Polymers and Polymerization
Before the introduction of acrylic polymers to dentistry in 1937, the principal polymer used was vulcanized rubber for denture bases. Polymers introduced since then have included vinyl acrylics, polystyrene, epoxies, polycarbonates, polyvinylacetatepolyethylene, cis- and trans-polyisoprene, polysulfides, silicones, polyethers, and polyacrylic acids. In addition, oligomers from bisphenol A and glycidyl methacrylate (such as dimethacrylates) and urethane dimethacrylates have been applied.
In terms of volume, the primary use of polymers has been in the construction of prosthetic components such as denture bases. However, they have also been used for highly important applications such as artificial teeth, tooth restoratives, cements, orthodontic space maintainers and elastics, fixed partial denture facings, obturators for cleft palates, impressions, dies, provisional restorations, root canal filling materials, and athletic mouth protectors.
BASIC NATURE OF POLYMERS
The term polymer denotes a molecule that is made up of many (poly) parts (mers). The mer ending represents the simplest repeating chemical structural unit from which the polymer is composed. Thus poly(methyl methacrylate) is a polymer having chemical structural units derived from methyl methacrylate, as indicated by the simplified reaction and structural formula I.
The molecules from which the polymer is constructed are called monomers (one part). Polymer molecules may be prepared from a mixture of different types of monomers. They are called copolymers if they contain two or more different chemical units and terpolymers if they contain three different units, as indicated by the structural formulas II and III.
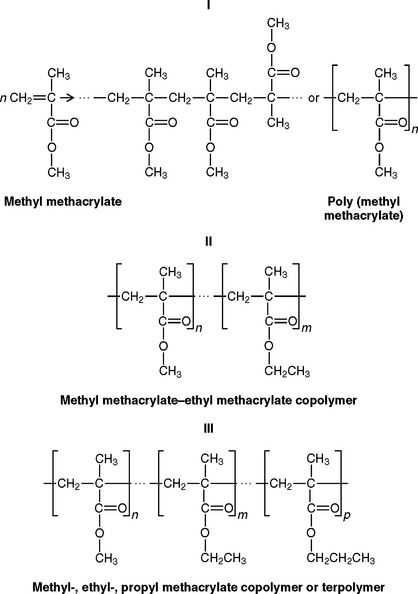
As a convenience in expressing the structural formulas of polymers, the mer units are enclosed in brackets, and subscripts such as n, m, and p represent the average number of the various mer units that make up the polymer molecules. Notice that in normal polymers the mer units are spaced in a random orientation along the polymer chain. It is possible, however, to produce copolymers with mer units arranged so that a large number of one mer type are connected to a large number of another mer type. This special type of polymer is called a block polymer. It also is possible to produce polymers having mer units with a special spatial arrangement with respect to the adjacent units; these are called stereospecific polymers.
MOLECULAR WEIGHT
The molecular weight of the polymer molecule, which equals the molecular weight of the various mers multiplied by the number of the mers, may range from thousands to millions of molecular weight units, depending on the preparation conditions. The higher the molecular weight of the polymer made from a single monomer, the higher the degree of polymerization. The term polymerization is often used in a qualitative sense, but the degree of polymerization is defined as the total number of mers in a polymer molecule. In general, the molecular weight of a polymer is reported as the average molecular weight because the number of repeating units may vary greatly from one molecule to another. As would be expected, the fraction of low-, medium-, and high-molecular-weight molecules in a material or, in other words, the molecular weight distribution has as pronounced an effect on the physical properties as the average molecular weight does. Therefore, two poly(methyl methacrylate) specimens can have the same chemical composition but greatly different physical properties because one of the specimens has a high percentage of low-molecular-weight molecules, whereas the other has a high percentage of high-molecular-weight molecules. Variation in the molecular weight distribution may be obtained by altering the polymerization procedure. These materials therefore do not possess any precise physical constants, such as melting point, as ordinary small molecules do. For example, the higher the molecular weight, the higher the softening and melting points and the stiffer the polymer.
SPATIAL STRUCTURE
In addition to chemical composition and molecular weight, the physical or spatial structure of the polymer molecules is also important in determining the properties of the polymer. There are three basic types of structures: linear, branched, and cross-linked. They are illustrated in Fig. 7-1 as segments of linear, branched, and cross-linked polymers. The linear homopolymer has mer units of the same type, and the random copolymer of the linear type has the two mer units randomly distributed along the chain. The linear block copolymer has segments, or blocks, along the chain where the mer units are the same. The branched homopolymer again consists of the same mer units, whereas the graft-branched copolymer consists of one type of mer unit on the main chain and another mer for the branches. The cross-linked polymer shown is made up of a homopolymer cross-linked with a single cross-linking agent.
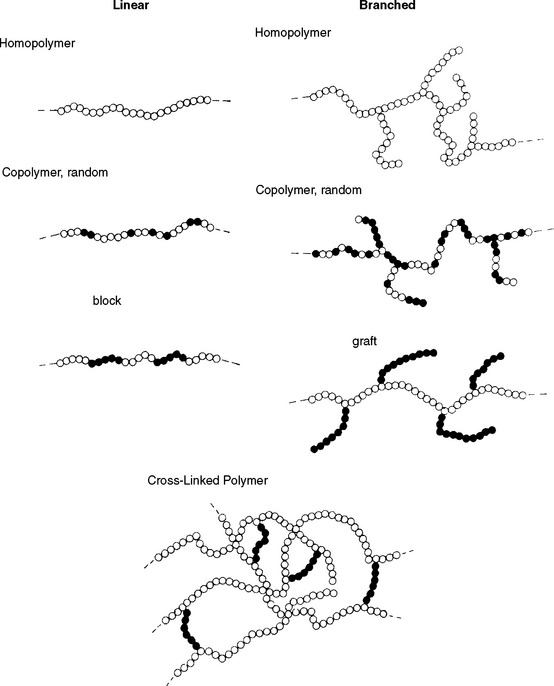
FIGURE 7-1 Linear, branched, and cross-linked homopolymers and copolymers. Open circles, one type of mer unit; solid circles, another type of mer unit; dashed lines, continuation of the polymer segment.
The linear and branched molecules are separate and discrete, whereas the cross-linked molecules are a network structure that may result in the polymer’s becoming one giant molecule. The spatial structure of polymers affects their flow properties, but generalizations are difficult to make because either the interaction between linear polymer molecules or the length of the branches on the branched molecules may be more important in a particular example. In general, however, the cross-linked polymers flow at higher temperatures than linear or branched polymers. Another distinguishing feature of some cross-linked polymers is that they do not absorb liquids as readily as either the linear or branched materials.
An additional method of classifying polymers other than by their spatial structure is according to whether they are thermoplastic or thermosetting. The term thermoplastic refers to polymers that may be softened by heating and solidify on cooling, the process being repeatable. Typical examples of polymers of this type are poly(methyl methacrylate), polyethylene-polyvinylacetate, and polystyrene. The term thermosetting refers to polymers that solidify during fabrication but cannot be softened by reheating. These polymers generally become nonfusible because of a cross-linking reaction and the formation of a spacial structure. Typical dental examples are cross-linked poly(methyl methacrylate), silicones, cis-polyisoprene, and bisphenol A diacrylates.
Polymers as a class have unique properties, and by varying the chemical composition, molecular weight, molecular-weight distribution, or spatial arrangement of the mer units, the physical and mechanical properties of polymers may be altered.
PREPARATION OF POLYMERS
Polymers are prepared by a process called polymerization, which consists of the monomer units becoming chemically linked together to form high-molecular-weight molecules. The polymerization process may take place by several different mechanisms, but most polymerization reactions fall into two basic types: addition polymerization and condensation polymerization. Important addition polymerization reactions are free-radical, ring-opening, and ionic reactions.
ADDITION POLYMERIZATION
Free-radical polymerization reactions usually occur with unsaturated molecules containing double bonds, as indicated by the following equation, where R represents any organic group, chlorine, or hydrogen.
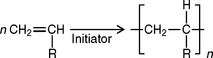
In this type of reaction, no byproduct is obtained. The reaction takes place in three stages, called the initiation, propagation, and termination stages. The reaction may be accelerated by heat, light, and traces of peroxides, as well as trialkyl borane and other chemicals. In any case, the reaction is initiated by a free radical, which may be produced by any of the methods mentioned, as shown in the following equation.
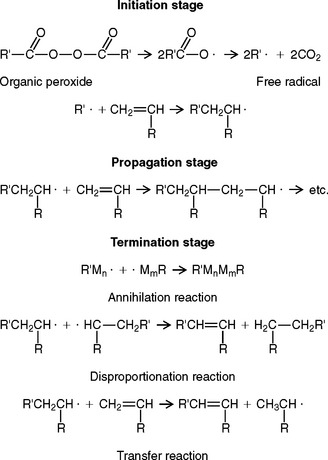
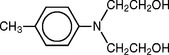
Sufficient free radicals for polymerization may be produced at room temperatures by the reaction of a chemical accelerator such as a tertiary amine or a sulfinic acid with the organic peroxide. N,N-dihydroxyethyl-para-toluidine, has commonly been used as an accelerator in dental products.
The initiation stage is followed by the rapid addition of other monomer molecules to the free radical and the shifting of the free electron to the end of the growing chain (see displayed reactions below), which describes the propagation stage.
This propagation reaction continues until the growing free radical is terminated. The termination stage may take place in several ways, as indicated, where M represents the mer unit and n and m represent the number of mer units.
A study of these termination reactions reveals how branched and cross-linked polymer molecules may be obtained.
Free-radical polymerization reactions can be inhibited by the presence of any material that will react with a free radical, thus decreasing the rate of initiation or increasing the rate of termination. Decreasing the rate of initiation retards the polymerization reaction, and increasing the rate of termination decreases the degree of polymerization or the molecular weight of the final polymer. Such materials as hydroquinone, eugenol, or large amounts of oxygen will inhibit or retard the polymerization. Small amounts of hydroquinone are used to protect the methyl methacrylate monomer from premature polymerization, which prolongs the shelf life of the monomer.
Another important free-radical polymerization reaction is responsible for the setting of resin restorative composites. The manufacturer prepares a compound from one molecule of bisphenol A and two molecules of glycidyl methacrylate, to produce 2,2-bis [4(2-Hydroxy-3-methacryloyloxy-propyloxy)phenyl]propane. The acronym Bis-GMA has been used to identify this compound. Because it is not, strictly speaking, a monomer, it is called an oligomer. A simplified structural formula is shown in formula IV.
Lower-molecular-weight difunctional monomers such as triethyleneglycol dimethacrylate are added to reduce the viscosity, and polymerization is accomplished using free radicals. Because the Bis-GMA molecule has reactive double bonds at each end, a highly cross-linked polymer is obtained.
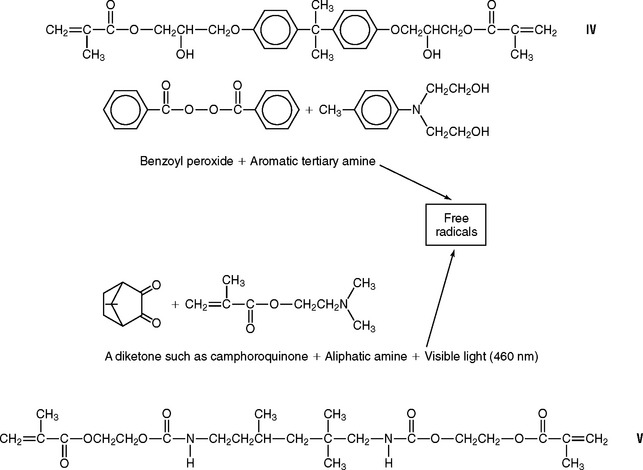
Free radicals needed to initiate the reaction are produced in composites by one of the two methods shown in formula IV.
Some composites contain oligomers that are urethane dimethacrylates, such as that shown in formula V.
Polymerization is accomplished by free-radical initiation with a peroxide-amine system or a diketone-amine system and exposure to blue visible light.
The free-radical polymerization of monomers or oligomers with unsaturated double bonds does not result in all the double bonds reacting. The term degree of conversion describes the percentage of double bonds that react and convert to single bonds; depending on the conditions, the value may vary from 35% at the air-inhibited layer to 80% in the bulk.
Photo-initiation polymerization has become highly popular in dentistry; it has been shown that the degree of conversion with photo-initiation ranges from about 65% to 80%, whereas chemical initiation results in values from 60% to 75%. Systems used to cement restorations often use both photo- and chemical-initiation (dual curing) because it is often difficult to expose regions of the material to sufficient light to reach the maximum degree of conversion and thus maximum strength. With these dual-curing materials, maximum degrees of conversion of 80% have been reported.
Ring-Opening Polymerization
Two important ring-opening polymerizations in dentistry are the epoxy and ethylene imine reactions. The former is used to produce dies from elastomeric impressions, and the latter is used in the setting reaction of polyether rubber impression materials.
The reactants for the epoxy system are a difunctional epoxide oligomer and a difunctional amine, as shown in the following simplified equation:
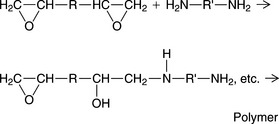
The amine opens the ring and cross-linking results in a rigid polymer. Water interferes with the setting reaction because it reacts with the epoxide. Therefore, alginate impressions are incompatible with this die material.
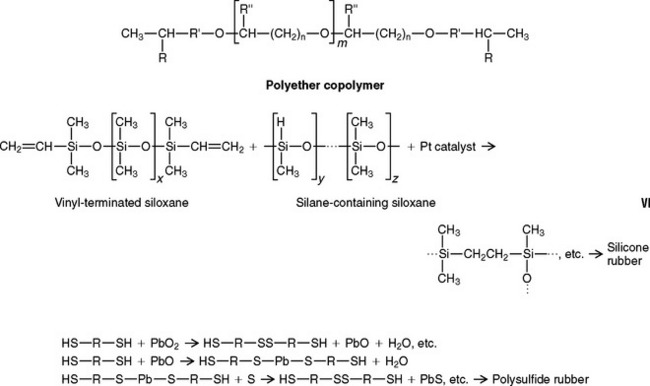
The polyether oligomer has a three-membered ring containing nitrogen shown as R in formula VI.
The ring is opened by the sulfonium fluoborate catalyst, and polymerization results in a cross-linked rubber. The oligomer and setting reaction are further discussed in Chapter 12, Impression Materials.
Hydrosilylation
One final example of an addition reaction is with a vinyl-terminated silicone and a silane (—H)-containing siloxane, as shown in formula VI. In this instance the platinum catalyst attacks the hydrogen in the silane-containing dimethyl siloxane, and this complex reacts with the vinyl-terminated dimethyl siloxane to form a cross-linked silicone rubber. Compounds used in the vulcanization of latex surgical gloves interfere in the polymerization of addition silicones, and thus contact should be avoided.
CONDENSATION POLYMERIZATION
Condensation reactions result in polymerization plus the production of low-molecular-weight byproducts. Polysulfide rubbers are formed by a condensation reaction, the most general reaction being between low-molecular-weight polysulfide polymers having mercaptan (—SH) groups and lead dioxide, as shown here by the simplified reactions.
Water and lead sulfide are byproducts of the reaction. Mercaptan groups are also along the chain and thus cross-linking occurs. The rate of the reaction is proportional to —SH, PbO2, and H2O.
Condensation polymerization of the mercaptan groups can also be accomplished by use of a Cu(OH)2, as shown by the following simplified reaction:
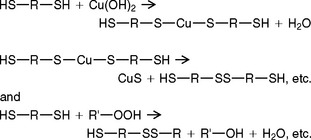
The polymerization with Cu(OH)2 avoids the dark color of PbO2 and the staining of fabric by the PbO2-containing polysulfide impression materials.
Silicones may be polymerized by a condensation reaction if they contain terminal hydroxy groups, as shown by the reaction:
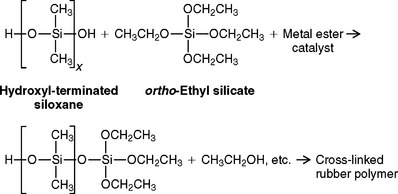
Metal esters used have been stannous octoate and dibutyl tin dilaurate. The ortho-ethyl silicate is used as a cross-linking agent and is more stable if not combined with the metal ester. Ethyl alcohol is the byproduct, and its evaporation from the set rubber accounts for a significant portion of the shrinkage of condensation silicones after setting. Organosilicon compounds with only two ethoxy groups can be substituted for the ortho-ethyl silicate, thus reducing the byproduct and shrinkage on setting.
Polymer acids are used successfully in dentistry to react with hydrated metal ions such as Zn2+, Ca2+, or Al3+. A copolymer of acrylic acid and itaconic acid in water is reacted with zinc oxide in an acid-base reaction to form a cement called zinc polyacrylate, as outlined:
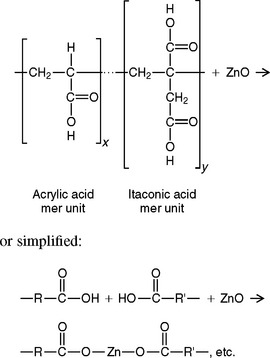
The copolymer acid can also be freeze-dried and included with the ZnO powder, and then only water is mixed with the powder.
A similar reaction with a copolymer of acrylic and itaconic acid in water solution is used with an aluminosilicate glass to form a glass ionomer. The copolymer is used rather than polyacrylic acid because the presence of itaconic acid prevents thickening of the water solution observed when only polyacrylic acid is used. Tartaric acid is also present in the formulation to increase the strength of the set ionomer by cross-linking from the difunctional acid groups.
A stronger diacid, maleic acid, has been used to increase the rate of reaction, resulting in a higher early strength and allowing finishing at the placement appointment.
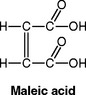
The copolymer acid reacts first with Ca2+ and then Al3+ dissolved out of the glass by an ionic reaction to form metal esters. The material is called an ionomer and is used as both a restorative and cement.
Materials called compomers use a combination of the ionomer reaction and free-radical polymerization. The polyacrylic acid molecules containing pendant methacrylate groups are dissolved in water along with 2-hydroxyethylmethacrylate and tartaric acid. The powder contains a glass and microencapsulated potassium persulfate and ascorbic acid catalyst. On mixing the powder and liquid, an acid-base ionomer reaction accompanied by a free-radical methacrylate reaction occurs.
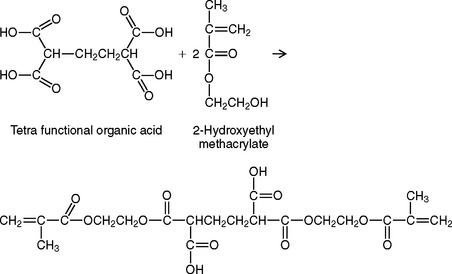
Some compomers are made by reacting a polyfunctional organic acid with hydroxyethylmethacrylate to form a compound that will undergo both a free-radical and an acid-base reaction.
As a result, compomers of the resin-modified ionomer type and ionomer-modified composites type are available. Also, some products are polymerized by chemical and light initiation and by an acid-base reaction; these products are called tri-cured materials. The greater the number polyacid groups, the more the material is like a glass ionomer; the fewer the number of polyacid groups, the more it is like a resin composite.
OTHER POLYMERS
A variety of polymers are used in fully polymerized form without any polymerization reaction carried out by the dentist or laboratory technician. Polyisoprene is available in two forms, cis and trans, and both are natural rubbers. These structures are shown as follows:
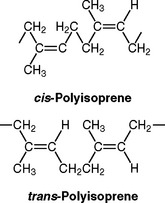
Notice that for the cis type the CH3 and H are on the same (cis) side, whereas for the trans type they are on opposite (trans) sides. cis-Polyisoprene is cross-linked by the process of vulcanization with sulfur or other chemicals such as peroxides. In the vulcanized form it is very flexible and is used in surgical gloves, rubber dams for restorative procedures, and elastics for orthodontic applications. trans-Polyisoprene is rigid; it is compounded mainly with zinc oxide (but also with some waxes and zinc silicate) and is used for endodontic points as part of the filling material for root canals. For this application it is called gutta-percha.
A copolymer of ethylene and vinyl acetate has the following structural formula:
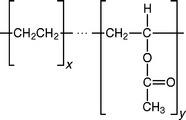
Copolymers containing 18% to 33% vinyl acetate are sold in fully polymerized sheets, which are heated to about 90° C and vacuum- or hand-formed over gypsum dental models to produce athletic mouth protectors. The higher the percentage of vinyl acetate, the softer the copolymer. The manufacturer uses a free-radical polymerization method to produce the polymer, which is then molded into sheets. No polymerization occurs in the dental processing of the mouth protector; they are processed as thermoplastics.
Allen, JG, Dart, EC, Jones, E, et al. Jones DG, ed.. Photochemistry. ICI Corporate Laboratory, Chemistry and Industry: Feb 7, 1976: 79. no 3, p, p 86
Asmussen, E. NMR—analysis of monomers in restorative resins. Acta Odontol Scand. 1975;33:129.
Braden, M. Characterization of the setting process in dental polysulfide rubbers. J Dent Res. 1966;45:1065.
Braden, M, Elliott, JC. Characterization of the setting process of silicone dental rubbers. J Dent Res. 1966;45:1016.
Braden, M, Causton, B, Clarke, RL. A polyether impression rubber. J Dent Res. 1972;51:889.
Brauer, GM, Antonucci, JM, Dental applications. ed 2. Encyclopedia of polymer science and engineering, 4.. Wiley: New York, 1986.
Cook, WD. Rheological studies of the polymerization of elastomeric impression materials. I. Network structure of the set state. J Biomed Mater Res. 1982;16:315.
Cook, WD. Rheological studies of the polymerization of elastomeric impression materials. II. Viscosity measurements. J Biomed Mater Res. 1982;16:331.
Cook, WD. Rheological studies of the polymerization of elastomeric impression materials. III. Dynamic stress relaxation modulus. J Biomed Mater Res. 1982;16:345.
Cook, WD. Photopolymerization kinetics of dimethacrylates using camphoroquinone/amine initiator system. Polymer. 1992;33:600.
Craig, RG. Chemistry, composition, and properties of composite resins. Dent Clin North Am. 1981;25:219.
Craig, RG. Photopolymerization of dental composite systems. In: Leinfelder KF, Taylor DF, eds. Posterior composites: Proceedings of the International Symposium on Posterior Composite Resins. NC: Chapel Hill, 1984.
Darr, AN, Jacobsen, PH. Conversion of dual cure luting cements. J Oral Rehabil. 1995;22:43.
Harashima, I, Nomata, T, Hirasawa, T. Degree of conversion of dual cured composite luting agents. Dent Mater. 1991;10:8.
Higashi, S, Yasuda, S, Horie, K, et al. Studies on rubber base impression materials. Discussions on the setting mechanism of polysulfide rubber as the dental impression material, chiefly viewed from variations of viscosity and molecular weight. J Nihon Univ Sch Dent. 1971;13:33.
Kitian, RJ. The application of photochemistry to dental materials. In: Gebelein CG, Koblitz FF, eds. Polymer science and technology, vol 14, Biochemical and dental applications of polymers. New York: Plenum, 1981.
McCabe, JR, Wilson, HJ. Addition curing silicone rubber impression materials. Br Dent J. 1978;145:17.
Phillips, D, Polymer photochemistry. Bryce-Smith, D, eds. Photochemistry, 1. London: The Chemical Society, Burlington House, 1970.
Ruyter, IE. International Symposium on Posterior Composite Resin Dental Restorative Materials, Netherlands. Peter Szulc Publ. 1985;6:109. Monomer systems and polymerization. In Vanherle G, Smith DC:
, Chapter 6.1.2. Ullmann’s Encyclopedia of Industrial Chemistry. ed 6. John Wiley and Sons: Elastomers, New Jersey, 2000.
Williams, JR, Craig, RG. Physical properties of addition silicones as a function of composition. J Oral Rehabil. 1988;15:639.