Complex Odontogenic Infections
Odontogenic infections are usually mild and readily treated with the appropriate surgical procedure with or without supplemental antibiotic therapy. Infections that spread beyond the teeth into the oral vestibule are usually managed by intraoral incision and drainage (I&D) procedures, plus dental extraction, root canal therapy, or gingival curettage, as appropriate. The principles of management of routine odontogenic infections are discussed in Chapter 15. Some odontogenic infections are serious and require management by oral and maxillofacial surgeons, who have extensive training and experience in this area. Even after the advent of antibiotics and improved dental health, serious odontogenic infections still sometimes result in death. These deaths occur when the infection reaches areas distant from the alveolar process. The purpose of this chapter is to present an overview of deep fascial space infections of the head and neck originating in the teeth, as well as several less common but important infections of the oral cavity.
DEEP FASCIAL SPACE INFECTIONS
The pathways of odontogenic infection extending from the teeth through bone and into surrounding soft tissues are discussed in Chapter 15. As a general rule, infection erodes through the thinnest bone and causes infection in the adjacent tissue. Whether this becomes a vestibular or a deeper fascial space abscess is determined primarily by the relationship of the attachment of the nearby muscles to the point at which the infection perforates the bony cortical plate. Most odontogenic infections penetrate the facial cortical plate of bone to become vestibular abscesses. On occasion, infections erode into other deep fascial spaces directly (Fig. 16-1). Fascial spaces are fascia-lined tissue compartments filled with loose, areolar connective tissue that can become inflamed when invaded by microorganisms. The resulting process of inflammation passes through stages that are seen clinically as edema (inoculation), cellulitis, and abscess. In healthy persons, the deep fascial spaces are only potential spaces that do not exist. The loose areolar tissue within these spaces serves to cushion the muscles, vessels, nerves, glands, and other structures that it surrounds and to allow relative movement between these structures. During an infection, this cushioning and lubricating tissue has the potential to become greatly edematous in response to the exudation of tissue fluid and then to become indurated as polymorphonuclear leukocytes, lymphocytes, and macrophages migrate from the vascular space into the infected interstitial spaces. Ultimately, liquefactive necrosis of white blood cells and this connective tissue leads to abscess formation, and spontaneous or surgical drainage typically leads to resolution. This is the pathophysiology of the stages of infection that clinicians see as edema, when the bacteria are inoculating the tissues of a particular anatomic space; cellulitis, when an intense inflammatory response causes all of the classic signs of inflammation; and abscess, when small areas of liquefactive necrosis coalesce centrally to form pus within the tissues.
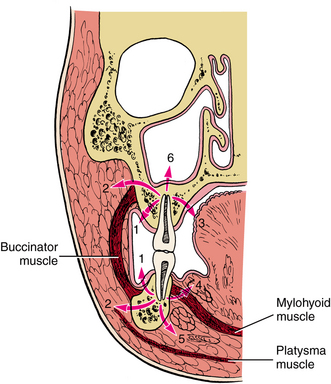
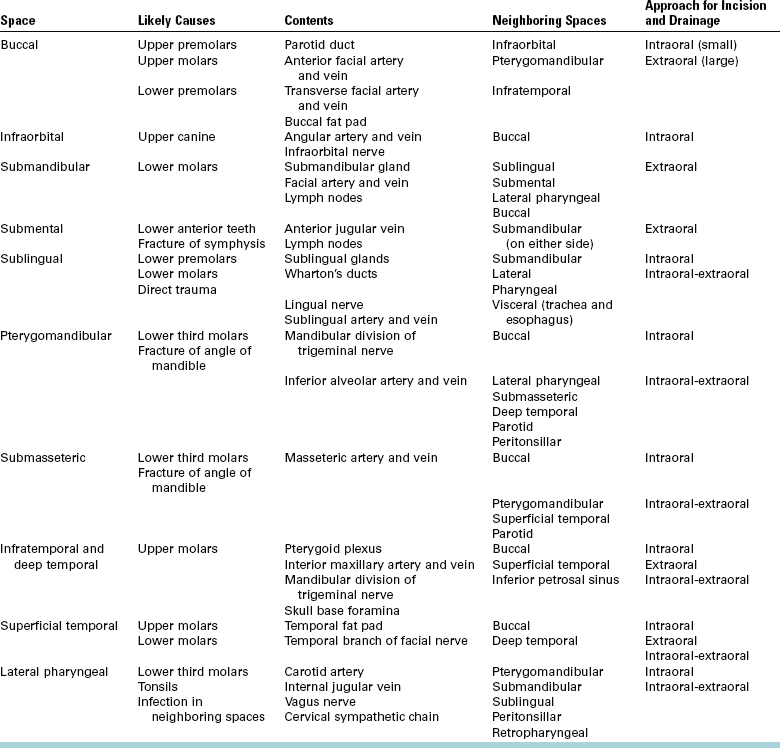
FIGURE 16-1 As infection erodes through bone, it can express itself in a variety of places depending on thickness of overlying bone and relationship of muscle attachments to site of perforation. This illustration notes six possible locations: vestibular abscess (1), buccal space (2), palatal abscess (3), sublingual space (4), submandibular space (5), and maxillary sinus (6). (From Cummings CW, Fredrickson JM, Harker LA, et al, editors: Otolaryngology: head and neck surgery, ed 3, vol 3, St Louis, 1998, Mosby.)
Based on the relationship between the point at which the infection erodes through the alveolar bone and the surrounding muscle attachments, infections arising from any maxillary or mandibular tooth can cause vestibular, buccal, or subcutaneous space infections. Infections passing beyond the alveolar process on the deep (toward the oral cavity) side of the nearby muscle of facial expression invade the vestibular space, and those that enter the soft tissues on the superficial (toward the skin) side of those muscles enter the buccal or the subcutaneous space. Infections arising from the maxillary teeth also tend to spread into the infraorbital, palatal, orbital, and infratemporal spaces, and the maxillary sinus (Box 16-1). Mandibular dental infections also tend to spread into the submandibular, sublingual, submental, and masticator spaces. Infections can extend beyond these primary spaces into the deeper fascial spaces of the neck, such as the lateral pharyngeal, retropharyngeal, carotid, and pretracheal spaces. From there, such infections can spread into the danger space and the mediastinum. In addition, infections can rise superiorly through the sinuses or vascular structures to invade the brain or the intracranial dural sinuses, such as the cavernous sinus.
Infections of the deep fascial spaces can be classified as having low, moderate, or high severity according to their likelihood of threatening the airway or other vital structures. Low-severity infections are not likely to threaten the airway or vital structures. Moderate-severity infections hinder access to the airway by causing trismus or elevation of the tongue, which can make endotracheal intubation difficult. High-severity infections can directly compress or deviate the airway or damage vital organs, such as the brain, heart, or lungs. Determining the exact anatomic location of infection is a key step in determining its severity. A classification of the deep fascial spaces according to their severity is listed in Box 16-2. Examples of low-, moderate-, and high-severity infections are shown in Figures 16-2 to 16-4. Table 16-1 lists the anatomic borders of the more commonly infected deep fascial spaces of the head and neck.
TABLE 16-1
Borders of the Deep Fascial Spaces of the Head and Neck
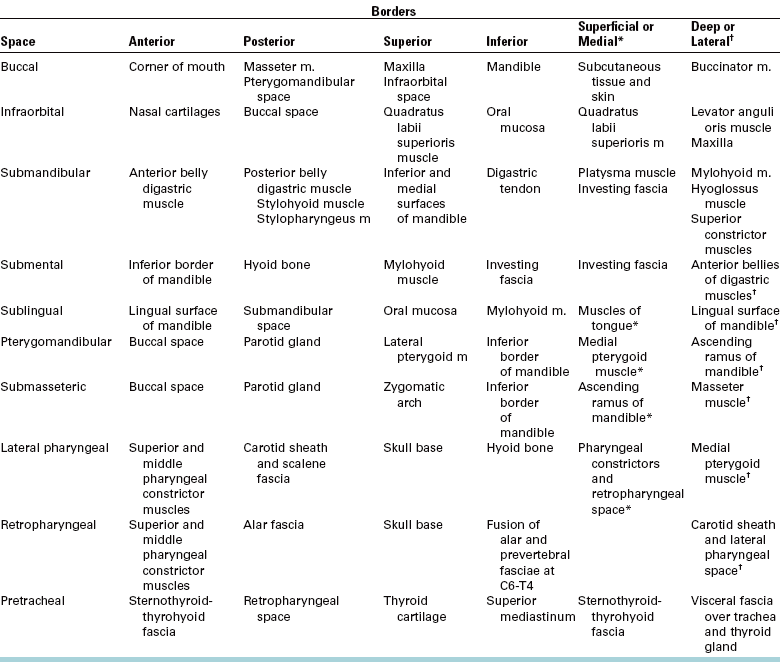
From Flynn TR: Anatomy of oral and maxillofacial infections. In Topazian RG, Goldberg MH, Hupp JR, editors: Oral and maxillofacial infections, ed 4, Philadelphia, 2002, WB Saunders.
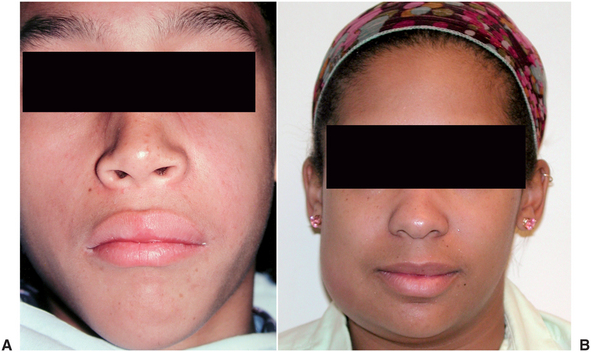
FIGURE 16-2 Low-severity infections that are not likely to threaten the airway or vital structures. A, Vestibular space abscess under the upper lip. B, Cellulitis of the space of the body of the mandible. (A from Flynn TR: Anatomy of oral and maxillofacial infections. In Topazian RG, Goldberg MH, Hupp JR, editors: Oral and maxillofacial infections, ed 4, Philadelphia, 2002, WB Saunders.)
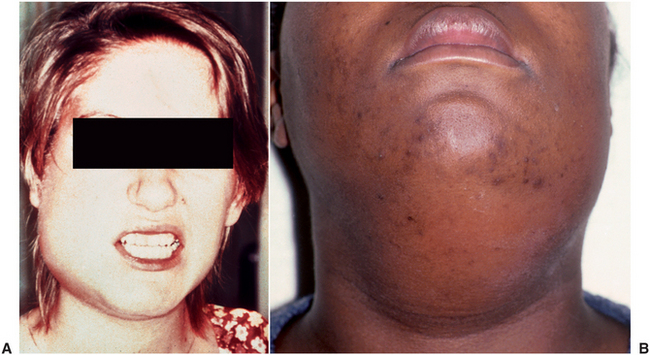
FIGURE 16-3 Moderate-severity infections that hinder access to the airway. A Submasseteric space abscess that is causing severe trismus. B Cellulitis of the submandibular and submental spaces. (A from Goldberg MH: Odontogenic infections and deep fascial space infections of odontogenic origin. In Topazian RG, Goldberg MH, Hupp JR, editors: Oral and maxillofacial infections, ed 4, Philadelphia, 2002, WB Saunders; B from Flynn TR: Surgical management of orofacial infections. Atlas Oral Maxillofac Surg Clin North Am 8:77-100, March 2000.)
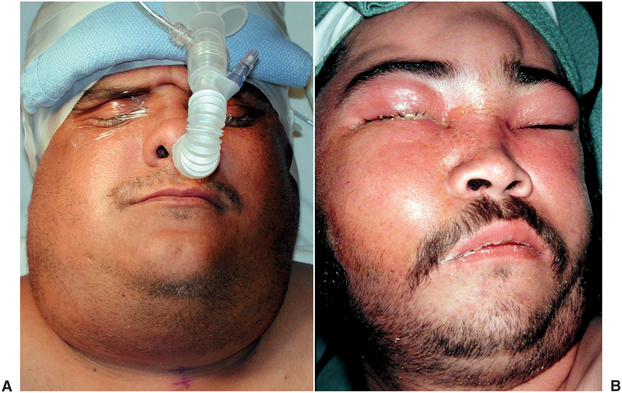
FIGURE 16-4 High-severity infections that are likely to obstruct the airway or threaten vital structures. A Lateral pharyngeal space abscess. B Cavernous sinus thrombsis. (From Flynn TR, Topazian RG: Infections of the oral cavity. In Waite D, editor: Textbook of practical oral and maxillofacial surgery, Philadelphia, 1987, Lea & Febiger.) Lea & Febiger
Infections Arising from Any Tooth
As discussed in Chapter 15 and previously in this chapter, maxillary or mandibular teeth can cause infections of the buccal, vestibular, or subcutaneous spaces. The buccal space is actually a portion of the subcutaneous space, which extends from head to toe. Thus a long-standing buccal space abscess tends to drain spontaneously through the skin at its inferior extent near the inferior border of the mandible. Table 16-2 lists the likely causative teeth, contents, neighboring spaces into which infection may spread, and surgical approaches for drainage of the more commonly infected deep fascial spaces of the head and neck.
Infections Arising from the Maxillary Teeth
Because the apices of the upper lateral incisors and the palatal roots of upper premolars and molars are closest to the palatal cortical plate, infection arising from these teeth can erode through the bone without perforating the periosteum. The potential subperiosteal space in the palate is the palatal space.
The infraorbital space is a thin potential space between the levator anguli oris and the levator labii superioris muscles. The infraorbital space becomes involved primarily as the result of infections from the maxillary canine tooth or by extension of infections from the buccal space. The canine root is often sufficiently long to allow erosion to occur through the alveolar bone superior to the origin of the levator anguli oris and below the origin of the levator labii superioris muscle. When this space is infected, swelling of the anterior face obliterates the nasolabial fold (Fig. 16-5). Spontaneous drainage of infections of this space commonly occurs near the medial or the lateral canthus of the eye because the path of least resistance is to either side of the levator labii superioris muscle, which attaches along the center of the inferior orbital rim.
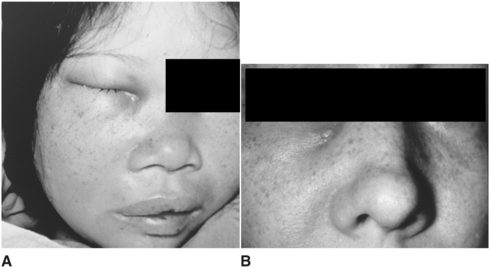
FIGURE 16-5 A Infraorbital space abscess that is about to drain medial to the attachment of the levator labii superioris muscle. B Infraorbital space abscess with chronic cutaneous drainage medial to the levator labii superioris muscle. (A and B from Goldberg MH: Odontogenic infections and deep fascial space infections of odontogenic origin. In Topazian RG, Goldberg MH, editors: Oral and maxillofacial infections, ed 3, Philadelphia, 1993, WB Saunders, p. 218, with permission.)
The buccal space is bounded by the overlying skin of the face on the lateral aspect and the buccinator muscle on the medial aspect (Fig. 16-6). This space may become infected from extensions of infection from maxillary teeth through the bone superior to the attachment of the buccinator on the alveolar process of the maxilla. The posterior maxillary teeth, most commonly the molars, cause most buccal space infections.
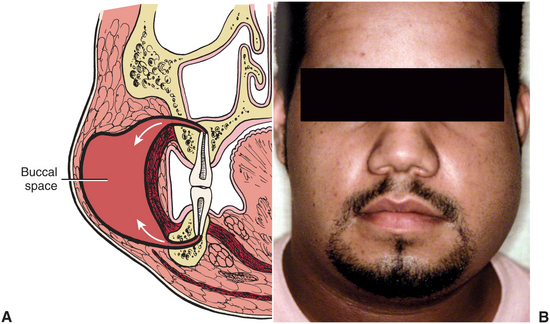
FIGURE 16-6 A Buccal space lies between buccinator muscle and overlying skin and superficial fascia. This potential space may become involved via maxillary or mandibular molars (arrows). B Typical buccal space infection, extending from the level of the zygomatic arch to the inferior border of the mandible and from the oral commissure to the anterior border of the masseter muscle.(From Flynn TR: The swollen face. Emerg Med Clin North Am 15:481-519, Aug 2000.)
Involvement of the buccal space usually results in swelling below the zygomatic arch and above the inferior border of the mandible. Infections may follow the extensions of the buccal fat pad into the superficial temporal space, the infratemporal space, the infraorbital space, and the periorbital space, as illustrated in Figure 16-7. Because the fascial layers that pass over the zygomatic arch are tightly bound down to the bone, swelling above and below the zygomatic arch can cause the relatively dimpled appearance over the left zygomatic arch in Figure 16-7. The zygomatic arch and the inferior border of the mandible remain palpable in buccal space infections.
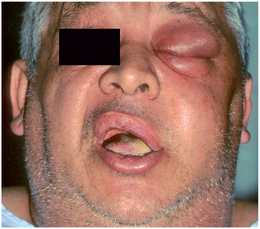
FIGURE 16-7 A buccal space infection that has followed the extensions of the buccal fat pad into the infraorbital, periorbital, and superficial temporal spaces. (From Flynn TR: The swollen face. Emerg Med Clin North Am 15:481-519, Aug 2000.)
The infratemporal space lies posterior to the maxilla. The space is bounded medially by the lateral pterygoid plate of the sphenoid bone and superiorly by the base of the skull. Laterally and superiorly, the infratemporal space is continuous with the deep temporal space (Fig. 16-8). Essentially, the infratemporal space is the bottom portion of the deep temporal space. The space contains branches of the internal maxillary artery and the pterygoid venous plexus. Importantly, emissary veins from the pterygoid plexus pass through foramina in the base of the skull to connect with the intracranial dural sinuses. Because the veins of the face and the orbit do not have valves, blood-borne infections may pass superiorly or inferiorly along their course. The infratemporal space is the origin of the posterior route by which infections may spread into the cavernous sinus (Fig. 16-9). The infratemporal space is rarely infected, but when it is, the cause is usually an infection of the maxillary third molar.
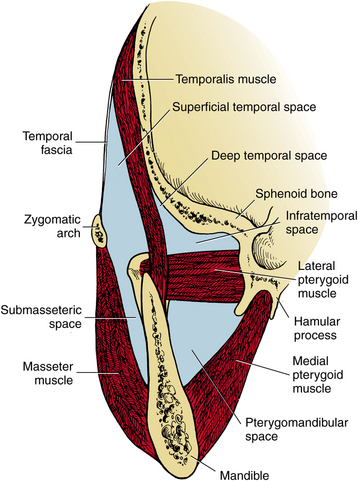
FIGURE 16-8 The masticator space is bounded by the fascia overlying the masseter muscle, medial pterygoid muscle, temporalis muscle, and the skull. The superficial and deep temporal spaces are separated from each other by the temporalis muscle. The lateral pterygoid muscle divides the pterygomandibular space from the infratemporal portion of the deep temporal space, and the zygomatic arch divides the submasseteric space from the superficial temporal space. (Redrawn from Cummings CW, Fredrickson JM, Harker LA et al, editors: Otolaryngology: head and neck surgery, vol 3, St Louis, 1998, Mosby.)
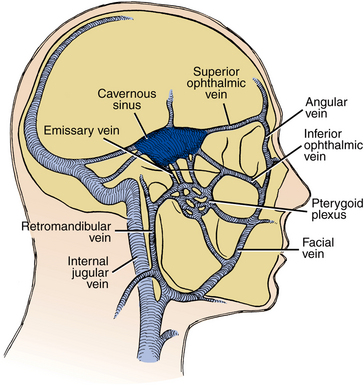
FIGURE 16-9 Hematogenous spread of infection from the jaw to the cavernous sinus may occur anteriorly via the inferior or superior ophthalmic vein or posteriorly via emissary veins from the pterygoid plexus. (From Cummings CW, Fredrickson JM, Harker LA et al, editors: Otolaryngology: head and neck surgery, vol 3, St Louis, 1998, Mosby.)
Periapical or periodontal infections of maxillary posterior teeth may erode superiorly through the floor of the maxillary sinus. Approximately 20% of cases of maxillary sinusitis are odontogenic. Odontogenic maxillary sinus infections may also spread superiorly through the ethmoid sinus or the orbital floor to cause secondary periorbital or orbital infections. Periorbital or orbital infection rarely occurs as the result of odontogenic infection, but when either does occur, the presentation is typical: redness and swelling of the eyelids and involvement of the vascular and neural components of the orbit. This is a serious infection and requires aggressive medical and surgical intervention from an oral and maxillofacial surgeon and other specialists. Figure 16-10 shows the clinical and radiographic appearance of a case of odontogenic infection extending from the maxillary sinus through the ethmoid sinus into the orbit.
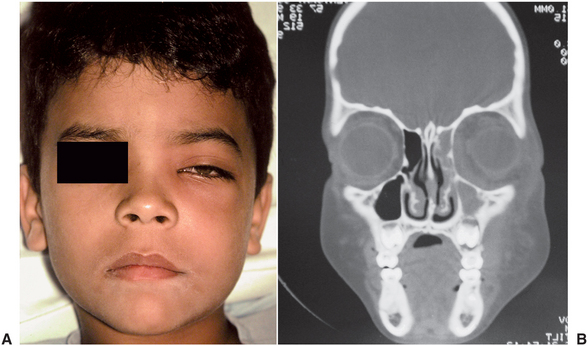
FIGURE 16-10 Subperiosteal orbital infection arising from an infected primary molar. A Note the periorbital erythema and the lateral displacement of the pupil. B Computed tomography scan demonstrating opacification of the right maxillary and ethmoid sinuses and subperiosteal thickening along the medial and inferior orbital walls. (From Flynn TR, Piecuch JF, Topazian RG: Infections of the oral cavity. In Feigin RD, Cherry JD, editors: Textbook of pediatric infectious diseases, ed 4, Philadelphia, 1998, JB Lippincott.)
When maxillary odontogenic infections erode into the infraorbital vein in the infraorbital space or the inferior ophthalmic vein via the sinuses, they can follow the common ophthalmic vein through the superior orbital fissure and extend directly into the cavernous sinus. This is the anterior route to the cavernous sinus. Intravascular inflammation caused by the invading bacteria stimulates the clotting pathways, resulting in a septic cavernous sinus thrombosis. Cavernous sinus thrombosis is an unusual occurrence that is rarely the result of an infected tooth. Like orbital cellulitis, cavernous sinus thrombosis is a serious, life-threatening infection that requires aggressive medical and surgical care. Cavernous sinus thrombosis has a high mortality even today. Figure 16-11 illustrates the rapid extension of a case of orbital cellulitis into cavernous sinus thrombosis; fortunately, the most vulnerable structure in the cavernous sinus, the abducens (sixth cranial) nerve, was spared.
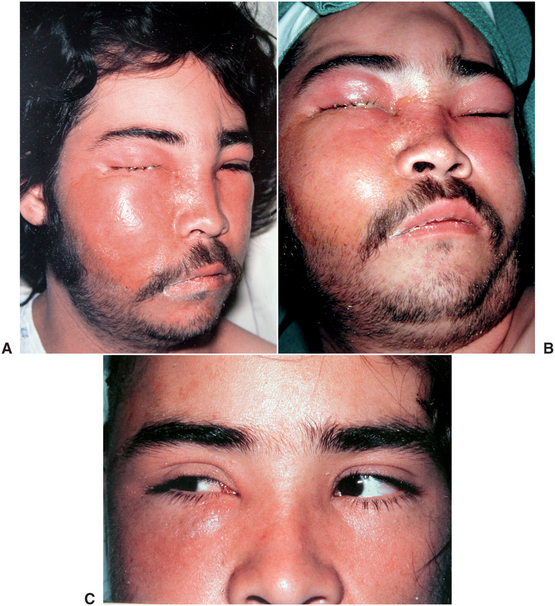
FIGURE 16-11 Cavernous sinus thrombosis. A Infraorbital and buccal space abscess with extension into the periorbital and orbital spaces. B The same patient, 4 hours later, with continued spread of the infection to the superficial and deep temporal spaces, cavernous sinus, and the opposite orbit. C The same patient, 2 weeks later. On voluntary gaze toward the affected right side, both eyes turn toward the right, demonstrating that the right abducens nerve was not injured. (A and B from Flynn TR, Topazian RG: Infections of the oral cavity. In Waite D, editor: Textbook of practical oral and maxillofacial surgery, Philadelphia, 1987, Lea & Febiger.) Lea & Febiger
Infections Arising from the Mandibular Teeth
Although many infections arising from the mandibular teeth erode into the vestibular space, they may also spread into other deep fascial spaces. Initially, such mandibular infections tend to enter the space of the body of the mandible, the submandibular, sublingual, submental, or masticator spaces. From there, severe infections can spread into the deep fascial spaces of the neck and even extend into the mediastinum to threaten the heart, lungs, and great vessels.
The space of the body of the mandible, like the palatal space, is a subperiosteal space. Thus if an infection erodes through the buccal cortical bone but does not perforate the periosteum, it can essentially peel the periosteal layer of soft tissue off the bony surface. Clinically, this results in a swelling that assumes the shape of the underlying mandible. It can appear as if the bone itself has been enlarged, as in Figure 16-12.

FIGURE 16-12 Space of the body of the mandible infection. It appears as if the mandible itself is enlarged by the subperiosteal collection of fluid. (From Flynn TR: The swollen face. Emerg Med Clin North Am 15:481-519, Aug 2000.)
If an infection arising from a mandibular posterior tooth perforates the buccal cortical bone and the periosteum inferior to the attachment of the buccinator muscle, then the buccal space is involved (see Fig. 16-6, A).
In their landmark work on the anatomy of the deep fascial spaces of the head and neck, Grodinsky and Holyoke1–3 identified the submaxillary space as one large space that encompasses the three anatomic spaces referred to separately today as the submandibular, sublingual, and submental spaces. One may collectively refer to them as the perimandibular spaces. The sublingual and submandibular spaces have the medial border of the mandible as their lateral boundary. These two spaces are involved primarily by lingual perforation of infection from the mandibular molars, although they may be involved by premolars as well. The factor that determines whether the infection is submandibular or sublingual is the attachment of the mylohyoid muscle on the mylohyoid ridge of the medial aspect of the mandible (Fig. 16-13). If the infection erodes through the medial aspect of the mandible above this line, the infection will be in the sublingual space. This is most commonly seen with premolars and the first molar. If the infection erodes through the medial aspect of the mandible inferior to the mylohyoid line, the submandibular space will be involved. The mandibular third molar is the tooth that most commonly involves the submandibular space primarily. The second molar may involve the sublingual or submandibular space, depending on the length of the individual roots.

FIGURE 16-13 Mylohyoid line is area of attachment of mylohyoid muscle. Linguocortical plate perforation by infection from premolars and first molar causes sublingual space infection, whereas infection from third molar involves submandibular space. (From Cummings CW, Fredrickson JM, Harker LA et al, editors: Otolaryngology: head and neck surgery, vol 3, St Louis, 1998, Mosby.)
The sublingual space lies between the oral mucosa of the floor of the mouth and the mylohyoid muscle (Fig. 16-14, A). The posterior border of the sublingual space is open, and therefore it freely communicates with the submandibular space. Clinically, little or no extraoral swelling is produced by an infection of the sublingual space, but much intraoral swelling is seen in the floor of the mouth on the infected side. The infection usually becomes bilateral, and the tongue becomes elevated (Fig. 16-14, B).
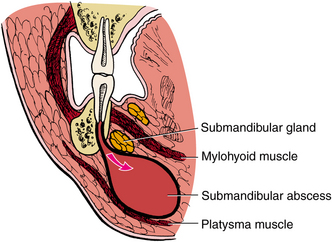
FIGURE 16-14 A The sublingual space lies between the oral mucosa and the mylohyoid muscle. The space is primarily involved by infection from mandibular premolars and first molar. B Severe sublingual space abscess that has elevated the tongue into the palate such that only the ventral surface of the tongue and floor of the mouth are visible. (From Flynn TR, Topazian RG: Infections of the oral cavity. In Waite D, editor: Textbook of practical oral and maxillofacial surgery, Philadelphia, 1987, Lea & Febiger.)
The submandibular space lies between the mylohyoid muscle and the overlying superficial layer of the deep cervical fascia (Fig. 16-15). The posterior extent of the submandibular space communicates with the deep fascial spaces of the neck. Infection of the submandibular space causes swelling that can look like an inverted triangle, with the base at the inferior border of the mandible, the sides determined by the anterior and posterior bellies of the digastric muscle, and the apex at the hyoid bone (Fig. 16-16).
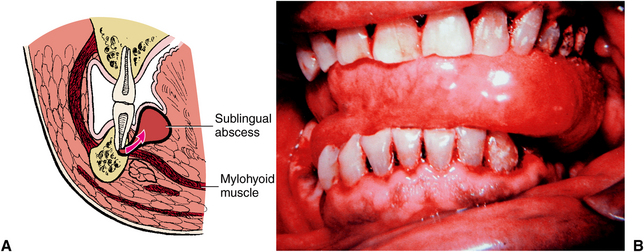
FIGURE 16-15 The submandibular space lies between the mylohyoid muscle and anterior layer of the deep cervical fascia, just deep to the platysma muscle, and includes the lingual and inferior surfaces of the mandible below the mylohyoid muscle attachment. (From Cummings CW, Fredrickson JM, Harker LA et al, editors: Otolaryngology: head and neck surgery, vol 3, St Louis, 1998, Mosby.)
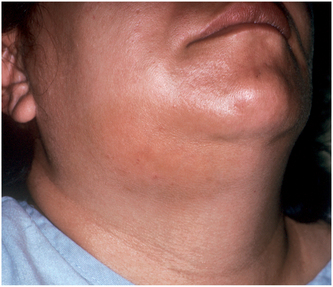
FIGURE 16-16 Typical submandibular space infection demarcated by both bellies of the digastric muscle, the inferior border of the mandible, and the hyoid bone. (From Flynn TR: The swollen face. Emerg Med Clin North Am 15:481-519, Aug 2000.)
The submental space lies between the anterior bellies of the right and left digastric muscles and between the mylohyoid muscle and the overlying fascia (Fig. 16-17). Isolated submental space infections are rare, caused by infections of the mandibular incisors. More commonly, submental space involvement is the result of the spread of a submandibular space infection, which can easily pass around the anterior belly of the digastric muscle to enter the submental space. Such an aggressive infection can then easily pass from the submental space to the contralateral submandibular space to involve all three spaces.
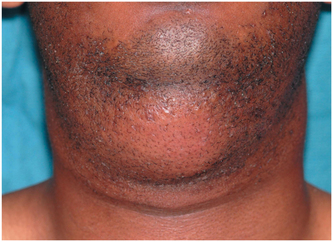
FIGURE 16-17 Submental space infection appears as discrete swelling in central area of submandibular region.
When the perimandibular spaces (submandibular, sublingual, and submental) are bilaterally involved in an infection, it is known as Ludwig’s angina. This infection is a rapidly spreading cellulitis that can obstruct the airway and commonly spreads posteriorly to the deep fascial spaces of the neck.
Severe swelling is almost always seen, with elevation and displacement of the tongue, and a tense, hard, bilateral induration of the submandibular region superior to the hyoid bone. The patient usually has trismus, drooling of saliva, and difficulty with swallowing and sometimes breathing. The patient often experiences severe anxiety concerning the inability to swallow and maintain an airway. This infection may progress with alarming speed and thus may produce upper airway obstruction that often leads to death. The most common cause of Ludwig’s angina is an odontogenic infection. In the 1940s, before penicillin was available, Williams4 and Williams and Guralnick5 were able to reduce the mortality of Ludwig’s angina from 54% to 10% by instituting a protocol of initially securing the airway and then performing early and aggressive I&D procedures. Their landmark studies established the principles of airway security and prompt, aggressive surgery and demonstrated that antibiotic therapy plays only a supportive role in the management of severe odontogenic infections.
The submandibular and sublingual spaces join together at the posterior edge of the mylohyoid muscle. At the posterior edge of this junction is the buccopharyngeal gap, where the styloglossus and stylohyoid muscles pass between the superior and middle pharyngeal constrictor muscles on their way to the tongue and the hyoid bone, respectively. Submandibular or sublingual space infections can pass through the buccopharyngeal gap to enter the lateral pharyngeal space, which is one of the deep fascial spaces of the neck. In addition, submandibular space infections can pass around the posterior belly of the digastric muscle to enter the lateral pharyngeal space directly. These are the pathways by which submandibular and sublingual space infections can spread into the deep fascial spaces of the neck and beyond.
In most of the reported case series of severe odontogenic infections, the submandibular space is the most frequently involved. In a recent series of severe odontogenic infections requiring hospitalization, however, the submandibular space was involved in 54% of cases, and the masticator space was involved in 78% of cases. The pterygomandibular portion of the masticator space was involved in 60% of cases. The mandibular third molar is the most commonly associated tooth, and it frequently causes infections of the pterygomandibular portion of the masticator space.6,7
The masticator space is formed by the splitting of the anterior layer of the deep cervical fascia, also called the superficial or the investing layer of the deep cervical fascia, to surround the muscles of mastication. This fascia separates at the inferior border of the mandible to pass laterally over the masseter muscle and medially over the medial surface of the medial pterygoid muscle. Medially, this fascia terminates at its attachment to the pterygoid plates and sphenoid bone. Laterally, this fascia, locally called the parotideomasseteric fascia, rises over the masseter muscle and fuses with the periosteum over the zygomatic arch. Above the zygomatic arch, this fascia, locally called the temporalis fascia, rises over the lateral surface of the temporalis muscle, and terminates at the insertion of the temporalis muscle on the cranium. This fascial envelope, and the surfaces of the skull that form its medial border, is the masticator space. Within this space, there are four compartments that are referred to as separate spaces. They are the submasseteric space, between the masseter muscle and the lateral surface of the ascending ramus of the mandible; the pterygomandibular space, between the medial pterygoid muscle and the medial surface of the ascending ramus; the superficial temporal space, between the temporalis fascia and the temporalis muscle; and the deep temporal space, between the temporalis muscle and the skull. The zygomatic arch separates the submasseteric and superficial temporal spaces, and the lateral pterygoid muscle separates the pterygomandibular and the deep temporal spaces. The infratemporal space is actually the inferior portion of the deep temporal space, between the lateral pterygoid muscle and the infratemporal crest of the sphenoid bone. These four compartments of the masticator space clinically behave like separate spaces because in most cases only one compartment of the masticator space becomes infected. However, particularly severe or long-standing masticator space infections can involve all four compartments, as illustrated in Figure 16-18.
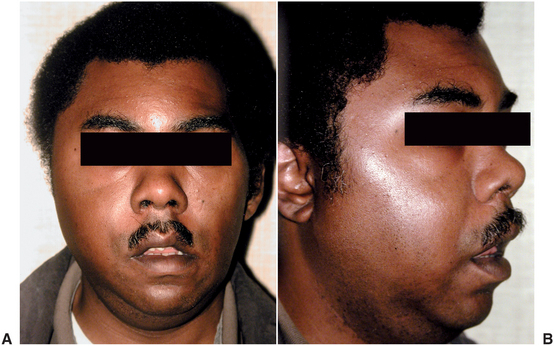
FIGURE 16-18 Masticator space infection, with all compartments involved. A Frontal view demonstrating the swelling anterior to and obscuring the ear and in the temporal region. B Oblique view demonstrating the dimpling of the swelling over the zygomatic arch, with temporal and submasseteric swelling above and below. (A from Flynn TR: Emerg Med Clin North Am 15:499, Aug 2000.)
The submasseteric space is involved by infection most commonly as the result of spread from the buccal space or from soft tissue infection around the mandibular third molar (pericoronitis). Occasionally, an infected mandibular angle fracture causes a submasseteric space infection. When the submasseteric space is involved, the masseter muscle also becomes inflamed and swollen, as seen clinically and radiographically in Figure 16-19. Because of the involvement of the masseter muscle, the patient also has moderate to severe trismus caused by inflammation of the masseter muscle.
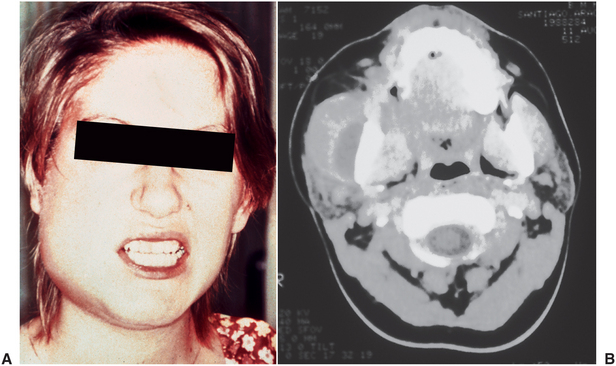
FIGURE 16-19 Submasseteric space abscess. A Severe trismus. B Computed tomography image of a submasseteric space abscess that illustrates the fluid between the edematous masseter muscle and the ascending ramus of the mandible. (A from Goldberg MH: Odontogenic infections and deep fascial space infections of odontogenic origin. In Topazian RG, Goldberg MH, Hupp JR, editors: Oral and maxillofacial infections, ed 4, Philadelphia, 2002, WB Saunders; B from Flynn TR: Anatomy of oral and maxillofacial infections. In Topazian RG, Goldberg MH, Hupp JR, editors: Oral and maxillofacial infections, ed 4, Philadelphia, 2002, WB Saunders.)
The pterygomandibular space is the site into which local anesthetic solution is injected when an inferior alveolar nerve block is performed. Infections of this space spread primarily from the mandibular third molar. When the pterygomandibular space alone is involved, little or no facial swelling is observed; however, the patient almost always has significant trismus. Therefore, trismus without swelling is a valuable diagnostic clue for pterygomandibular space infection. On physical examination, with a good light and a tongue depressor, the clinician can see swelling and erythema of the anterior tonsillar pillar on the affected side and deviation of the uvula to the opposite side. On computed tomography examination, fluid collection may be detected between the medial pterygoid muscle and the mandible; the airway is often compressed and deviated by the swelling, as shown in Figure 16-20. Occasionally, this clinical picture is caused by needle tract infection from a mandibular block.
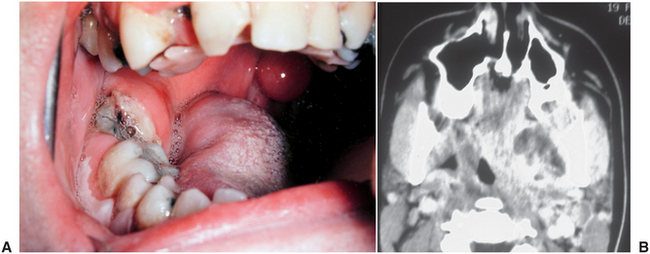
FIGURE 16-20 A Pterygomandibular space abscess due to a carious lower third molar, with swelling of the anterior tonsillar pillar and deviation of the uvula to the opposite side. B Computed tomography scan of a pterygomandibular space abscess due to a lower third molar. Note the fluid collection between the distended medial pterygoid muscle and the ascending ramus of the mandible, as well as the displacement and compression of the airway. (A from Flynn TR, Topazian RG: Infections of the oral cavity. In Waite D, editor: Textbook of practical oral and maxillofacial surgery, Philadelphia, 1987, Lea & Febiger; B from Flynn TR: The swollen face. Emerg Med Clin North Am 15:481-519, Aug 2000.)
The superficial and deep temporal spaces rarely become infected and usually only in severe infections. When these spaces are involved, the swelling that occurs is evident in the temporal region, superior to the zygomatic arch and posterior to the lateral orbital rim. The tight attachment of the anterior layer of the deep cervical fascia to the zygomatic arch prevents swelling there, so that when either of the temporal spaces plus the submasseteric space are infected, an hourglass shape can be detected in the frontal view, as shown in Figures 16-7 and 16-18.
Deep Cervical Fascial Space Infections
Extension of odontogenic infections beyond the spaces described before is an uncommon occurrence. However, when it does happen, involvement of the deep cervical spaces may have serious life-threatening sequelae. Infection of the deep fascial spaces of the neck can compress, deviate, or completely obstruct the airway, invade vital structures such as the major vessels, and allow extension of the infection into the mediastinum and the vital structures it contains.
Infection extending posteriorly from the pterygomandibular, submandibular, or sublingual spaces first encounters the lateral pharyngeal space. This space extends from the base of the skull at the sphenoid bone to the hyoid bone inferiorly. The space is medial to the medial pterygoid muscle and lateral to the superior pharyngeal constrictor muscle (Fig. 16-21). The space is bounded anteriorly by the pterygomandibular raphe and extends posteromedially to the retropharyngeal space. The styloid process and associated muscles and fascia divide the lateral pharyngeal space into an anterior compartment, which contains primarily loose connective tissue, and a posterior compartment, which contains the carotid sheath and cranial nerves IX (glossopharyngeal), X (vagus), and XII (hypoglossal).
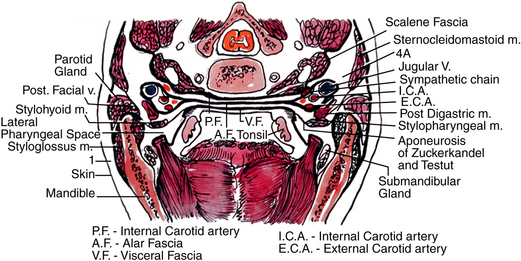
FIGURE 16-21 The lateral pharyngeal space is located between medial pterygoid muscle laterally and superior pharyngeal constrictor medially. The retropharyngeal and danger spaces lie between the pharyngeal constrictor muscles and the prevertebral fascia. The retropharyngeal space lies between the superior constrictor muscle and the alar fascia. The danger space lies between the alar layer and the prevertebral fascia. (From Flynn TR: Anatomy and surgery of deep fascial space infections. In Kelly JJ, editor: Oral and maxillofacial surgery knowledge update 1994, Rosemont, IL, 1994, American Association of Oral and Maxillofacial Surgeons.)
The clinical findings of lateral pharyngeal space infection include trismus as the result of inflammation of the medial pterygoid muscle; lateral swelling of the neck, especially between the angle of the mandible and the sternocleidomastoid muscle; and swelling of the lateral pharyngeal wall, toward the midline. Patients who have lateral pharyngeal space infections have difficulty swallowing and usually have a high temperature and become very sick. Figure 16-22 illustrates the clinical and computed tomography radiographic appearance of patients with lateral pharyngeal space infection.
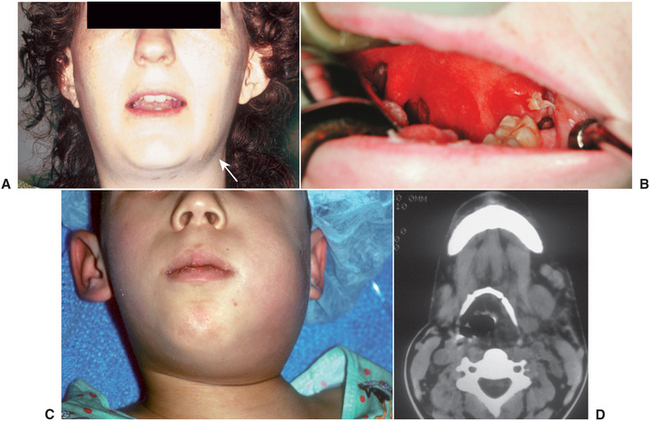
FIGURE 16-22 Lateral pharyngeal space abscess. A Left lateral pharyngeal space abscess with extraoral swelling (arrow) and trismus. B Intraoral view of the same patient, illustrating swelling of the anterior tonsillar pillar and blunting of the palatouvular fold. C A boy with a left lateral pharyngeal space abscess who is deviating his head toward the right shoulder in order to place the upper airway over his deviated trachea. D Computed tomography at the level of the hyoid bone, showing a lateral pharyngeal space infection that is deviating the airway to the opposite side. (From Flynn TR: Anatomy of oral and maxillofacial infections. In Topazian RG, Goldberg MH, Hupp JR, editors: Oral and maxillofacial infections, ed 4, Philadelphia, 2002, WB Saunders.)
Patients who have infection of the lateral pharyngeal space have several serious potential problems. When the lateral pharyngeal space is involved, the odontogenic infection is severe and may be progressing at a rapid rate. Another possible problem is the direct effect of the infection on the contents of the space, especially those of the posterior compartment. These problems include thrombosis of the internal jugular vein, erosion of the carotid artery or its branches, and interference with cranial nerves IX, X, and XII. A third serious complication arises if the infection progresses from the lateral pharyngeal space to the retropharyngeal space or beyond.
The retropharyngeal space lies behind the soft tissue of the posterior aspect of the pharynx. The retropharyngeal space is bounded anteriorly by the pharyngeal constrictor muscles and the retropharyngeal fascia and posteriorly by the alar fascia (see Fig. 16-21).
The retropharyngeal space begins at the base of the skull and ends inferiorly at a variable point between the sixth cervical (C6) and fourth thoracic (T4) vertebrae, where the alar fascia fuses anteriorly with the retropharyngeal fascia (Fig. 16-23). The retropharyngeal space contains only loose connective tissue and lymph nodes, so it provides little barrier to the spread of infection from one lateral pharyngeal space to the other to encircle the airway, as illustrated in Figure 16-24. Moreover, when the retropharyngeal space becomes involved, the major concern is that the infection can rupture the alar fascia posteriorly to enter the danger space (Fig. 16-25).
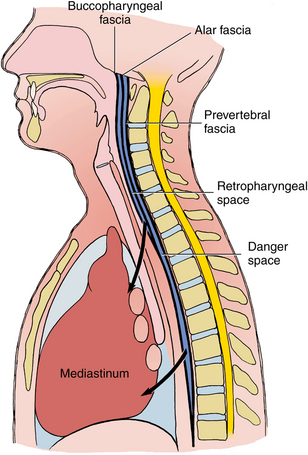
FIGURE 16-23 The retropharyngeal and the alar fascia fuse at a variable level between the C6 and T4 vertebrae, which forms a pouch at the inferior extent of the retropharyngeal space. If infection passes through the alar fascia to the danger space, the posterosuperior mediastinum will most likely soon become involved. The inferior boundary of the danger space is the diaphragm, which puts the entire mediastinum at risk.
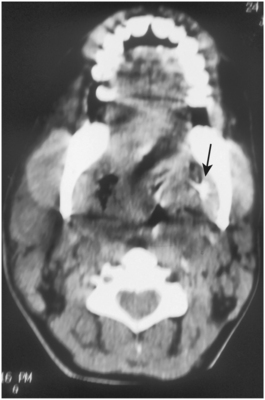
FIGURE 16-24 Postoperative computed tomography scan of a patient with a previously placed drain (arrow) in the left pterygomandibular space. The infection has now spread through the left lateral pharyngeal space and the retropharyngeal space to the right lateral pharyngeal space, thus encircling, compressing, and deviating the airway. (From Flynn TR: Surgical management of orofacial infections. Atlas Oral Maxillofac Surg Clin North Am 8:77-100, March 2000.)
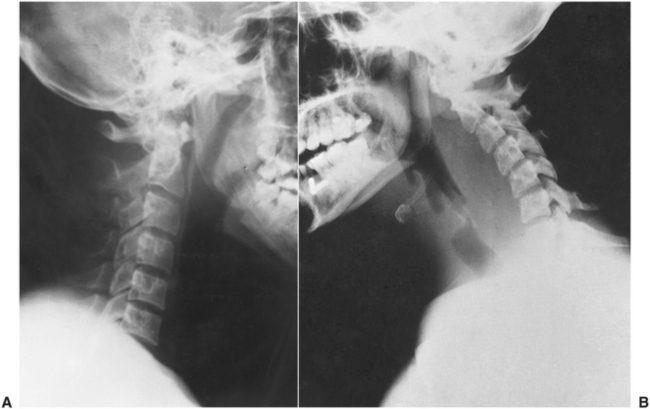
FIGURE 16-25 A The normal retropharyngeal soft tissue shadow is narrow (6 mm or less) at C2 and 20 mm or less at C6 on plain x-ray films. B When the retropharyngeal space is infected, the soft tissue becomes substantially thicker, and the width of the oropharyngeal air shadow decreases. (From Cummings CW, Fredrickson JM, Harker LA et al, editors: Otolaryngology: head and neck surgery, vol 3, St Louis, 1998, Mosby.)
The danger space lies between the alar fascia anteriorly and the prevertebral fascia posteriorly. The danger space extends from the base of the skull to the diaphragm, and it is continuous with the posterior mediastinum (Fig. 16-23). The prevertebral space is rarely involved in odontogenic infections because the prevertebral fascia fuses with the periosteum of the vertebral bodies. Prevertebral space infections are usually caused by osteomyelitis of the vertebrae.
The mediastinum is the space between the lungs, and it contains the heart, the phrenic and vagus nerves, the trachea and the main stem bronchi, the esophagus, and the great vessels, including the aorta and the inferior and superior vena cava. A patient with mediastinitis can have an overwhelming infection that compresses the heart and lungs; interferes with the neurologic control of heart rate and respiration; ruptures into the lung, trachea, or esophagus; and even spreads into the abdominal cavity. The mortality of mediastinitis is high, even with modern methods of care, such as open thoracic surgical drainage and close follow-up with serial computed tomography scans.
Management of Fascial Space Infections
Management of infections, mild or severe, always has five general goals: (1) medical support of the patient, with special attention to protection of the airway and correcting host defense compromises where they exist; (2) surgical removal of the source of infection as early as possible; (3) surgical drainage of the infection, with proper placement of drains; (4) administration of correct antibiotics in appropriate doses; and (5) frequent reevaluation of the patient’s progress toward resolution. Although the intensity of treatment is greater in complex odontogenic infections, the principles of surgical and medical management of fascial space infections are the same as those for less serious infections; these principles are described in detail in Chapter 15 and are summarized in Box 16-3. Conscientious application of these principles cannot guarantee an ideal result in any given case, but it should ensure that the standard of care has been met.
The patient’s airway must be continually monitored, and endotracheal intubation or tracheotomy should be performed if warranted. Airway security is the prime concern in the management of severe odontogenic infections. Medical management of the patient with a serious infection must include a thorough assessment and support of host defense mechanisms, including analgesics, fluid requirements, and nutrition. High-dose bactericidal antibiotics are usually necessary and are almost always administered intravenously.
Several large studies, performed as early as the 1950s, have shown that extraction of teeth in the presence of infection hastens the resolution of the infection and reduces the morbidity of the infection by measures such as decreased time out of work, shortened or avoided hospitalization, and reduced need for extraoral I&D. Occasionally, tooth extraction has been blamed for subsequent severe infections requiring hospitalization. In actuality, the infection that necessitated the tooth extraction had most likely become severe enough to warrant hospital care and more aggressive surgery.
Surgical management of fascial space infections almost always requires a generous incision and aggressive exploration of the involved fascial spaces with a hemostat. One or more drains are usually required to provide adequate drainage and decompression of the infected area. Because the I&D must be extensive, it is usually done in an operating room, with the patient under general anesthesia. The locations of various I&D sites are depicted in Figure 16-26. Ample clinical experience and experimental evidence indicate that, even if no abscess formation can be detected by palpation, needle aspiration, radiographic examination, or open surgical drainage, an infection in the cellulitis stage will resolve more rapidly if incised and drained. The surgeon must not wait for unequivocal evidence of pus formation. In the preantibiotic era, surgical treatment was the only method of therapy for infections, and early and aggressive surgical therapy was frequently curative for these severe infections. One must remember that aggressive surgical exploration is still the primary method of therapy for serious odontogenic infections of the head and neck.
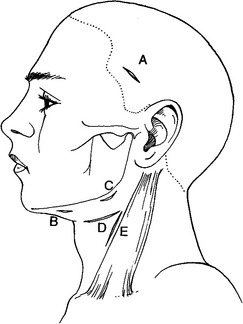
FIGURE 16-26 Placement of incisions for extraoral drainage of deep space infections. A, Superficial or deep temporal. B, Submental or submandibular. C, Submandibular, submasseteric, or submandibular. This incision may also be used with incision B for through-and-through drainage of the submental space or with incision A for through-and-through drainage of the temporal spaces. D, Lateral pharyngeal or the superior portion of the retropharyngeal space. E, Retropharyngeal space or carotid sheath. This incision may also be combined with incision D. (From Flynn TR: Surgical management of orofacial infections. Atlas Oral Maxillofac Surg Clin North Am 8:77-100, March 2000.)
OSTEOMYELITIS
The term osteomyelitis literally means inflammation of the bone marrow. Clinically, osteomyelitis implies an infection of the bone. Osteomyelitis usually begins in the medullary cavity, involving the cancellous bone; then it extends and spreads to the cortical bone and eventually to the periosteum. Invasion of bacteria into the cancellous bone causes soft tissue inflammation and edema within the closed bony marrow spaces. As with the dental pulp, soft tissue edema that is enclosed by unyielding calcified tissue results in increased tissue hydrostatic pressure that rises above the blood pressure of the feeding arterial vessels. The resulting severe compromise of the blood supply then causes soft tissue necrosis. The failure of microcirculation in the cancellous bone is a critical factor in the establishment of osteomyelitis because the involved area becomes ischemic and the cellular component of the bone becomes necrotic. Bacteria can then proliferate because normal blood-borne defenses do not reach the tissue, and the osteomyelitis spreads until it is arrested by medical and surgical therapy.
Although the maxilla can also become involved in osteomyelitis, it does so rarely compared with the mandible. The primary reason for this is that the blood supply to the maxilla is much richer and is derived from several arteries, which form a complex network of feeder vessels. Because the mandible tends to draw its primary blood supply from the inferior alveolar artery and because the dense overlying cortical bone of the mandible limits penetration of periosteal blood vessels, the mandibular cancellous bone is more likely to become ischemic and therefore infected.
In spite of the many opportunities that bacteria have to enter into the cancellous bone via dental infections, osteomyelitis of the mandible rarely occurs if the host defenses are reasonably intact. The major predisposing factors for osteomyelitis of the jaws are preceding odontogenic infections and fractures of the mandible (Fig. 16-27). Even these two events rarely cause infections of the bone unless the host defenses are suppressed by problems such as diabetes, alcoholism, intravenous drug abuse, malnutrition, and myeloproliferative diseases, such as the leukemias, sickle cell disease, or chemotherapy-treated cancer.
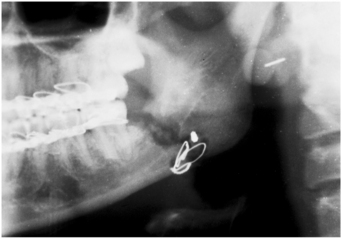
FIGURE 16-27 Osteomyelitis occurred in the area of a fracture of the mandible of a patient who was poorly nourished and abused ethanol. The sequestrum is surrounded by the radiolucency.
Recent carefully performed investigations on the microbiology of osteomyelitis of the mandible have adequately demonstrated that the primary bacteria of concern are similar to those causing odontogenic infections, that is, streptococci, anaerobic cocci such as Peptostreptococcus spp., and anaerobic gram-negative rods such as those of the genera Fusobacterium and Prevotella. Traditional investigation of the microbiology of osteomyelitis of the jaws has used culture specimens from surface drainage of pus (contaminated with Staphylococcus organisms) and not anaerobic culture techniques (and thereby have not grown anaerobes). Thus, osteomyelitis of the mandible differs substantially from osteomyelitis of other bones in which staphylococci are the predominant bacteria.
Acute suppurative osteomyelitis shows little or no radiographic change because at least 10 to 12 days are required for lost bone to be detectable radiographically. Chronic osteomyelitis usually demonstrates bony destruction in the area of infection. The appearance is one of increased radiolucency, which may be uniform in its pattern or patchy, with a moth-eaten appearance. Areas of radiopacity also may occur within the radiolucency. These radiopaque areas represent islands of bone that have not been resorbed and are known as sequestra. In long-standing chronic osteomyelitis, there may actually be an area of increased radiodensity surrounding the area of radiolucency called an involucrum. This is the result of a reaction in which bone production increases as a result of the inflammatory reaction.
Treatment of osteomyelitis is medical and surgical. Because patients with osteomyelitis may have depressed host defense mechanisms, the clinician must take these compromises into account during the treatment and seek medical consultation when necessary.
Acute osteomyelitis of the jaws is primarily managed by the administration of appropriate antibiotics. The precipitating event, condition, or both must also be carefully managed. If the event is a fracture of the mandible, careful attention must be given to its treatment. The antibiotics of choice include clindamycin, the penicillins, and the fluoroquinolones because of their effectiveness against the flora of odontogenic infections and their good to excellent bone penetration. If the patient has a serious acute osteomyelitis, hospitalization may be required for intravenous administration of antibiotics, which can then be followed by home intravenous therapy via a peripherally inserted central catheter or oral therapy, especially with the fluoroquinolones, which are very well absorbed orally. Surgical treatment of acute or chronic suppurative osteomyelitis consists primarily of removing obviously nonvital teeth in the area of the infection, any wires or bone plates that may have been used to stabilize a fracture in the area, or any obviously loose pieces of bone. Bone specimens are sent for aerobic and anaerobic culture and sensitivity testing and histopathologic examination. In addition, corticotomy (removal or perforation of the bony cortex) and excision of necrotic bone (until actively bleeding bone tissue is encountered) may be necessary. For acute osteomyelitis that results from jaw fracture, the surgeon must stabilize the mobile segments of the mandible, usually by open reduction and rigid internal fixation. Immobility of the fracture segments aids in the resolution of osteomyelitis.
Chronic osteomyelitis requires not only aggressive antibiotic therapy but also aggressive surgical therapy. Because of the severe compromise in the blood supply to the area of osteomyelitis, the patient is usually admitted to the hospital and given high-dose intravenous antibiotics to control the infection. The surgeon should obtain culture material at the time of surgery so that the selection of an antibiotic can be based on the specific microbiology of the infection.
Therapy for acute and chronic osteomyelitis, most authorities agree, should ensure that antibiotics are continued for a much longer time than is usual for odontogenic infections. For mild acute osteomyelitis that has responded well, antibiotics should be continued for at least 6 weeks after resolution of symptoms. For severe chronic osteomyelitis that has been difficult to control, antibiotic administration may continue for up to 6 months. This is especially true in actinomycotic osteomyelitis, which has a propensity to recur after long, symptom-free intervals.
Osteomyelitis of the mandible is a severe infection that may result in loss of a large portion of the mandible. Therefore a clinician who has the training and experience to handle the problem expeditiously should manage this infection. In addition, it is likely that medical consultation will be required to help correct any underlying compromise of host defenses.
ACTINOMYCOSIS
Actinomycosis is a relatively uncommon infection of the hard and soft tissues of the head and neck. Actinomycosis is usually caused by Actinomyces israelii but may also be caused by A. naeslundii or A. viscosus. Actinomyces is an endogenous bacterium of the oral cavity that was once thought to be an anaerobic fungus. However, it has now been clearly established that the actinomycetes are anaerobic bacteria.
Actinomycosis is a relatively uncommon disease because the bacteria have a low degree of virulence. For the infection to become established, the bacteria must be inoculated into an area of injury or locally increased susceptibility, such as areas of recent tooth extraction, severely carious teeth, bone fracture, or minor oral trauma. The infection is primarily one of soft tissue and progresses by direct extension into adjacent tissues and bone.
Unlike other infections, actinomycosis does not follow usual anatomic planes but rather burrows through them and becomes a lobular “pseudotumor.” If the infection erodes through a cutaneous surface, which is common with orofacial actinomycosis, multiple sinus tracts typically develop. Once drainage is established, the patient has minimal pain, although the sinus tracts continue to drain spontaneously until the infection is brought under control (Fig. 16-28).
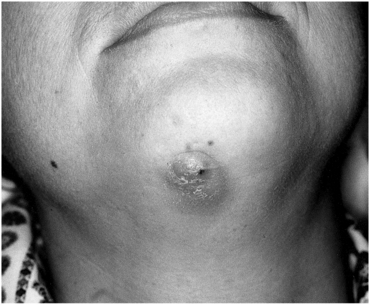
FIGURE 16-28 This actinomycosis had multiple recurrences. The patient experienced a small amount of swelling with multiple small sinus tracts.
A definitive diagnosis depends on laboratory identification. Actinomyces is an anaerobic bacterium and therefore must be incubated in an anaerobic environment, usually on brain-heart agar or blood agar, for 4 to 6 days. In up to 50% of all actinomycotic infections, the organism is not grown. However, the clinical presentation of the patient with actinomycosis is characteristic. The diagnosis is often made on histopathologic examination of a pus specimen because of the presence of typical colonies of actinomyces that look like sulfur granules within the exudate. The patient has an atypical infection of the jaws that responds well to antibiotic therapy initially; however, after the antibiotic is stopped, the infection often recurs. The patient with this disease has frequently had multiple episodes of recurrent infection in the same area.
Therapy of actinomycosis includes surgical I&D and excision of all sinus tracts. This portion of the treatment is important to ensure that adequate amounts of antibiotic are actually delivered to the infected area.
The antibiotic of choice for actinomycosis in the nonallergic patient is one of the intravenous penicillins initially, followed by long-term oral therapy. The reason for the prolonged administration of the antibiotic is to prevent the recurrence of the infection.
The alternative antibiotics include doxycycline or clindamycin. An advantage of doxycycline is that it can be administered orally once per day for long-term therapy.
In summary, actinomycosis is an indolent infection that tends to erode through tissues rather than to follow the typical fascial planes and spaces. Actinomycosis is difficult to eradicate using short-term antibiotic regimens. Therefore, I&D of any accumulation of pus and excision of chronic sinus tracts and necrotic bone and foreign bodies must be accomplished. Finally, high-dose antibiotic administration is recommended for initial control of the infection, with long-term antibiotic therapy to prevent recurrence of actinomycosis.
CANDIDIASIS
The organism Candida albicans is a naturally occurring yeast in the oral cavity. Candida rarely causes disease unless the patient’s health becomes compromised. The two most common causes of compromise are administration of antibiotics, especially penicillin, for prolonged periods and immune system compromise, such as acquired immunodeficiency syndrome (AIDS) or chemotherapy for leukemias and other forms of cancer. In these situations, Candida organisms overgrow in the oral cavity and cause a superficial infection. Three forms of oral candidiasis have been described: pseudomembranous, which usually appears intraorally as distinct white patches that can be easily rubbed off with gauze to expose an underlying red, raw surface; erythematous, which appears simply as a raw surface, or as loss of the filiform papillae of the tongue; and angular cheilitis, which appears as white or ulcerated patches in the corners of the mouth. The pseudomembranous and erythematous types are visible in Figure 16-29. Candida spp. can be diagnosed by culture and by their typical appearance on Gram stain.
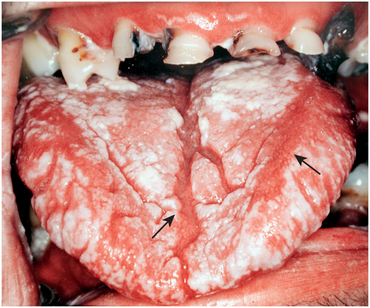
FIGURE 16-29 Candidiasis of the tongue. The white patches are the pseudomembranous type, and the eroded, glossy areas (arrows) are the erythematous type. (From Flynn TR, Piecuch JF, Topazian RG: Orofacial infections. In McMillan JA, DeAngelis CD, Feigin RD, Warshaw JB, editors: Oski’s pediatrics: principles and practice, ed 4, Philadelphia, 2006, Lippincott Williams & Wilkins.)
Angular cheilitis can be caused by Candida organisms. Most patients who have this problem are edentulous and have decreased vertical dimension between the upper and lower jaws, resulting in chronic wetness at the corner of the mouth and subsequent yeast growth.
Topical antifungal agents can usually deliver effective therapy for oral candidiasis. The two most commonly used drugs for this purpose are nystatin and clotrimazole. Both drugs are prepared as lozenges and are delivered by sucking on the lozenge until it is totally dissolved. Nystatin is preferred for initial therapy because of low toxicity and cost.
Clotrimazole, a newer antifungal, has a very low risk of toxicity and better taste but higher cost. The usual course of either preparation is one lozenge allowed to dissolve in the mouth 4 or 5 times per day for 2 weeks. Patients usually experience rapid resolution of the signs and symptoms of candidiasis but must be informed that there will be a recurrence of the infection unless they continue the therapy for the entire 14 days. If the patient has a denture, it should also be treated to eliminate yeast because it can serve as a reservoir of yeast organisms. Treatment of the denture involves removal of a thin layer of acrylic from the internal surface of the denture and antiseptic soaks overnight for the entire time of active treatment and periodically thereafter.
Systemically administered new antifungals, such as fluconazole, ketoconazole, and itraconazole, are used to treat oropharyngeal candidiasis in immunocompromised individuals, such as in AIDS. These antifungal drugs are effective, especially against some of the highly resistant strains of Candida albicans and other species such as C. glabrata that are seen more frequently in immunocompromised individuals. Because of their expense and occasionally life-threatening drug interactions, these newer, highly potent antifungals are generally reserved for immunocompromised individuals and use by specialists.
One must remember that candidiasis occurs most commonly in medically compromised patients. Patients without histories of recent antibiotic therapy, poorly controlled diabetes, AIDS, cancer chemotherapy, or other types of immunocompromise should be suspected of having an underlying systemic immunocompromising disease. Therefore, referral for evaluation and control of underlying medical conditions is important in the management of a patient with oral candidiasis that cannot be controlled by local means.
REFERENCES
1. Grodinsky, M. Retropharyngeal and lateral pharyngeal abscesses. Ann Surg. 1939;110:177.
2. Grodinsky, M. Ludwig’s angina: an anatomical and clinical study with review of the literature. Surgery. 1939;5:678.
3. Grodinsky, M, Holyoke, EA. The fasciae and fascial spaces of the head, neck, and adjacent regions. Am J Anat. 1938;63:367.
4. Williams, AC. Ludwig’s angina. Surg Gynecol Obstet. 1940;70:140.
5. Williams, AC, Guralnick, WC. The diagnosis and treatment of Ludwig’s angina: a report of twenty cases. N Engl J Med. 1943;228:443.
6. Flynn, TR, Shanti, RM, Hayes, C. Severe odontogenic infections, part two: prospective outcomes study. J Oral Maxillofac Surg. 2006;64:1104–1113.
7. Flynn, TR, Shanti, RM, Levy, M, et al. Severe odontogenic infections, part one: prospective report. J Oral Maxillofac Surg. 2006;64:1093–1103.
Balcerak, RJ, Sisto, JM, Bosack, RC. Cervicofacial necrotizing fasciitis: report of three cases and literature review. J Oral Maxillofac Surg. 1988;46:450.
Bennett, JD, Flynn, TR. Anesthetic considerations in orofacial infections. In Topazian RG, Goldberg MH, Hupp JR, eds.: Oral and maxillofacial infections, ed 4, Philadelphia: WB Saunders, 2002.
Carey, JW, Dodson, TB. Hospital course of HIV-positive patients with odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(1):23–27.
Flynn, TR. Anatomy of oral and maxillofacial infections. In Topazian RG, Goldberg MH, Hupp JR, eds.: Oral and maxillofacial infections, ed 4, Philadelphia: WB Saunders, 2002.
Flynn, TR. Deep fascial space infections. In: Laskin DM, Abubakar AO, eds. Decision making in oral and maxillofacial surgery. Chicago: Quintessence, 2007.
Flynn, TR. Odontogenic infections. Oral Maxillofac Surg Clin North Am. 1991;3:311–329.
Flynn, TR. Principles of management of odontogenic infections. In Miloro M, ed.: Peterson’s principles of oral and maxillofacial surgery, ed 2, Hamilton, Ontario: BC Decker, 2005.
Flynn, TR. Surgical management of orofacial infections. Atlas Oral Maxillofac Surg Clin North Am. March 2000;8:77–100.
Flynn, TR. The swollen face. Emerg Med Clin North Am. Aug 2000;15:481–519.
Flynn, TR. The timing of incision and drainage. In: Piecuch JF, ed. Oral and maxillofacial surgery knowledge update 2002. Rosemont, Ill: American Association of Oral and Maxillofacial Surgeons, 2002.
Flynn, TR. Use of antibiotics. In: Laskin DM, Abubaker AO, eds. Decision making in oral and maxillofacial surgery. Chicago: Quintessence, 2007.
Flynn, TR, Halpern, LR. Antibiotic selection in head and neck infections. Oral and Maxillofacial Surgery Clinics of North America. Feb 2003;15:17–38.
Freeman, RK, Vallieres, E, Verrier, ED, et al. Descending necrotizing mediastinitis: an analysis of the effects of serial surgical debridement on patient mortality. J Thorac Cardiovasc Surg. 2000;119(2):260–267.
Gidley, PW, Ghorayeb, BY, Stiernberg, CM. Contemporary management of deep neck space infections. Otolaryngol Head Neck Surg. 1997;116:16–22.
Hall, HD, Gunter, JW, Jamison, HC, et al. Effect of time of extraction on resolution of odontogenic cellulitis. J Am Dent Assoc. 1968;77:626.
Haug, RH, Picard, U, Indresano, AT. Diagnosis and treatment of the retropharyngeal abscess in adults. Br J Oral Maxillofac Surg. 1990;28:34–38.
Heimdahl, A, VonKonow, L, Satoh, T, et al. Clinical appearance of orofacial infections of odontogenic origin in relation to microbiological findings. J Clin Microbiol. 1985;22:299.
Hought, RT, Fitzgerald, BE, Latta, JE, et al. Ludwig’s angina: report of two cases and review of the literature from 1945 to January 1979. J Oral Surg. 1980;38:849.
Kim, Y, Flynn, TR, Donoff, RB, et al. The gene: the polymerase chain reaction and its clinical application. J Oral Maxillofac Surg. 2002;60:808–815.
Krogh, HW. Extraction of teeth in the presence of acute infections. J Oral Surg. 1951;9:136.
Langford, FPJ, Moon, RE, Stolp, BW, et al. Treatment of cervical necrotizing fasciitis with hyperbaric oxygen therapy. Otolaryngol Head Neck Surg. 1995;112:274–278.
LeBlanc, DJ, Flynn, TR, Simos, C, et al. Antibiotics and the treatment of infectious diseases. In: Lamont RJ, Burne RA, Lantz MS, et al, eds. Oral microbiology and immunology. Washington, DC: ASM Press, 2006.
Marra, S, Hotaling, AJ. Deep neck infections. Am J Otol. 1996;17:287–298.
Martis, CS, Karakasis, DT. Extractions in the presence of acute infections. J Dent Res. 1975;54:59.
Marx, RE. Chronic osteomyelitis of the jaws. Oral and Maxillofacial Surgery Clinics of North America. 1991;3:367.
Miller, EJ, Jr., Dodson, TB. The risk of serious odontogenic infections in HIV-positive patients: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:406–409.
Miller, WD, Furst, IM, Sandor, GKB, et al. A prospective blinded comparison of clinical examination and computed tomography in deep neck infections. Laryngoscope. 1999;109:1873–1879.
O’Ryan, F, Diloreto, D, Barber, HD, et al. Orbital infections: clinical and radiographic diagnosis and surgical treatment. J Oral Maxillofac Surg. 1988;46:991.
Peterson, LJ. Principles of surgical and antimicrobial infection management. In Topazian RG, Goldberg MH, Hupp JR, eds.: Oral and maxillofacial infections, ed 4, Philadelphia: WB Saunders, 2002.
Scully, C, McCarthy, G. Management of oral health in persons with HIV infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1992;73:215.
Telford, G. Postoperative fever. In Condon RE, Nyhus LM, eds.: Manual of surgical therapeutics, ed 6, Boston: Little, Brown, 1985.
Umeda, M, Minamikawa, T, Komatsubara, H, et al. Necrotizing fasciitis caused by dental infection: a retrospective analysis of 9 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:283–290.