Biocompatibility of Dental Materials
ANATOMICAL AND PATHOLOGICAL ASPECTS OF ORAL TISSUES
Tests for Cell Metabolism or Cell Function
Tests That Use Barriers (Indirect Tests)
CORRELATION AMONG IN VITRO, ANIMAL, AND USAGE TESTS
Biocompatibility is formally defined as the ability of a material to elicit an appropriate biological response in a given application in the body. Inherent in this definition is the idea that a single material may not be biologically acceptable in all applications. For example, a material that is acceptable as a full cast crown may not be acceptable as a dental implant. Also implicit in this definition is an expectation for the biological performance of the material. In a bone implant, the expectation is that the material will allow the bone to integrate with the implant. Thus an appropriate biological response for the implant is osseointegration. In a full cast crown, the expectation is that the material will not cause inflammation of pulpal or periodontal tissues, but osseointegration is not an expectation. Whether or not a material is biocompatible is therefore dependent on the physical function we ask of the material and the biological response we require from it. Using this definition, it makes little sense to say that any given material is or is not biocompatible—we need to define how the material will be used before we can assess this.
In this regard, biocompatibility is much like color. Color is a property of a material interacting with its environment (light), and the color of a material depends on the light source and the observer of the light. Similarly, biocompatibility is a property of a material interacting with its environment. The biological response will change if changes occur in the host, the application of the material, or the material itself (Fig. 5-1).
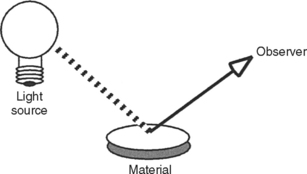
FIGURE 5-1 Like color, biocompatibility is not a property of just a material, but rather a property of how a material interacts with its environment. A material’s color depends on the character of the light source, how the light interacts with the material, and how the observer interprets the reflected light. In this sense, the material’s color depends on its environment. The biocompatibility of a material is similar in the sense that it depends on its environment.
Dentistry shares concerns about biocompatibility with other fields of medicine, such as orthopedics, cardiology, and vascular biology, among others. In the development of any biomaterial, one must consider the strength, esthetics, and functional aspects of the material, as well as its biocompatibility. Furthermore, demands for appropriate biological responses are increasing as we require that materials perform more sophisticated functions in the body for longer time periods. Thus, considerations of biocompatibility are important to manufacturers, practitioners, scientists, and patients. The field of biocompatibility is interdisciplinary, and draws on knowledge from materials science, bioengineering, biochemistry, molecular biology, tissue engineering and other fields.
This chapter surveys the tests used for evaluating biocompatibility of dental materials, the specifications that govern such testing, and the strengths and weaknesses of the testing methods. In addition, biocompatibility of various materials used in dentistry is discussed within this framework of principles. In order to understand the concepts involved in biocompatibility assessment, one must understand the biological system into which that material is placed. Therefore, this chapter will first summarize the anatomical and pathological aspects of the oral tissues relevant to dental materials.
ANATOMICAL AND PATHOLOGICAL ASPECTS OF ORAL TISSUES
Enamel
Mature human enamel is highly mineralized (96% by weight), with 1% of its weight being composed of organic molecules and 3% being water. The organic matrix of enamel consists of at least two types of glycoproteins: amelogenins and enamelins. After synthesis by ameloblasts, the calcified organic matrix of enamel does not appear to be maintained in any way by cellular synthetic mechanisms, contrary to other calcified tissues such as dentin, bone, and cementum. Enamel rods have a specific orientation with each other, and this orientation provides maximal strength. Because of its high mineral (hydroxylapatite) content, enamel is much more brittle than dentin and is solubilized to a greater extent by acid solutions. This property is used to advantage with bonding agents, when acids are used to etch the enamel to provide micromechanical retention of resin composites. The differential etching that occurs is a consequence of the different orientation of enamel rods on the enamel surface. Permeability of enamel to most oral molecules is quite low, and in this sense enamel “seals” the tooth to outside agents. However, recent evidence indicates that enamel is not totally impermeable. Peroxides in bleaching agents have been shown to penetrate intact enamel in just a few seconds.
Dentin and Pulp
Because of their intimate anatomical relationship, most researchers consider dentin and pulp to be a single tissue. The dentinal matrix (both calcified and uncalcified) forms the greatest bulk of the tooth. Calcified dentin is about 20% organic, 70% inorganic, and 10% aqueous by weight. Collagen constitutes approximately 85% of the organic portion of dentin, and hydroxyapatite is the main inorganic compound. The dentinal matrix also contains many proteins, including collagenous proteins (mainly type I collagen with smaller amounts of type V and type I trimer collagens), noncollagenous dentinspecific proteins (phosphophoryns, dentin sialoprotein, and dentin matrix protein-1), and several nonspecific proteins associated with mineralized tissues (e.g., osteocalcin and osteopontin).
The dentinal matrix surrounds dentinal tubules that are filled with odontoblastic processes. These processes stem from cells that reside in the pulp of the tooth. The tubules traverse the region between the dentoenamel junction (DEJ) and the pulp. The numbers of tubules per cross-sectional area range from about 20,000/mm2 near the DEJ to 50,000/mm2 near the pulp. Tubule diameter varies from about 0.5 μm at the DEJ to about 2.5 μm near the pulp (Fig. 5-2). Some odontoblastic processes extend through the dentin tubules to the DEJ, but the percentage of processes that reach the DEJ is a matter of some conjecture.
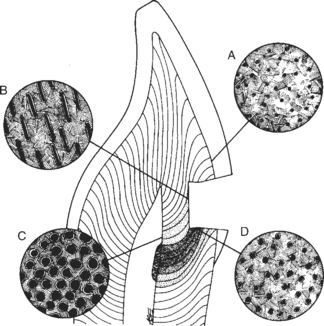
FIGURE 5-2 Diagram of dentinal tubules. Dentinal tubules occur throughout the dentin, but their size and number vary as a function of proximity to the pulp of the tooth. Near the dentin-enamel junction (A), the tubules are small in diameter and relatively few in number per centimeter squared. As the depth approaches the pulp (B, D), the tubules become larger in diameter and are denser in number. At the pulp (C), the tubules are very dense and have the largest diameters. Furthermore, the tubules do not follow a straight path from the pulp to the enamel, but curve as a function of the shape of the tooth. Thus a cavity preparation may section the tubules in either a cross-section (D) or a longitudinal section (B). The tubular structure of dentin is critical to biocompatibility because components of materials may use the tubules as conduits to pulpal tissues. (Courtesy Avery JK: Ann Arbor, 1987, University of Michigan School of Dentistry.) University of Michigan School of Dentistry
A serumlike fluid fills the dentinal tubules. This fluid has continuity with the extracellular fluid of the pulp tissue. Pulpal circulation maintains an intercellular hydraulic pressure of about 24 mm Hg (32.5 cm H2O), which causes fluid flow in the tubules to be directed from the pulp outward toward the DEJ when enamel is removed. External hydrostatic and osmotic pressures can also cause fluid movement toward or away from the pulp. The positive or negative displacement of this fluid through exposed dentinal tubules is capable of affecting either odontoblasts or pulpal nerve endings. These effects are the basis of the hydrodynamic theory of hyperalgesia (pulpal hypersensitivity).
During cavity preparation by a dentist, a “smear layer” is formed by the action of the bur or hand instruments on the calcified dentin matrix (Fig. 5-3). This mat of organic and inorganic particles occludes the dentinal tubules to some extent. The smear layer is quite effective in reducing hydrostatic pressures, but less effective in reducing diffusion, especially if the layer is interrupted or defective. The smear layer can be removed by acid etching, which also demineralizes the openings of the tubules (Fig. 5-4). The dentinal tubules establish continuity with the pulpal fluid to facilitate the diffusion of molecules, both natural or from materials, into and out of the pulp. The smear layer, dentinal tubules, and dentinal matrix are all important in the application of dentinal bonding agents and the ability of components of the bonding agents to reach and affect pulpal tissues.
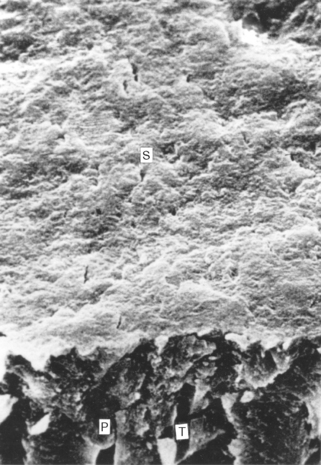
FIGURE 5-3 Scanning electron micrograph of cut dentin. When a dentin surface is cut with a bur, a layer of debris, called the smear layer (S), remains on the surface. The smear layer consists of organic and inorganic debris that covers the dentinal surface and the tubules (T). Often, the debris fills the distal part of the tubules in a smear plug (P). (From Brännström M: Dentin and pulp in restorative dentistry, Stockholm, 1981, Dental Therapeutics AB.) Dental Therapeutics AB
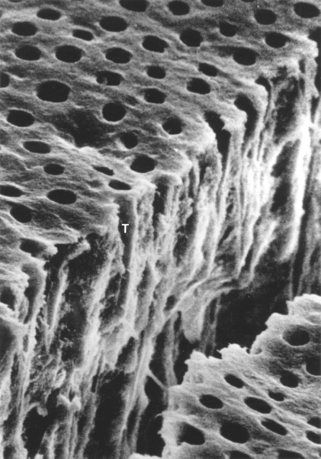
FIGURE 5-4 Scanning electron micrograph of acid-etched dentin. When a smear layer (as seen in Figure 5-3) is etched with an acid, the smear debris is removed. The rate of etching depends on the acid and the character of the dentin. The layer shown above was etched for 5 seconds with 37% phosphoric acid. The smear layer is completely gone and the tubules (T) are open. Further etching will open the tubules even further. (From Brännström M: Dentin and pulp in restorative dentistry, Stockholm, 1981, Dental Therapeutics AB.) Dental Therapeutics AB
Moderately deep cavity preparation will damage odontoblasts by severing the odontoblastic processes. Deep cavity preparation may destroy most of the dentin and kill the primary odontoblasts. A number of investigators think that the extracellular matrix (ECM) of dentin and pulp is largely responsible for the differentiation of secondary odontoblasts that form reparative dentin. The source of secondary odontoblasts is not known, but much of the proliferative activity of granulation tissue following pulpal insult is found in perivascular areas proximal to the core of the pulp. In monkeys, the minimal amount of time between pulp injury and replacement odontoblast differentiation is about 5 days. Nerves and blood vessels, which arborize from the core of the pulp as they approach the odontoblastic layer, may influence the extent of the inflammatory response and the amount of new dentinal matrix formed during dentin repair.
In the absence of a smear layer, ions released from materials and/or bacterial products diffuse toward the pulp against the pressure gradient (diffusional permeability, see later discussion). Bacteria can sometimes be seen within tubules below a carious lesion or at the base of a prepared cavity, with or without a restoration (Fig. 5-5). When toxic bacterial or chemical products traverse the dentin, odontoblasts and the pulpal connective tissue usually respond first by focal necrosis (0 to 12 hours), which may be followed by an acute, but more widespread, pulpitis (12 hours to several days). This response may resolve naturally if the injurious agent is removed or the tubules are blocked. If the pulpitis does not resolve, it may spread to more completely involve the pulp in liquefaction necrosis (especially if the pulpitis results from bacterial products) or in chronic inflammation. Finally, both acute complete pulpitis and acute exacerbation of chronic pulpitis may lead to sequelae such as dental periapical lesions and osteomyelitis. These conditions may be reviewed in oral pathology textbooks.
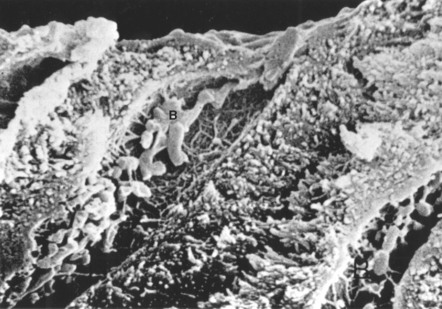
FIGURE 5-5 Scanning electron micrograph of bacteria in dentinal tubules. The dentinal tubules may form a conduit for bacteria and bacterial products to reach the pulp of the tooth. Bacteria (B) are visible in the tubules under the smear layer, which is visible in cross-section at the top of the picture. (From Brännström M: Dentin and pulp in restorative dentistry, Stockholm, 1981, Dental Therapeutics AB, and London, 1982, Wolfe Medical.) Wolfe Medical
Dentin Permeability
Much has been learned about dentin permeability in the last three decades. In practical terms, two types of dentin permeability occur. The first is fluid convection; that is, movement of fluid through the dentinal tubules. Fluid convection toward the pulp will occur under positive hydraulic pressure when a crown or inlay is being seated. If the dentinal tubules are open, this produces a sharp, localized pain in the pulp from stimulation of A-fibers. Fluid convection away from the pulp will occur with negative osmotic pressures when concentrated solutions, such as sucrose or saturated calcium chloride, are exposed to open dentinal tubules. Clinically, this situation occurs with cervical abrasion or carious lesions. Convection of fluids across dentin varies with the fourth power of the radius of the dentinal tubule (r 4), and thus is very sensitive to the diameter of the tubules. In general, coronal dentin exhibits greater convective permeability than root dentin. Axial wall dentin is more permeable than dentin in the floor of cavities, and dentin near pulp horns (where tubule diameter is greatest) is more permeable than dentin at a distance from the pulp horns. The presence of a smear layer or of cavity liners, sealers, resin bonding agents, crystals such as calcium oxalate, and even debris and bacteria in the dentinal tubules can dramatically reduce fluid convection.
The second type of dentin permeability is diffusion. Patent dentinal tubules, no matter how small the diameter, establish a diffusion gradient through which ions and molecules can move, even against positive hydraulic pressure. Diffusion is proportional to the length of the dentinal tubules and thus, roughly, to the thickness of the dentin between cavity preparation and the pulp. Smear layers created in cavity preparation are better than cavity liners and sealers at limiting diffusional permeability. However, if the smear layer is incomplete, interrupted, or removed, or if there is a disruption in a cavity liner, sealer, or base, then diffusion of molecules toward the pulp will occur.
Diffusion of natural and synthetic molecules through dentin has been studied. In general, diffusion through a given thickness of dentin is proportional to the molecular size of the molecule. Consequently, molecules the size of urea (molecular weight [mw] 60), phenol (mw 94), and glucose (mw 180) diffuse more easily than molecules the size of dextran (mw 20,000) and albumin (bovine serum albumin, mw 68,000). Through diffusion, small or globular molecules such as albumin, gamma globulin, and bis-glycidyldimethacrylate (Bis-GMA, an oligomer of resin composites) are diluted 2000 to 10,000 times on the pulpal side of the dentin by 0.3 to 0.4 mm of dentin. Large, fibrous molecules such as fibrinogen are diluted 25,000 to 125,000 times by the same dentin thickness. Most molecules are probably adsorbed to some extent by dentin. Some molecules and atoms or ions, such as tetracycline, zinc, H2O2, and fluorescein, are adsorbed to a greater extent than the biological molecules and resin monomers mentioned earlier. Finally, the capillary beds and vascular dynamics in most healthy pulp are probably capable of removing relatively large amounts of cytotoxic chemicals and bacterial products once they diffuse through the dentin. However, if the pulp is already damaged (inflamed because of caries or trauma), edema and sluggish circulation probably compromise the removal of these materials. Much still needs to be learned about the dynamics and significance of diffusion and adsorption of both host and foreign molecules through dentin.
BONE
Bone is an extracellular matrix (ECM) with accompanying cells and tissue. The ECM of bone is a mineralized tissue composed of about 23% organic substances and about 77% hydroxyapatite. Like dentin, most (86%) of this organic matrix is type I collagen, which gives elastic and viscoelastic qualities to bone. The hydroxyapatite crystals are smaller and less well formed than those in dentin. Because of the vascularity of bone, the mineral phase serves as a major reservoir of calcium and phosphate ions for the body’s metabolic processes.
The ECM of bone (osteoid) is synthesized by osteoblasts that constitute the innermost layer of the periosteum and endosteum. The osteoblasts also initiate mineralization of this ECM. As bone is formed, osteoblasts are trapped within the ECM and become osteocytes that reside in lacunae, communicate with cells in other lacunae through canaliculi, and maintain the vitality of bone. These cells die if surgical manipulation destroys the vascular supply or heats the bone above 45° C for more than a few minutes. Another bone-cell type, the osteoclast, decalcifies the ECM and resorbs the organic portion of bone. It also responds to both physiological stimuli and injury. The coupling of osteoblastic and osteoclastic activity directs remodeling and occurs almost continuously throughout life.
Bone has an excellent capacity for self-repair. In bony defects caused by tooth extraction or bone fracture, the site initially fills with blood. The fibrin cascade results in a blood clot that fills this site and attaches to the walls of the alveolar bony socket. Subsequently, mesenchymal cells and endothelial cells grow into the blood clot from the surrounding connective tissue of the alveolar bone and create a young, vascular granulation tissue. With succeeding weeks and months, new osteoblasts differentiate from the granulation tissue and fabricate an ECM that eventually mineralizes. Through osteoblastic and osteoclastic influence, the new bone remodels itself to the general shape and architecture of the surrounding bone. Usually, however, the lack of strain from tensile forces generated by the teeth and functional periodontal ligament results in the loss of alveolar bone height and width.
PERIODONTIUM
The periodontium is a combination of tissues, including the periodontal ligament (PDL), cementum, and alveolar bone. The cementum and alveolar bone are mineralized extracellular matrices with the associated cells responsible for generating and maintaining them.
Collagenous fibers of the PDL extend from cementum to fibrous connective tissue above the alveolar crest, to alveolar cortical bone, or to the cementum of adjacent teeth. The ends of these collagenous fibers are anchored in a calcified ECM synthesized by cementoblasts (cementoid) or osteoblasts (osteoid). The orientation of the fibers translates compressive forces of mastication to tensile forces on the cementum and alveolar bone. The tensile stress stimulates low-grade cementogenesis and osteogenesis and maintains fairly constant alveolar bone heights, cementum thicknesses, and PDL widths. In contrast, lack of tensile forces in the physiologic range on alveolar bone and cementum (e.g., in orthodontic movement) results in PDL necrosis and an active biological resorption that removes portions of alveolar bone, cementum, and dentin from the tooth root.
Cellular synthetic processes maintain the PDL and its attachment to alveolar bone and teeth. At least in some animal species, there appears to be some compartmentalization of differentiated cells within the PDL, with fibroblasts in the tooth half of the PDL moving mesially with the constantly erupting incisor. Thus, specific populations of differentiated, predifferentiated, or precursor cells probably give rise to and maintain the matrices of cementum, alveolar bone, and PDL. When cells that maintain the PDL are destroyed during injury and have no source of progenitor cells, ankylosis may result between tooth and bone (e.g., after tooth transplantation or placement of dental implant).
Regeneration of PDL, epithelial attachment, and alveolar bone around periodontally diseased teeth is an important issue in dentistry. In an attempt at regeneration, gingival epithelium replaces crevicular epithelium, which was originally responsible for the epithelial attachment of the tooth. Following scaling and curettage of periodontal pockets, this gingival epithelium proliferates faster than the PDL fibers can reattach in newly formed cementum. Although fibrous reattachment to alveolar bone appears to occur quite readily, the original orientation of fiber to tooth surface seems quite difficult to achieve. This results in epithelium-lined subcrestal pockets in the PDL space between alveolar bone and tooth surface. As this process advances, it has the potential effect of exfoliating the tooth. Clinicians and researchers have investigated chemical and surgical methods to limit epithelial down-growth of the gingiva, in order to enhance PDL reattachment to tooth and bone surfaces.
GINGIVA AND MUCOSA
The linings of the oral cavity are composed of gingiva and oral mucosa. The gingiva is a connective tissue with an epithelial surface that covers the alveolar ridge, surrounds the cervices of the teeth, and fills interproximal spaces between teeth. Gingiva is divided into attached and free gingiva. The attached gingiva forms a junction with the alveolar oral mucosa toward the vestibule of the mouth and with the free gingival margin toward the crowns of the teeth. The free gingiva fuses with the attachment epithelium, which surrounds the tooth at its cervix in the young, healthy tooth. Some of the crevicular epithelium and all the attachment epithelium, at least in young individuals, are derived embryologically from reduced enamel epithelium. The oral mucosa is composed of a loose fibroelastic connective tissue with a well-vascularized and innervated lamina propria and submucosa and is covered mainly by a parakeratinized stratified squamous epithelium.
Dental materials can chemically or physically injure the oral mucous membrane. If the injury is short-term (acute) and leads to loss of tissue but does not involve infection by pathogenic microorganisms, the connective tissue defect is filled in with granulation tissue within 3 to 4 days. The epithelium then regenerates over the surface within a week. The tissue is remodeled and appears nearly normal by the end of 2 to 3 weeks. As with other body tissues, the ability to heal depends on the metabolic status of the patient and the removal of external irritating factors. Occasionally, immune hypersensitivity to materials or pharmaceutical agents may delay the healing response. The presence of microorganisms or immune hypersensitivity reactions results in an infiltration of acute or chronic inflammatory cells and delay of healing.
Its association with the tooth may complicate the gingival response to injury. Calculus deposits on the tooth, malocclusion, and faulty restorations may enhance the destructive effects of microorganisms. Crevicular epithelium then becomes vulnerable to endotoxin and various exogenous and endogenous chemicals. The resulting breakdown of tissue leads to an acute inflammatory response by the gingival connective tissue called acute gingivitis. This condition often reverses if the injurious agent is removed and the reaction is limited to the connective tissue above the alveolar bony crest. If the insult continues, however, the inflammatory infiltrate becomes mixed and then predominantly mononuclear. Inflamed epithelium-lined granulation tissue gradually spreads apically below the alveolar bony crest. At this point the condition is described as chronic periodontal disease, a progressive disease process that is probably reinforced by immune mechanisms. The relationship between periodontal disease and dental materials that are in close contact with the gingiva is not known but is an active area of research.
The reaction of gingival tissues to oral implants is also an important area of research. Transmucosal implants present special problems, including epithelial ingrowth, encystification, and exfoliation of the implant. There is also the problem of maintaining a close epithelial attachment between the implant and soft tissue to exclude bacteria. Thus, the implant material should ideally encourage firm attachment of epithelial cells on its surface, but only limited growth and migration of these cells downward. An inflammatory disease around implants, called periimplantitis, has been described and is probably similar in etiology and progression to periodontal disease. Peri-implantitis occurs in response to bacteria that attach to implants and exist near the gingiva. The role of materials in altering the peri-implantitis process is not currently known.
Dental materials that are antigenic can cause immune hypersensitivity reactions in oral mucosa and gingiva. Local binding of antigens to membranes of white blood cells (i.e., lymphocytes, macrophages, basophils, mast cells) or Langerhans cells of skin and oral mucosal epithelium plays a role in activating these various reactions. Although a few of the mucosal reactions are documented as Type I reactions (wherein vasoactive substances are released from mast cells because of antigen-IgE reactions), most reactions to dental materials are classified as type IV (T-cell-mediated) reactions. This type of reaction is sometimes called contact mucositis. Skin testing can be used to help document type I and type IV reactions to environmental antigens, metallic elements used in alloys, and byproducts from polymers. In vitro tests for cell-mediated hyperimmunity are occasionally performed and include transformation of the patient’s lymphocytes and production of a migration-inhibition factor by these cells in response to the antigenic stimulus.
MEASURING BIOCOMPATIBILITY
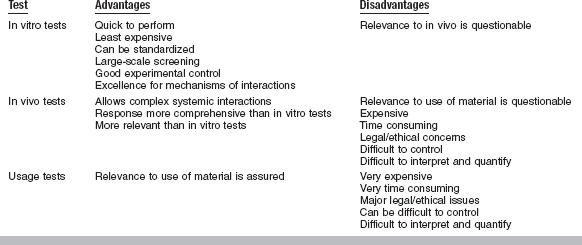
Measuring the biocompatibility of a material is not simple, and the methods of measurement are evolving rapidly as more is known about the interactions between dental materials and oral tissues and as technologies for testing improve. Historically, new materials were simply tested in humans to assess their biocompatibility. However, this practice has not been acceptable for many years, and current materials must be extensively screened for biocompatibility before they are used in humans. Several varieties of tests are currently used to ensure that new materials are biologically acceptable. These tests are classified as in vitro, animal, and usage tests. These three testing types include clinical trials, which is really a special case of a usage test in humans. The remainder of this section will discuss several of each type of test, their advantages and disadvantages, how the tests are used together, and standards that rely on these tests to regulate the use of materials in dentistry.
IN VITRO TESTS
In vitro tests for biocompatibility are done in a test tube, cell-culture dish, or otherwise outside a living organism. These tests require placement of a material or a component of a material in contact with a cell, enzyme, or some other isolated biological system. The contact can be either direct, when the material contacts the cell system without barriers, or indirect, when there is a barrier of some sort between the material and the cell system. Direct tests can be further subdivided into those in which the material is physically present with the cells and those in which some extract from the material contacts the cell system. In vitro tests can be roughly subdivided into those that measure cytotoxicity or cell growth, those that measure some metabolic or other cell function, and those that measure the effect on the genetic material in a cell (mutagenesis assays). Often there is overlap in what a test measures. In vitro tests have a number of significant advantages over other types of biocompatibility tests (Table 5-1). They are relatively quick to perform, generally cost less than animal or usage tests, can be standardized, are well suited to large-scale screening, and can be tightly controlled to address specific scientific questions. The overriding disadvantage of in vitro tests is their questionable relevance to the final in vivo use of the material (see later section on correlation between tests). Another significant disadvantage includes the lack of inflammatory and other tissue-protective mechanisms in the in vitro environment. It should be emphasized that in vitro tests alone cannot entirely predict the overall biocompatibility of a material.
Standardization of in vitro tests is a primary concern for those trying to evaluate materials. Two types of cells can be used for in vitro assays. Primary cells are those cells taken directly from an animal and cultured. These cells will grow for only a limited time in culture but usually retain many of the characteristics of cells in vivo. Continuous cells or cell lines are primary cells that have been transformed previously to allow them to grow more or less indefinitely in culture. Because of this transformation, these cells do not retain all in vivo characteristics, but they do consistently exhibit any features that they do retain. Primary cell cultures seem to be more relevant than continuous cell lines for measuring cytotoxicity of materials. However, primary cells have their restrictions as well. Being from a single individual, the cells have limited genetic variability, may harbor viral or bacterial agents that alter their behavior, and often rapidly lose their in vivo functionality once placed in culture. Furthermore, the genetic and metabolic stability of continuous cell lines contributes significantly toward standardizing assay methods. In the end, both primary and continuous cells play important roles in in vitro testing; both should be used to assess any material.
Cytotoxicity Tests
Cytotoxicity tests assess cell death caused by a material by measuring cell number or growth before and after exposure to that material. Cells are plated in a well of a cell-culture dish, where they attach. The material is then placed in the test system. If the material is not cytotoxic, cells will remain attached to the well and will proliferate over time. If the material is cytotoxic, cells may stop growing, exhibit cytopathic features (Figs. 5-6 and 5-7), or detach from the well. If the material is a solid, then the density (number of cells per unit area) of cells may be assessed at different distances from the material, and a “zone” of inhibited cell growth may be described (Fig. 5-8). Cell density can be assessed qualitatively, semiquantitatively, or quantitatively. Substances such as Teflon or cell-culture treated polystyrene can be used as negative (noncytotoxic) control materials, whereas materials such as plasticized polyvinyl chloride can be used as positive (cytotoxic) control materials. Control materials should be well defined and commercially available to facilitate comparisons among other testing laboratories.
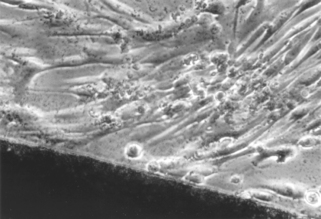
FIGURE 5-6 Light microscopic view of a noncytotoxic interaction between a material (dark image at bottom of the picture) and periodontal ligament fibroblasts in a cell culture (in vitro) test. The morphology of the fibroblasts indicates that they are alive and are not suffering from a toxic response (see Fig. 5-7 for contrast). The material in this case was a calcium hydroxide pulp-capping agent.
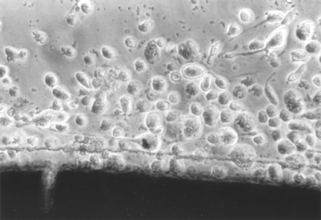
FIGURE 5-7 Light microscopic view of a cytotoxic interaction between a material (dark image at the bottom of the picture) and periodontal ligament fibroblasts in a cell culture test. The fibroblasts are rounded and detached (see Fig. 5-6 for contrast), indicating that they are either dead or dying. The material is a type of calcium hydroxide pulp-capping agent, different from the one shown in Fig. 5-6.
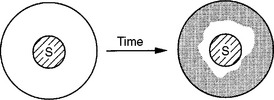
FIGURE 5-8 A material sample (S) is placed in the center of a cell culture well, and cells and medium are added. After 1 to 3 days, the cells have multiplied in areas where the material has not inhibited their growth. The area devoid of growth is often referred to as a ring of inhibition. Several methods are available to assess the amount of cellular growth around the samples.
Another group of tests is used to measure cytotoxicity by a change in membrane permeability (Fig. 5-9). Membrane permeability is the ease with which a dye can pass through a cell membrane. This test is used on the basis that a loss in membrane permeability is equivalent to or very nearly equivalent to cell death. The advantage of the membrane permeability test is that it identifies cells that are alive (or dead) under the microscope. This feature is important because it is possible for cells to be physically present, but dead (when materials fix the cells). There are two basic types of dyes used. Vital dyes are actively transported into viable cells, where they are retained unless cytotoxic effects increase the permeability of the membrane. It is important to establish that the dye itself does not exhibit cytotoxicity during the time frame of the test. Nonvital dyes are not actively transported, and are only taken up if membrane permeability has been compromised by cytotoxicity. Many types of vital dyes have been used, including neutral red and Na251CrO4. The use of neutral red and Na2[51Cr]O4 are particularly advantageous because they are neither synthesized nor metabolized by the cell. Examples of nonvital dyes include trypan blue and propidium iodide.
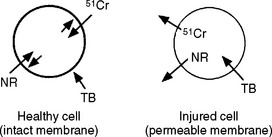
FIGURE 5-9 The selective permeability of cell membranes is the basis for several cytotoxicity tests. Compounds such as Na51CrO4 (51Cr), and neutral red (NR) are actively sequestered by healthy cells. These compounds will leach out of the cell if the cell is injured and cannot maintain its membrane integrity. Other compounds such as trypan blue (TB) are excluded by a healthy cell, but can diffuse through the membrane of an injured cell.
Tests for Cell Metabolism or Cell Function
Some in vitro tests for biocompatibility use the biosynthetic or enzymatic activity of cells to assess cytotoxic response. Tests that measure deoxyribonucleic acid (DNA) synthesis or protein synthesis are common examples of this type of test. The synthesis of DNA or protein by cells is usually analyzed by adding radioisotope-labeled precursors to the medium and quantifying the radioisotope (e.g., 3H-thymidine or 3H-leucine) incorporated into DNA or protein. A commonly used enzymatic test for cytotoxicity is the MTT test. This test measures the activity of cellular dehydrogenases, which convert a chemical called MTT, via several cellular reducing agents, to a blue, insoluble formazan compound (Fig. 5-10). If the dehydrogenases are not active because of cytotoxic effects, the formazan will not form. Production of formazan is quantified by dissolving it and measuring the optical density of the resulting solution. Alternatively, the formazan can be localized around the test sample by light or electron microscopy. Other formazan-generating chemicals have been used, including NBT, XTT, and WST.
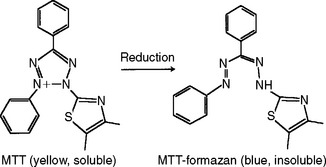
FIGURE 5-10 MTT is a yellow, soluble molecule that can be used to assess cellular enzymatic activity. If the cell is able to reduce the MTT, the resulting formazan is blue and insoluble and deposits in the cell. The amount of formazan formed is proportional to the enzymatic activity. The activity of a number of cellular enzymes can be assessed in this manner.
Recently developed indicator dyes such as alamarBlue have been formulated to quantitatively measure cell proliferation using an oxidationreduction (redox) indicator that yields a colorimetric change and a fluorescent signal in a response to a metabolic activity. These new generation dyes permit continuous monitoring of cells over time. Additional in vitro tests to measure gene activation, gene expression, cellular oxidative stress, and other specific cell functions have been proposed. However, these tests are not yet routinely used to assess the biocompatibility of materials.
Tests That Use Barriers (Indirect Tests)
Most of the cytotoxicity tests presented thus far are performed with the material in direct contact with the cell culture. Researchers have long recognized that in vivo, direct contact often does not exist between cells and the materials. Separation of cells and materials may occur from keratinized epithelium, dentin, or extracellular matrix. Thus, several in vitro barrier tests have been developed to mimic in vivo conditions. One such test is the agar overlay method (Fig. 5-11) in which a monolayer of cultured cells is established before adding 1% agar or agarose (low melting temperature) plus a vital stain, such as neutral red, to fresh culture media. The agar forms a barrier between the cells and the material, which is placed on top of the agar. Nutrients, gas, and soluble toxic substances can diffuse through the agar. Solid test samples or liquid samples adsorbed onto filter paper can be tested with this assay for up to 24 hours. This assay correlates positively with the direct-contact assays described previously and the intramuscular implantation test in rabbits. However, the agar may not adequately represent barriers that occur in vivo. Furthermore, because of variability of the agar’s diffusion properties, it is difficult to correlate the intensity of color or width of the zone around a material with the concentration of leachable toxic products.
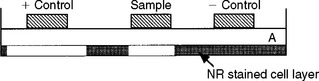
FIGURE 5-11 The agar overlay method has been used to evaluate the cytotoxicity of dental materials. The cell layer, which has been previously stained with neutral red (NR), is covered with a thin layer of agar (A). Samples are placed on top of the agar for a time. If the material is cytotoxic, it will injure the cells and the neutral red will be released, leaving a zone of inhibition.
A second barrier assay is the Millipore filter assay. This technique establishes a monolayer of cells on filters made of cellulose esters. The culture medium is then replaced with medium containing about 1% agar, and this mixture is allowed to gel over the cells. Finally, the filter-monolayer-gel is detached and turned over so that the filter is on top for placement of solid or soluble test samples for 2 or more hours. After exposure to the test samples, the filter is removed and an assay is used to determine the effect of the sample on a cellular metabolic activity. The succinyl dehydrogenase assay described previously can be used with this test. Like the agar overlay test and the cell contact tests, toxicity in the Millipore filter test is assessed by the width of the cytotoxic zone around each test sample. This test also has the drawback of arbitrarily influencing the diffusion of leachable products from the test material. The agar diffusion and Millipore filter tests can provide, at best, a qualitative cytotoxic ranking among materials.
Dentin barrier tests have shown improved correlation with the cytotoxicity of dental materials in usage tests in teeth, and are gradually being developed for screening purposes (Fig. 5-12). A number of studies have shown that dentin forms a barrier through which toxic materials must diffuse to reach pulp tissue. Thus pulpal reaction to zinc oxide–eugenol is relatively mild as compared with the more severe reactions to the same material in direct contact with cells in in vitro assays and tissue in implantation tests. The thickness of the dentin correlates directly with the protection offered to the pulp. Thus, assays have been developed that incorporate dentin disks between the test sample and the cell assay system. The use of dentin disks offers the added advantage of directional diffusion between the restorative material and the culture medium.
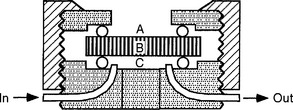
FIGURE 5-12 A dentin disk used as a barrier in cytotoxicity tests that attempt to predict the toxicity of materials placed on dentin in vivo. The material is placed on one side (A) of the dentin disk (B) in the device used to hold the dentin disk. Collection fluid (cell culture medium or saline) is on the other side of the disk (C). Cells can also be grown in the collection side. Components of the material may diffuse through the dentin and the effect of the medium on cell metabolism can then be measured. To assess the rate of diffusion, the collection fluid can be circulated into and out of the collection chamber (C).
Other Assays for Cell Function
In vitro assays to measure immune function or other tissue reactions have also been used. The in vivo significance of these assays is yet to be ascertained, but many show promise for being able to reduce the number of animal tests required to assess the biocompatibility of a material. These assays measure cytokine production by lymphocytes and macrophages, lymphocyte proliferation, chemotaxis, or T-cell rosetting to sheep red blood cells. Other tests measure the ability of a material to alter the cell cycle or activate complement. The activation of complement is of particular concern to researchers working on artificial or “engineered” blood vessels and other tissues in direct contact with blood. Materials that activate complement may generate inflammation or thrombi, and may propagate a chronic inflammatory response. Whereas concerns about these types of response to dental materials are fewer, it is possible that activation of complement by resins or metals or their corrosion products may prolong inflammation in the gingiva or pulp.
Mutagenesis Assays
Mutagenesis assays assess the effect of a biomaterial on a cell’s genetic material. There are a wide range of mechanisms by which a material can affect a cell’s genes. Genotoxic mutagens directly alter cell DNA through various types of mutations. Each chemical may be associated with a specific type of DNA mutation. Genotoxic chemicals may be mutagens in their native states, or may require activation or biotransformation to be mutagens, in which case they are called promutagens. Epigenetic mutagens do not alter the DNA themselves, but support tumor growth by altering the cell’s biochemistry, altering the immune system, acting as hormones, or other mechanisms. Carcinogenesis is the ability to cause cancer in vivo. Mutagens may or may not be carcinogens, and carcinogens may or may not be mutagens. Thus, quantitation and relevance of tests that measure mutagenesis and carcinogenesis are extremely complex. A number of government-sponsored programs evaluate the ability of in vitro mutagenesis assays to predict carcinogenicity.
The Ames test is the most widely used short-term mutagenesis test and the only short-term test that is considered thoroughly validated. It uses mutant stocks of Salmonella typhimurium that require exogenous histidine. Native stocks of bacteria do not require exogenous histidine. Exclusion of histidine from the culture medium allows a chemical to be tested for its ability to convert the mutant strain to a native strain. Chemicals that significantly increase the frequency of reversion back to the native state have a reportedly high probability of being carcinogenic in mammals because they significantly alter genetic material. Performance of this test requires experience in the field and special strains of Salmonella to produce meaningful results. Several strains of Salmonella are used, each to detect a different type of mutation transformation. Furthermore, chemicals can be “metabolized” in vitro using homogenates of liver enzymes to simulate the body’s action on chemicals before testing for mutagenicity.
A second test for mutagenesis is the Styles’ cell transformation test. This test on mammalian cells was developed to offer an alternative to bacterial tests (Ames test), which may not be relevant to mammalian systems. This assay quantifies the ability of potential carcinogens to transform standardized cell lines so they will grow in soft agar. Untransformed fibroblasts normally will not grow within an agar gel, whereas genetically transformed cells will grow below the gel surface. This characteristic of transformed fibroblasts is the only characteristic that correlates with the ability of cells to produce tumors in vivo. At least four different continuous cell lines (Chang, BHK, HeLa, WI-38) have been used. In 1978, Styles claimed 94% “accuracy in determining carcinogenic or noncarcinogenic activity” when testing 120 compounds in two cell lines. However, there has been some difficulty in reproducing these results.
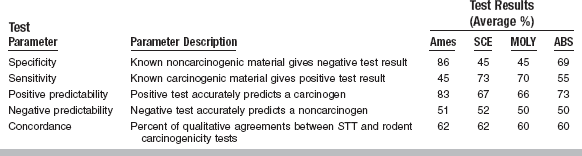
In a recent report, four short-term tests (STTs) for gene toxicity were compared (Table 5-2). The Ames test was the most specific (86% of noncarcinogens yielding a negative result). The Ames test also had the highest positive predictability (83% of positives were actually carcinogens) and displayed negative predictability equal to that of other STTs (i.e., 51% of all Ames test negatives were noncarcinogenic). However, the results were in agreement (concordance) with rodent carcinogenicity tests for only 62% of the chemicals. Also, the Ames test was sensitive to only 45% of the carcinogens; that is, it missed over half of the known carcinogens. The other three STTs were assays for chromosomal aberration, sister chromatid exchange in CHO cells, and the mouse lymphoma L5178Y cell mutagenesis assay. The sister chromatid exchange method, the mouse lymphoma mutagenesis assay, and the Ames test had 73%, 70%, and 45% sensitivity, respectively. However, because the Ames test is widely used, extensively described in the literature, and technically easier to conduct in a testing laboratory than the other tests, it is most often conducted in a screening program. These studies suggest that not all carcinogens are genotoxic (mutagenic) and not all mutagens are carcinogenic. Thus, although STTs for mutagenesis are helpful for predicting some carcinogens, STTs cannot predict all of them.
ANIMAL TESTS
Animal tests for biocompatibility are usually used in mammals such as mice, rats, hamsters, or guinea pigs, although many types of animals have been used. Animal tests are distinct from usage tests (which are also often done in animals) in that the material is not placed in the animal with regard to its final use. The use of an animal allows many complex interactions between the material and a functioning, complete biological system to occur. For example, an immune response may occur or complement may be activated in an animal system in a way that would be difficult to mimic in a cell-culture system. Thus, the biological responses in animal tests are more comprehensive and may be more relevant than in vitro tests, and these features are the major advantages of these tests (see Table 5-1). The main disadvantages of animal tests are that they can be difficult to interpret and control, are expensive, may be time consuming, and often involve significant ethical concerns and paperwork. Furthermore, the relevance of the test to the in vivo use of a material can be quite unclear, especially in estimating the appropriateness of an animal species to represent a human. A variety of animal tests have been used to assess biocompatibility, and a few are discussed in detail here.
The mucous membrane irritation test determines if a material causes inflammation to mucous membranes or abraded skin. During this test, test materials, positive controls, and negative controls are all placed into contact with hamster cheek-pouch tissue or rabbit oral tissue. After several weeks of contact, the controls and test sites are examined, and the gross tissue reactions in the living animals are recorded and photographed in color. The animals are then sacrificed, and biopsy specimens are prepared for histological evaluation of inflammatory changes.
In the skin sensitization test in guinea pigs (guinea pig maximization test), the materials are injected intradermally to test for development of skin hypersensitivity reactions. Freund’s adjuvant can be used to augment the reaction. This injection is followed by secondary treatment with adhesive patches containing the test substance. If hypersensitivity developed from the initial injection, the patch will elicit an inflammatory response. The skin-patch test can result in a spectrum from no reaction to intense redness and swelling. The degree of reaction in the patch test and the percentage of animals that show a reaction are the bases for estimating the allergenicity of the material.
Animal tests that measure the mutagenic and carcinogenic properties of materials have been developed by toxicologists. These tests are employed with a strategy called the decision-point approach. Using this strategy, tests are applied in a specific order, and testing is stopped when any one indicates mutagenic potential of the material or chemical. Issues of species, tissue, gender, and other factors affect the validity of these tests. Decision-point tests are generally divided into limited-term in vivo tests and long-term or lifetime tests. Limited-term in vivo tests measure altered liver function or increased tumor induction when animals are exposed to the chemicals for a fraction of their lifetimes. Long-term in vivo tests, on the other hand, keep the chemical in contact with the animal over the majority of its lifetime.
Implantation tests are used to evaluate materials that will contact subcutaneous tissue or bone. The location of the implant site is determined by the use of the material and may include connective tissue, bone, or muscle. Although amalgams and alloys are tested because the margins of the restorative materials contact the gingiva, most subcutaneous tests are used for materials that will directly contact soft tissue during implantation, endodontic, or periodontal treatment. Short-term implantation is studied by aseptically placing the compounds in small, open-ended, polyethylene tubes into the tissue. The test samples and controls are placed at separate sites and allowed to remain for 1 to 11 weeks. Alternatively, an empty tube is embedded first, and the inflammatory reaction from surgery is allowed to subside. The implant site is then reopened, and the test material is placed into this healed site or is packed into the tube that was placed previously. At the appropriate time, the areas are excised and prepared for microscopic examination and interpretation. The tissue response can be evaluated by normal histological, histochemical, or immunohistochemical methods. Implantation tests of longer duration, for identification of either chronic inflammation or tumor formation, are performed in a manner similar to that of short-term tests except the materials remain in place for 1 to 2 years before examination.
USAGE TESTS
Usage tests may be done in animals or in human volunteers. They are distinct from other animal tests because they require that the material be placed in a situation identical to its intended clinical use. The usefulness of a usage test for predicting biocompatibility is directly proportional to the fidelity with which the test mimics the clinical use of the material in every regard, including time, location, environment, and placement technique. For this reason, usage tests in animals usually employ larger animals that have similar oral environments to humans, such as dogs or monkeys. If humans are used, the usage test is a clinical trial. The overwhelming advantage for a usage test is its relevance (see Table 5-1). These tests are the gold standard, in that they give the ultimate answer to whether or not a material will be biocompatible. One might ask, then, why bother with in vitro or animal tests at all. The answer is in the significant disadvantages of the usage test. These tests are extremely expensive, last for long periods, involve many ethical and often legal concerns, and are exceptionally difficult to control and interpret accurately. The statistical analysis of these tests is often a daunting process. In dentistry, dental pulp, periodontium, and gingival or mucosal tissues are generally the targets of usage tests.
Dental Pulp Irritation Tests
Generally, materials to be tested on the dental pulp are placed in class 5 cavity preparations in intact, noncarious teeth of monkeys or other suitable animals. Care is taken to prepare uniformly sized cavities. After anesthesia and a thorough prophylaxis of teeth, cavities are prepared under sterile conditions with an efficient water-spray coolant to ensure minimal trauma to the pulp. The compounds are placed in an equal number of anterior and posterior teeth of the maxilla and mandible to ensure uniform distribution in all types of teeth. The materials are left in place from 1 to 8 weeks. Zinc oxide–eugenol (ZOE) and silicate cement have been used as negative and positive control materials, respectively.
At the conclusion of the study, the teeth are removed and sectioned for microscopic examination. The tissue sections are evaluated by the investigators without knowledge of the identity of the materials, and necrotic and inflammatory reactions are classified according to the intensity of the response. The thicknesses of the remaining dentin and reparative dentin for each histological specimen is measured with a photomicrometer and recorded. The response of the pulp is evaluated based on its appearance after treatment. The severity of the lesions is based on disruption of the structure of the tissue and the number of inflammatory cells (usually both acute and chronic) present. Pulpal response is classified as either slight (mild hyperemia, few inflammatory cells, slight hemorrhage in odontoblastic zone), moderate (definite increase in number of inflammatory cells, hyperemia, and slight disruption of odontoblastic zone), or severe (decided inflammatory infiltrate, hyperemia, total disruption of odontoblastic layer in the zone of cavity preparation, reduction or absence of predentin, and perhaps even localized abscesses). As with dental caries, the mononuclear cells are usually most prominent in the inflammatory response. If neutrophils are present, the presence of bacteria or bacterial products must be suspected. Some investigators now use ZOE cements to “surface-seal” the restorations to eliminate the effects of microleakage on the pulp.
Until recently, most dental-pulp irritation tests have involved intact, noncarious teeth, without inflamed pulps. There has been increased concern that inflamed dental pulp tissue may respond differently than normal pulps to liners, cements, and restorative agents. Efforts have been made to develop techniques that identify bacterial insults to the pulp. Usage tests that study teeth with induced pulpitis allow evaluation of types and amount of reparative dentin formed and will probably continue to be developed.
Dental Implants in Bone
At present, the best estimations of the success and failure of implants are gained from three tests: (1) penetration of a periodontal probe along the side of the implant, (2) mobility of the implant, and (3) radiographs indicating either osseous integration or radiolucency around the implant. Currently, an implant is considered successful if it exhibits no mobility, no radiographic evidence of peri-implant radiolucency, minimal vertical bone loss, and absence of persistent peri-implant soft-tissue complications. Previously, investigators argued that formation of a fibrous connective tissue capsule around a subperiosteal implant or root cylinder was the natural reaction of the body to a material. They argued that this was actually an attachment similar to the periodontal ligament and should be considered a sign of an acceptable material. However, in most cases it resembled the wall of a cyst, which is the body’s attempt to isolate the implanted material as the material slowly degrades and leaches its components into tissue. Currently, for implants in bone, implants should be completely encased in bone, the most differentiated state of that tissue. Fibrous capsule formation is a sign of irritation and chronic inflammation.
Mucosa and Gingival Usage Tests
Because various dental materials contact gingival and mucosal tissues, the tissue response to these materials must be measured. Materials are placed in cavity preparations with subgingival extensions. The materials’ effects on gingival tissues are observed at 7 days and again after 30 days. Responses are categorized as slight, moderate, or severe. A few mononuclear inflammatory cells (mainly lymphocytes) in the epithelium and adjacent connective tissue constitute a slight response. A moderate response is indicated by numerous mononuclear cells in the connective tissue and a few neutrophils in the epithelium. A severe reaction evokes a significant mononuclear and neutrophilic infiltrate and thinned or absent epithelium.
A difficulty with this type of study is the frequent presence of some degree of preexisting inflammation in gingival tissue. Bacterial plaque is the most important factor in causing this inflammation. Secondary factors are surface roughness of the restorative material, open or overhanging margins, and overcontouring or undercontouring of the restoration. One way to reduce the interference of inflammation caused by plaque is to perform dental prophylaxis before preparing the cavity and placing the material. However, prophylaxis and cavity preparation will themselves cause some inflammation of soft tissues. Thus, if margins are placed subgingivally, time for healing (typically 8 to 14 days) must be allowed before assessing the effects of the restorative agents.
CORRELATION AMONG IN VITRO, ANIMAL, AND USAGE TESTS
In the field of biocompatibility, some scientists question the usefulness of in vitro and animal tests in light of the apparent lack of correlation with usage tests and the clinical history of materials. However, lack of correlation is not surprising in light of differences among these tests. In vitro and animal tests often measure aspects of biological response that are more subtle or less prominent than those observed during a material’s clinical use. Furthermore, barriers between the material and tissues may exist in usage tests or clinical use, but may not exist in the in vitro or animal tests. Thus, it is important to remember that each type of test has been designed to measure different aspects of biological response to a material, and correlation is not always to be expected.
The best example of a barrier that occurs in use but not during in vitro testing is the dentin barrier. When restorative materials are placed in teeth, dentin will generally be interposed between the material and the pulp. The dentin barrier, although possibly only a fraction of a millimeter thick, is effective in modulating the toxic effect of a dental material. This dentin barrier effect is illustrated by the following classic study (Table 5-3). Three methods were used to evaluate the following materials: ZOE cement; resin composite; and silicate cement. The evaluation methods included (1) four different cell culture tests, (2) an implantation test, and (3) a usage test in class 5 cavity preparations in monkey teeth. The results of the four cell culture tests were relatively consistent, with silicate having only a slight effect on cultured cells, composite a moderate effect, and ZOE a severe effect. These three materials were also embedded subcutaneously in connective tissue in polyethylene tubes (secondary test), and observations were made at 7, 30, and 90 days. Reactions at 7 days could not be determined because of inflammation caused by the operative procedure. At 30 days, ZOE caused a more severe reaction than silicate cement. The inflammatory reactions at 90 days caused by ZOE and silicate were slight, while the reaction to resin composites was moderate. When the three materials were evaluated in class 5 cavity preparations under prescribed conditions of cavity size and depth (usage test), the results were quite different from those obtained by the other methods. The silicate was found to have the most severe inflammatory reaction, the composite had a moderate to slight reaction, and the ZOE had little or no effect.
TABLE 5-3
Comparison of Reactions of Three Materials by Screening and Usage Tests
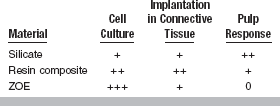
+ + + = Severe; + + = Moderate; + = Slight; 0 = No reaction; ZOE, zinc oxide–eugenol.
From Mjör IA, Hensten-Pettersen A, Skogedal O: Int Dent J 27:127, 1977.
Apparent contradictions in this study are explained by considering the components that were released from the materials and the environments into which they were released. The silicate cement released hydrogen ions that were probably buffered in the cell culture and implantation tests but were not adequately buffered by the dentin in the usage tests. Microleakage of bacteria or bacterial products may have added to the inflammatory reaction in those usage tests. Thus this material appeared to be the most toxic in the usage test. The composites released low-molecular-weight resins, and the ZOE released eugenol and zinc ions. In the cell culture tests, these compounds had direct access to cells and probably caused the moderate to severe cytotoxicity. In the implantation tests, the released components may have caused some cytotoxicity, but the severity may have been reduced because of the capacity of the surrounding tissue to disperse the toxins. In usage tests, these materials probably were less toxic because the diffusion gradient of the dentin barrier reduced concentrations of the released molecules to low levels. The slight reaction observed with the composites may also have been caused in part by microleakage around these restorations. The ZOE did not show this reaction, however, because the eugenol and zinc probably killed bacteria in the cavity, and the ZOE may have reduced microleakage.
Another example of the lack of correlation of usage tests with implantation tests is the inflammatory response of the gingiva at the gingival and interproximal margins of restorations that accumulate bacterial plaque and calculus. Plaque and calculus cannot accumulate on implanted materials and therefore the implantation test cannot hope to duplicate the usage test. However, connective tissue implantation tests are of great value in demonstrating the cytotoxic effects of materials and evaluating materials that will be used in contact with alveolar bone and apical periodontal connective tissues. In these cases, the implant site and the usage sites are sufficiently similar to compare the test results of the two sites.
USING IN VITRO, ANIMAL, AND USAGE TESTS TOGETHER
For about 25 years, scientists, industry, and the government have recognized that the most accurate and cost-effective means to assess biocompatibility of a new material is a combination of in vitro, animal, and usage tests. Implicit in this philosophy is the concept that no single test will be adequate to completely characterize biocompatibility of a material. The ways in which these tests are used together, however, are controversial and have evolved over many years as knowledge has increased and new technologies were developed (see Figs. 5-1 and 5-13). This evolution can be expected to continue as we ask materials to perform more sophisticated functions for longer periods.
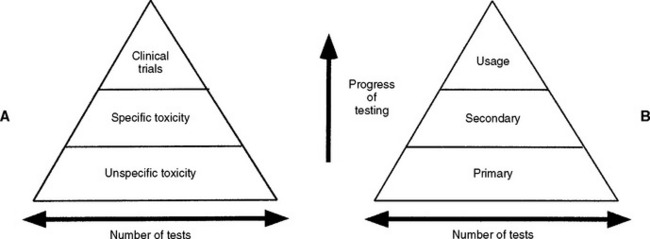
FIGURE 5-13 Early and contemporary strategies for the use of biocompatibility tests to assess the safety of materials. Testing begins at the bottom of the pyramid and works up. The number of tests needed decreases with the progress of testing because unacceptable materials are theoretically eliminated in the early testing stages. A, The earliest strategy, in which the testing strategy is focused on toxicity only. Unspecific toxicity refers to tests not necessarily related to the use of the material, whereas tests under specific toxicity were more relevant. Clinical trials are equivalent to usage tests in this scheme. B, The contemporary strategy used in most standards documents. Primary tests are in vitro and in vivo tests, but not necessarily related to the use of the material. Secondary tests are more advanced biological tests that may be partly related to the use of the material. Usage tests are either clinical trials in humans or a close model of the use of a material in higher animals. In both of these testing strategies, the major problem is the inability of the early tests to accurately predict problems with the materials. Thus, good materials might be screened out and poor materials might be advanced.
Early combination schemes proposed a pyramid testing protocol, in which all materials were tested at the bottom of the pyramid and materials were “weeded out” as the testing continued toward the top of the pyramid (see Fig. 5-13). Tests at the bottom of the pyramid were “unspecific toxicity” tests of any type (in vitro or animal) with conditions that did not necessarily reflect those of the material’s use. The next tier shows specific toxicity tests that presumably dealt with conditions more relevant to the use of the material. The final tier was a clinical trial of the material. Later, another pyramid scheme was proposed that divided tests into initial, secondary, and usage tests. The philosophy was similar to the first scheme, except the types of tests were broadened to encompass biological reactions other than toxicity, such as immunogenicity and mutagenicity. The concept of a usage test in an animal was also added (versus a clinical trial in a human). There are several important features of these early schemes. First, only materials that “passed” the first tier of tests were graduated to the second tier, and only those that passed the second tier were graduated to the clinical trials.
Presumably, then, this scheme fed safer materials into the clinical trials area and eliminated unsafe materials. This strategy was welcomed because clinical trials are the most expensive and time-consuming aspect of biocompatibility testing. Second, any material that survived all three tiers of tests was deemed acceptable for clinical use. Third, each tier of the system put a great deal of weight on the tests used to accurately screen in or out a material. Although still used in principle today, the inability of in vitro and animal tests to unequivocally screen materials in or out has led to development of newer schemes in biocompatibility testing.
Two newer testing schemes have evolved in the past 5 years with regard to using combinations of biocompatibility tests to evaluate materials (Fig. 5-14). Both of these newer schemes accommodate several important ideas. First, all tests (in vitro, animal, and usage) continue to be of value in assessing biocompatibility of a material during its development and even in its clinical service. For example, tests of inflammatory response in animals may be useful not only during the development of a material, but also if a problem is noted with the material after it has been on the market for a time. Second, these new schemes recognize the inability of current testing methods to accurately and absolutely screen in or out a material. Finally, they incorporate the philosophy that assessing the biocompatibility of a material is an ongoing process. Undoubtedly, we will see still newer strategies in the use of combinations of biocompatibility tests as the roles of materials change and the technologies for testing improve.
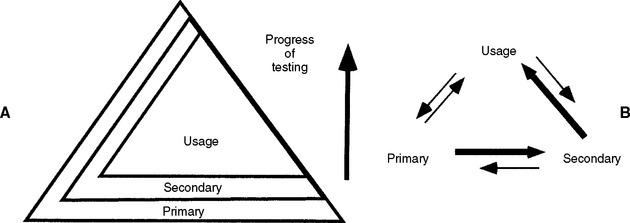
FIGURE 5-14 Two suggested future strategies for biocompatibility testing of materials. A, The pyramid scheme of Fig. 5-13 is retained, but it is acknowledged that primary and secondary tests will play a continuing (but decreased) role as the progress of the testing continues. B, The usage test has the most stature and the most common progression of tests is from primary to secondary to usage, but the need to go through several iterations between testing types is acknowledged. Furthermore, the ongoing nature of biocompatibility is recognized by the need to use primary and secondary tests after clinical evaluation of a material. In this scheme the order of testing is ultimately determined as the testing and clinical use of the material continue to provide new data.
STANDARDS THAT REGULATE THE MEASUREMENT OF BIOCOMPATIBILITY
The first efforts of the ADA to establish guidelines for dental materials came in 1926 when scientists at the National Bureau of Standards, now the National Institute of Science and Technology, developed specifications for dental amalgam. Unfortunately, recommendations on materials and conditions for biological compatibility have not kept pace with the technological development of dental materials. Reasons for this are (1) the fast advance of cellular and molecular biology, (2) the variety of tests available for assessing biocompatibility of materials, and (3) the lack of standardization of these tests.
Standardization is a difficult and lengthy process, made more difficult by disagreement on the appropriateness and significance of particular tests. One of the early attempts to develop a uniform test for all materials was the study by Dixon and Rickert in 1933, in which the toxicity of most dental materials in use at that time was investigated by implanting the materials into pockets in subdermal tissue. Small, standard-sized pieces of gold, amalgam, gutta-percha, silicates, and copper amalgam were sterilized and placed in uniformly sized pockets within skeletal muscle tissue. Biopsy specimens were evaluated microscopically after 6 months. Other early attempts to standardize techniques were carried out by Mitchell (1959) on connective tissue and by Massler (1958) on tooth pulp. Not until the passage of the Medical Device Bill by Congress in 1976 was biological testing for all medical devices (including dental materials) given a high priority. In 1972 the ADA Council on Dental Materials, Instruments, and Equipment (now the Council on Scientific Affairs) approved Specification No. 41 for Recommended Standard Practices for Biological Evaluation of Dental Materials. The committee that developed this document recognized the need for standardized methods of testing and for sequential testing of materials to reduce the number of compounds that would need to be tested clinically. In 1982, an addendum was made to this document, including an update of the Ames test for mutagenic activity.
ANSI/ADA Specification 41
Three categories of tests are described in the 1982 ANSI/ADA Specification: initial, secondary, and usage tests. This document uses the testing scheme shown in Fig. 5-13, B. The initial tests include in vitro assays for cytotoxicity, red blood cell membrane lysis (hemolysis), mutagenesis and carcinogenesis at the cellular level, and in vivo acute physiological distress and death at the level of the organism. Based on the results of these initial tests, promising materials are tested by one or more secondary tests in small animals (in vivo) for inflammatory or immunogenic potential (e.g., dermal irritation, subcutaneous and bony implantation, and hypersensitivity tests). Finally, materials that pass secondary tests and still hold potential are subjected to one or more in vivo usage test (placement of the materials in their intended contexts, first in larger animals, often primates, and finally, with Food and Drug Administration approval, in humans). The ANSI/ADA Specification No. 41, 1982 Addendum has two assays for mutagenesis—the Ames test and the Styles cell transformation test.
ISO 10993
In the 1980s, international efforts were initiated by several organizations to develop international standards for biomedical materials and devices. Several multinational working groups, including scientists from ANSI and the International Standards Organization (ISO) were formed to develop these standards. The final document (ISO 10993) was published in 1992. Further revision of the dental components of this document resulted in the publication of ISO 7405:1997 (Preclinical evaluation of biocompatibility of medical devices used in dentistry—Test methods for dental materials). This is the most recent standard available for biological testing methods for dental materials. The original ISO 10993 contained 12 parts, each dealing with a different aspect of biological testing for all types of medical and dental devices. For example, part 2 addressed animal welfare requirements; part 3 addressed tests for genotoxicity, carcinogenicity, and reproductive toxicity; and part 4 dealt with tests for interactions with blood. The standard divided tests into “initial” and “supplementary” tests to assess the biological reaction to materials. Initial tests are tests for cytotoxicity, sensitization, and systemic toxicity. Some of these tests are done in vitro, others in animals in nonusage situations. Supplementary tests assessed things such as chronic toxicity, carcinogenicity, and biodegradation. Most of the supplementary tests are to be done in animal systems, many in usage situations. The selection of tests for a specific material is left up to the manufacturer, who must present and defend the testing results. Guidelines for the selection of tests are given in part 1 of the standard and are based on how long the material will be present; whether it will contact body surface only, blood, or bone; and whether the device communicates externally from the body.
The current version of the ISO standard is available from the International Organization for Standardization (www.iso.ch), Case Postale 56, CH-1211 Geneva 20, Switzerland, with reference to document ISO 7405:1997—Dentistry (Preclinical evaluation of biocompatibility of medical devices used in dentistry—Test methods for dental materials). ANSI/ADA Specification No. 41 was also recently revised to conform to the ISO standard, and was reaffirmed in 2001 as ANSI/ADA Specification 41-1979 (Reaffirmed 2001) (Recommended Standard Practices for Biological Evaluation of Dental Materials and 41a, Addendum). The ANSI/ADA specification, which governs biocompatibility testing in the United States, is available from the Council on Scientific Affairs, American Dental Association (www.ada.org), 211 E. Chicago Avenue, Chicago, IL 60611, or the American National Standards Institute (www.ansi.org), 1819 L Street NW, Washington, DC 20036.
BIOCOMPATIBILITY OF DENTAL MATERIALS
Microleakage
There is evidence that restorative materials may not bond to enamel or dentin with sufficient strength to resist the forces of contraction during polymerization, wear, or thermal cycling. If a bond does not form, or debonding occurs, bacteria, food debris, or saliva may be drawn into the gap between the restoration and the tooth by capillary action. This effect has been termed microleakage. The importance of microleakage in pulpal irritation has been extensively studied. Several early studies reported that various dental restorative materials irritated pulp tissue in animal tests. However, several other studies hypothesized that the products of microleakage, not the restorative materials, caused the irritation. Subsequently, numerous studies showed that bacteria present under restorations and in dentinal tubules might be responsible for pulpal irritation. Other studies showed that bacteria or bacterial products such as lipopolysaccharides could cause pulp irritation within hours of being applied to dentin.
Finally, a classic animal study shed light on the roles of restorative materials and microleakage on pulpal irritation. Amalgam, composite, zincphosphate cement, and silicate cement were used as restorative materials in class 5 cavity preparations in monkey teeth. The materials were placed directly on pulp tissues. Half of the restorations were surface-sealed with ZOE cement. Although some irritation was evident in all restorations at 7 days, after 21 days, the sealed restorations showed less pulpal irritation than those not sealed, presumably because microleakage had been eliminated. Only zinc phosphate cement elicited a long-term inflammatory response. Furthermore, the sealed teeth exhibited a much higher rate of dentin bridging under the material. Only amalgam seemed to prevent bridging. This study suggests that microleakage plays a significant role in pulpal irritation, but that the materials can also alter normal pulpal and dentinal repair.
Recently, the concept of nanoleakage has been put forward. Like microleakage, nanoleakage refers to the leakage of saliva, bacteria, or material components through the interface between a material and tooth structure. However, nanoleakage refers specifically to dentin bonding, and may occur between mineralized dentin and a bonded material in the very small spaces of demineralized collagen matrix into which the bonded material did not penetrate. Thus nanoleakage can occur even when the bond between the material and dentin is intact. It is not known how significant a role nanoleakage plays in the biological response to materials, but it is thought to play at least some role and it is suspected of contributing to the hydrolytic degradation of the dentin-material bond, leading ultimately much more serious microleakage.
The full biological effects of restorative materials on the pulp are still not clear. Restorative materials may directly affect pulpal tissues, or may play an auxiliary role by causing sublethal changes in pulpal cells that make them more susceptible to bacteria or neutrophils. It is clear, however, that the design of tests measuring pulpal irritation to materials must include provisions for eliminating bacteria, bacterial products, and other microleakage. Furthermore, the role of dentin in mitigating the effects of microleakage remains to be fully understood. Recent research has focused on the effects that resin components have on the ability of odontoblasts to form secondary dentin. Other research has established the rates at which these components traverse the dentin (see the next section on dentin bonding).
Dentin Bonding
Traditionally, the strength of bonds to enamel have been higher than those to dentin. Bonding to dentin has proved more difficult because of its composition (being both organic and inorganic), wetness, and lower mineral content. The wettability of demineralized dentin collagen matrix has also been problematic. Because the dentinal tubules and their resident odontoblasts are extensions of the pulp, bonding to dentin also involves biocompatibility issues.
After being cut, such as in a cavity preparation, the dentin surface that remains is covered by a 1- to 2-μm layer of organic and inorganic debris. This layer has been named the smear layer (see Fig. 5-3). In addition to covering the surface of the dentin, the smear layer debris is also deposited into the tubules to form dentinal plugs. The smear layer and dentinal plugs, which appear impermeable when viewed by electron microscopy, reduce the flow of fluid (convective transport) significantly. However, research has shown that diffusion of molecules as large as albumin (66 kDa) will occur through the smear layer. The presence of this smear layer is important to the strength of bonds to restorative materials and to the biocompatibility of those bonded materials.
Numerous studies have shown that removing the smear layer improves the strength of the bond between dentin and restorative materials with contemporary dentin bonding agents, although earlier research with older bonding agents showed the opposite effect. A variety of agents have been used to remove the smear layer, including acids, chelating agents such as ethylenediaminetetraacetic acid (EDTA), sodium hypochlorite, and proteolytic enzymes. Removing the smear layer increases the wetness of the dentin and requires that the bonding agent be able to wet dentin and displace dentinal fluid. The precise mechanism by which bonding occurs remains unclear. However, it appears that the most successful bonding agents are able to penetrate into the layer of collagen that remains after acid etching. There, they create a “hybrid layer” of resin and collagen in intimate contact with dentin and dentinal tubules. The strength of the collagen itself has also been shown to be important to bond strengths.
From the standpoint of biocompatibility, the removal of the smear layer may pose a threat to the pulp for three reasons. First, its removal juxtaposes resin materials and dentin without a barrier, and therefore increases the risk that these materials can diffuse and cause pulpal irritation. Second, the removal of the smear layer makes any microleakage more significant because a significant barrier to the diffusion of bacteria or bacterial products toward the pulp is removed. Third, the acids used to remove the smear layer are a potential source of irritation themselves. Nevertheless, removal of the smear layer is now a routine procedure because superior bond strengths are achieved. Some recent techniques that etch and “bond” direct pulp exposures make biocompatibility of the bonding agents even more critical, because the dentinal barrier between the materials and the pulp is totally absent.
Biocompatibility of acids used to remove the smear layer has been extensively studied. Numerous acids, including phosphoric, hydrochloric, citric, and lactic acids, have been used to remove the smear layer. The effect of these acids on pulp tissues depends on a number of factors, including thickness of dentin between the restoration and the pulp, strength of the acid, and degree of etching. Most studies have shown that dentin is a very efficient buffer of protons, and that most of the acid never reaches the pulp if sufficient dentin remains. A dentin thickness of 0.5 mm has proved adequate in this regard. Citric or lactic acids are not as well buffered, probably because these weak acids do not dissociate as efficiently. Usage tests that have studied the effects of acids have shown that phosphoric, pyruvic, and citric acids produce moderate pulpal inflammatory responses, but this resolves after 8 weeks. Recent research has shown that in most cases the penetration depth of acid into the dentin is less than 100 μm. However, the possibility of adverse effects of these acids cannot be ruled out, because odontoblastic processes in the tubules may be affected even though the acids do not reach the pulp itself.
Bonding Agents
Numerous bonding agents have been developed and are applied to cut dentin during restoration of the tooth. There have been a number of studies of biocompatibility of these bonding systems. Many of these reagents are cytotoxic to cells in vitro if tested alone. However, when placed on dentin and rinsed with water between applications of subsequent reagents as prescribed by the manufacturer, cytotoxicity is reduced. Longer-term in vitro studies suggest, however, that components of the bonding agents may penetrate up to 0.5 mm of dentin and cause significant suppression of cellular metabolism for up to 4 weeks after application. This suggests that residual unbound constituents may cause adverse reactions.
Hydroxyethyl methacrylate (HEMA), a hydrophilic resin contained in several bonding systems, is at least 100 times less cytotoxic in tissue culture than Bis-GMA. Studies using long-term in vitro systems have shown, however, that adverse effects of resins occur at much lower concentrations (by a factor of 100 or more) when exposure times are increased to 4 to 6 weeks. Many cytotoxic effects of resin components are reduced significantly by the presence of a dentin barrier. However, if the dentin in the floor of the cavity preparation is thin (<0.1 mm), there is some evidence that HEMA is cytotoxic in vivo.
Other studies have established the in vitro cytotoxicity of most of the common resins in bonding agents, such as Bis-GMA, triethylene glycol dimethacrylate, urethane dimethacrylate (UDMA), and others. Combinations of HEMA and other resins found in dentin bonding agents may act synergistically to cause cytotoxic effects in vitro. There have been very few clinical studies of diffusion of hydrophilic and hydrophobic resin components through dentin. These studies indicated that some diffusion of these components occurs in vivo as well. Interestingly, there has been one report that some resin components enhance the growth of oral bacteria. If substantiated, this would cause concern about the ability of resin-based materials to increase plaque formation.
Resin-Based Materials
For tooth restorations, resin-based materials have been used as cements and restorative materials. Because they are a combination of organic and inorganic phases, these materials are called resin composites. In vitro, freshly set chemically cured and light-cured resins often cause moderate cytotoxic reactions in cultured cells over 24 to 72 hours of exposure, although several newer systems seem to have minimal toxicity. The cytotoxicity is significantly reduced 24 to 48 hours after setting and by the presence of a dentin barrier. Several studies have shown that some materials are persistently cytotoxic in vitro even up to 4 weeks, whereas others gradually improve, and a few newer systems show little toxicity even initially. In all cases, cytotoxicity is thought to be mediated by resin components released from the materials. Evidence indicates that the light-cured resins are less cytotoxic than chemically cured systems, but this effect is highly dependent on the curing efficiency of the light and the type of resin system. In vivo, usage tests have been used to assess the biological response to resin composites. The pulpal inflammatory response to hemically cured and light-cured resin composites is low to moderate after 3 days when they were placed in cavities with approximately 0.5 mm of remaining dentin. Any reaction diminished as the postoperative periods increased to 5 to 8 weeks and was accompanied by an increase in reparative dentin (Fig. 5-15). With a protective liner or a bonding agent, the reaction of the pulp to resin composite materials is minimal. The longer-term effects of resins placed directly on pulpal tissue are not known, but are suspected to be less favorable.
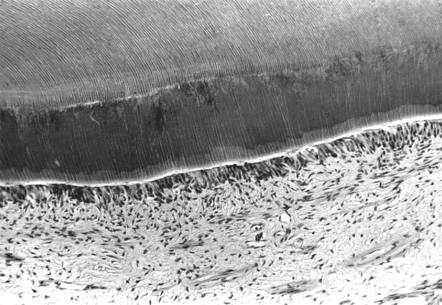
FIGURE 5-15 Light micrograph of the dentinal and pulpal response to unlined composite at 5 to 8 weeks in a monkey. The primary dentin is the lighter layer seen at the top. The tubules are evident. Secondary dentin is occurring (the dark, wide middle layer), and it is closely approximated by intact odontoblasts in the pulp. Few inflammatory cells are present. The response seen in this micrograph is indicative of a favorable response to the material. (Courtesy AK Avery, Ann Arbor, Michigan)
Amalgam and Casting Alloys
Dental amalgam has been used extensively for dental restorations. Biocompatibility of amalgam is thought to be determined largely by the corrosion products released while in service. Corrosion, in turn, depends on the type of amalgam, whether it contains the γ2 phase, and its composition. In cell culture screening tests, free or nonreacted mercury from amalgam is toxic. With the addition of copper, amalgams become toxic to cells in culture, but low-copper amalgam that has set for 24 hours does not even inhibit cell growth. Implantation tests show that low-copper amalgams are well tolerated, but high-copper amalgams cause severe reactions when in direct contact with tissue. In usage tests, the response of the pulp to amalgam in shallow cavities or in deeper but lined cavities is minimal, and amalgam rarely causes irreversible damage to the pulp. However, pain results from using amalgams in deep, unlined cavity preparations (0.5 mm or less remaining dentin), with an inflammatory response occurring after 3 days.
In cavities with 0.5 to 1.0 mm of dentin remaining in the floor, the cavity preparation should be lined for two other reasons. First, thermal conductivity with amalgam is significant and can be a problem clinically. Second, margins of newly placed amalgam restorations show significant microleakage. Marginal leakage of corrosion and microbial products is probably enhanced by the natural daily thermal cycle in the oral cavity, although long-term sealing of the margins occurs through the buildup of these corrosion products.
A number of high-copper amalgams are in clinical use. Usage tests reported that after 3 days, the pulpal response to these materials appears similar to that elicited by low-copper amalgams in deep, unlined cavities. At 5 weeks they provoke only slight pulpal response. At 8 weeks the inflammatory response was reduced. Bacterial tests on the high-copper amalgam pellets have revealed little inhibitory effect on serotypes of Streptococcus mutans, thus suggesting that the elements were not released in amounts necessary to kill these microorganisms. Although the high-copper amalgams seem biologically acceptable in usage tests, liners are suggested for all deep cavities.
Although not a commonly used material, amalgams based on gallium rather than mercury have been developed to provide direct restorative materials that are mercury-free. In cell culture, these alloys appear to be no more cytotoxic than traditional high-copper amalgams. These restorations release significant amounts of gallium in vitro, but the biological significance of this release is not known. In implantation tests, gallium alloys caused significant foreign body reaction. Clinically, these materials show much higher corrosion rates than standard amalgams. This leads to surface roughness and discoloration. There are few reports of pulpal responses to these materials.
Cast alloys have been used for single restorations, fixed partial dentures, ceramic-metal crowns, and removable partial dentures. The gold content in these alloys ranges from 0 wt% to 85 wt%. These alloys contain several other noble and non-noble metals that may have an adverse effect on cells if they are released from the alloys. However, metal ions released from these materials are most likely to contact gingival and mucosal tissues, while the pulp is more likely to be affected by the cement retaining the restoration.
Glass Ionomers
Glass ionomer has been used as both a cement (luting agent) and as a restorative material. Light-cured ionomer systems have been introduced; these systems use Bis-GMA or other oligomers as pendant chains on the polyacrylate main chain. In screening tests, freshly prepared ionomer is mildly cytotoxic, but this effect is reduced over time. The fluoride release from these materials, which is probably of some therapeutic value, causes cytotoxicity in vitro. Some researchers have reported that certain systems are more cytotoxic than others, but the reasons for this are not clear. The overall pulpal biocompatibility of glass ionomer materials has been attributed to the weak nature of the polyacrylic acid. It is unable to diffuse through dentin due to its high molecular weight. In usage tests the pulp reaction to these cements is mild, and histological studies show that any inflammatory infiltrate from ionomer is minimal or absent after 1 month. There have been several reports of pulpal hyperalgesia for short periods (days) after placing glass ionomers in cervical cavities. This effect is probably the result of increased dentin permeability after acid etching.
Liners, Varnishes, and Nonresin Cements
Calcium hydroxide cavity liners come in many forms, ranging from saline suspensions with a very alkaline pH (above 12) to modified forms containing zinc oxide, titanium dioxide, and resins. Resin-containing preparations can be polymerized chemically, but light-activated systems have are also available. The high pH of calcium hydroxide in suspension leads to extreme cytotoxicity in screening tests. Calcium hydroxide cements containing resins cause mild-to-moderate cytotoxic effects in tissue culture in both the freshly set and long-term set conditions. Inhibition of cell metabolism is reversible in tissue culture by high levels of serum proteins, suggesting that protein binding or buffering in inflamed pulp tissue may play an important role in detoxifying these materials in vivo. The initial response after exposing pulp tissue to these highly alkaline aqueous pulp-capping agents is necrosis to a depth of 1 mm or more. The alkaline pH also helps to coagulate any hemorrhagic exudate of the superficial pulp. Shortly after necrosis occurs, neutrophils infiltrate into the subnecrotic zone. After 5 to 8 weeks, only a slight inflammatory response remains. Within weeks to months, however, the necrotic zone undergoes dystrophic calcification, which appears to be a stimulus for dentin bridge formation. When resins are incorporated into the compound, these calcium hydroxide compounds become less irritating and are able to stimulate reparative dentin bridge formation more quickly than the Ca(OH)2 suspension alone. Significantly, this occurs with no zone of necrosis, and reparative dentin is laid down adjacent to the liner (Fig. 5-16). This indicates that replacement odontoblasts form the dentin bridge in contact with the liner. However, some of these materials evidently break down with time and create a gap between the restoration and the cavity wall. Resin-containing calcium hydroxide pulp capping agents are the most effective liners now available for treating pulp exposures, and after treatment, the uninfected pulp undergoes a relatively uncomplicated wound-healing process.
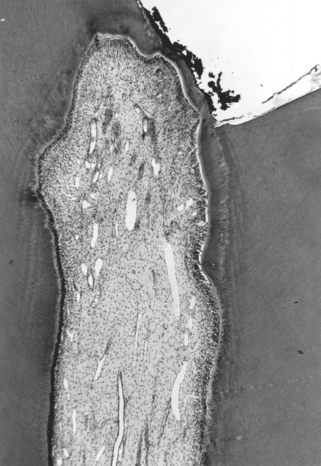
FIGURE 5-16 Light micrograph of a dentin bridge that has formed between a material and the pulp in a monkey. Initially, the pulp of the tooth was purposely exposed (top right) with a bur. The exposure was covered with a calcium hydroxide pulp-capping agent for 5 weeks before histological evaluation. A layer of secondary dentin has formed at the site of the pulp exposure, forming a dentin bridge. Some inflammatory cells are evident under the bridge, but the pulpal response is generally favorable. (Courtesy DR Heys, Ann Arbor, Michigan.)
Numerous investigators have analyzed the effects of applying thin liners such as copal varnishes and polystyrenes under restorations. These materials are not generally used under resin-based materials, because resin components dissolve the thin film of varnish. Because liners are used in such thin layers, they do not provide thermal insulation, but do initially isolate the dentinal tubule contents from the cavity preparation. They may also reduce penetration of bacteria or chemical substances for a time. However, because of the thinness of the film and formation of pinpoint holes, the integrity of these materials is not as reliable as that of other cavity liners.
Zinc phosphate has been widely used as a cement for castings and orthodontic bands, and as a cavity preparation base. Because the thermal conductivity of this cement is approximately equal to that of enamel (considerably less than that of metals), it has also been used to build up remaining tooth structure. In vitro screening tests indicate that zinc phosphate cement elicits strong-to-moderate cytotoxic reactions that decrease with time. Leaching of zinc ions and a low pH may explain these effects. The dilution of leached cement products by dentin filtration has been shown to protect the pulp from most of these cytotoxic effects. Focal necrosis, observed in implantation tests with zinc phosphate cements injected into rat pulp, confirm the cytotoxic effects of this cement when it contacts pulp tissue. In usage tests in deep cavity preparations, moderate-to-severe localized pulpal damage is produced within 3 days, probably because of the initial low pH (4.2 at 3 minutes). However, the pH of the set cement approaches neutrality after 48 hours. By 5 to 8 weeks, only mild chronic inflammation is present, and reparative dentin has usually formed. Because of the initially painful and damaging effects on the pulp of this cement when placed in deep cavity preparations, the placement of a protective layer of a dentin bonding agent, ZOE, varnish, or calcium hydroxide is recommended under the cement.
Zinc polyacrylate cements (polycarboxylate cements) were developed to combine the strength of zinc phosphate cements with the adhesiveness and biocompatibility of ZOE cements. In short-term tissue culture tests, cytotoxicity of freshly set and completely set cements has correlated with both the release of zinc and fluoride ions into the culture medium and with a reduced pH. Some researchers suggest that this cytotoxicity is an artifact of tissue culture because the phosphate buffers in the culture medium encourage zinc ions to leach from the cement. Supporting this theory, cell growth inhibition can be reversed if EDTA (which chelates zinc) is added to the culture medium. Furthermore, inhibition of cells decreases as the cement sets. In addition, concentrations of polyacrylic acid above 1% appear to be cytotoxic in tissue culture tests. On the other hand, subcutaneous and bone implant tests over a 1-year period have not indicated long-term cytotoxicity of these cements. Thus, other mechanisms such as buffering and protein-binding of these materials may neutralize these effects in vivo over time. Polyacrylate cements evoke a pulpal response similar to that caused by ZOE, with a slight-to-moderate response after 3 days and only mild, chronic inflammation after 5 weeks. Reparative dentin formation is minimal with these cements, and thus they are recommended only in cavities with intact dentin in the floors of the cavity preparations.
ZOE cements have been used in dentistry for many years. In vitro, eugenol from ZOE fixes cells, depresses cell respiration, and reduces nerve transmission with direct contact. Surprisingly, it is relatively innocuous in usage tests with class 5 cavity preparations. This is not contradictory for a number of reasons. The effects of eugenol are dose dependent and diffusion through dentin dilutes eugenol by several orders of magnitude. Thus, although the concentration of eugenol in the cavity preparations just below the ZOE has been reported to be 10−2 M (bactericidal), the concentration on the pulpal side of the dentin may be 10−4 M or less. This lower concentration reportedly suppresses nerve transmission and inhibits synthesis of prostaglandins and leukotrienes (anti-inflammatory). In addition and as described before, ZOE may form a temporary seal against bacterial invasion. In cavity preparations in primate teeth (usage tests), ZOE caused only a slight-tomoderate inflammatory reaction within the first week. This was reduced to a mild, chronic inflammatory reaction, with some reparative dentin formation (within 5 to 8 weeks), when cavities were deep. For this reason, it has been used as a negative control substance for comparison with restorative procedures in usage tests.
Bleaching Agents
Bleaching agents have been used on nonvital and vital teeth for many years, but their use on vital teeth has increased astronomically in recent years. These agents usually contain some form of peroxide (generally carbamide or hydrogen peroxide) in a gel that can be applied to the teeth either by a dentist or at home by a patient. The agents may be in contact with teeth for several minutes to several hours depending on the formulation of the material. Home bleaching agents may be applied for weeks to even months in some cases. In vitro studies have shown that peroxides can rapidly (within minutes) traverse the dentin in sufficient concentrations to be cytotoxic. The cytotoxicity depends to a large extent on the concentration of the peroxide in the bleaching agent. Other studies have even shown that peroxides can rapidly penetrate intact enamel and reach the pulp in a few minutes. In vivo studies have demonstrated adverse pulpal effects from bleaching, and most reports agree that a legitimate concern exists about the long-term use of these products on vital teeth. In clinical studies, the occurrence of tooth sensitivity is very common with the use of these agents, although the cause of these reactions is not known. Bleaching agents will also chemically burn the gingiva if the agent is not sequestered adequately in the bleaching tray. This is not a problem with a properly constructed tray, and long-term, low-dose effects of peroxides on the gingival and periodontal tissues are not known.
REACTION OF OTHER ORAL SOFT TISSUES TO RESTORATIVE MATERIALS
Restorative materials may cause reactions in the oral soft tissues such as the gingiva. It is not clear how much of the in vivo cytotoxicity observed is caused by the restorative materials and how much is caused by products of bacterial plaque that accumulate on teeth and restorations. In general, conditions that promote retention of plaque, such as rough surfaces or open margins, increase inflammatory reactions around these materials. However, released products from restorative materials also contribute either directly or indirectly to this inflammation. This is particularly true in areas where the washing effects of saliva are minimal, such as in interproximal areas, in deep gingival pockets, or under removable appliances. Several studies have documented increased inflammation or recession of gingiva adjacent to restorations where plaque indexes are low. In these studies, released products from materials could cause inflammation in the absence of plaque or could inhibit formation of plaque and cause inflammation in gingiva. In vitro research has shown that components from dental materials and plaque may synergize to enhance inflammatory reactions.
Cements exhibit some soft-tissue cytotoxicity in the freshly set state, but this decreases substantially over time. The buffering and protein-binding effects of saliva appear to mitigate these cytotoxic effects.
Resin composites in direct contact with fibroblasts are initially very cytotoxic in vitro. This cytotoxicity most likely results from unpolymerized components in the air-inhibited layer that leach out from the materials. Other in vitro studies, which have aged the composites in artificial saliva for up to 6 weeks, have shown that toxicity diminishes with some materials but remains high for others. Some of the newer composites with non-Bis-GMA and non-UDMA matrices have significantly lower cytotoxicity in vitro, presumably because of lower amounts of leached components. Polished composites show markedly less cytotoxicity in vitro, although some materials are persistently toxic even in the polished state.
Recently, there has been significant controversy about the ability of bisphenol A and bisphenol A dimethacrylate to cause estrogen-like responses in vitro. These compounds are basic components of many commercial composites. However, there is no evidence that xenoestrogenic effects are a concern in vivo from any commercial resin. Relatively little is known about other in vivo effects of released components of composites on soft tissues, although the concerns are similar to those regarding denture base resin and soft liners (see later discussion, this section). There is some evidence that methacrylate-based composite components may cause significant rates of hypersensitivity, although few clinical trials exist.
Amalgam restorations carried into the gingival crevice may cause inflammation of the gingiva because of products of corrosion or bacterial plaque. Seven days after placing an amalgam, a few inflammatory cells appear in the gingival connective tissue, and hydropic degeneration of some epithelial cells may be seen. Some proliferation of epithelial cells into the connective tissue may also occur by 30 days, and chronic mononuclear cell infiltration of connective tissue is evident. Increased vascularity persists, with more epithelial cells invaginating into the connective tissue. Some of these changes may be a chronic response of the gingiva to plaque on the margins of the amalgam. Nevertheless, corrosion products from amalgam cannot be ruled out at this time because implanted amalgams produce similar responses in connective tissues in animals. In addition, although copper enhances the physical properties of amalgam and is bactericidal, it is also toxic to host cells and causes severe tissue reactions in implantation tests. Animal implantation studies have shown severe reactions to gallium-based alloys that have been used as amalgam replacements.
There is a report in the literature in which amalgam and resin composite restorations were placed in cavity preparations in monkey central incisors that had been extracted for less than 1 hour. The cavities, with depths of about 2 mm, were placed halfway between the cementoenamel junction and the root tip. The teeth were immediately replanted after restoration, and the animals were sacrificed at intervals up to 6 months. Repair of the PDL took place in a normal fashion except for the presence of an intense inflammatory infiltrate in the PDL adjacent to the amalgams through 2 weeks. A similar effect was observed through 3 to 6 months with the resin composites. This result suggests that resin composites and amalgam release cytotoxic materials that cause tissue responses, at least at sites of implantation. For materials placed where they are rinsed in saliva, these cytotoxic agents are probably washed away before they harm the gingiva.
However, rough surfaces on these types of restorations have been associated with increased inflammation in vivo. Usage tests in which restorations were extended into the gingival crevice have shown that finished materials gave a much milder inflammatory response than unfinished materials. The detrimental effect of surface roughness has been attributed to the increased plaque retention on these surfaces. However, rough surfaces on alloy restorations also caused increased cytotoxic effects in vitro, where plaque was absent. This and other in vitro studies again would suggest that the cytotoxic response to alloys may be associated with release of elements from the alloys, and that the increased surface area of a rough surface may enhance release of these elements.
Casting alloys have a long history of in vivo use with a generally good record of biocompatibility. Some questions about the biological liability of elemental release from many of the formulations developed in the past 10 years have arisen, but there is no clinical evidence that elemental release is a problem, aside from hypersensitivity. Nickel allergy is a relatively common problem, occurring in 10% to 20% of females. It is a significant risk from nickel-based alloys, because release of nickel ions from these alloys is generally higher than for high-noble or noble alloys. Palladium sensitivity has also been a concern in some countries, although the incidence of true palladium allergy is one third that of nickel allergy. However, it has been clinically documented that patients with palladium allergy are virtually always allergic to nickel. The converse is not true, however.
In vitro, there have been numerous articles published on the effects of metal ions on cells in the gingival tissues, such as epithelial cells, fibroblasts, and macrophages. For the most part, the concentrations of metal ions required to cause problems with these cells in vitro are greater than those released from most casting alloys. However, some recent research has shown that extended exposures to low doses of metal ions may also have biological liabilities. These new data are noteworthy because the low-dose concentrations approach those known to be released from some alloys. Clinical significance of this research, however, is not known.
Denture base materials, especially methacrylates, have been associated with immune hypersensitivity reactions of gingiva and mucosa more than any other dental material. The greatest potential for hypersensitization is for dental and laboratory personnel who are exposed repeatedly to a variety of unreacted components. Hypersensitivity has been documented to the acrylic and diacrylic monomers, certain curing agents, antioxidants, amines, and formaldehyde. For patients, however, most of these materials have undergone the polymerization reaction, and the incidence of hypersensitization is quite low. Screening tests for sensitization potential include testing the unreacted ingredients, the polymeric substance after reaction, and oil, saline, or aqueous extracts of the polymer using the in vitro tests previously described. In addition to hypersensitivity, visible light-cured denture base resins and denture base resin sealants have been shown to be cytotoxic to epithelial cells in culture.
Soft-tissue responses to soft denture liners and denture adhesives are of concern because these materials are used in intimate contact with the gingiva. Plasticizers, incorporated into some materials to make them soft and flexible, are released in vivo and in vitro. Cell culture tests have shown that some of these materials are extremely cytotoxic and affect a number cellular metabolic reactions. In animal tests, several of these materials have caused significant epithelial changes, presumably from the released plasticizers. In usage, the effects of the released plasticizers are probably often masked by the inflammation already present in the tissues onto which these materials are placed. Denture adhesives have been evaluated in vitro and show severe cytotoxic reactions. Several had substantial formaldehyde content. The adhesives also allowed significant microbial growth. Newer formulations that add antifungal or antibacterial agents have not yet been shown to be clinically effective.
REACTION OF BONE AND SOFT TISSUES TO IMPLANT MATERIALS
Interest in the biocompatibility of implant materials has grown as the use of implants in clinical practice has increased dramatically in the past 10 years. Successful dental implant materials either promote osseointegration or biointegration (see Chapter 22).
Reactions to Ceramic Implant Materials
Most ceramic implant materials have very low toxic effects on tissues, because they are in an oxidized state and are corrosion resistant. As a group, not only are they minimally toxic, but they are nonimmunogenic and noncarcinogenic. However, they are brittle and lack impact and shear strength.
Reactions to Implant Metals and Alloys
Pure metals and alloys are the oldest type of oral implant materials. All metal implants share the quality of strength. Initially, metallic materials were selected on the basis of ease of fabrication. Over time, however, biocompatibility with bone and soft tissue and the longevity of the implant have become more important. Although a variety of implant materials have previously been used (including stainless steel and chromium-cobalt-molybdenum), the only metallic dental implant materials in common use today are titanium and titanium alloys.
Titanium is a pure metal, at least when first cast. In less than a second the surface forms a thin film of various titanium oxides. This oxide layer is corrosion resistant and allows bone to osseointegrate. The major disadvantage of this metal is that it is difficult to cast. It has been wrought into various forms, but this process introduces metallic impurities into the surface that may adversely affect bony response unless extreme care is taken during manufacturing. Titanium implants have been used with success as root forms that are left unloaded under the mucosa for several months before they are used to support a prosthesis. With frequent recall and good oral hygiene, implants have been maintained in healthy tissue for longer than two decades. Titanium-aluminum-vanadium alloys (Ti-6Al-4V) have been used successfully in this regard as well, but questions remain about the liability of released aluminum and vanadium.
Although titanium and titanium alloy implants have corrosion rates that are markedly less than other metallic implants, they do release titanium into the body. Currently there is no evidence that these released elements are a problem locally or systemically. The issue remains unresolved.
In soft tissue, the bond epithelium forms with titanium is morphologically similar to that formed with the tooth, but this interface has not been fully characterized. Connective tissue apparently does not bond to the titanium, but does form a tight seal that seems to limit ingress of bacteria and bacterial products. Techniques are being developed to limit down-growth of the epithelium and loss of bone height around the implant, as this will ultimately cause implant failure. Peri-implantitis is now a documented disease around implants and involves many of the same bacteria as periodontitis. The role of the implant material or its released components in the progression of peri-implantitis is not known.
SUMMARY
Biocompatibility of a dental material depends on its composition, location, and interactions with the oral cavity. Metal, ceramic, and polymer materials elicit different biological responses because of differences in composition. Furthermore, diverse biological responses to these materials depend on whether they release their components and whether those components are toxic, immunogenic, or mutagenic at the released concentrations. The location of a material in the oral cavity partially determines its biocompatibility. Materials that are biocompatible in contact with the oral mucosal surface may cause adverse reactions if they are implanted beneath it. Materials that are toxic in direct contact with the pulp may be essentially innocuous if placed on dentin or enamel. Finally, interactions between the material and the body influence the biocompatibility of the material. A material’s response to changes in pH, application of force, or the effect of biological fluids can alter its biocompatibility. Surface features of a material may promote or discourage attachment of bacteria, host cells, or biological molecules. These effects also determine whether the material will promote plaque retention, integrate with bone, or adhere to dentin.
Abdul Razak, AA. Mercury toxicity and its implications—a review of the literature. Dent J Malays. 1988;10:5.
ADA Council on Scientific Affairs. Dental amalgam: update on safety concerns. J Am Dent Assoc. 1998;129:494.
AAMI Standards and Recommended Practices. Biological Evaluation of Medical Devices, 4 . Association for the Advancement of Medical Instrumentation: Arlington, VA, 1994.
Addy, M, Martin, MV. Systemic antimicrobials in the treatment of chronic periodontal diseases: a dilemma. Oral Dis. 2003;9:38.
al-Dawood, A, Wennberg, A. Biocompatibility of dentin bonding agents. Endod Dent Traumatol. 1993;9:1.
Aoba, T, Fejerskov, O. Dental fluorosis: chemistry and biology. Crit Rev Oral Biol Med. 2002;13:155.
Autian, J, Dillingham, E. Toxicogenic potentials of biomaterials and methods for evaluating toxicity. Med Instrum. 1973;7:125.
Banerjee, R, Nageswari, K, Puniyani, RR. Hematological aspects of biocompatibility—review article. J Biomater Appl. 1997;12:57.
Barile, FA. In vitro cytotoxicity: mechanisms and methods. Boca Raton: CRC Press, 1994.
Bauer, JG, First, HA. The toxicity of mercury in dental amalgam. J Calif Dent Assoc. 1982;10:47.
Beltran-Aguilar, ED, Goldstein, JW, Lockwood, SA. Fluoride varnishes. A review of their clinical use, cariostatic mechanism, efficacy and safety. J Am Dent Assoc. 2000;31:589.
Bergenholtz, G. In vivo pulp response to bonding of dental restorations. Trans Acad Dent Mater. 1998:123.
Berkenstock, OL. Issues concerning possible cobaltchromium carcinogenicity: a literature review and discussion. Contemp Orthop. 1992;24:265.
Bouillaguet, S, Wataha, JC. Future directions in bonding resins to the dentine-pulp complex. J Oral Rehabil. 2004;31:385.
Bouillaguet, S, Ciucchi, B, Holz, J. Potential risks for pulpal irritation with contemporary adhesive restorations: an overview. Acta Med Dent Helv. 1996;1:235.
Brännström, M. Dentin and pulp in restorative dentistry. London: Wolfe Medical, 1982.
Brodin, P. Neurotoxic and analgesic effects of root canal cements and pulp-protecting dental materials. Endod Dent Traumatol. 1988;4:1.
Browne, RM. Animal tests for biocompatibility of dental materials—relevance, advantages and limitations. J Dent. 1994;22:S21.
Browne, RM. The in vitro assessment of the cytotoxicity of dental materials—does it have a role? Int Endod J. 1988;21:50.
Brune, D. Metal release from dental biomaterials. Biomaterials. 1986;7:163.
Chai, J. Some myths and legends about dental implants. Ann Acad Med Singapore. 1995;24:43.
Clarkson, TW. The toxicology of mercury. Crit Rev Clin Lab Sci. 1997;34:369.
Cook, SD, Dalton, JE. Biocompatibility and biofunctionality of implanted materials. Alpha Omegan. 1992;85:41.
Counter, SA, Buchanan, LH. Mercury exposure in children: a review. Toxicol Appl Pharmacol. 2004;15:209.
Cox, CF, Hafez, AA. Biocomposition and reaction of pulp tissues to restorative treatments. Dent Clin North Am. 2001;45:31.
Cox, CF, Keall, CL, Keall, HJ, Ostro, EO. Biocompatibility of surface-sealed dental materials against exposed pulps. J Prosthet Dent. 1987;57:1.
Cox, CF, Suzuki, S, Suzuki, SH. Biocompatibility of dental adhesives. J Calif Dent Assoc. 1995;23:35.
Cox, CF. Evaluation and treatment of bacterial microleakage. Am J Dent. 1994;7:293.
Dadoun, MP, Bartlett, DW. Safety issues when using carbamide peroxide to bleach vital teeth—a review of the literature. Eur J Prosthodont Restor Dent. 2003;11:9.
Dahl, JE, Pallesen, U. Tooth bleaching—a critical review of the biological aspects. Crit Rev Oral Biol Med. 2003;14:292.
Davies, JE. In vitro assessment of bone biocompatibility. Int Endod J. 1988;21:178.
Dodes, JE. Amalgam toxicity: a review of the literature. Oper Dent. 1988;13:32.
Dunne, SM, Gainsford, ID, Wilson, NH. Current materials and techniques for direct restorations in posterior teeth. Part 1: Silver amalgam. Int Dent J. 1997;47:123.
Ecobichon, DJ. The basis of toxicity testing. Boca Raton: CRC Press, 1992.
Edgerton, M, Levine, MJ. Biocompatibility: its future in prosthodontic research. J Prosthet Dent. 1993;69:406.
Eggleston, DW. Dental amalgam: a review of the literature. Compendium. 1989;10:500.
Eley, BM, Cox, SW. “Mercury poisoning” from dental amalgam—an evaluation of the evidence. J Dent. 1988;16:90.
Eliades, T, Athanasiou, AE. In vivo aging of orthodontic alloys: implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod. 2002;72:222.
Enwonwu, CO. Potential health hazard of use of mercury in dentistry:critical review of the literature. Environ Res. 1987;42:257.
Ferracane, JL. Elution of leachable components from composites. J Oral Rehabil. 1994;21:441.
Fry, BW. Toxicology of fluorides. Symp Pharmacol Ther Toxicol Group. 1974;21:26.
Fung, YK, Molvar, MP. Toxicity of mercury from dental environment and from amalgam restorations. J Toxicol Clin Toxicol. 1992;30:49.
Geurtsen, W, Leyhausen, G. Biological aspects of root canal filling materials—histocompatibility, cytotoxicity, and mutagenicity. Clin Oral Investig. 1997;1:5.
Geurtsen, W, Leyhausen, G. Chemical-biological interactions of the resin monomer triethyleneglycol-dimethacrylate (TEGDMA). J Dent Res. 2001;80:2046.
Geurtsen, W. Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med. 2000;11:333.
Geurtsen, W. Biocompatibility of root canal filling materials. Aust Endod J. 2001;27:12.
Geurtsen, W. Substances released from dental resin composites and glass ionomer cements. Eur J Oral Sci. 1998;106:687.
Glantz, PO, Mjor, IA. On amalgam toxicity. Int J Technol Assess Health Care. 1990;6:363.
Glantz, PO. Intraoral behaviour and biocompatibility of gold versus non precious alloys. J Biol Buccale. 1984;12:3.
Gochfeld, M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol Environ Saf. 2003;56:174.
Goldstein, GR, Kiremidjian-Schumacher, L. Bleaching: is it safe and effective. J Prosthet Dent. 1993;69:325.
Grimaudo, NJ. Biocompatibility of nickel and cobalt dental alloys. Gen Dent. 2001;49:498.
Gross, UM. Biocompatibility—the interaction of biomaterials and host response. J Dent Educ. 1988;52:798.
Haeffner-Cavaillon, N, Kazatchkine, MD. Methods for assessing monocytic cytokine production as an index of biocompatibility. Nephrol Dial Transplant. 1994;9:112.
Hammesfahr, PD. Biocompatible resins in dentistry. J Biomater Appl. 1987;1:373.
Hanks, CT, Wataha, JC, Sun, Z. In vitro models of biocompatibility: a review. Dent Mater. 1996;12:186.
Hanson, M, Pleva, J. The dental amalgam issue. A review. Experientia. 1991;15:9.
Hauman, CH, Love, RM. Biocompatibility of dental materials used in contemporary endodontic therapy: a review. Part 1. Intracanal drugs and substances. Int Endod J. 2003;36:75.
Hauman, CH, Love, RM. Biocompatibility of dental materials used in contemporary endodontic therapy: a review. Part 2. Root-canal-filling materials. Int Endod J. 2003;36:147.
Hayden, J, Jr. Considerations in applying to man the results of drug effects observed in laboratory animals. J Am Dent Assoc. 1977;95:777.
Haywood, VB, Heymann, HO. Nightguard vital bleaching: how safe is it? Quint Int. 1991;22:515.
Hensten-Pettersen, A. Comparison of the methods available for assessing cytotoxicity. Int Endod J. 1988;21:89.
Hensten-Pettersen, A. Skin and mucosal reactions associated with dental materials. Eur J Oral Sci. 1998;106:707.
Hodgson E, Levi PE, eds. A textbook of modern toxicology. New York: Elsevier Science, 1987.
Horsted-Bindslev, P. Amalgam toxicity—environmental and occupational hazards. J Dent. 2004;32:359.
Hubbard, MJ. Calcium transport across the dental enamel epithelium. Crit Rev Oral Biol Med. 2000;11:437.
Hume, WR, Gerzia, TM. Bioavailability of components of resin-based materials which are applied to teeth. Crit Rev Oral Biol Med. 1996;7:172.
Hume, WR, Massey, WL. Keeping the pulp alive: the pharmacology and toxicology of agents applied to dentine. Aust Dent J. 1990;35:32.
Hume, WR. A new technique for screening chemical toxicity to the pulp from dental restorative materials and procedures. J Dent Res. 1985;64:1322.
International Standards Organization. Biological evaluation of medical devices ISO 10993, ed 1. Geneva, Switzerland: ISO, 1992.
International Standards Organization. Preclinical evaluation of biocompatibility of medical devices used in dentistry—Test methods for dental materials ISO 7405:1997. Geneva, Switzerland: ISO, 1997.
Jansen, JA, Caulier, H, Van’t Hof, MA, Naert, I. Evaluation of Ca-P coatings in animal experiments: importance of study design. J Invest Surg. 1996;9:463.
Jarup, L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167.
Jones, PA, Taintor, JF, Adams, AB. Comparative dental material cytotoxicity measured by depression of rat incisor pulp respiration. J Endod. 1979;5:48.
Jontell, M, Okiji, T, Dahlgren, U, et al. Immune defense mechanisms of the dental pulp. Crit Rev Oral Biol Med. 1998;9:179.
Jorge, JH, Giampaolo, ET, Machado, AL, et al. Cytotoxicity of denture base acrylic resins: a literature review. J Prosthet Dent. 2003;90:190.
Kanca, J, III. Pulpal studies: biocompatibility or effectiveness of marginal seal. Quint Int. 1990;21:775.
Kawahara, H, Yamagami, A, Nakamura, M. Biological testing of dental materials by means of tissue culture. Int Dent J. 1968;18:443.
Kirkpatrick, CJ, Bittinger, F, Wagner, M, et al. Current trends in biocompatibility testing. Proc Inst Mech Eng [H]. 1998;212:75.
Laurencin, CT, Pierre-Jacques, HM, Langer, R. Toxicology and biocompatibility considerations in the evaluation of polymeric materials for biomedical applications. Clin Lab Med. 1990;10:549.
Leggat, PA, Kedjarune, U, Smith, DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207.
Leggat, PA, Kedjarune, U. Toxicity of methyl methacrylate in dentistry. Int Dent J. 2003;53:126.
Lemons, J, Natiella, J. Biomaterials, biocompatibility, and peri-implant considerations. Dent Clin North Am. 1986;30:3.
Levy, M. Dental amalgam: toxicological evaluation and health risk assessment. J Can Dent Assoc. 1995;61:667.
Lewis, B. Formaldehyde in dentistry: a review for the millennium. J Clin Pediatr Dent. 1998;22:167.
Li, Y. Tooth bleaching using peroxide-containing agents: current status of safety issues. Compend Contin Educ Dent. 1998;19:783.
Li, Y. Peroxide-containing tooth whiteners: an update on safety. Compend Contin Educ Dent Suppl. 2000;28:S4.
Lutz, J, Kettemann, M, Racz, I, Noth, U. Several methods utilized for the assessment of biocompatibility of perfluorochemicals. Artif Cells Blood Substit Immobil Biotechnol. 1995;23:407.
Mackenzie, R, Holmes, CJ, Jones, S, et al. Clinical indices of in vivo biocompatibility: the role of ex vivo cell function studies and effluent markers in peritoneal dialysis patients. Kidney Int Suppl. 2003;88:S84.
Mackert, JR, Bergland, A. Mercury exposure from dental amalgam filling: absorbed dose and the potential for adverse health effects. Crit Rev Oral Biol Med. 1997;8:410.
Mackert, JR, Jr., Wahl, MJ. Are there acceptable alternatives to amalgam. J Calif Dent Assoc. 2004;32:601.
Mackert, JR, Jr. Dental amalgam and mercury. J Am Dent Assoc. 1991;122:54.
Mackert, JR, Jr. Side-effects of dental ceramics. Adv Dent Res. 1992;6:90.
Marigo, L, Vittorini Orgeas, G, Piselli, D, et al. Pulpo-dentin protection: the biocompatibility of materials most commonly used in restorative work. A literature review. Minerva Stomatol. 1999;48:373.
Marshall, MV, Cancro, LP, Fischman, SL. Hydrogen peroxide: a review of its use in dentistry. J Periodontol. 1995;66:786.
Meryon, SD. The influence of dentine on the in vitro cytotoxicity testing of dental restorative materials. J Biomed Mater Res. 1984;18:771.
Messer, RL, Bishop, S, Lucas, LC. Effects of metallic ion toxicity on human gingival fibroblasts morphology. Biomaterials. 1999;20:1647.
Mjör, IA, Hensten-Pettersen, A, Skogedal, O. Biologic evaluation of filling materials: a comparison of results using cell culture techniques, implantation tests and pulp studies. Int Dent J. 1977;27:124.
Mjör, IA. Current views on biological testing of restorative materials. J Oral Rehabil. 1990;17:503.
Moienafshari, R, Bar-Oz, B, Koren, G. Occupational exposure to mercury. What is a safe level. Can Fam Physician. 1999;45:43.
Mongkolnam, P. The adverse effects of dental restorative materials—a review. Aust Dent J. 1992;37:360.
Mutter, J, Naumann, J, Sadaghiani, C, et al. Amalgam studies: disregarding basic principles of mercury toxicity. Int J Hyg Environ Health. 2004;207:391.
Natiella, JR. The use of animal models in research on dental implants. J Dent Educ. 1988;52:792.
National Institutes of Health. Consensus development statement on dental implants, June 13–15, 1988. J Dent Educ. 1988;52:824.
Nicholson, JW, Braybrook, JH, Wasson, EA. The biocompatibility of glass-poly(alkenoate) (glass-ionomer) cements: a review. J Biomater Sci Polym Ed. 1991;2:277.
Nicholson, JW. Glass-ionomers in medicine and dentistry. Proc Inst Mech Eng [H]. 1998;212:121.
Northup, SJ. Strategies for biological testing of biomaterials. J Biomater Appl. 1987;2:132.
Osborne, JW, Albino, JE. Psychological and medical effects of mercury intake from dental amalgam. A status report for the American Journal of Dentistry. Am J Dent. 1999;12:151.
Pariente, JL, Bordenave, L, Bareille, R, et al. Cultured differentiated human urothelial cells in the biomaterials field. Biomaterials. 2000;21:835.
Peterson, DE. Oral toxicity of chemotherapeutic agents. Semin Oncol. 1992;19:478.
Pierce, LH, Goodkind, RJ. A status report of possible risks of base metal alloys and their components. J Prosthet Dent. 1989;62:234.
Pillai, KS, Stanley, VA. Implications of fluoride—an endless uncertainty. J Environ Biol. 2002;23:81.
Pizzoferrato, A, Ciapetti, G, Stea, S, et al. Cell culture methods for testing biocompatibility. Clin Mater. 1994;15:173.
Polyzois, GL. In vitro evaluation of dental materials. Clin Mater. 1994;16:21.
Pretorius, E, Naude, H. Dental amalgam and mercury toxicity: should dentists be concerned. SADJ. 2003;58:366.
Ratcliffe, HE, Swanson, GM, Fischer, LJ. Human exposure to mercury: a critical assessment of the evidence of adverse health effects. J Toxicol Environ Health. 1996;25:221.
Ratner, BD. Replacing and renewing: synthetic materials, biomimetics, and tissue engineering in implant dentistry. J Dent Educ. 2001;65:1340.
Reinhardt, JW. Risk assessment of mercury exposure from dental amalgams. J Public Health Dent. 1988;48:172.
Rogers, KD. Status of scrap (recyclable) dental amalgams as environmental health hazards or toxic substances. J Am Dent Assoc. 1989;119:159.
Rubel, DM, Watchorn, RB. Allergic contact dermatitis in dentistry. Australas J Dermatol. 2000;41:63.
Santerre, JP, Shajii, L, Leung, BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136.
Santini, A. Biocompatibility of dentine-bonding agents. 1. Factors associated with function. Prim Dent Care. 1998;5:15.
Santini, A. Biocompatibility of dentine-bonding agents. 2. Pulpal considerations. Prim Dent Care. 1998;5:69.
Schmalz, G. Concepts in biocompatibility testing of dental restorative materials. Clin Oral Investig. 1997;1:154.
Schmalz, G. The biocompatibility of non-amalgam dental filling materials. Eur J Oral Sci. 1998;106:696.
Schmalz, G. Use of cell cultures for toxicity testing of dental materials—advantages and limitations. J Dent. 1994;22:S6.
Schuster, GS, Lefebvre, CA, Wataha, JC, et al. Biocompatibility of posterior restorative materials. J Calif Dent Assoc. 1996;24:17.
Schuurs, AH. Reproductive toxicity of occupational mercury. A review of the literature. J Dent. 1999;27:249.
Shabalovskaya, SA. On the nature of the biocompatibility and on medical applications of NiTi shape memory and superelastic alloys. Biomed Mater Eng. 1996;6:267.
Sidhu, SK, Schmalz, G. The biocompatibility of glass-ionomer cement materials. A status report for the American Journal of Dentistry. Am J Dent. 2001;14:387.
Smith, GE. Is fluoride a mutagen. Sci Total Environ. 1988;68:79.
Smith, GE. Toxicity of fluoride-containing dental preparations: a review. Sci Total Environ. 1985;43:41.
Soderholm, KJ, Mariotti, A. Bis-GMA–based resins in dentistry: are they safe? J Am Dent Assoc. 1999;130:201.
Stadtler, P. Dental amalgam. III: Toxicity. Int J Clin Pharmacol Ther Toxicol. 1991;29:168.
Stanley, HR. Biological evaluation of dental materials. Int Dent J. 1992;42:37.
Stanley, HR. Effects of dental restorative materials: local and systemic responses reviewed. J Am Dent Assoc. 1993;124:76.
Stanley, HR. Local and systemic responses to dental composites and glass ionomers. Adv Dent Res. 1992;6:55.
Stanley, HR. Pulpal responses to ionomer cements—biological characteristics. J Am Dent Assoc. 1990;120:25.
van der Bijl, P, Dreyer, WP, van Wyk, CW. Mercury in dentistry: a review. J Dent Assoc S Afr. 1987;42:537.
van Zyl, I. Mercury amalgam safety: a review. J Mich Dent Assoc. 1999;81:40.
Veron, MH, Couble, ML. The biological effects of fluoride on tooth development: possible use of cell culture systems. Int Dent J. 1992;42:108.
Wahl, MJ. Amalgam—resurrection and redemption. Part 2: The medical mythology of anti-amalgam. Quint Int. 2001;32:696.
Ward, RA. Phagocytic cell function as an index of biocompatibility. Nephrol Dial Transplant. 1994;9:46.
Wataha, JC, Hanks, CT, Craig, RG. Precision of and new methods for testing in vitro alloy cytotoxicity. Dent Mater. 1992;8:65–71.
Wataha, JC, Hanks, CT, Strawn, SE, et al. Cytotoxicity of components of resins and other dental restorative materials. J Oral Rehabil. 1994;21:453.
Wataha, JC, Hanks, CT. Biological effects of palladium and risk of using palladium in dental casting alloys. J Oral Rehabil. 1996;23:309.
Wataha, JC. Biocompatibility of dental casting alloys: a review. J Prosthet Dent. 2000;83:223.
Wataha, JC. Principles of biocompatibility for dental practitioners. J Prosthet Dent. 2001;86:203.
Watts, A, Paterson, RC. Initial biological testing of root canal sealing materials—a critical review. J Dent. 1992;20:259.
Whitford, GM. Fluoride in dental products: safety considerations. J Dent Res. 1987;66:1056.
Whitford, GM. The physiological and toxicological characteristics of fluoride. J Dent Res. 1990;69:539.
Williams, DF, Toxicology of ceramics. Williams, DF, eds. Fundamental aspects of biocompatibility, 2. Boca Raton: CRC Press, 1981.
Ziff, MF. Documented clinical side-effects to dental amalgam. Adv Dent Res. 1992;6:131.