations in which resistant anaerobic bacteria are suspected. Metronidazole may be useful, especially when anaerobic bacteria are suspected. It may be used in combination with another antibiotic that kills the facultative and aerobic oral pathogens. Moxifloxacin, because of the need to limit the development of resistance and due to its toxicities and drug interactions, should be restricted to use by specialists in the treatment of severe infections.
Principle 7: Administer Antibiotic Properly
Once the decision is made to prescribe an antibiotic to the patient, the drug should be administered in the proper dose and at the proper dose interval. The manufacturer usually recommends the proper dosage and administration. Provision of plasma levels that are sufficiently high to kill the bacteria that are sensitive to the antibiotic but are not so high as to cause toxicity is adequate. The peak plasma level of the drug should usually be at least 4 or 5 times the minimal inhibitory concentration for the bacteria involved in the infection.
Clearly, some patients stop taking their antibiotics after acute symptoms have subsided and rarely take their drugs as prescribed after 4 or 5 days. Therefore the antibiotic that would have the highest compliance would be the drug that could be given once a day for 4 or 5 days. One study has shown that for odontogenic infections a 4-day course of penicillin, combined with the appropriate surgery, was as effective as a 7-day course of the antibiotic.
Additional administration of antibiotics may be necessary in some infections that have not resolved rapidly at the clinical follow-up examination. The clinician must make it clear to the patient that the entire prescription must be taken. If for some reason the patient is advised to stop taking the antibiotic early, all remaining pills or capsules should be discarded. Keeping small amounts of unused antibiotics in medicine cabinets for the anticipated sore throat next winter should be strongly discouraged. Casual self-administration of antibiotics is not useful and may be hazardous to the health of the individual and the community.
Principle 8: Evaluate Patient Frequently
Once the patient has been treated by surgery and antibiotic therapy has been prescribed, the patient should be followed carefully to monitor response to treatment and complications. In most situations the patient should be asked to return to the dentist 2 days after the original therapy. Typically, the patient is much improved. If therapy is successful, swelling and pain decreases dramatically. The dentist should check the I&D site to determine whether the drain should be removed at this time. Other parameters, such as temperature, trismus, swelling, and the patient’s subjective feelings of improvement, should also be evaluated.
If there is not an adequate response to treatment, the patient should be examined carefully for clues to the reason for failure (Box 15-9). The most common cause of treatment failure is inadequate surgery. A tooth may have to be reevaluated for extraction, or an extension of the infection into an area not detected at the first treatment may have to be incised and drained. It may be necessary to admit such a patient to the hospital for purposes of airway security, further surgery, and intravenous antibiotic therapy.
A second reason for failure is depressed host defense mechanisms. A review of the patient’s medical history should be performed, and more careful probing questions should be asked. In addition to immunocompromising diseases, conditions that diminish physiologic reserves, such as dehydration, malnutrition, and pain, should also be considered and corrected if necessary.
A third reason for treatment failure is the presence of a foreign body. Although this is unlikely in an odontogenic infection, the dentist may consider taking a careful history and a periapical radiograph of the area to help ensure that a radiopaque foreign body is not present. Dental implants are increasingly common foreign bodies, and the ability of bacteria to find shelter from the immune system in their surface gaps and irregularities can perpetuate the infection until the implant is successfully débrided or removed.
Finally, there may be problems with the antibiotic that was given to the patient. The dentist first ascertains whether the patient has been compliant. The patient must have the prescription filled and must take the antibiotic according to directions. Many patients fail to follow the orders of their dentists as carefully as they should. Sometimes, a patient may not have the prescription filled because he or she cannot afford it. The dentist should use the most cost-effective antibiotic available and can frankly ask whether a particular prescription is affordable to the patient. Another problem to consider is whether the antibiotic reached the infected area. The penetration of antibiotics into abscess cavities is poor. Failure of the antibiotic to reach the area may be related to inadequate surgery or drainage, inadequate blood supply to the local area, or a dose that is too low to be effective against the bacteria. Another antibiotic-related problem is an incorrect bacterial diagnosis. If a culture was not performed at the initial surgical treatment or if no surgical treatment was done at the initial therapy, the dentist should obtain a specimen for C&S testing. Finally, it is possible that the wrong antibiotic was prescribed for the infection, which may be because of an inaccurate bacterial diagnosis or the increasing antibiotic resistance of oral bacteria. For example, 25% to 35% of Prevotella organisms are resistant to penicillin but rarely cause persistent infection if penicillin is given and adequate surgery is done. However, if the patient has a persistent, low-grade infection that does not resolve despite adequate surgery, prescribing an antianaerobic antibiotic such as clindamycin is appropriate.
The clinician must also examine the patient to look specifically for toxicity reactions and untoward side effects. Patients may report complaints such as nausea and abdominal cramping but may fail to associate watery diarrhea with the drug administration. Specific questioning about the expected toxicities is important to their early recognition.
The dentist should also be aware of the possibility of secondary or superinfections. The most common secondary infection encountered by dentists is oral or vaginal candidiasis. This is the result of an overgrowth of Candida organisms because the normal oral flora has been altered by the antibiotic therapy. Other secondary infections may arise as normal host flora is altered, but they are not seen with any degree of frequency in the management of odontogenic infections.
Finally, the dentist should follow the patient carefully once the infection has resolved to check for recurrent infection. Recurrence would be seen in a patient who had incomplete therapy for the infection. A variety of reasons may account for this. For example, the patient may have stopped taking the antibiotic too early. The drain may have been removed too early and the drainage site sealed too early, which reestablished the infectious process. If infection does recur, surgical intervention and reinstitution of antibiotic therapy should be considered.
PRINCIPLES OF PREVENTION OF INFECTION
The use of antibiotics to treat an established infection is a well-accepted and well-defined technique. These drugs provide major assistance for the patient in overcoming an established infection. The use of antibiotics for prevention (i.e., prophylaxis) of infection is less widely accepted. The final section of this chapter discusses the use of antibiotics for prophylaxis of two distinct types of infection. The use of antibiotics to prevent wound infection after surgery is presented first, followed by a discussion of antibiotic use to prevent metastatic infection.
PRINCIPLES OF PROPHYLAXIS OF WOUND INFECTION
The use of antibiotics for prophylaxis of postoperative wound infections may be effective and desirable in certain situations. On the other hand, there is little scientific evidence that demonstrates the effectiveness of prophylactic antibiotics in dentistry and oral and maxillofacial surgery. If, however, prophylactic antibiotics are effective in preventing postoperative wound infections and blood-borne infections of distant sites, they would have three distinct advantages. First, prophylactic antibiotics may reduce the incidence of postoperative infection and thereby reduce postoperative morbidity. When a patient becomes infected after surgery, wound healing and recovery are substantially delayed. Second, appropriate and effective antibiotic prophylaxis may reduce the cost of health care. By decreasing the incidence of postoperative infection, the patient can be saved the additional expense of returning to the dentist, buying additional antibiotics, and missing additional days of work. Third, appropriate use of prophylactic antibiotics requires a shorter-term administration than therapeutic use, thereby possibly decreasing the total amount of antibiotics used by the population. On the other hand, when they are used inappropriately, prophylactic antibiotics are actually associated with an increased risk of postoperative infection, typically by a bacterium resistant to the prophylactic antibiotic.
The use of prophylactic antibiotics also has several disadvantages: First, they may alter host flora. The body is populated with a variety of bacteria that have a symbiotic relationship with the host. When antibiotics are administered, some of these bacteria are eliminated, allowing the overgrowth of antibiotic-resistant and perhaps more pathogenic bacteria that may then cause infection. Second, several studies have shown that antibiotic administration in one patient allows antibiotic-resistant organisms to spread to the patient’s family and community. Third, the antibiotic may provide no benefit, which means that in certain situations the risk of infection is so low that the antibiotic provides no additional decrease in the incidence of infection. Fourth, the use of prophylactic antibiotics may encourage lax surgical and aseptic technique on the part of the dentist. The attitude of, “Oh well, the patient is on antibiotics,” is an unacceptable excuse when the principles of atraumatic tissue handling and surgical asepsis are violated. Fifth, the cost of the antibiotic must be considered. Although for a single event for a single patient the cost may be small, the cost for many surgeries for many patients can be enormous. Finally, the toxicity of the drug to the patient must be also kept in mind. All drugs have the potential to cause injury to the patient. Although most antibiotics used by dentists have low toxicity, the possibility of toxicity is always present. The principles of prophylactic antibiotic use are summarized in Box 15-10.
Principle 1: Procedure Should Have Significant Risk of Infection
For prophylactically administered antibiotics to reduce the incidence of infection, the surgical procedure must have a high enough incidence of infection to be reduced with antibiotic therapy. Clean surgery done with strict adherence to basic surgical principles usually has an incidence of infection of about 3%. Infection rates of 10% or more are usually considered unacceptable, and the prophylactic use of antibiotics must be strongly considered for such infection-prone procedures. For the dentist doing routine office surgery, this means that most office procedures performed on healthy patients do not require prophylactically administered antibiotics. The incidence of infection after tooth extraction, frenectomy, biopsy, minor alveoloplasty, and torus reduction is extremely low; therefore, antibiotics would provide no benefit. This is true even in the presence of periapical infection, severe periodontitis, and multiple extractions.
However, several surgical factors may influence the dentist to strongly consider the use of antibiotic prophylaxis (Box 15-11): The first and most obvious factor that may lead to infection is a bacterial inoculum of sufficient size. The usual surgical procedure performed in the mouth rarely involves sufficient bacterial inoculation to cause infection, unless an acute infection with cellulitis or abscess is already present. The second factor is surgical procedures that require prolonged surgery. In hospital surgeries, the incidence of postoperative infection increases significantly with operations lasting longer than 4 hours. A third factor that may suggest the use of antibiotics is the insertion or presence of a foreign body, most commonly a dental implant. Most data seem to suggest that the use of antibiotics may decrease the incidence of infection when foreign bodies, such as dental implants, are inserted into the jaws.
The final and most important factor for most dentists in determining which patients should receive prophylactically administered antibiotics is whether the patient has depressed host defenses. Patients who have a compromised ability to defend themselves against infection should receive antibiotics prophylactically because they are likely to have a higher incidence of more severe infection. All patients receiving cancer chemotherapy or immunosuppressives should receive antibiotics prophylactically, even when minor surgical procedures are performed. Patients receiving immunosuppressives for organ transplant will be taking these drugs for the remainder of their lives and should be given preventive antibiotics accordingly. Patients receiving cancer chemotherapy will receive cytotoxic drugs for 1 year or less but should be given antibiotics prophylactically for at least a 1-year period after the cessation of their chemotherapy. This is also wise for those receiving radiation therapy to the jaw. End-stage renal disease, including patients receiving kidney dialysis, is an immunocompromising disease that requires antibiotic prophylaxis for oral surgical procedures. The most common immunocompromising disease, however, is diabetes mellitus. The incidence of postoperative infection in diabetic persons is directly correlated with elevations of blood sugar. The oral surgical management of diabetic persons based on their blood glucose is summarized in Table 15-5. The glycosylated hemoglobin test, or hemoglobin A1c, is a good measure of the level of diabetes control over the previous 3 to 4 months. Before complex reconstructive oral surgery, such as the placement of dental implants, the dentist is wise to ensure that acceptable intermediate-term control of blood sugar has been achieved, as measured by a hemoglobin A1c of 8% or less. The American Diabetes Association recommends a therapeutic target hemoglobin A1c level of 7%. Withholding dental implant therapy and working with the patient’s physician can help motivate the patient to achieve good long-term control of diabetes. Although prophylactically administered antibiotics are helpful in diabetic patients, perioperative control of blood sugar levels is paramount.
TABLE 15-5
Dental Treatment for Diabetics Based on Fingerstick Blood Glucose Testing
| Finger Stick Blood Glucose (mg/dL%) | Dental Treatment |
| Less than 85 | Administer glucose; postpone elective treatment |
| 85-200 | Stress reduction; consider antibiotic prophylaxis for extraction |
| 200-300 | Stress reduction; antibiotic prophylaxis; referral to primary care physician |
| 300-400 | Avoid elective treatment; referral to primary care physician or emergency room at nearby hospital |
| Greater than 400 | Avoid elective treatment; send to emergency room at nearby hospital |
Principle 2: Choose Correct Antibiotic
The choice of antibiotic for prophylaxis against infections after surgery of the oral cavity should be based on the following criteria: First, the antibiotic should be effective against the organisms most likely to cause infection in the oral cavity. As previously discussed, the facultative streptococci are usually the originally invading organism in oral infection. Second, the antibiotic chosen should be a narrow-spectrum antibiotic.
By using a narrow-spectrum antibiotic, the disadvantage of altering host flora is minimized. Third, the antibiotic should be the least toxic antibiotic available for the patient. Finally, the drug selected should be a bactericidal antibiotic. Because many of the routine prophylactic uses of antibiotics in the dental office are for patients with compromised host defenses, it is important that the antibiotic effectively kill the bacteria.
Taking into account these four criteria, the antibiotic of choice for prophylaxis before oral surgery is penicillin or amoxicillin. These two antibiotics are effective against the causative organism (i.e., Streptococcus), are narrow-spectrum, have low toxicity, and are bactericidal. For patients allergic to penicillin, the best choice is clindamycin. Clindamycin is fairly effective against oral streptococci and is narrow-spectrum, but it is bacteriostatic. The third choice for oral administration for prophylaxis is azithromycin. Azithromycin is reasonably effective against the usual organisms and is narrow-spectrum, but it is also bacteriostatic.
Principle 3: Antibiotic Plasma Level Must Be High
When antibiotics are used prophylactically, the antibiotic level in the plasma must be higher than when antibiotics are used therapeutically. The peak plasma levels should be high to ensure diffusion of the antibiotic into all of the fluid and tissue spaces where the surgery is going to be performed. The usual recommendation for prophylaxis is that the drug be given in a dose at least 2 times the usual therapeutic dose. Use of the same prophylactic doses recommended by the American Heart Association for prophylaxis of infective endocarditis is reasonable. For penicillin or amoxicillin, this is 2 g; for clindamycin, 600 mg; and for azithromycin, 500 mg.
Principle 4: Time Antibiotic Administration Correctly
For the antibiotic to be maximally effective in preventing postoperative infection, the antibiotic must be given 2 hours or less before the surgery begins. The time of dosing before surgery varies by the route used, allowing for absorption of the antibiotic into the tissues at the time of wounding. For the oral route, this is usually 1 hour; with the intravenous route, a much shorter preoperative dosing interval is possible. This principle has been clearly established in many animal and human clinical trials. Antibiotic administration that occurs after surgery is greatly decreased in its efficacy or has no effect at all on preventing infection; there is evidence to indicate that prophylactically administered antibiotics given 2 hours or more after surgery may increase the risk of wound infection.
If the surgery is prolonged and an additional antibiotic dose is required, intraoperative dose intervals should be shorter (i.e., one half the usual therapeutic dose interval). Therefore, penicillin and clindamycin should be given every 3 hours. This ensures that the peak plasma levels will stay adequately high and avoids periods of inadequate antibiotic levels in the tissue fluids.
Principle 5: Use Shortest Antibiotic Exposure That Is Effective
For the antibiotic prophylaxis to be effective, the antibiotic must be given before the surgery begins, and adequate plasma levels must be maintained during the surgical procedure. Once the surgical procedure is completed, continued antibiotic administration produces little if any benefit. If the procedure is a short operation, a single preoperative dose of antibiotics is adequate. A plethora of animal and human clinical data demonstrates that the prophylactic use of antibiotics is necessary only for the time of surgery; after closure of the wounds and formation of the blood clots, migration of bacteria into the wound and underlying tissues occurs at such a low level that additional antibiotics are not necessary.
Summary
The use of antibiotics for prophylaxis of postoperative wound infection may be effective. It may reduce patient pain, morbidity, cost, and total antibiotic use. Appropriate antibiotic prophylaxis does little to alter host flora. Most dental procedures on healthy patients do not require antibiotic prophylaxis. A few select patients who are to undergo long surgical procedures or the insertion of foreign bodies, such as dental implants, should be considered for prophylaxis. Patients who have compromised host defenses because of poorly controlled metabolic diseases or certain diseases that interfere with host defenses or who are taking drugs that suppress the immune system should also be given prophylactic antibiotics. The drug of choice is a narrow-spectrum antibiotic that is effective against causative organisms, is nontoxic, and is bactericidal. Penicillin fits these criteria the best.
When the antibiotic is given, it should be taken before the surgery begins, at a normal dose twice that of therapeutically administered antibiotics. If the surgery is prolonged, interim doses at half the normal dose interval should be used. High plasma levels should be maintained during the surgical procedure, but no additional antibiotics are necessary after surgery.
PRINCIPLES OF PROPHYLAXIS AGAINST METASTATIC INFECTION
Metastatic infection is defined as infection that occurs at a location physically separate from the portal of entry of the bacteria. The classic and most widely understood example of this phenomenon is bacterial endocarditis, which may arise from bacteria that are introduced into the circulation as a result of tooth extraction. The incidence of metastatic infection can be reduced if antibiotic administration is used to eliminate the bacteria before they can establish an infection at the remote site.
For metastatic infection to occur, several conditions must be met (Box 15-12). The first and most important is that there must be a susceptible location in which an infection can be established. An example of this is the deformed heart valve with its altered endothelial surface onto which an irregularly surfaced vegetation has formed.
Bacterial seeding of the susceptible area must also take place. This seeding occurs as the result of a bacteremia in which bacteria from the mouth are carried to the susceptible site. Most likely, a quantitative factor is involved in this seeding process because the body experiences multiple episodes of small bacteremias as a result of normal daily activities, such as chewing and tooth brushing. Turbulent blood flow across a deformed heart valve can traumatize the endothelial lining of the valve, which can then precipitate the deposition of platelets and fibrin, resulting in nonbacterial thrombotic endocarditis (NBTE). Subsequently, bacterial proteins called adhesins recognize the fibrin and platelet matrix of NBTE. Some staphylococci and oral streptococci, especially Streptococcus sanguis, Streptococcus mitis, and Streptococcus oralis, have these adhesins, which explains their association with infective endocarditis.
Also necessary for the establishment of metastatic infection is some impairment of the local host defenses. Once bacteria have attached to NBTE, they are protected from white blood cell phagocytosis by a thin coating of fibrin and an extracellular matrix synthesized by the bacteria, resulting in biofilm. More than 90% of the bacteria existing within a mature cardiac vegetation are in a metabolically inactive state, which also renders them less susceptible to bactericidal antibiotics. Bacteria inhabiting such a biofilm coating on foreign bodies, such as prosthetic joint or dental implants, are not easily phagocytized by white blood cells or killed by antibiotics, as in endocarditis.
Prophylaxis Against Infectious Endocarditis
Historically, the rationale for antibiotic prophylaxis of infectious endocarditis (IE) after dental procedures has been based on the following facts: bacteremia has been shown to cause IE; viridans-group streptococci are part of the normal oral flora and have been commonly found in IE; dental procedures can cause bacteremias because of Streptococcus viridans; a large number of case reports associate dental procedures with subsequent IE; S. viridans is generally susceptible to the antiobiotics recommended for prophylaxis of IE; antibiotic prophylaxis prevents experimental endocarditis in animals because of S. viridans; and the risk of significant adverse reaction to the antibiotic is low in an individual patient, and the morbidity and mortality of IE are high. When this occurs, the patient must be treated in the hospital with high doses of intravenous (IV) antibiotics for prolonged periods. Often, the damaged native heart valve must be surgically replaced with a prosthetic valve. Although initial recovery from bacterial endocarditis approaches 100%, recurrent episodes reduce the 5-year survival rate of patients with this disease to approximately 60%.
Recent evidence puts into question the likelihood, however, that prophylactic antibiotics prevent IE in human beings. Antibiotics do no consistently prevent bacteremias after dental procedures. Bacteremias after chewing, toothbrushing, and other daily activities occur far more frequently than after dental procedures. Endocarditis has been shown to occur despite appropriate antibiotic prophylaxis for dental procedures. Only a small proportion of IE cases are from dental procedures, and very few cases of IE would be prevented by antibiotic prophylaxis for dental procedures, even it if were 100% effective.
The American Heart Association has had formal recommendations for the prevention of IE after dental treatments since 1960. The latest formal recommendations appeared in May 2007. Dentists must stay abreast of revised recommendations as they are published by the American Heart Association and the American Dental Association. These new guidelines take into account that a very small number of cases of IE are caused by dental procedures and can be prevented by antibiotic prophylaxis. New emphasis has been correctly placed on the establishment and maintenance of optimum oral health in patients with increased risk for IE.
The new guidelines indicate prophylaxis only for the patients at highest risk of endocarditis, including those with previous endocarditis, prosthetic heart valves, cyanotic congenital heart defects that have not been repaired or have remaining partial defects after repair, and heart transplant patients with valvulopathy. This will significantly decrease the number of dental patients for whom prophylaxis is indicated. A list of the conditions that pose the highest risk of endocarditis can be found in Box 15-13.
Recent evidence also indicates that the magnitude of bacteremia caused by a given dental procedure is not necessarily correlated with the incidence of IE. Therefore the new guidelines have simplified the description of dental procedures for which antibiotic prophylaxis should be used to the following description: “All dental procedures that involve manipulation of gingival tissue or the periapical region of teeth or perforation of the oral mucosa” (Box 15-14). Prophylaxis is not required for routine local anesthetic injections through noninfected tissue, dental radiographs, placement of removable prosthodontic or orthodontic appliances, adjustment of orthodontic appliances, placement of orthodontic brackets, shedding of deciduous teeth, and bleeding from trauma to the lips or oral mucosa (Box 15-15).
Bacterial endocarditis prophylaxis is achieved for most routine conditions with the administration of 2 g of amoxicillin orally ½ to 1 hour before the procedure (Table 15-6). Amoxicillin is the drug of choice because it is better absorbed from the gastrointestinal tract and provides higher and more sustained plasma levels. Amoxicillin is an effective killer of viridans-group streptococci, which include the organisms that most commonly causes IE after dental procedures.
TABLE 15-6
Antibiotic Regimens for Prophylaxis of Bacterial Endocarditis
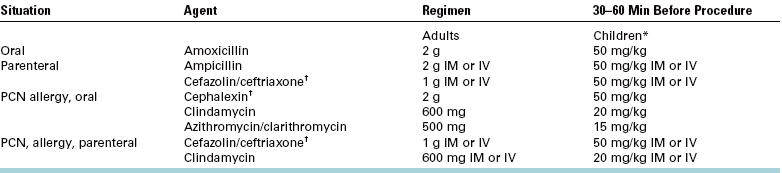
IM, Intramuscularly; IV, intravenously.
*Total children’s dose should not exceed adult dose.
†Cephalosporins should not be used in patients with immediate-type hypersensitivity reaction to penicillins. Other first- or second-generation oral cephalosporins may be substituted in equivalent adult or pediatric doses.
For patients who are allergic to penicillin, two alternative drugs have been recommended. The first recommended drug is clindamycin, with a dose of 600 mg orally 1 hour before the surgery. If the patient’s allergy to penicillin is mild and not of an anaphylactic type, a first-generation cephalosporin, such as cephalexin, may be prescribed. Although erythromycin is no longer recommended, the newer macrolide antibiotics, azithromycin or clarithromycin, are acceptable alternative drugs. If the patient is unable to take oral medication, parenteral administration can be used.
For the pediatric patient, the dose of the drugs that are given must be reduced. The recommendations include clear guidelines for proper pediatric dosing (see Table 15-6).
Some patients at risk for bacterial endocarditis may be taking daily doses of penicillin to prevent recurrence of rheumatic fever or may already be taking an antibiotic for other reasons. In these patients, the streptococci may be relatively resistant to penicillin. The recommendation for this situation is that the dentist should use clindamycin, azithromycin, or clarithromycin for endocarditis prophylaxis. The cephalosporins should be avoided because of possible cross-resistance with the penicillins. If possible, the procedure should be postponed until 10 or more days after the antibiotic is completed, thus allowing a more normal oral flora to be reestablished.
If a particular patient requires a series of dental treatments that requires antibiotic prophylaxis, a period of 10 or more days between appointments is appropriate. The reason for the interval is that the administration of antibiotics for several days or more continuously may promote colonization of the patient by bacteria that are resistant to the antibiotic being given, thus making prophylaxis more likely to fail. The 10-or-more day antibiotic-free period may allow antibiotic-sensitive organisms to repopulate the oral flora. On the other hand, it has been shown that baseline antibiotic resistance levels are not reestablished for several months after a course of antibiotics. For this reason, the number of dental visits should also be minimized, consistent with the patient’s tolerance level.
Occasionally, unexpected bleeding may occur during dental treatment in a patient who is at risk for endocarditis, or a patient may not inform the dentist of the indication for antibiotic prophylaxis before the beginning of the procedure. In this situation, appropriate antibiotic prophylaxis should be administered as soon as possible but definitely within 2 hours after the procedure. Prophylaxis given longer than 4 hours after the bacteremia has limited prophylactic benefit.
Patients at risk for IE should have a comprehensive prophylaxis program that includes excellent oral hygiene with excellent periodic professional care. Special care should be taken for the establishment of an effective preventive program, and all incipient dental and periodontal disease should be treated. If surgery is required, the mouth can be rinsed preoperatively with an antibacterial agent, such as chlorhexidine. Preoperative oral antiseptic rinses have been shown to reduce the magnitude of bacteremias (the number of bacteria entering the bloodstream), although they are not a substitute for antibiotic prophylaxis.
Finally, it is important for the dentist to understand that even when appropriate measures are taken to prevent bacterial endocarditis, it may still occur. Patients should be informed of this and advised to return to the dentist or to their primary care physician if any of the signs and symptoms of bacterial endocarditis, especially fever and malaise, occur.
Prosthetic valve endocarditis occurs when the tissue around the cardiac valve implant becomes infected. Such infections are caused by the same bacteria that cause typical native valve endocarditis. Prosthetic valve endocarditis is a much more serious illness than native valve endocarditis because the loosening of the heart valve may result in death. The 1-year survival rate for patients who have prosthetic valve endocarditis is about 50%. The American Heart Association currently states that the standard oral regimens are adequate for most patients with prosthetic heart valves.
Prophylaxis in Patients with Other Cardiovascular Conditions
Several other cardiovascular conditions require the clinician to consider the administration of prophylactic antibiotics for the prevention of metastatic infection. In coronary artery bypass grafting (CABG), the coronary arteries are reconstructed with vascular grafts. Because CABG does not predispose patients to metastatic infection, these patients should not be given prophylactic antibiotics before a dental procedure is performed.
Patients with a transvenous pacemaker have a battery pack implanted in their chests, with a thin wire that runs through the superior vena cava into the right side of the heart. These patients usually do not require prophylactic antibiotics when dental procedures are performed. Similarly, coronary artery angioplasty with or without stent placement is not an indication for endocarditis prophylaxis. However, consultation with the patient’s cardiologist may be used to confirm that this is the best management.
Patients receiving renal dialysis frequently have an arteriovenous shunt surgically constructed in their forearms to provide the dialysis team ready access to the bloodstream. Metastatic infection may occur in these shunts after bacteremia. Therefore the dentist should contact the patient’s nephrologist or renal dialysis team to discuss the best management.
Patients who have hydrocephaly may have decompression with ventriculoatrial shunts. Because these shunts may induce valvular dysfunction, antibiotic prophylaxis may be required. Consultation with the patient’s neurosurgeon should be considered.
Patients who have had severe atherosclerotic vascular disease and have had alloplastic vascular grafts placed to replace portions of their arteries do not appear to be at risk for metastatic infection from dental procedures. Therefore the American Heart Association does not recommend antibiotic prophylaxis for nonvalvular cardiovascular devices, including coronary artery stents and vena caval filters. The exception to this rule is for incision and drainage of abscesses at other sites, including the oral cavity.
Prophylaxis Against Total Joint Replacement Infection
Patients who have undergone total replacement of a joint with a prosthetic joint may be at risk for hematogenous spread of bacteria and subsequent infection. These late prosthetic joint infections result in severe morbidity because the implant is usually lost when infections occur. There has been great concern that the bacteremia caused by tooth extraction may result in such infections. However, the recent literature suggests that bacteremias from oral procedures are not likely to cause prosthetic joint infections. It appears that the bacteremia after oral surgery is of a transient nature and does not expose the implant and periimplant tissues to bacteria long enough to cause infection.
Instead it appears that the hematogenous spread of prosthetic joint infections is caused by chronic infections elsewhere in the body that result in chronic septicemias. These infections are typically urogenital, gastrointestinal, pulmonary, or skin infections, but established odontogenic infections may also cause a septicemia of sufficient magnitude to cause a total joint infection. Patients with orthopedic pins, plates, and screws do not need antibiotic prophylaxis.
In July of 2003 the American Dental Association (ADA) and the American Academy of Orthopaedic Surgeons (AAOS) issued a revised joint recommendation concerning the management of patients with prosthetic total joints. The recommendations of the ADA and AAOS recognize that most patients with a prosthetic joint are not at risk for joint infection after a dental surgical procedure. Instead the guidelines identify the high-risk patients who are potentially susceptible to such infections (Box 15-16). Likewise, it identifies those procedures that are most likely to cause joint infections and therefore require prophylaxis (Box 15-17). The joint statement recommends specific antibiotic regimens to help prevent infection in the susceptible patient who is undergoing one of the procedures that require prophylaxis (Table 15-7). Ultimately however, the revised guidelines make the following statement: “Practitioners must exercise their own clinical judgment in determining whether or not antibiotic prophylaxis is appropriate.”
TABLE 15-7
Antibiotic Regimens for Prophylaxis of Total Joint Replacement Infection
| Regimen | Drug | Dose |
| Standard oral prophylaxis | Amoxicillin, cephalexin, or cephradine | 2 g orally 1 hour before procedure |
| Penicillin-allergic oral prophylaxis | Clindamycin | 600 mg orally 1 hour before procedure |
| Parenteral prophylaxis | Cefazolin or ampicillin | 1 g IV 1 hour before procedure |
| 2 g IV 1 hour before procedure | ||
| Penicillin-allergic parenteral prophylaxis | Clindamycin | 600 mg IV 1 hour before procedure |
When the dentist decides to provide antibiotic prophylaxis of prosthetic joint infection, the recommended antibiotics are a first-generation cephalosporin and amoxicillin. For patients who are allergic to penicillin, clindamycin is recommended. As with bacterial endocarditis prophylaxis, only a single preoperative dose is recommended, with no follow-up doses. If patients are unable to take oral medication, a parenteral regimen is also suggested (Box 15-18).
If a patient who has a total joint replacement needs treatment of an infection, aggressive therapy for the infection is necessary to prevent seeding of the bacteria into the prosthesis, causing odontogenic infection of the prosthetic joint. This aggressive treatment should include extraction, I&D, and the use of high-dose bactericidal antibiotics, possibly given IV. The clinician should strongly consider performing C&S testing because if a prosthetic joint infection does occur, it would be useful to know which bacteria are likely the causative organisms along with their antibiotic sensitivity.
When there is disagreement between the dentist, the patient, or the patient’s physician over the need for antibiotic prophylaxis, clear communication between all parties is required to clarify all relevant facts and scientific data and to arrive at a consensus. Ultimately, the dentist is responsible for his or her own treatment decisions and should not render care that he or she thinks is not in the best interest of the patient.
Bibliography
ADA Council on Scientific Affairs. Combating antibiotic resistance. JADA. 2004;135:484–487.
American Dental Association, American Academy of Orthopaedic Surgeons. Antibiotic prophylaxis dental patients with total joint replacements. J Am Dent Assoc. 2003;134:895–899.
Brook, I, Frazier, EH, Gher, ME. Aerobic and anaerobic microbiology of periapical abscess. Oral Microbiol Immunol. 1991;6:123–125.
Chow, AW, Roser, SM, Brady, FA. Orofacial odontogenic infections. Ann Intern Med. 1978;88:392.
Conover, MA, Kaban, LB, Mulliken, JB. Antibiotic prophylaxis for major maxillocraniofacial surgery. J Oral Maxillofac Surg. 1985;43:865.
Conover, MA, Kaban, LB, Mulliken, JB. Antibiotic prophylaxis for major maxillofacial surgery: one-day vs five-day therapy. Otolaryngology. 1986;95:554.
Dajani, AS, Taubert, KA, Wilson, W, et al. Prevention of bacterial endocarditis: recommendations by the American Heart Association. JAMA. 1997;277:1794–1801.
Doern, GV, Ferraro, MJ, Brueggemann, AB, Ruoff, KL. Emergence of high rates of antimicrobial resistance among viridans group streptococci in the United States. Antimicrob Agents Chemother. 1996;40:891–894.
Fazakerley, MW, McGowan, P, Hardy, P, Martin, MV. A comparative study of cephradine, amoxycillin and phenoxymethylpenicillin in the treatment of acute dentoalveolar infection. Br Dent J. 1993;174:359–363. [(see comments).].
Field, EA, Martin, MV. Prophylactic antibiotics for patients with artificial joints undergoing oral and dental surgery: necessary or not? Br J Oral Maxillofac Surg. 1991;29:341–346.
Flynn, TR, Halpern, LR. Antibiotic selection in head and neck infections. Oral Maxillofac Surg Clin North Am. 2003;15:17–38.
Flynn, TR, Shanti, RM, Hayes, C. Severe odontogenic infections, part two: prospective outcomes study. J Oral Maxillofac Surg. 2006;64:1104–1113.
Flynn, TR, Shanti, RM, Levy, M, et al. Severe odontogenic infections, part one: prospective report. J Oral Maxillofac Surg. 2006;64:1093–1103.
Fouad, AF, Rivera, EM, Walton, RE. Penicillin as a supplement in resolving the localized acute apical abscess. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:590–595.
Gilmore, WC, Jacobus, NV, Gorbach, SL, et al. A prospective double-blind evaluation of penicillin versus clindamycin in the treatment of odontogenic infections. J Oral Maxillofac Surg. 1988;46:1065–1070.
Heimdahl, A, Nord, CE. Treatment of orofacial infections of odontogenic origin. Scand J Infect Dis. 1985;46(suppl):101.
Heimdahl, A, Von Konow, L, Satoh, T, et al. Clinical appearance of orofacial infections of odontogenic origin in relation to microbiological findings. J Clin Microbiol. 1985;22:299.
Jacobson, JJ, Schweitzer, SO, Kowalski, CJ. Chemoprophylaxis of prosthetic joint patients during dental treatment: a decision-utility analysis. Oral Surg Oral Med Oral Pathol. 1991;72:167.
Kaye, D. Prophylaxis for infective endocarditis: an update. Ann Intern Med. 1986;104:419.
Kim, Y, Flynn, TR, Donoff, RB, et al. The gene: the polymerase chain reaction and its clinical application. J Oral Maxillofac Surg. 2002;60:808–815.
Kuriyama, T, Absi, EG, Williams, DW, Lewis, MA. An outcome audit of the treatment of acute dentoalveolar infection: impact of penicillin resistance. Br Dent J. 2005;198:759–763.
Kuriyama, T, Karasawa, T, Nakagawa, K, et al. Antimicrobial susceptibility of major pathogens of orofacial odontogenic infections to 11 beta-lactam antibiotics. Oral Microbiol Immunol. 2002;17(5):285–289.
Kuriyama, T, Nakagawa, K, Karasawa, T, et al. Past administration of beta-lactam antibiotics and increase in the emergence of beta-lactamase-producing bacteria in patients with orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:186–192.
Laskin, DM. Anatomic considerations in diagnosis and treatment of odontogenic infections. J Am Dent Assoc. 1964;69:308.
Lewis, MA, Carmichael, F, MacFarlane, TW, Milligan, SG. A randomised trial of co-amoxiclav (Augmentin) versus penicillin V in the treatment of acute dentoalveolar abscess. Br Dent J. 1993;175:169–174.
Lewis, MAO, MacFarlane, TW, McGowan, DA. Quantitative bacteriology of acute dento-alveolar abscesses. J Med Microbiol. 1986;2:101.
Lewis, MAO, Parkhurst, CL, Douglas, CW, et al. Prevalence of penicillin-resistant bacteria in acute suppurative oral infection. J Antimicrob Chemother. 1995;35:785–791.
Martin, C, Karabouta, I. Infection after orthognathic surgery, with and without preventative antibiotics. Int J Oral Surg. 1984;13:490.
Nager, C, Murphy, AA. Antibiotics and oral contraceptive pills. Semin Reprod Endocrinol. 1989;7:220.
Pallasch, TJ. Antibiotic prophylaxis: problems in paradise. Dent Clin North Am. 2003;47:665–679.
Paterson, SA, Curzon, MEJ. The effect of amoxicillin versus penicillin V in the treatment of acutely abscessed primary teeth. Br Dent J. 1993;174:443.
Peterson, LJ. Antibiotic prophylaxis against wound infections in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1990;48:617.
Peterson, LJ. Microbiology of head and neck infections. Atlas Oral Maxillofac Surg Clin North Am. 1991;3:247.
Peterson, LJ. Contemporary management of deep infections of the neck. J Oral Maxillofac Surg. 1993;51:226.
Polk, HC, Jr., Simpson, CJ, Simmons, BP, Alexander, JW. Guidelines for prevention of surgical wound infection. Arch Surg. 1983;118:1213–1217.
Sakamoto, H, Kato, H, Sato, T, Sasaki, J. Semiquantitative bacteriology of closed odontogenic abscesses. Bull Tokyo Dent Coll. 1998;39:103–107.
Sclar, DA, Tartaglione, TA, Fine, MJ. Overview of issues related to medical compliance with implications for outpatient management of infectious disease. Infect Agents Dis. 1994;3:266.
Takai, S, Kuriyama, T, Yanagisawa, et al. Incidence and bacteriology of bacteremia associated with various oral and maxillofacial surgical procedures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:292–298.
Wilson, W, Taubert, KA, Gewitz, M, et al, Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007. doi:10.1161/CIRCULATIONAHA. 106.183095. Published online before print April 19, 2007. http://circ.ahajournals.org./cgi/content/abstract/CIRCULATIONAHA.106.183095v1. Accessed May 19, 2007.