Dental Implants
The practice of restorative dentistry seeks to replace the form and function of missing tooth structure. It was therefore expected for dentistry to follow orthopedic medicine in the use of implants to anchor prosthetic devices. In 2000, over 910,000 dental implants were placed in the United States. These numbers are expected to grow at an annual compounded rate of 18% through year 2010. Nearly 5 million implant procedures per year are predicted by the end of the decade. In 2004, the global implant market was $575 million.
Dental implants are fixtures that serve as replacements for the root of a missing natural tooth. Implants may be placed in the mandible or maxilla. When properly designed and placed, dental implants bond with bone over time and serve as an anchor for dental prostheses. Dental implants are used to replace a single missing tooth or many teeth, or to support a complete removable denture.
Worldwide, modern single-tooth implants have a success rate of nearly 95% survival at 15 years. Many implants placed over 35 years ago are still in function. Implants are permanent devices, surgically anchored in the oral cavity, that often provide significant advantages over other fixed or removable prosthodontic options. Implants are often more conservative than traditional fixed partial dentures because they conserve tooth structure by eliminating the need for reduction of adjacent abutment teeth and they support the maintenance of healthy bone in the region.
HISTORY
Dental implants are not a new invention. For thousands of years, people have been attempting to replace their lost or missing teeth with various materials. Early writings from ancient Greece, Egypt, and South America have described these attempts, and skulls with seashells, stones, pieces of wood, jade, and metal forced into the mandible and maxilla have been discovered in archeological sites.
Starting around the second century and continuing until as recently as the 1960s, transplantation of animal and human teeth became relatively common. It even became something of a status symbol to have animal teeth implanted in the anterior of one’s mandible during the eighteenth and nineteenth centuries. Of course, the periodontal ligament attached to these teeth was antigenic and was quickly attacked by the recipient’s immune system. The teeth became ankylosed and their roots were eventually resorbed, resulting in failure and loss of the tooth.
The inevitable failure of these “natural implants” encouraged Maggiolo in the early 1800s to begin the switch back to the use of artificial roots. He designed and implanted gold tooth roots into fresh extraction sockets. A crown was attached to the root only after they were allowed to heal. Although all of these implants also failed after some time in function, this ushered in a period of controlled experimental use of various metals and other alloplastic materials to replace missing teeth.
As scientific experimentation on tissue biocompatibility and bone-material interactions continued, Vitallium (a cobalt-chromium-molybdenum alloy) became the first long-term successful implant metal in the 1930s. It proved to be successfully retained for periods of up to 40 years. However, the success rate and service life for these implants were greatly variable and unpredictable.
The modern era of implant dentistry began around 1967, when two groups independently developed the endosteal blade implant from Vitallium. It could be restored within a month of placement and had wide applicability owing to its slender body design. It was the first implant to fit into narrow spaces such as the posterior edentulous ridge. This technology was quickly adopted, and for a time it became the most widely used device in both the United States and Europe.
In the 1950s a Swedish orthopedic surgeon, Per-Ingvar Bränemark, observed the fusion of bone with the titanium chambers he had placed into the femurs of rabbits. He named this phenomenon osseointegration. Over the next several years, he developed a two-stage implant system using titanium cylinders, and performed a series of controlled, prospective clinical studies to assess their survivability. In 1978, Dr. Bränemark presented his research at the Toronto Conference on Osseointegration in Clinical Dentistry, and revolutionized dental implant science by catalyzing the acceptance and use of dental implants in North America.
The original Bränemark design included five components: implant, abutment, abutment screw, crown, and retaining screw. The prosthetic components were designed to be removable from the implant to facilitate access and maintenance of the implant body. This design rapidly replaced all previous systems and the titanium implant is still the most widely used today. Current designs and systems are modifications of the original Bränemark design.
CLASSIFICATION
Historically, dental implants have been classified according to their design. This design was in turn based on the way in which they are surgically implanted. The three types of implants commonly used for the past 40 years are the subperiosteal implant, the transosteal implant, and the endosseous implant (Table 22-1).
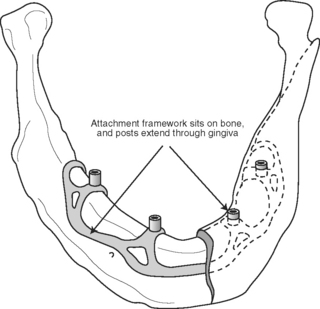
SUBPERIOSTEAL IMPLANT
Subperiosteal implants are designed to rest directly on the bone underlying the periosteum (Fig. 22-1). They typically employ a custom-cast metal framework that adapts closely to the bone surface. Attached to this framework are transmucosal posts upon which a superstructure is fabricated. These implants are most often composed of cobalt-chromium-molybdenum alloy. In this alloy, the continuous phase that provides the basic properties is the cobalt. The addition of chromium not only provides corrosion resistance, by the formation of an oxide surface, but also contributes to the formation of a secondary phase, which provides additional strength. The molybdenum also contributes to additional strength, and enhances bulk corrosion resistance.
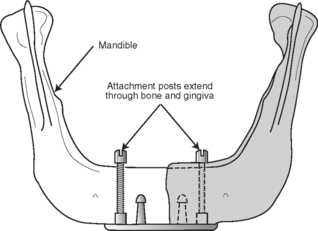
TRANSOSTEAL IMPLANT
This type of implant is limited to the anterior mandible only, as it extends completely through the mandible, penetrating both the inferior and superior cortical plates (Fig. 22-2). Posts on this implant design extend through the mucosa and provide retention for the restorative superstructure. With the predictable success rates for root-form implants, this design has mostly fallen out of favor.
ENDOSSEOUS IMPLANT
Endosseus implants are by far the most common type of implant placed today. Implants are placed directly into the mandible or maxilla (Fig. 22-3). A pilot hole is drilled into the alveolar or basal bone beneath (in cases in which the alveolar bone has been partially or completely resorbed), and the implant body is inserted into this site. The top of the implant is positioned so that it either protrudes slightly through the cortical plate or is flush with the surface of the bone. Typically a superstructure containing a prosthetic tooth or teeth connects to the implant body through an abutment that is screwed into the body directly through the mucosa.
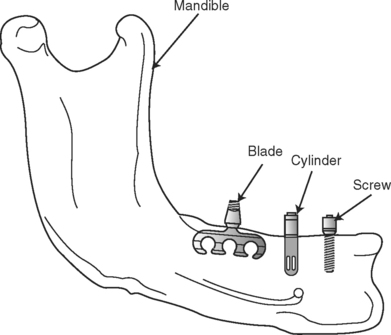
FIGURE 22-3 Endosteal implant design. Shown here are three different endosseous implant designs. Notice that all of the designs are implanted directly within the bone. Although the blade design has fallen out of use, the cylinder and screw-shaped versions continue to be the most widely placed implant designs in use today.
OSSEOINTEGRATION AND BIOINTEGRATION
A major issue for implant design is the development of materials that are physically and biologically compatible with alveolar bone. Ideally, bone should integrate with the material, substance, or device and remodel the bone structure around it, rather than responding to the material as a foreign substance by encapsulating it with fibrous tissue. Under optimum circumstances, bone differentiation should occur directly adjacent to the material (osseointegration). Ideally, this osseointegration provides a stable bone-implant connection that can support a dental prosthesis and transfer applied loads without concentrating stresses at the interface between bone and the implant.
Osseointegration is now formally defined as the close approximation of bone to an implant material (Fig. 22-4). To achieve osseointegration, the bone must be viable, the space between the bone and implant must be less than 10 nm and contain no fibrous tissue, and the bone-implant interface must be able to survive loading by a dental prosthesis. In current practice, osseointegration is an absolute requirement for the successful implant-supported dental prosthesis. To achieve osseointegration between an implant and bone, a number of factors must be correct. The bone must be prepared in a way that does not cause necrosis or inflammation (see Chapter 5). The implant must be allowed to heal for a time without a load. Finally, the proper material must be implanted, because not all materials will promote osseointegration.
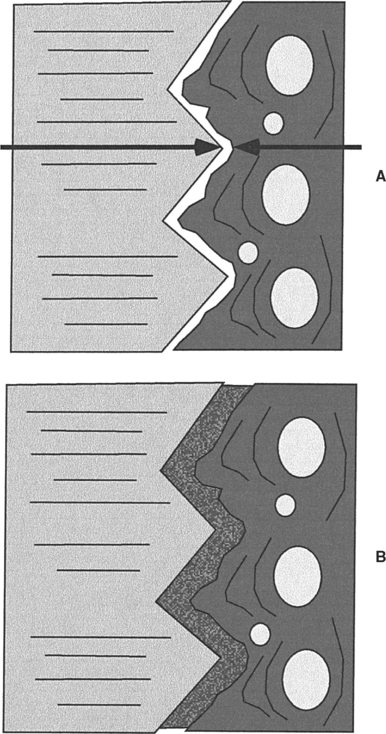
FIGURE 22-4 Osseointegration and biointegration. A, In osseointegration, the implant material (left) and the bone (right) closely approximate one another. This approximation must be closer than 10 nm (arrows). In the intervening space, there can be no fibrous tissue. B, In biointegration, the implant and bone are fused and continuous with one another. Osseointegration commonly occurs with titanium alloys, whereas biointegration occurs with ceramics and ceramic-coated metallic implants.
In recent years, various surface configurations have been proposed as means of improving the cohesiveness of the implant/tissue interface, maximizing load transfer, minimizing relative motion between the implant and tissue, minimizing fibrous integration and loosening, and lengthening the service life of the construct. Because of the necessity of developing a stable interface before loading, effort has been placed on developing materials and methods to accelerate tissue apposition to the implant surface. Surface-roughened implants and ceramic coatings have been implemented into clinical practice. Other, more experimental techniques include electrical stimulation, bone grafting, and the use of growth factors and other tissue engineering approaches described in Chapter 23.
The application of bioactive ceramics as implant materials was traditionally limited to their use as bone bonding and augmentation materials. Recently, there has been interest in coating titanium alloys with bioactive materials to promote an implant bone connection. Bioactive ceramic materials are more than just biocompatible. The use of the term “bioactive” implies that they have the ability to elicit a favorable tissue response when implanted in vivo. These ceramics form a direct chemical bond with natural tissues and are most often designed to bioresorb or biodegrade, having high solubility. Commonly implanted dental ceramics include the calcium phosphates with various calcium-to-phosphorus ratios (e.g., hydroxyapatite, and tricalcium phosphate), bioactive glasses (mixtures of SiO2, CaO, P2O5, and sometimes Na2O, and MgO), and glass ceramics.
Important examples of bioactive glasses and glass ceramics include Bioglass (a glass containing a mixture of silica, phosphate, calcia, and soda); Ceravital (which has a different alkali oxide concentration compared to Bioglass); Biogran (which has a different physical conformation compared to Bioglass); and glass ceramic A-W (a glass ceramic–containing crystalline oxyapatite and fluorapatite (Ca10[PO4]6[O,F2]) and β-wollastonite (SiO2CaO) in a MgO-CaO-SiO2 glassy matrix). Additionally, many other glass and glass-ceramic compositions, based on recently developed sol-gel synthesis methods, are being developed. Calcium phosphate ceramics vary in composition, depending on processing-induced physical and chemical changes. Among this group are the apatite ceramics, and of particular interest is hydroxyapatite (HA). This is the synthetic version of the inorganic phase found in tooth and bone, and is the bioactive ceramic material that has been most extensively investigated.
The impetus for using synthetic HA as a biomaterial stems from the perceived advantage of using a material similar to the mineral phase in natural tissues for replacing these materials. Because of this similarity, better tissue bonding is expected. Additional perceived advantages of HA and other bioactive ceramics include low thermal and electrical conductivity, elastic properties similar to those of bone, control of in vivo degradation rates through control of material properties, and the possibility of the ceramic functioning as a barrier to metallic corrosion products when it is coated onto a metal substrate.
However, temperature-induced phase transformations while processing HA provoke considerable changes in its in vitro dissolution behavior, and the altered structure changes the biological reaction to the material. Given the multitude of chemical compositions and structures resulting from processing bioactive ceramics and the resultant fact that pure HA is rarely used, the broader term calcium phosphate ceramics (CPCs) has been proposed in lieu of the more specific term hydroxyapatite. Each individual CPC is then defined by its own unique set of chemical and physical properties.
Although calcium phosphate ceramics are too brittle and too stiff to serve as stand-alone dental implants for prosthetic tooth replacement, there has been continuing interest in using a thin (50 to 75 μm) layer of ceramic materials to coat the surface of metallic implants. This provides the beneficial osseointegration characteristics of the ceramic combined with the high strength of the metallic alloy. Most manufacturers provide implants coated with CPC for use in sites where poor bone quality exists. A major limitation in using this concept in all clinical situations, however, has revolved around the inability to predict and maintain the bond strength of the coating to the metal. When the bioactive ceramic material resorbs in vivo, an unpredictable change occurs in the implant-bone interface, and implant micromotion and loosening may occur. This makes the long-term stability of these implants uncertain.
If successful, the ceramic coating becomes completely fused with the surrounding bone. In this case, the interface is called biointegration rather than osseointegration and there is no intervening space between the bone and the implant (see Fig. 22-4). A number of ceramic coatings have been used in this manner.
Typically, these coatings have been applied to the surface of an implant via a plasma-spray deposition process. This results in a complex mixture of HA, tricalcium phosphate, and tetracalcium phosphate in the coating, rather than a recapitulation of the starting powder mixture. Physical properties of importance to the functionality of CPCs include powder particle size, particle shape, pore size, pore shape, pore-size distribution, specific surface area, phases present, crystal structure, crystal size, grain size, density, coating thickness, hardness, and surface roughness.
The long-term integrity of the ceramic coating in vivo is not known, but evidence indicates that these coatings will resorb over time. Additionally, results of ex vivo push-out tests indicate that the ceramic-metal bond fails before the ceramic-tissue bond and is the weak link in the system. Thus, the weak ceramic-metal bond and the integrity of that interface over a lengthy service life of functional loading is reason for concern.
FACTORS AFFECTING THE ENDOSTEAL IMPLANT
Two primary objectives influence a patient’s decision to pursue dental implant treatment: aesthetics and function. To fulfill these objectives over an extended period, a dental implant must be capable of withstanding the occlusal stresses generated in the oral environment and in turn transfer this load to the supporting tissues. Not only must loads be transferred, they should also be of an appropriate direction and magnitude so tissue viability is maintained. In this respect, the implant principally acts to minimize and distribute the biomechanical forces. The forces are characterized by their magnitude, duration, and type. The ability to transfer force largely depends on attaining interfacial fixation. The interface between the implant and bone must stabilize in as short a time as possible postoperatively, and once stable, must remain stable throughout its service life. Designing an “optimal” implant that meets all the foregoing objectives requires the integration of material, physical, chemical, mechanical, biological, and economic factors.
MAGNITUDE OF THE FORCE
The amount of load applied during normal chewing varies greatly, depending on location and state of the patient’s dentition. Bite force values reported in the literature range from about 40 to 1250 N. The magnitude of force is greatest in the molar region as this area acts like the hinge of a lever (Fig. 22-5). The incisor region, in comparison, experiences about 10% of the magnitude seen in the posterior segment. This difference in load borne by the teeth and supporting bone dictates differences in mechanical requirements between anterior and posterior implants. Because stress depends not only on the applied load, but also on the area over which this load is distributed, the loss of some teeth by a patient will greatly increase the stresses applied to the remaining teeth and implants in partially edentulous patients.
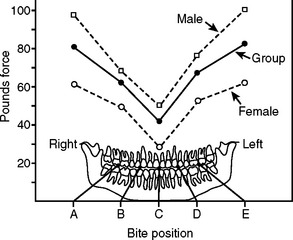
FIGURE 22-5 Mean adult bite force at different positions in the jaw. (From Rugh SD, Solberg WK: Behav Res Meth Instrum 4:125, 1972.)
A prime requirement for any dental implant is adequate supporting bone height, width, and density. It is well established that bone grows in response to strain, and the presence of an increasing magnitude of stress applied to the bone will result in an increasing magnitude of resorption or loss of bone. However, in the absence of a critical level of strain for normal bone maintenance, the bone will also resorb. Therefore, if the patient has been edentulous for a prolonged time, the underlying bone will have resorbed and become less dense. It is common to place implants preferentially in the anterior mandible, because this region has the greatest trabecular bone density when compared with the premolar or molar regions in both dentate and edentulous patients. When planning implant treatment, careful consideration must be given to the load distribution.
A great majority of the materials considered to be biocompatible are not suitable for use as implants, because their ultimate strength is not high enough to withstand the forces to which they are subjected during normal function. But, in order to survive and continue to function effectively, it is not only the ultimate strength, but also the modulus of elasticity (or stiffness) of the material that must be considered. Unless the bone experiences at least 50 microstrain on a routine basis, it will begin to resorb. Most ceramic materials are extremely stiff, for example, polycrystalline aluminum oxide has a modulus of ∼372 GPa. This stiffness is too high to transfer an adequate amount of an applied force to the bone. Instead, the stiffer implant material will carry a disproportionate amount of the load, causing stress shielding of the bone. In contrast, titanium has a modulus of ∼100 GPa, still too high to be ideal, but much closer to that of bone (∼20 GPa). It will permit normal physiological loading of the bone.
DURATION OF THE FORCE
When considering repetitive loading such as occurs during mastication, it is more appropriate to consider the endurance limit of a material rather than its ultimate strength. The endurance limit is the highest amount of stress to which a material may be repeatedly subjected without failing. This limit is typically only about half of the ultimate strength for the material.
The tooth root-form implant is designed to be loaded parallel to the long axis and is vulnerable to fatigue failure from cyclic bending loads. These bending loads often result from premature contact, bruxism, inappropriate occlusal schemes, and the use of angled abutments. Off-axis loading should be avoided in design of the implant superstructure.
TYPE OF FORCE
An implant experiences three types of loads in function: tensile forces, compressive forces, and shear forces. As discussed, a well-designed implant transfers and distributes these forces to the supporting bone. Bone is composed of both inorganic and organic constituents, and the inorganic components render it strongest when loaded in compression. Bone is about 30% weaker when placed in tension, and nearly 70% weaker when subjected to shear forces. Therefore, occlusion is a crucial consideration in designing the implant loading.
Smooth-sided cylindrical implant designs place the interface between the implant and the bone in nearly pure shear, the weakest possible loading scenario. These implant designs rely either on microscopic texturing of the implant body to offer some mechanical interlocking and provide retention, or else they rely on a coating. If the coating on these fails or resorbs, bone loss usually results from the lack of load transfer.
In contrast, screw-shaped implants have threads to engage the bone in compression and transfer the applied load. The thread designs have been extensively researched to provide a minimum of shear forces and maximal compression to the bone.
The concept of osseointegration around cylindrical or screw-shaped implants represents a situation of bone ongrowth. An alternative method of implant fixation is based on bone tissue ingrowth into roughened or three-dimensional porous surface layers. Such a system has been shown to have a higher bone/metal shear strength than have other types of fixation. Increased interfacial shear strength results in a better stress transfer from the implant to the surrounding bone, a more uniform stress distribution between the implant and bone, and lower stresses in the implant. In principle, the result of a stronger interfacial bond is a decreased propensity for implant loosening. A progression of surfaces from the lowest implant/tissue shear strength to the highest is as follows: smooth, textured, screw threaded, plasma sprayed, and porous coated.
IMPLANT DIAMETER
An increase in implant length or diameter increases the total surface area of the implant. As a consequence, the area for distribution of the occlusal forces is increased and the stress on the bone is decreased. The bending fracture resistance (and hence rigidity) of the implant increases greatly as the implant width increases and is related to the implant radius raised to the fourth power. This dramatic increase can be deleterious if the diameter chosen causes stress shielding by reducing bone strain to subphysiological levels.
IMPLANT LENGTH
As with increases the width, increases in implant length also increase the surface area and reduce the bone stress. However, a careful consideration of the bone quality is advised. In the highly dense type of bone usually found in the anterior mandible, overheating of the bone while drilling is a major cause of future failure. Preparation of an extra long implant site tends to increase heating in this type of bone. Any immediate stability advantages provided by this long implant are transitory, because once the implant osseointegrates, the apical region of the implant receives minimal stress transfer. Most of the stresses are still concentrated around the upper cortical plate through which the implant emerges. Conversely, in regions of poor bone quality, typically found in the posterior mandible and maxilla, anatomical considerations dictate the length of implant placed.
SURFACES AND BIOCOMPATIBILITY
In analyzing an implant/tissue system, three aspects are important: (1) the individual constituents, namely the implant materials and tissues; (2) the effect of the implant and its breakdown products on the local and systemic tissues; and (3) the interfacial zone between the implant and tissue. Regarding the ultrastructure of the implant-tissue interface, it is important to understand that, although this zone is relatively thin (on the order of angstroms), the constituents of the zone (heterogeneous metallic oxide, proteinaceous layer, and connective tissue) have a substantial effect on the maintenance of interfacial integrity. Furthermore, interfacial integrity depends on material, mechanical, chemical, surface, biological, and local environmental factors, all of which change as functions of time in vivo. Thus, implant success is a function of biomaterial and biomechanical factors, as well as of surgical techniques, tissue healing, and a patient’s overall medical and dental status.
ION RELEASE
Implant materials may corrode or wear, leading to the generation of particulate debris, which may in turn elicit both local and systemic biological responses. Metals are more susceptible to electrochemical degradation than are ceramics. Therefore, a fundamental criterion when choosing a metallic implant material is that it must not elicit significant adverse biological response. Titanium alloys are well tolerated by the body because of their passive oxide layers. The main elemental ingredients, as well as the minor alloying constituents, are endured by the body in trace amounts. However, larger amounts of any metal cannot be tolerated. Therefore, minimizing mechanical and chemical breakdown of implant materials is a primary objective.
Titanium and other implant metals are in their passive state under typical physiological conditions, and breakdown of passivity should not occur. Both cp Ti and Ti-6Al-4V possess excellent corrosion resistance for a full range of oxide states and pH levels. It is the extremely coherent oxide layer and the fact that titanium repassivates almost instantaneously through surface controlled-oxidation kinetics that renders titanium so corrosion resistant. The low dissolution rate and near chemical inertness of titanium dissolution products allow bone to thrive and therefore osseointegrate with titanium. However, even in its passive condition, titanium is not inert. Titanium ion release does occur as a result of the chemical dissolution of titanium oxide.
SURFACES
Analysis of the implant surface is necessary to ensure a twofold requirement. First, implant materials cannot adversely affect local tissues, organ systems, or organ functions. Second, the in vivo environment cannot degrade the implant and compromise its long-term function. The interface zone between an implant and the surrounding tissue is therefore the most important entity in defining the biological response to the implant and the response of the implant to the body.
The success of any implant depends on its bulk and surface properties, the site of implantation, tissue trauma during surgery, and motion at the implant-tissue interface. Surface analysis in implantology therefore aids in material characterization, determining structural and composition changes occurring during processing, identifying biologically induced surface reactions, and analyzing environmental effects on the interfaces.
The surface of a material is always different in chemical composition, form, and structure from the bulk material. These differences arise from the molecular arrangement, surface reactions, and contamination. In this regard, interface chemistry is primarily determined by the properties of the metal oxide and not as much by the metal itself. Little or no similarity is found between the properties of the metal and the properties of the oxide, but the adsorption and desorption phenomena can still be influenced by the properties of the underlying metal. Therefore, characterization of surface composition, binding state, form, and function are important in the analysis of implant surfaces and implant-tissue interfaces.
In an effort to improve the in vivo performance of dental implants, research is being conducted to investigate the effects of macro- and micro-scale textures on osseointegration. Although many of these research reports are contradictory and few absolutes have been identified, a couple of guiding principles are slowly emerging. The first of these is that cells in vitro undergo contact guidance on the implant surface. In other words, cells grow preferentially in and along nanometer- to micrometer-sized groove and ridge patterns on the surface of an implant. These minute grooves influence cell behavior by causing the cells to align themselves in the direction of the groove and migrate guided by the surface grooves (Fig. 22-6). Furthermore, cell shape and proliferation rate also depend on the texture of the implant surface to a great extent. Whether this nano- to micro-scale texture plays a significant role in implant anchorage in vivo is yet unclear.
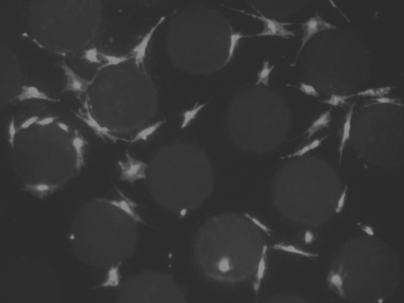
FIGURE 22-6 Light microscope image of cells grown on a patterned surface. Notice the contact guidance of the cells provided by the surface texture.
A second principle is that varying the macro-scale surface texture of an implant material significantly affects the interface between the implant and bone in vivo. In general, rough-surfaced implants exhibit greater shear bond strengths than corresponding smooth-surfaced implants. Upon microscopic examination, rough implants exhibit direct bone apposition, whereas smooth implants exhibit various degrees of fibrous tissue encapsulation. Because this direct bone apposition is the method by which dental implants are retained in the jaw, they appear to be significantly affected by macro-scale surface texture. The exact dimensions or degree of roughness that is optimal remain ambiguous at this time.
Finally, it is clear that the type of metallic oxide at the implant surface dictates the type of cellular and protein binding onto that surface. Surface oxides are continually altered by the inward diffusion of oxygen, and by hydroxide formation and the outward diffusion of metallic ions. Thus, a single oxide stoichiometry does not exist.
IMPLANT MATERIALS AND PROCESSING
In general, two basic classes of materials (ceramics and metals) are used as implants, either alone or in hybrid fashion. Metallic implant materials are largely titanium based—either CP Ti or Ti-6Al-4V. However, it is essential to note that synergistic relationships between processing, composition, structure, and properties of the bulk metals and their surface oxides effectively leaves more than two metals. Casting, forging, and machining to form near-net-shaped end products alters the bulk microstructure, surface chemistry, and properties. Similarly, densification of ceramics and deposition of ceramic and metal coatings by hot isostatic pressing or sintering can change bulk and surface composition, structure, and properties. Thus, the many material processing sequences necessary to yield the end-stage dental implant have a strong influence on the properties and functionality of the implant, primarily through temperature and pressure effects.
Metallic dental implants are almost exclusively titanium-based alloys, although cobalt-based alloys have been used historically and experimentally in dentistry. The attributes of titanium, namely, corrosion resistance and high strength, have been discussed in Chapter 16.
The initial rationale for using ceramics in dentistry was based on the relative biological inertness of ceramics as compared with that of metals. Ceramics are fully oxidized materials and therefore chemically stable. Thus, ceramics are less likely to elicit an adverse biological response than metals, which oxidize only at their surface. As discussed previously, a greater emphasis has been placed recently on bioactive and bioresorbable ceramics, materials that not only elicit normal tissue formation, but that may also form an intimate bond with bone tissue and even be replaced by tissue over time.
CHALLENGES AND THE FUTURE
Although there is no consensus regarding methods of evaluating dental implants and what criteria are most important, clinical evaluations have generally shown that dental implants are successful in about 75% of the cases, 5 years after implantation. Despite advances in synthesis and processing of materials, surgical technique, and clinical protocols, clinical failures occur at rates of approximately 2% to 5% per year. Causes of failure and current problems with dental implants include (1) early loosening from a lack of initial osseointegration; (2) late loosening, or loss of osseointegration; (3) bone resorption; (4) infection; (5) fracture of the implant or abutment; and (6) delamination of the coating from the bulk implant in the case of coated implants. The most common failure mechanism is alveolar crest resorption due to overloading. This inevitably leads to progressive periodontal lesions, decreased areas of supporting tissues, and ultimately to implant loosening. Aseptic failures are most often the cumulative result of more than one of the aforementioned factors.
Changes in implant materials and design will be accomplished by groups composed of dental researchers and the implant manufacturers themselves. They will continue to perform careful, multi-institutional clinical trials and prospective studies of compatibility, stress shielding, and bone loading among other factors. These three areas in particular present a major motivation for change in implant materials. Although very biocompatible, the current alloys suffer from a large elastic modulus mismatch with the supporting bone. Research work is under way to create composite materials that are biologically compatible and have the same elastic modulus as bone. Similar modulii will result in a stress distribution that more closely mimics that seen physiologically. Additionally, the work being performed to investigate implant texture effects is sure to reveal additional fundamental principles that will incrementally improve implant designs.
However, in spite of these new research directions, the future of implant dentistry lies in the hands of the restorative dentist. After an implant has been placed and healed, it is the restorative dentist who is responsible for designing and delivering the restoration or prosthesis to the patient. Patient satisfaction of the function and esthetics of the implantsupported prosthesis defines the success, or failure, of an implant case. Implants are in the mainstream of routine dental care in many countries. Their clinical success justifies the offering of implants along with more traditional therapies of fixed and removable partial dentures for restoring edentulous spaces.
Albrektsson, T, Branemark, PI, Hansson, HA, et al. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155.
Ameen, AP, Short, RD, Johns, R, et al. The surface analysis of implant materials. 1. The surface composition of a titanium dental implant material. Clin Oral Implants Res. 1993;4:144.
Aparicio, C, Gil, FJ, Fonseca, C, et al. Corrosion behaviour of commercially pure titanium shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials. 2003;24:263.
Arvidson, K, Fartash, B, Moberg, LE, et al. In vitro and in vivo experimental studies on single crystal sapphire dental implants. Clin Oral Implants Res. 1991;2:47.
Baschong, W, Suetterlin, R, Hefti, A, et al. Confocal laser scanning microscopy and scanning electron microscopy of tissue Ti-implant interfaces. Micron. 2001;32:33.
Berglundh, T, Abrahamsson, I, Lang, NP, et al. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003;14:251.
Block, MS, Kent, JN. Sinus augmentation for dental implants: the use of autogenous bone. J Oral Maxillofac Surg. 1997;55:1281.
Boggan, RS, Strong, JT, Misch, CE, et al. Influence of hex geometry and prosthetic table width on static and fatigue strength of dental implants. J Prosthet Dent. 1999;82:436.
Botticelli, D, Berglundh, T, Lindhe, J. The influence of a biomaterial on the closure of a marginal hard tissue defect adjacent to implants. An experimental study in the dog. Clin Oral Implants Res. 2004;15:285.
Branemark, PI, Adell, R, Breine, U, et al. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3:81.
Branemark, PI, Albrektsson, T. Titanium implants permanently penetrating human skin. Scand J Plast Reconstr Surg. 1982;16:17.
Catledge, SA, Fries, MD, Vohra, YK, et al. Nanostructured ceramics for biomedical implants. J Nanosci Nanotechnol. 2002;2:293.
Cook, SD, Dalton, JE. Biocompatibility and biofunctionality of implanted materials. Alpha Omegan. 1992;85:41.
Cook, SD, Klawitter, JJ, Weinstein, AM. A model for the implant-bone interface characteristics of porous dental implants. J Dent Res. 1982;61:1006.
Cook, SD, Weinstein, AM, Klawitter, JJ, et al. Quantitative histologic evaluation of LTI carbon, carbon-coated aluminum oxide and uncoated aluminum oxide dental implants. J Biomed Mater Res. 1983;17:519.
Cooper, LF, Masuda, T, Yliheikkila, PK, et al. Generalizations regarding the process and phenomenon of osseointegration. Part II. In vitro studies. Int J Oral Maxillofac Implants. 1998;13:163.
de Lavos-Valereto, IC, Deboni, MC, Azambuja, N, Jr., et al. Evaluation of the titanium Ti-6Al-7Nb alloy with and without plasma-sprayed hydroxyapatite coating on growth and viability of cultured osteoblast-like cells. J Periodontol. 2002;73:900.
De Maeztu, MA, Alava, JI, Gay-Escoda, C. Ion implantation: surface treatment for improving the bone integration of titanium and Ti6Al4V dental implants. Clin Oral Implants Res. 2003;14:57.
Denissen, HW, Klein, CP, Visch, LL, et al. Behavior of calcium phosphate coatings with different chemistries in bone. Int J Prosthodont. 1996;9:142.
Dubruille, JH, Viguier, E, Le Naour, G, et al. Evaluation of combinations of titanium, zirconia, and alumina implants with 2 bone fillers in the dog. Int J Oral Maxillofac Implants. 1999;14:271.
Galgut, PN, Waite, IM, Brookshaw, JD, et al. A 4-year controlled clinical study into the use of a ceramic hydroxylapatite implant material for the treatment of periodontal bone defects. J Clin Periodontol. 1992;19:570.
Galgut, PN, Waite, IM, Tinkler, SM. Histological investigation of the tissue response to hydroxyapatite used as an implant material in periodontal treatment. Clin Mater. 1990;6:105.
Gatti, AM, Zaffe, D, Poli, GP, et al. The evaluation of the interface between bone and a bioceramic dental implant. J Biomed Mater Res. 1987;21:1005.
Gineste, L, Gineste, M, Ranz, X, et al. Degradation of hydroxylapatite, fluorapatite, and fluorhydroxyapatite coatings of dental implants in dogs. J Biomed Mater Res. 1998;48:224.
Glantz, PO. The choice of alloplastic materials for oral implants: does it really matter. Int J Prosthodont. 1998;11:402.
Guy, SC, McQuade, MJ, Scheidt, MJ, et al. In vitro attachment of human gingival fibroblasts to endosseous implant materials. J Periodontol. 1993;64:542.
Haas, R, Donath, K, Fodinger, M, et al. Bovine hydroxyapatite for maxillary sinus grafting: comparative histomorphometric findings in sheep. Clin Oral Implants Res. 1998;9:107.
Hall, EE, Meffert, RM, Hermann, JS, et al. Comparison of bioactive glass to demineralized freeze-dried bone allograft in the treatment of intrabony defects around implants in the canine mandible. J Periodontol. 1999;70:526.
Haman, JD, Scripa, RN, Rigsbee, JM, et al. Production of thin calcium phosphate coatings from glass source materials. J Mater Sci Mater Med. 2002;13:175.
Hatano, N, Shimizu, Y, Ooya, K. A clinical long-term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/xenograft mixture and simultaneous placement of dental implants. Clin Oral Implants Res. 2004;15:339.
Hedia, HS, Mahmoud, NA. Design optimization of functionally graded dental implant. Biomed Mater Eng. 2004;14:133.
Hobkirk, JA. Endosseous implants: the host-implant surface. Ann Acad Med Singapore. 1986;15:403.
Hodosh, M, Shklar, G. A polymethacrylate-silica composite material for dental implants. J Biomed Mater Res. 1977;11:893.
Holden, CM, Bernard, GW. Ultrastructural in vitro characterization of a porous hydroxyapatite/bone cell interface. J Oral Implantol. 1990;16:86.
Hucke, EE, Fuys, RA, Craig, RG. Glassy carbon: a potential dental implant material. J Biomed Mater Res. 1973;7:263.
Hurson, S. Threaded implant design criteria. Int J Dent Symp. 1994;2:38.
Jarcho, M. Retrospective analysis of hydroxyapatite development for oral implant applications. Dent Clin North Am. 1992;36:19.
Jokstad, A, Braegger, U, Brunski, JB, et al. Quality of dental implants. Int Dent J. 2003;53:409.
Kamel, I. A porous and potentially tough dental implant material. J Dent Res. 1976;55:1143.
Karoussis, IK, Bragger, U, Salvi, GE, et al. Effect of implant design on survival and success rates of titanium oral implants: a 10-year prospective cohort study of the ITI Dental Implant System. Clin Oral Implants Res. 2004;15:8.
Kasemo, B, Gold, J. Implant surfaces and interface processes. Adv Dent Res. 1999;13:8.
Kay, JF. Calcium phosphate coatings for dental implants. Current status and future potential. Dent Clin North Am. 1992;36:1.
Kohal, RJ, Bachle, M, Emmerich, D, et al. Hard tissue reaction to dual acid-etched titanium implants: influence of plaque accumulation. A histological study in humans. Clin Oral Implants Res. 2003;14:381.
Kohal, RJ, Weng, D, Bachle, M, et al. Loaded custom-made zirconia and titanium implants show similar osseointegration: an animal experiment. J Periodontol. 2004;75:1262.
Kohn, DH. Overview of factors important in implant design. J Oral Implantol. 1992;18:204.
Kononen, M, Hormia, M, Kivilahti, J, et al. Effect of surface processing on the attachment, orientation, and proliferation of human gingival fibroblasts on titanium. J Biomed Mater Res. 1992;26:1325.
Lacefield, WR. Current status of ceramic coatings for dental implants. Implant Dent. 1998;7:315.
Lemons, JE. Dental implant biomaterials. J Am Dent Assoc. 1990;121:716.
Linder, L, Albrektsson, T, Branemark, PI, et al. Electron microscopic analysis of the bone-titanium interface. Acta Orthop Scand. 1983;54:45.
Massaro, C, Rotolo, P, De Riccardis, F, et al. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: chemical composition. J Mater Sci Mater Med. 2002;13:535.
Meyer, U, Wiesmann, HP, Fillies, T, et al. Early tissue reaction at the interface of immediately loaded dental implants. Int J Oral Maxillofac Implants. 2003;18:489.
Morris, HF, Ochi, S. Hydroxyapatite-coated implants: a case for their use. J Oral Maxillofac Surg. 1998;56:1303.
Mueller, WD, Gross, U, Fritz, T, et al. Evaluation of the interface between bone and titanium surfaces being blasted by aluminium oxide or bioceramic particles. Clin Oral Implants Res. 2003;14:349.
Muller-Mai, C, Schmitz, HJ, Strunz, V, et al. Tissues at the surface of the new composite material titanium/glass-ceramic for replacement of bone and teeth. J Biomed Mater Res. 1989;23:1149.
Najjar, TA, Lerdrit, W, Parsons, JR. Enhanced osseointegration of hydroxylapatite implant material. Oral Surg Oral Med Oral Pathol. 1991;71:9.
O’Neal, RB, Sauk, JJ, Somerman, MJ. Biological requirements for material integration. J Oral Implantol. 1992;18:243.
Piattelli, A, Scarano, A, Piattelli, M, et al. Histologic aspects of the bone and soft tissues surrounding three titanium non-submerged plasma-sprayed implants retrieved at autopsy: a case report. J Periodontol. 1997;68:694.
Piattelli, M, Scarano, A, Paolantonio, M, et al. Bone response to machined and resorbable blast material titanium implants: an experimental study in rabbits. J Oral Implantol. 2002;28:2.
Piddock, V. Production of bioceramic surfaces with controlled porosity. Int J Prosthodont. 1991;4:58.
Puleo, DA, Nanci, A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20:2311.
Rahal, MD, Branemark, PI, Osmond, DG. Response of bone marrow to titanium implants: osseointegration and the establishment of a bone marrow-titanium interface in mice. Int J Oral Maxillofac Implants. 1993;8:573.
Roberts, WE. Bone dynamics of osseointegration, ankylosis, and tooth movement. J Indiana Dent Assoc. 1999;78:24.
Rugh, SD, Solberg, WK. The measurement of human oral forces. Behav Res Meth Instrum. 1972;4:125.
Ruhling, A, Hellweg, A, Kocher, T, et al. Removal of HA and TPS implant coatings and fibroblast attachment on exposed surfaces. Clin Oral Implants Res. 2001;12:301.
Rupprecht, S, Bloch, A, Rosiwal, S, et al. Examination of the bone-metal interface of titanium implants coated by the microwave plasma chemical vapor deposition method. Int J Oral Maxillofac Implants. 2002;17:778.
Scarano, A, Pecora, G, Piattelli, M, et al. Osseointegration in a sinus augmented with bovine porous bone mineral: histological results in an implant retrieved 4 years after insertion. A case report. J Periodontol. 2004;75:1161.
Schlegel, AK, Donath, K. BIO-OSS—a resorbable bone substitute. J Long Term Eff Med Implants. 1998;8:201.
Schwarz, MS. Mechanical complications of dental implants. Clin Oral Implants Res. 2000;11:156.
Smith, DC. Dental implants: materials and design considerations. Int J Prosthodont. 1993;6:106.
Stanford, CM, Brand, RA. Toward an understanding of implant occlusion and strain adaptive bone modeling and remodeling. J Prosthet Dent. 1999;81:553.
Steflik, DE, Corpe, RS, Young, TR, et al. The biologic tissue responses to uncoated and coated implanted biomaterials. Adv Dent Res. 1999;13:27.
Steflik, DE, McKinney, RV, Jr., Koth, DL, et al. The biomaterial-tissue interface: a morphological study utilizing conventional and alternative ultrastructural modalities. Scan Electron Microsc. 1984;2:547.
Steinemann, SG. Titanium—the material of choice. Periodontol. 1998;17:7.
Sullivan, DY, Sherwood, RL, Mai, TN. Preliminary results of a multicenter study evaluating a chemically enhanced surface for machined commercially pure titanium implants. J Prosthet Dent. 1997;78:379.
Sun, L, Berndt, CC, Gross, KA, et al. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res. 2001;58:570.
Tamura, Y, Yokoyama, A, Watari, F, et al. Surface properties and biocompatibility of nitrided titanium for abrasion resistant implant materials. Dent Mater J. 2002;21:355.
Taylor, TD, Laney, WR. Dental implants: Are they for me?, ed 2. Carol Stream, IL: Quintessence Publishing Co, 1993.
Triplett, RG, Frohberg, U, Sykaras, N, et al. Implant materials, design, and surface topographies: their influence on osseointegration of dental implants. J Long Term Eff Med Implants. 2003;13:485.
Vercaigne, S, Wolke, JG, Naert, I, et al. Bone healing capacity of titanium plasma-sprayed and hydroxylapatite-coated oral implants. Clin Oral Implants Res. 1998;9:261.
Wagner, WC. A brief introduction to advanced surface modification technologies. J Oral Implantol. 1992;18:231.
Wataha, JC. Materials for endosseous dental implants. J Oral Rehabil. 1996;23:79.
Weinlaender, M. Bone growth around dental implants. Dent Clin North Am. 1991;35:585.