Odontogenic Diseases of the Maxillary Sinus
EMBRYOLOGY AND ANATOMY
The maxillary sinuses are air-containing spaces that occupy the maxillary bone bilaterally. The maxillary sinuses are the first of the paranasal sinuses (e.g., maxillary, ethmoid, frontal, and sphenoid) to develop embryonically and begin in the third month of fetal development as mucosal invaginations or pouching of the ethmoid infundibula. The initial maxillary sinus development, also termed primary pneumatization, progresses as the invagination expands into the cartilaginous nasal capsule.1 Secondary pneumatization begins in the fifth month of fetal development as the initial invaginations expand into the developing maxillary bone.
After birth the maxillary sinus expands by pneumatization into the developing alveolar process and extends anteriorly and inferiorly from the base of the skull, closely matching the growth rate of the maxilla and the development of the dentition. As the dentition develops, portions of the alveolar process of the maxilla, vacated by the eruption of teeth, become pneumatized.2 By the time a child reaches age 12 or 13, the sinus will have expanded to the point at which its floor will be on the same horizontal level as the floor of the nasal cavity. In adults the apices of the teeth may extend into the sinus cavity and can be identified in anatomic specimens or through computed tomography imaging.3 Expansion of the sinus normally ceases after the eruption of the permanent teeth, but on occasion the sinus will pneumatize further, after the removal of one or more posterior maxillary teeth, to occupy the residual alveolar process. In many of the cases the sinus often extends virtually to the crest of the edentulous ridge. The maxillary sinus is significantly larger in adult patients who are edentulous in the posterior maxilla compared with patients with complete posterior dentition.4
The maxillary sinus is the largest of the paranasal sinuses. The maxillary sinus is also known as the antrum or the antrum of Highmore. Antrum is derived from the Greek word meaning cave. Dr. Nathaniel Highmore, an English physician in the 1600s, described a sinus infection associated with a maxillary tooth, and his name has long been associated with sinus nomenclature.
The maxillary sinus is described as a four-sided pyramid, with the base lying vertically on the medial surface and forming the lateral nasal wall. The apex extends laterally into the zygomatic process of the maxilla. The upper wall, or roof, of the sinus is also the floor of the orbit. The posterior wall extends the length of the maxilla and dips into the maxillary tuberosity. Anteriorly and laterally the sinus extends to the region of the first bicuspid or cuspid teeth. The floor of the sinus forms the base of the alveolar process (Figs. 19-1 and 19-2). The adult maxillary sinus averages 34 mm in anteroposterior direction, 33 mm in height, and 23 mm in width. The volume of the sinus is approximately 15 to 20 mL.
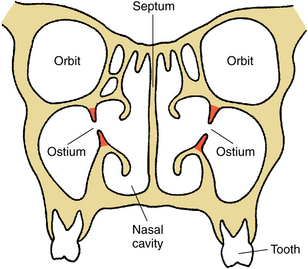
FIGURE 19-1 Frontal diagram of midface at ostium or opening of maxillary sinuses into middle meatus of nasal cavity. Ostium is in upper third of sinus cavity.
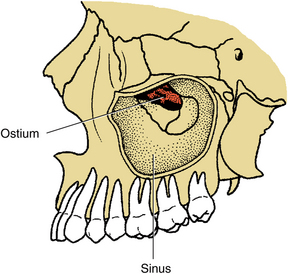
FIGURE 19-2 Lateral diagram of left maxillary sinus with zygoma removed. Medial sinus wall (i.e., lateral nasal wall) is seen in depth of sinus, as is the ostium. Maxillary sinus is pyramidal, with its apex directed into base of zygoma.
The sinuses are primarily lined by respiratory epithelium, a mucus-secreting, pseudostratified, ciliated, columnar epithelium. The cilia and mucus are necessary for the drainage of the sinus because the sinus opening, or ostium, is not in a dependent (inferior) position but lies two thirds the distance up the medial wall and drains into the nasal cavity (Figs. 19-1 and 19-2). The maxillary sinus opens into the posterior, or inferior, end of the semilunar hiatus, which lies in the middle meatus of the nasal cavity, between the inferior and middle nasal conchae. Beating of the cilia moves the mucus produced by the lining epithelium and any foreign material contained within the sinus toward the ostium, from which it drains into the nasal cavity. The cilia beat at a rate of up to 1000 strokes per minute and can move mucus a distance of 6 mm per minute.5 The environment within the sinus is a constantly moving thin layer of mucus that is transported along the walls of the sinus, through the ostium and into the nasopharynx.
CLINICAL EXAMINATION OF THE MAXILLARY SINUS
Clinical evaluation of a patient with suspected maxillary sinusitis should begin with a careful visual examination of the patient’s face and intraoral vestibule for swelling or redness. Nasal discharge may be evident during the initial evaluation. The examination of the patient with suspected maxillary sinus disease should also include tapping of the lateral walls of the sinus externally over the prominence of the cheek bones and palpation intraorally on the lateral surface of the maxilla between the canine fossa and the zygomatic buttress. The affected sinus may be very tender to gentle tapping or palpation. In some cases, there may be erosion of the lateral wall of the sinus/maxilla with a palpable defect. Patients with maxillary sinusitis frequently complain of dental pain, and pain to percussion of several maxillary posterior teeth is often indicative of an acute sinus infection.
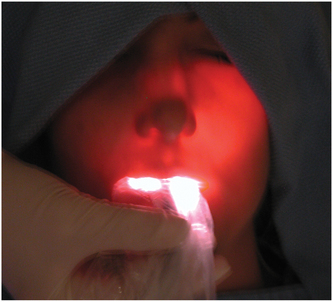
Further examination may include transillumination of the maxillary sinuses. Transillumination of the maxillary sinus is done by placing a bright fiberoptic light against the mucosa on the palatal or facial surfaces of the sinus and observing the transmission of light through the sinus in a darkened room (Fig. 19-3). In unilateral disease, one sinus may be compared with the sinus on the opposite side. The involved sinus shows decreased transmission of light because of the accumulation of fluid, debris, or pus and the thickening of the sinus mucosa. These simple tests may help to distinguish sinus disease, which may cause pain in the upper teeth, from abscess or other pain of dental origin associated with the molar and premolar teeth.
RADIOGRAPHIC EXAMINATION OF THE MAXILLARY SINUS
Radiographic examination of the maxillary sinus may be accomplished with a wide variety of exposures readily available in the dental office or radiology clinic. Standard dental radiographs that may be useful in evaluating the maxillary sinus include periapical, occlusal, and panoramic views. A periapical radiograph is limited in that only a small portion of the inferior aspect of the sinus can be visualized. In some cases the apices of the roots of the posterior maxillary teeth may be seen to project into the sinus floor (Fig. 19-4). Panoramic radiographs may provide a “screening” view of the maxillary sinuses (Fig. 19-5). This projection is the best radiograph available in most dental offices to provide a view of both maxillary sinuses for comparison. Because a panoramic radiograph provides a focused image within a limited focal trough, structures outside of this area may not be clearly delineated.
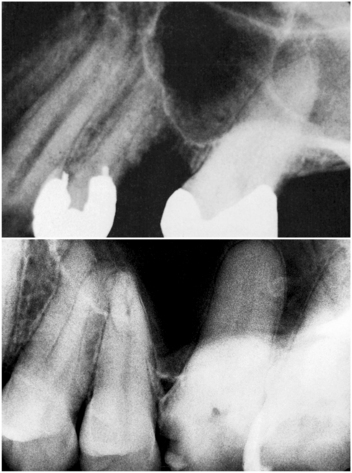
FIGURE 19-4 Periapical radiographs showing inferior portion of pneumatized maxillary sinus. Molar roots appear to be protruding into the sinus because sinus has pneumatized around roots.
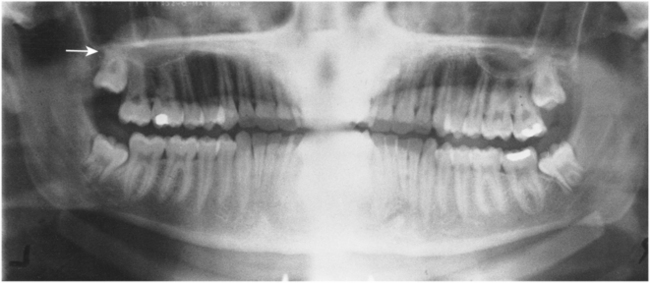
FIGURE 19-5 Panoramic radiograph showing mucous retention phenomenon on the floor of the right maxillary sinus (arrow).
Periapical, occlusal, and occasionally panoramic radiographs are of value in locating and retrieving foreign bodies within the sinus—particularly teeth, root tips, or osseous fragments—that have been displaced by trauma or during tooth removal (Fig. 19-6). These radiographs should also be used for the careful planning of surgical removal of teeth adjacent to the sinus.
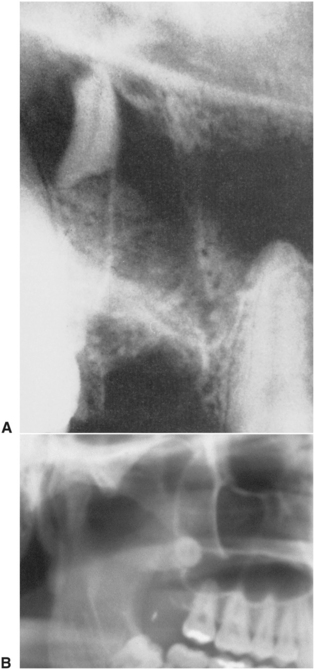
FIGURE 19-6 A, Periapical radiograph showing apical one third of palatal root of maxillary first molar, which was displaced into maxillary sinus during removal of the tooth. B, Close-up view of panoramic view of right maxillary sinus with third molar displaced superiorly, lying against the posterior wall of the sinus.
If additional radiographic information is required, the Waters’ and lateral views are two plain film radiographs that are frequently useful.6 The Waters’ view is taken with the head tipped 37 degrees to the central beam (Fig. 19-7). This projection places the maxillary sinus area above the petrous portion of the temporal bones, allowing for a clearer view of the sinuses than a standard posterior-anterior view of the skull. The lateral view can be obtained in a standard cephalometric machine with the patients head tipped slightly toward the cassette (Fig. 19-8). Tipping of the patient’s head avoids superimposition of the walls of the sinus.
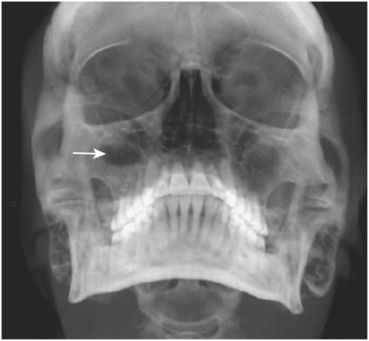
FIGURE 19-7 Waters’ view radiograph demonstrating right maxillary sinus with air-fluid level (arrow) and increased opacity of the left sinus because of fluid, significant thickening of the mucosa, or both.
Computed tomography is a useful technique for imaging of the maxillary sinuses and other facial bony structures.7 The decreased cost and more accessibility combined with clear, easily visualized images has made computed tomography scans increasingly popular for evaluating all types of facial bone pathologic conditions including abnormalities of the maxillary sinus (Fig. 19-9).
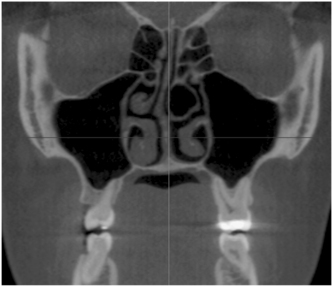
FIGURE 19-9 Computed tomography scan, coronal view, showing normal maxillary sinus anatomy with thin bony walls without any thickening of the mucosal lining, masses, or fluid.
Interpretation of radiographs of the maxillary sinus is not difficult. The findings in the normal antrum are those to be expected of a rather large, air-filled cavity surrounded by bone and dental structures. The body of the sinus should appear radiolucent and should be outlined in all peripheral areas by a well-demarcated layer of cortical bone. Comparison of one side with the other is helpful when examining the radiographs. One should not see evidence of thickened mucosa on the bony walls, air-fluid levels (caused by accumulation of mucus, pus, or blood), or foreign bodies lying free. Partial or complete opacification of the maxillary sinus may be caused by the mucosal hypertrophy and fluid accumulation of sinusitis, by filling with blood following trauma, or by neoplasia. Radiographic changes are to be expected with acute maxillary sinusitis. Mucosal thickening caused by infections may obstruct the ostium of the sinus and allow accumulation of mucus, which will become infected and produce pus. The characteristic radiographic changes may include an air-fluid level in the sinus (Fig. 19-7), thickened mucosa on any or all of the sinus walls (Fig. 19-10), or complete opacification of the sinus cavity. The radiographic changes indicative of chronic maxillary sinusitis include mucosal thickening, sinus opacification, and nasal or antral polyps. Air-fluid levels in the sinuses are more characteristic of acute sinus disease but may be seen in chronic sinusitis in periods of acute exacerbation.
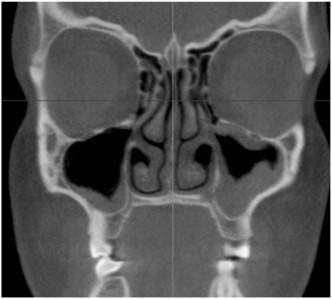
FIGURE 19-10 Computed tomography scan showing right maxillary sinus with thickened mucosa at the inferior portion of the sinus. The patient’s left side has significant mucosal thickening along the entire lining of the sinus.
Disruption of the cortical outline may be a result of trauma, tumor formation, an infectious process with abscess and fistula formation (Fig. 19-11), or a surgical procedure that violates the sinus walls. Expansion of the bony walls may also be apparent (Fig. 19-12). Dental pathologic conditions such as cysts or granulomas may produce radiolucent lesions that extend into the sinus cavity. These conditions may be distinguished from normal sinus anatomy by their association with the tooth apex, the clinical correlation with the dental examination, and the presence of a cortical osseous margin on the radiograph, which generally separates the area in question from the sinus itself.
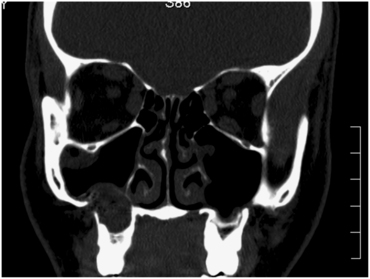
FIGURE 19-11 Perforation of the lateral wall of the right sinus as a result of an odontogenic infection associated with a molar tooth. The abscess expanded into the floor of the sinus and eroded the lateral wall of the sinus.
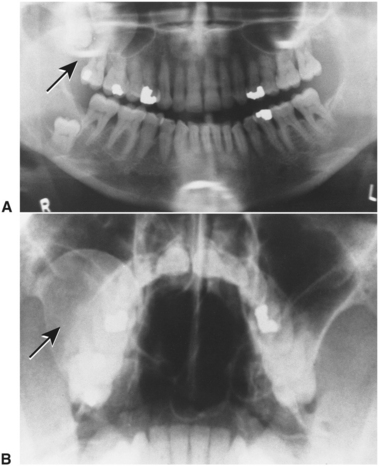
FIGURE 19-12 A, Panoramic radiograph shows large odontogenic keratocyst associated with impacted right maxillary third molar tooth (arrow). Cyst has impinged on right maxillary sinus as it expanded. Sinus cavity is almost totally obstructed by lesion. Another odontogenic keratocyst is seen associated with impacted right mandibular third molar. B, Waters’ view radiograph demonstrates the odontogenic keratocyst (seen in A). Lesion is also seen to have expanded lateral wall of right maxillary sinus (arrow).
NONODONTOGENIC INFECTIONS OF THE MAXILLARY SINUS
Historically, the consensus has been that the maxillary sinus is usually not colonized by any bacteria and is essentially sterile.8 More recent studies using updated techniques have occasionally shown that some bacteria may be cultured from a healthy paranasal sinus.9 Even though there may be some microorganisms present in the normal sinus, this appears to be minimal, and the dynamic nature of the sinus with active epithelium and a constant moving mucus layer prevents any significant colonization.
The mucosa of the sinus is susceptible to infectious, allergic, and neoplastic diseases. Inflammatory diseases of the sinus, such as infection or allergic reactions, cause hyperplasia and hypertrophy of the mucosa and may cause obstruction of the ostium. If the ostium becomes obstructed, the mucus produced by the secretory cells lining the sinus is collected over long periods. Bacterial overgrowth may then produce an infection resulting in the signs and symptoms of sinusitis, as well as the radiographic changes seen with these conditions.
When inflammation develops in any of the paranasal sinuses, whether caused by infection or allergy, the condition is described as sinusitis. Inflammation of most or all of the paranasal sinuses simultaneously is known as pansinusitis and is usually caused by infection. Similar conditions of individual sinuses are known, for example, as maxillary sinusitis or frontal sinusitis.
Acute maxillary sinusitis may occur at any age. The onset is usually described by the patient as a rapidly developing sense of pressure, pain, and/or fullness in the vicinity of the affected sinus. The discomfort rapidly increases in intensity and may be accompanied by facial swelling and erythema, malaise, fever, and drainage of foul smelling mucopurulent material into the nasal cavity and nasopharynx.
Chronic maxillary sinusitis is usually a result of bacterial or fungal infections that are low grade and recurrent, obstructive nasal disease or allergy. Chronic maxillary sinusitis is characterized by episodes of sinus disease that respond initially to treatment, only to return, or that remain symptomatic in spite of treatment.
Aerobic, anaerobic, or mixed bacteria may cause infections of the maxillary sinuses. The organisms usually associated with maxillary sinusitis of nonodontogenic origin include those organisms usually found within the nasal cavity. Mucostasis that occurs within the sinus allows for colonization of these organisms. The causative bacteria are primarily aerobic, with a few anaerobes. The important aerobes are Streptococcus pneumoniae, Haemophilus influenzae, and Branhamella catarrhalis. Anaerobes include Streptococcus viridans, Staphylococcus aureus, Enterobacteriaceae, Porphyromonas, Prevotella, Peptostreptococcus, Veillonella, Propionibacterium, Eubacterium, and Fusobacterium.
ODONTOGENIC INFECTIONS OF THE MAXILLARY SINUS
Maxillary sinusitis is occasionally a result of odontogenic sources because of the anatomic juxtaposition of the teeth and the maxillary sinus (Fig. 19-13). Odontogenic sources account for approximately 10% to 12% of all maxillary sinusitis.10 This condition may readily spread to involve the other paranasal sinuses if left untreated or inadequately treated. In rare cases, these infections become life threatening and can include orbital cellulitis, cavernous sinus thrombosis, meningitis, osteomyelitis, intracranial abscess, and death.
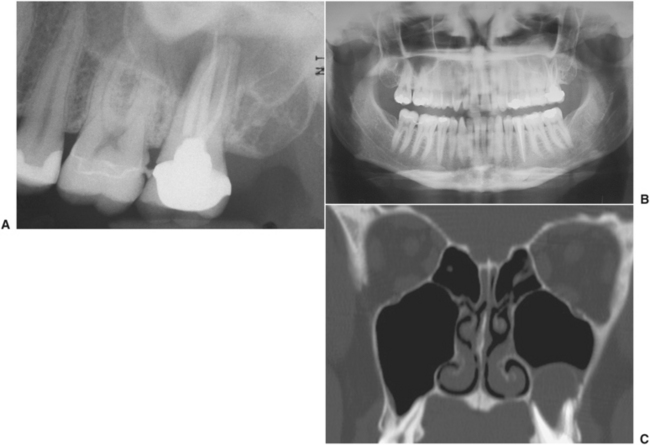
FIGURE 19-13 A, Periapical radiograph showing endodontically treated molar tooth. The tooth has a periapical abscess affecting the maxillary sinus, not seen well on this radiograph but more apparent on the panoramic radiograph and computed tomography scan. B, Panoramic radiograph showing significant opacification of inferior portion of left maxillary sinus. C, Computed tomography scan clearly shows fluid-filled mass associated with roots of second molar tooth.
Sources of odontogenic infections that involve the maxillary sinus include acute and chronic periapical disease and periodontal disease. Infection and sinusitis may also result from trauma to the dentition or from surgery in the posterior maxilla, including removal of teeth, alveolectomy, tuberosity reduction, sinus lift grafting and implant placement, or other procedures that create an area of communication between the oral cavity and the maxillary sinus.
Maxillary sinus infections of odontogenic origin are more likely to be caused by anaerobic bacteria, as is the usual odontogenic infection. Rarely does H. influenzae or S. aureus cause odontogenic sinusitis. The predominant organisms are aerobic and anaerobic streptococci and anaerobic Bacteroides, Enterobacteriaceae, Peptococcus, Peptostreptococcus, Porphyromonas, Prevotella, and Eubacterium.
TREATMENT OF MAXILLARY SINUSITIS
Early treatment of maxillary sinusitis consists of humidification of inspired air to loosen and aid in the removal of dried secretions from the nasal passage and the sinus ostium. Systemically administered decongestants such as pseudoephedrine (Sudafed) and nasal spray containing vasoconstrictors, such as 2% ephedrine or 0.25% phenylephrine, decrease nasal and sinus congestion and help facilitate normal drainage. Patients with sinus infections often experience moderate to severe pain, and prescribing a nonsteroidal or narcotic analgesic may be appropriate.11
Many cases of sinusitis are caused by allergies that result in congestion and altered natural drainage of the sinus. Allergic sinusitis often responds to the measures described before. However, when sinusitis is a result of an infectious process, the use of antibiotics is indicated. Knowledge of the bacteria most likely to be isolated in sinusitis is important in selecting an antibiotic. In cases of nonodontogenic sinusitis, the most likely organisms are H. influenzae and S. aureus, Streptococcus pneumonia, and a variety of anaerobic streptococci. Antibiotic choices for treatment of nonodontogenic maxillary sinusitis include amoxicillin, trimethoprim-sulfamethoxazole, amoxicillin/clavulanate, azithromycin, and cefuroxime.
Odontogenic sinusitis usually involves organisms that are associated with common odontogenic infections, including aerobic and anaerobic streptococci and anaerobes such as Bacteroides and Enterobacteriaceae. Therefore, antibiotics generally effective for odontogenic infections such as penicillin, clindamycin, and metronidazole are effective for sinusitis of odontogenic origin.
Because of the wide variety of microorganisms that can contribute to infections of the maxillary sinus, it is important to obtain purulent material for culture and sensitivity testing whenever possible. Sensitivity testing may suggest a change to another antibiotic if resistant organisms are cultured from the sinus and if the infection is failing to respond to appropriate initial treatment.
If the patient fails to respond to this initial treatment regimen within 72 hours, it is necessary to reassess the treatment and the antibiotic. If the cause of the problem has not been identified and eliminated, this must be carefully reevaluated. The results of the culture and sensitivity tests should be evaluated, and changes should be made if indicated. As many as 25% of the organisms cultured from acute sinus infections are b-lactamase producers, and many may be anaerobic, especially if the infection is odontogenic.11 If the organism or organisms causing the infection are b-lactamase producers, another antibiotic, such as the combination agent trimethoprim-sulfamethoxazole (Bactrim, Septra), may be effective. Cefaclor or a combination of amoxicillin and clavulanate potassium (Augmentin) has also been shown to be effective.
Acute maxillary sinusitis is a painful, potentially serious condition that requires immediate attention and aggressive medical and surgical care. Patients suspected of having maxillary sinusitis should be referred to an oral and maxillofacial surgeon or another specialist, such as an otolaryngologist. The referring clinician should send radiographs, the results of clinical procedures, the results of culture and sensitivity tests of purulent drainage, and any other pertinent diagnostic information to the surgeon.
Diagnosis and treatment of chronic maxillary sinusitis is difficult and may include allergy testing, nasal or septal surgery, and surgical débridement of the sinuses. The goal of sinus surgery is to remove abnormal tissue from within the sinus cavity and restore normal drainage through the ostium. Traditionally, this was accomplished with an open approach to the sinus known as a Caldwell-Luc procedure (Fig. 19-14).12 In this technique the anterior wall of the sinus is accessed in the area of the canine fossa through a vestibular approach. The sinus is opened, and abnormal tissue or foreign bodies can be removed. The ostiomeatal area can be evaluated and opened, or a new opening for more dependent drainage into the nose (termed an antrostomy) can be created near the floor of the sinus. Newer techniques allow exploration and surgical treatment of the sinus with less invasive endoscopic approaches (Fig. 19-15).12,13
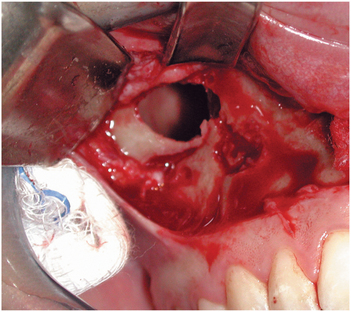
FIGURE 19-14 Caldwell-Luc exposure of the maxillary sinus through a vestibular incision and bony window created in the anterior maxillary wall.
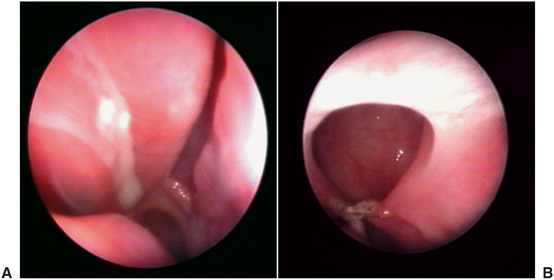
FIGURE 19-15 A, View of ostium and surrounding inflamed mucosa as seen through the endoscope. B, Ostium and surrounding healthy sinus mucosa. (From Costa F, Emanuelli E, Robiony M et al: Endoscopic surgery for maxillary sinusitis, J Oral Maxillofac Surg 65: 225-226, 2007, with permission.)
Sinus lift procedures, done primarily as preprosthetic surgical procedures to improve the posterior maxillary alveolar base for secondary or simultaneous endosseous implant placement, occasionally contribute to sinus infections. In most cases, careful elevation of the schneiderian (i.e., sinus) membrane creates a space into which particulate grafts of autologous bone, allogeneic bone, alloplastic materials, or combinations of these can be placed. If the procedure is done carefully, complications resulting from sinus lift operations are rare. Complications became more frequent in at least two instances: (1) when the sinus membrane is severely lacerated or avulsed or (2) when the sinus is overfilled.
Significant disruption of the sinus membrane allows exposure of the graft material to the open sinus and possible contamination by nasal bacteria. Disruption also allows particulate material from the sinus grafts or implants to become free foreign bodies within the sinus, which can cause foreign-body rejection responses from the sinus mucosa or outright infection. Lacerated sinus membranes may also interfere with normal nasal epithelial ciliary motility and thereby impede physiologic sinus drainage. Finally, fragments of sinus mucosa or graft material may obstruct the sinus ostium, further preventing normal sinus drainage.
When these situations occur, treatment consists of infection control and removal of contaminated or devitalized graft materials. This treatment also includes removal of foreign-body free segments and debulking of overly extended grafts. These procedures are usually accomplished through a Caldwell-Luc lateral sinus wall surgical approach or, rarely, with nasal access endoscopic sinus surgery. Antibiotic therapy alone may temporarily improve the acute problem, but the ultimate treatment will require sinus exploration and débridement.
ANTRAL PSEUDOCYSTS
Pseudocysts, mucoceles, and retention cysts are benign accumulations of fluid underneath or surrounded by sinus epithelium. The term mucocele has often been used to describe any type of localized fluid accumulation, but this is not accurate.14 Although each of these may appear as a round, faint radiopacity within the sinus, the cause of each is different, as is the histology.
The antral pseudocyst is seen in 2% to 10% of panoramic radiographs. This pseudocyst is a result of accumulation of serum (not sinus mucus) under the sinus mucosa. The cause of these accumulations is not clear but may be related to inflammation of the sinus lining. These lesions are of no clinical consequence, require no treatment, and often disappear over time.
Sinus mucoceles are actually cystic lesions in that they are lined by epithelium. One of the most common causes of true mucoceles is surgery on the sinus that results in separation of a portion of the sinus lining from the main portion of the sinus. This area can then become filled with mucus and walled off, forming a separate cystic lesion. These lesions are termed surgical ciliated cysts or postoperative maxillary cysts. These lesions can become expansile and may expand or erode walls of the sinus and must be differentiated, usually through removal and biopsy, from more aggressive and even malignant lesions of the sinus.
Retention cysts in the maxillary sinus result from blockage of ducts within the mucus-secreting glands within the sinus. The accumulated mucin becomes surrounded by epithelium, forming a true cystic lesion. These lesions are usually so small as not to be seen on radiographic images.
COMPLICATIONS OF ORAL SURGERY INVOLVING THE MAXILLARY SINUS
The most common dental complications of oral surgical procedures that subsequently involve the maxillary sinus include displacement of teeth, roots, or instrument fragments into the sinus or the creation of a communication between the oral cavity and the sinus during surgery of the posterior maxilla. Retrieval of a tooth, root fragment, or broken instrument can be accomplished in several ways. In many cases the opening created during initial displacement can be enlarged slightly, and the tooth or other object can be visualized and retrieved with small forceps or with the use of suction. Irrigating or flooding the sinus followed by suction can often accomplish retrieval or position the object close to the opening for easy recovery. In some cases, however, the sinus needs to be opened through a Caldwell-Luc approach and the object retrieved.
A sinus perforation resulting from a tooth extraction most commonly occurs when a maxillary molar with widely divergent roots that is adjacent to edentulous spaces requires extraction. In this instance the sinus is likely to have become pneumatized into the edentulous alveolar process surrounding the tooth, which weakens the entire alveolus and brings the tooth apices into a closer relationship with the sinus cavity. Other causes of perforation into the sinus include abnormally long roots, destruction of a portion of the sinus floor by periapical lesions, perforation of the floor and sinus membrane with injudicious use of instruments, forcing a root or tooth into the sinus during attempted removal, and removal of large cystic lesions that encroach on the sinus cavity.
In many cases the opening is small and primary closure can be easily accomplished with adequate healing. In some cases a larger perforation or communication is apparent, and routine closure is not possible or not adequate to cover the opening.
The treatment of oroantral communications is accomplished immediately, when the opening is created, or later, as in the instance of a long-standing fistula or failure of an attempted primary closure.
Oroantral Communications: Immediate Treatment
The best treatment of a potential sinus exposure is avoiding the problem through careful observation and treatment planning. Evaluation of high-quality radiographs before surgery usually reveals the presence or absence of an excessively pneumatized sinus or widely divergent or dilacerated roots, which have the potential of having a communication with the sinus or causing fractures in the bony floor of the antrum during removal. If this observation is made, surgery may be altered to section the tooth and remove it one root at a time (see Chapter 8).
When exposure and perforation of the sinus result, the least invasive therapy is indicated initially. If the opening to the sinus is small and the sinus is disease free, efforts should be made to establish a blood clot in the extraction site and preserve it in place. Additional soft tissue flap elevation is not required. Sutures are placed to reposition the soft tissues, and a gauze pack is placed over the surgical site for 1 to 2 hours. The patient is instructed to use nasal precautions for 10 to 14 days. These include opening the mouth while sneezing, not sucking on a straw or cigarettes, and avoiding nose blowing and any other situation that may produce pressure changes between the nasal passages and oral cavity. The patient is placed on an antibiotic, usually a penicillin; an antihistamine; and a systemic decongestant for 7 to 10 days to prevent infection, to shrink mucous membranes, and to lessen nasal and sinus secretions. The patient is seen postoperatively at 48- to 72-hour intervals and is instructed to return if an oroantral communication becomes evident by leakage of air into the mouth or fluid into the nose or if symptoms of maxillary sinusitis appear.
The majority of patients treated in this manner heal uneventfully if there is no evidence of preexisting sinus disease. If larger perforations occur, it may be necessary to cover the extraction site with some type of flap advancement to provide primary closure in an attempt to cover the sinus opening. The most commonly used flap procedure involves elevating a buccal flap, releasing the periosteum and advancing the flap to cover the extraction site (Fig. 19-16). The most important aspects of flap advancement for closure include elevating a broad-based flap with adequate width to cover the communication with margins of the flap positioned over bone rather than directly over the defect of area of communication. The flap must be free of any tension. To accomplish this, the periosteum usually must be incised and released at the height of the dissection. Following closure, the patient is instructed to follow the sinus precautions as described previously.
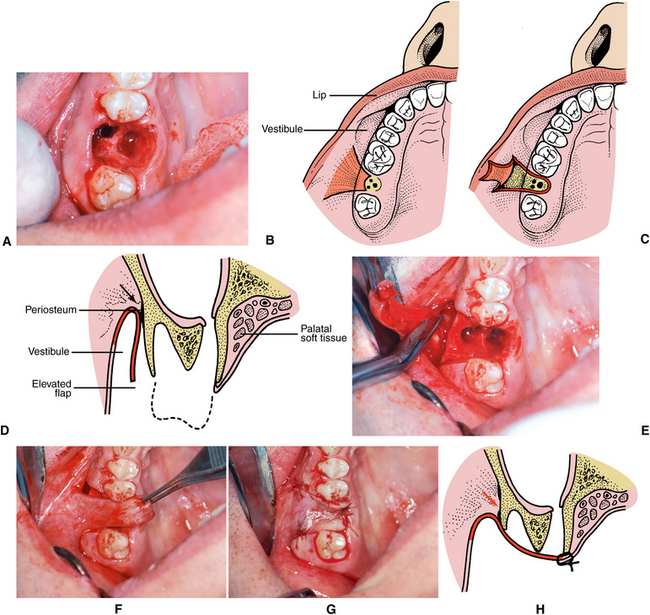
FIGURE 19-16 Closure of large oroantral communication. A, Clinical photograph of large oroantral fistula in molar region of right maxilla. B, Diagram of flap design. C, Illustration of flap elevated to depth of vestibule. D, Cross-section view of flap elevation. Periosteum must be incised in at the height of dissection (arrow) in the vestibule, releasing flap attachment in this area to allow tissue flap to be positioned without tension across extraction site. E, Clinical photograph showing elevation and flap. Scissors used to incise periosteum at height of dissection. F, Passive repositioning of flap across extraction site. G, Flap sutured in position. Note flap margins extend well beyond extraction site and communication defect. H, Cross section of closure. In some cases a small amount of bone reduction may be necessary over the facial aspect to facilitate flap closure.
Oroantral Fistulae: Delayed Treatment
Successful treatment and closure of the oroantral fistula requires more extensive medical and surgical treatment. Before closure of an oroantral fistula, it is imperative to eliminate any acute or chronic infection within the sinus. This may require frequent irrigation of the fistula and sinus combined with the use of antibiotics and decongestants. It may also be helpful to construct a temporary appliance to cover the fistula to prevent food and other oral contaminants from getting into the sinus. If sinus disease persists, it may be necessary to remove diseased tissues from the sinus using a Caldwell-Luc procedure through the lateral maxillary wall above the apices of the remaining teeth.
Adjacent teeth must be carefully evaluated for possible involvement. If the fistula has developed in approximation to the root of an adjacent tooth, closure is further complicated; and to be successful, removal of the tooth may be necessary.
Methods of closing oroantral fistulae include buccal flap advancement (Fig. 19-17), palatal flap advancement (Fig. 19-18), and advancement of palatal and facial flaps over a membrane of alloplastic material (Fig. 19-19). The buccal flap procedure is performed in a manner similar to the buccal advancement described before for immediate closure of an oroantral communication.15 In the case of a chronic fistula, however, the fistulous tract will be lined with epithelium that must be excised or elevated from the bony walls of the fistula, sutured together if possible, and inverted into the sinus cavity (Fig. 19-17). This should be accomplished before elevating the buccal flap so that the actual size of the bony defect can be inspected and the size of the flap designed appropriately to allow the flap to cover the entire defect with the margins lying over bone. The flap is elevated, the periosteum is released, and the flap is then extended over the defect and carefully sutured in place. A similar technique has been described of elevating a larger buccal flap but then using a pedicled portion of the buccal fat pad to cover the defect directly with partial closure of the mucoperiosteal flap.16 Regardless of the technique used, one must remember that the osseous defect surrounding the fistula is always much larger than the clinically apparent soft tissue deformity.17 Surgical planning of closure technique must be adjusted accordingly.
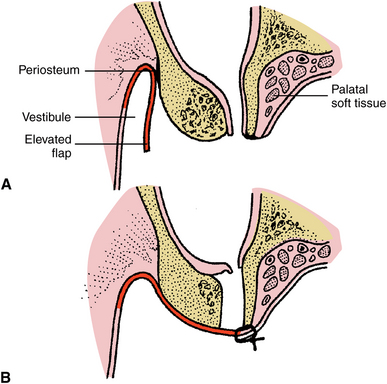
FIGURE 19-17 Buccal flap closure of oroantral fistula. A, Cross-section illustration of oroantral fistula in molar region. Buccal flap has been elevated. B, Epithelium lining the fistula has been excised, the periosteum has been released at the vestibular height of the dissection, and the tension-free flap has been closed across the defect with margins of the flap resting over bone.
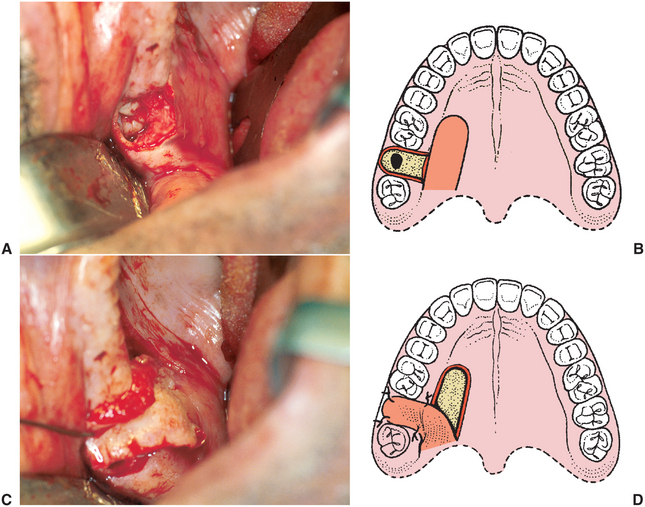
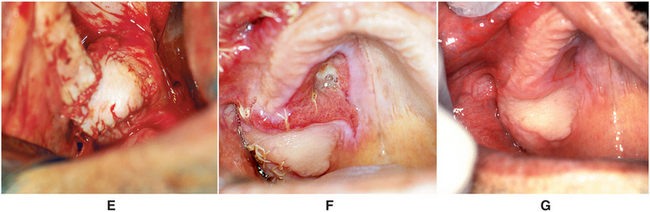
FIGURE 19-18 Palatal flap closure of oroantral fistula. A, Clinical photograph of fistula resulting from removal of lone standing molar in posterior maxilla where sinus was pneumatized. B, Soft tissues surrounding oroantral opening are excised, exposing underlying alveolar bone around osseous defect. Full-thickness palatal flap is outlined, incised, and elevated from anterior to posterior. Flap should be full thickness of mucoperiosteum, should have broad posterior base, and should include the palatine artery. Flap width should be sufficient to cover entire defect around oroantral opening, and its length must be adequate to allow rotation of flap and repositioning over defect without placing undue tension on flap. C, Flap rotated to cover to ensure that there is no tension on flap when positioned to cover osseous defect. D, Illustration of flap rotation and closure. E, Clinical photograph of closure. F, Healing at 1 week postoperatively. G, Three weeks postoperatively.
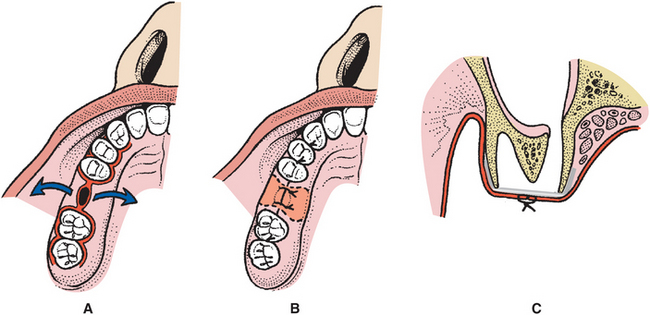
FIGURE 19-19 Membrane-assisted closure of oroantral communications. A, Diagrammatic illustration of oroantral fistula in right maxillary alveolar process in region of missing first molar tooth, which is to be closed with subperiosteal placement of alloplastic material such as gold or titanium foil or a resorbable collagen membrane. Facial and palatal mucoperiosteal flaps are developed. Extension of the flaps along the gingival sulcus one or two teeth anterior and posterior allows some stretching of the flap to facilitate advancement for closure over the defect. Fistulous tract is excised. Osseous margins must be exposed 360 degrees around bony defect to allow placement of membrane beneath mucoperiosteal flaps. Flap is supported on all sides by underlying bone. B, Diagram of closure. Ideally, the flaps can be approximated over the defect. In some small cases a small gap between the flaps will heal over the membrane by secondary intention. Even if the intraoral mucosa does not heal primarily, the sinus lining usually heals and closes, and the membrane is then exfoliated or resorbed and mucosal healing progresses. C, Cross-sectional diagram of membrane closure technique. Buccal and palatal mucoperiosteal flaps are elevated to expose osseous defect and large area of underlying alveolar bone around oroantral communication. Membrane overlaps all margins of the defect, and the facial and palatal flaps are sutured over the membrane.
Rotation of a palatal flap is often used to close an oroantral fistula.18 The advantages of using a full-thickness palatal flap are that a large amount of tissue can be elevated with good blood supply from palatal vessels and the thickness and keratinized nature of palatal tissue more closely resemble crestal ridge tissue than thinner, less keratinized tissue in the buccal vestibule. The disadvantage of this flap is the large area of exposed bone that results from elevation of the flap. The size of the flap must allow for passive rotation of the flap to cover the entire defect with flap margins extending past the bony margins of the defect. Once the fistula is excised and the flap is elevated, rotated, and sutured in place, the palatal defect will eventually heal with granulation and secondary epithialization (Fig. 19-18). In some cases the defect can be covered with a temporary obturator with some type of soft tissue conditioning liner; however, it is important that no pressure be applied over the flap area because this may decrease blood supply and cause tissue necrosis.
Another technique for fistula closure uses excision of the fistula, elevation of flaps on the facial and palatal aspect of the defect, covering of the defect with some type of alloplastic material, and approximation of the flaps as closely as possible over the alloplast. Thin metallic foil such as gold foil or thin titanium has been used for this purpose and must be closely adapted to the contour of the bony surface.19 The sinus lining and in some cases crestal bone heals over the superior surface of the metal. In some cases the foil remains permanently, but more frequently a small portion of the metal eventually becomes exposed and the material is gradually exfoliated. An identical closure technique may also be performed using material such as a collagen membrane that is eventually resorbed.20,21
In rare cases, larger defects, particularly those resulting from surgical removal of pathologic lesions, may require larger flaps to accomplish closure and may include the use of pedicle flaps from the tongue or temporalis muscle.
REFERENCES
1. Moss-Salentijn, L. Anatomy and embryology. In: Blitzer A, Lawson W, Friedman WH, eds. Surgery of the paranasal sinuses. Philadelphia: WB Saunders, 1991.
2. Anon, JB, Rontal, M, Zinreich, SJ. Maxillary sinus anatomy. In: Anon JG, Rontal MK, Zinreich SJ, eds. Anatomy of the paranasal sinuses. New York: Thieme, 1996.
3. Eberhardt, JA, Torabinejad, M, Christiansen, EL. A computed tomographic study of the distances between the maxillary sinus floor and the apices of the maxillary posterior teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1992;73:345.
4. Harorh, A, Bacutoglu, O. The comparison of vertical height and width of maxillary sinus by means of Waters’ view radiograms taken from dentate and edentulous cases. Ann Dent. 1995;54:47.
5. McCafferey, TF, Kern, EB. Clinical evaluation of nasal obstruction. Arch Otolaryngol Head Neck Surg. 1979;105:542.
6. Som, PM, Brandwein, M. Anatomy, physiology, and plain film normal anatomy. In Som P, Curtin HD, eds.: Head and neck imaging, ed 3, St Louis: Mosby, 1996.
7. Zinreich, SJ, Benson, JL, Oliverio, PJ. Sinonasal cavities: CT normal anatomy, imaging of the osteomeatal complex and functional endoscopic surgery. In Som P, Curtin HD, eds.: Head and neck imaging, ed 3, St Louis: Mosby, 1996.
8. Gwaltney, JM, Jr. Acute community-acquired sinusitis. Clin Infect Dis. 1996;23:1209–1225.
9. Weymouth, LA. Microbiology of the maxillary sinus. Oral and Maxillofacial Clinics of North America. 1999;11:21–33.
10. Brook, I. Sinusitis of odontogenic origin. Otolaryngol Head Neck Surg. 2006;135:349–355.
11. Okeson, J, Falace, D. Nonodontogenic toothache. Dent Clin North Am. 1997;41:367.
12. Nariki-Makela, M, Qvarnberg, Y. Endoscopic sinus surgery or Caldwell-Luc operation in the treatment of chronic and recurrent maxillary sinusitis. Acta Otolaryngol. 1997;529:177.
13. Costa, F, Emanuelli, E, Robiony, M, et al. Endoscopic surgical treatment of chronic maxillary sinusitis of dental origin. J Oral Maxillofac Surg. 2007;65:223–228.
14. Gardner, DG, Gullane, PJ. Mucoceles of the maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1986;62:538–543.
15. Killey, H, Kay, LW. An analysis of 250 cases of oroantral fistula treated by the buccal flap operation. J Oral Surg. 1967;24:726.
16. Hanazawa, Y, Itoh, K, Mabashi, T, et al. Closure of oroantral communications using a pedicled buccal fat pad graft. J Oral Maxillofac Surg. 1995;53:771.
17. Juselius, H, Katollio, K. Closure of antroalveolar fistulae. J Laryngol Otol. 1991;85:387.
18. Awang, MN. Closure of oroantral fistula. Int J Oral Maxillofac Surg. 1988;17:110.
19. Mainous, EG, Hammer, DD. Surgical closure of oroantral fistula using the gold foil technique. J Oral Surg. 1974;32:528.
20. Mitchell, R, Lamb, J. Immediate closure of oroantral communications with a collagen implant: a preliminary report. Br Dent J. 1983;154:171.
21. Van Minnen, B, Stegenga, B, vanLeeuwen, MBM, et al. Nonsurgical closure of oroantral communications with a biodegradable polyurethane foam: a pilot study in rabbits. J Oral Maxillofac Surg. 2007;65:218.