Bonding to Dental Substrates
Every dental restoration requires retention by some system of connection or attachment. Dentures are held in place by the combination of tissue irregularities, saliva, and adhesives. Crowns traditionally are held in place by luting cement that fills minor irregularities along the crown and dentin surfaces. Partial dentures are retained by clasps. Undercut regions in the cavity preparation retain dental amalgam. All these methods of retention rely predominantly on macroscopic mechanisms. Even so, there has always been a strong desire to develop a dental adhesive that could provide a bonded and sealed interface. The scientific beginnings of dental adhesion originated in the early 1950s with studies of bonding to enamel and dentin. Now, 55 years later, bonding agents are used routinely in restorative and preventive dentistry. This chapter focuses on the science, systems, and success of bonding systems for dental substrates with emphasis on bonding resin composites to tooth structure.
PRINCIPLES OF ADHESION
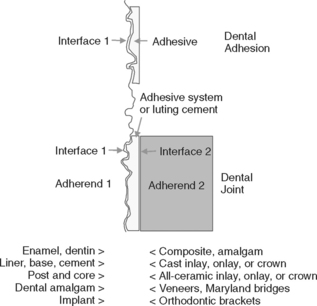
Adhesion or bonding is the process of forming an adhesive joint. The initial substrate is called the adherend, whereas the material producing the interface is generally called the adhesive. If two substrates are being joined, the adhesive produces two interfaces as part of the adhesive joint, as illustrated in Fig. 10-1. In dentistry, most adhesive joints involve two interfaces. A dental sealant attached to enamel is a simple adhesive joint with one interface. A bonded composite restoration is a more complex joint. In dentistry, there may be several steps in creating the adhesive layer, and these stages may involve separate components. These components are called a bonding agent.
ADHESION VERSUS BOND STRENGTH
Dentistry relies on the process of forming a joint and the resistance of the joint to failure. The science of adhesion studies the formation of an adhesive joint. There is a specific energy of adhesion determined by the chemical, physical, and mechanical attributes of the substrate and adhesive. Once formed, the joint’s resistance to failure depends on the extent of defects along the interface that allow cracks to form, grow, and rupture the joint. Failure processes depend on the bulk properties of the adherend and adhesive, the environment of the bond, and time. Strengths of the bonded system are measured by bond tests.
INTERFACE FORMATION FOR ADHESION
Formation of an optimally bonded interface requires that (1) the surface of the substrate be clean; (2) the adhesive wet the substrate well, have a low contact angle, and spread onto the surface; (3) adaptation to the substrate produce intimate approximation of the materials without entrapped air or other intervening materials; (4) the interface include the sufficient physical, chemical, and mechanical strength to resist intraoral forces of debonding; and (5) the adhesive be well cured under the conditions recommended for use. These events are schematically summarized in Fig. 10-2.
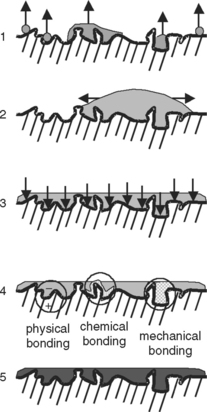
FIGURE 10-2 Examples of the microscopic steps involved in the formation of an adhesive joint. 1, Good adherend; 2, good wetting; 3, intimate adaptation; 4, bonding; 5, good curing.
Cleaning the surface of the substrate and then keeping it clean until the adhesive is applied are technical problems within a patient’s mouth. Dental surfaces exposed to the oral environment contain a pellicle of adsorbed materials from saliva. The pellicle may be contaminated by the formation of plaque or the deposition of components of food, such as stains. This material must be removed before bonding. Once a surface is cleaned, its surface energy is higher, and it is more likely to adsorb material from the surrounding air, such as moisture or saliva droplets, to decrease its energy. Therefore, the surface must be protected and the next step in a bonding procedure should proceed promptly.
Enamel and dentin prepared with rotary instruments contain a debris layer that is smeared onto their surfaces, called the smear layer. This layer is usually a few micrometers thick and adheres weakly to the substrate. Thus, it is essential to either remove this layer or penetrate it with adhesives. The most common approach to removing a smear layer is to chemically dissolve part or all of it.
When adhesive is applied to a substrate, it must wet the surface well. Good wetting is evidenced by a small contact angle and spreading of the adhesive onto the substrate (see Chapter 2). Clean dentin is hydrophilic and will be wet best by an adhesive that is also hydrophilic. In addition, the adhesive must flow in a practical time. Adding solvent to the adhesive promotes lower viscosity and good flow. However, it is not always possible to apply adhesive materials carefully in poorly accessible areas and in thin films.
Once good wetting is achieved, the adhesive should intimately contact the substrate to produce physical, chemical, or mechanical bonding. For effective chemical bonding, the distance between the adhesive and substrate must be less than a few angstroms, and a high density of new bonds must form along the interface. Because this is rarely the case, bonding of restorative materials involves mostly mechanical bonding. Mechanical bonds (gross mechanical retention and micro-mechanical retention) involve adhesive interlocking with surface irregularities. Cavity preparation produces some irregularities, and in other cases, surface roughness is increased by sandblasting or etching.
The final practical consideration for bonding is the method of curing (polymerizing) the adhesive. Most contemporary bonding agents harden by chemical reactions initiated by visible light, although self-cured and dual-cured systems are also available. If curing does not continue to a sufficient degree, the undercured adhesive may not provide good retention and sealing.
MECHANISMS OF INTERFACIAL DEBONDING
Debonding of dental joints occurs by a process of crack formation and propagation and subsequent joint failure. Cracks form at defects along the interface. Examples of defects include sites of interfacial contamination, excess moisture, trapped air bubbles, voids formed during solvent evaporation, places of poor wetting, bubbles within the adhesive, and curing shrinkage pores (Fig. 10-3). The bonded system includes the outermost layers of the substrate, which may have been altered during bonding techniques, the adhesive layer, and the restorative material interface. For all practical purposes, the bulk properties of the tooth substrates (enamel, dentin) and restorative substrates (composites, ceramics) are much stronger than the bond strength of the restorations (Table 10-1). Therefore, cracks that form generally remain in the bonded interfacial zone.
TABLE 10-1
Examples of Bulk Shear Strengths Versus Shear Bond Strengths of Materials Involved in Dental Bonding
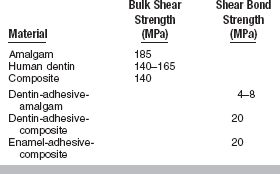
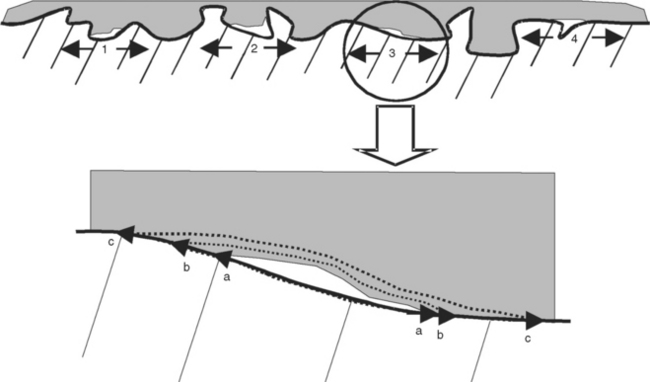
FIGURE 10-3 Defects in dental joints caused by (1) poor wetting, (2) poor adaptation, (3) inadequate bonding, and (4) inadequate curing. Polymerization shrinkage may cause an inadequately bonded area to debond further (ac) as shown in the enlargement of 3.
As cracks grow, they contribute to stress concentrations within the substrates. The final failure may often extend for short distances through portions of tooth structure or restorative material. The amount of substrate fractured is often indicative of the relative strength of the joint. Failed surfaces should be examined carefully with moderate magnification to identify the origin of the critical crack, although this crack may not be readily apparent. Therefore, failures are reported as being within the substrate (cohesive), between the adhesive and substrate (adhesive), within the restorative material (cohesive), or mixed. Generally, this information is not very useful to understanding why the failure occurred.
MEASUREMENTS OF BOND STRENGTH
Bond strength testing is one of most popular analyses conducted in evaluations of dental materials. Available tests accommodate differences in specimen size, test configurations for loading, and patterns of loading. There is no absolute bond strength; rather the measured bond strength is influenced by the concentrations of defects created in forming the interface and experimental testing variables, which may or may not be controlled. Different research investigators and different testing designs produce different values. Although the ADA and ISO have attempted to standardize the procedures (ADA SCDP WG 1.1 Test methods for the adhesion of restorative materials, ISO 11405), different values of bond strength are measured in different laboratories. It is therefore critical to include a control along with test results. Generally, the control for bond strength to dentin is the bond strength to enamel.
Bond strength can be studied using prospective or retrospective clinical models or in vitro using simulated clinical models or bonding to a standardized substrate. In a simulated clinical model, for example, a ceramic restoration is bonded with resin cement to extracted human or bovine teeth, and in vitro bond strength is tested in shear or tension. The advantage of this model is that both the tooth and ceramic restoration are present, so the test appears to be clinically relevant. The disadvantage is that often bond failures occur at both interfaces, so it is difficult to isolate the weak link in the tooth-resin cement-ceramic system. A more fundamental test is the isolated interface model, in which, for example, bonding of the tooth-resin cement interface is studied separately from the ceramic-resin cement interface.
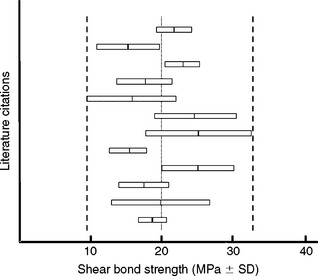
Shear bond strength is the most prevalent bond test (Fig. 10-4). It is difficult to control the position of the knife edge in this test, so there is some bending involved that causes variability. A reproducible tensile bond test for the isolated interface model is the inverted, truncated cone test (Fig. 10-5). Diameters of bonded test interfaces typically range from 3.0 to 4.5 mm. It appears that tests that use 3-mm joints produce less variation in bond strength. Shear and tensile tests report bond strengths of clinically successful composites bonded to human enamel and dentin to be in the range of 15 to 35 MPa. Coefficients of variation for shear bond tests range from 20% to 60%, whereas those for tensile bond strength tests range from 20% to 40%.
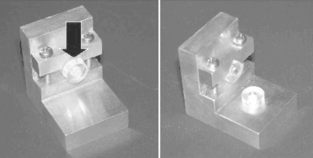
FIGURE 10-4 Composite bonded to dentin within plastic rings and debonded by shear bond strength (SBS) testing along the interface. (Courtesy SC Bayne, University of North Carolina School of Dentistry, Chapel Hill, NC.)
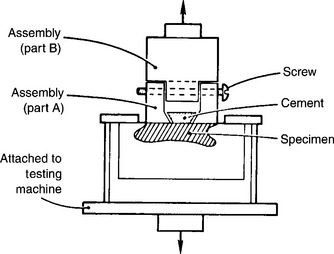
FIGURE 10-5 Tensile bond strength test using inverted, truncated cone in an isolated interface test model. (Adapted from Barakat MM, Powers JM: Aust Dent J 31:415, 1986.)
During the mid-1990s, there were numerous attempts to minimize the problems of in vitro bond strength testing and allow testing with fewer numbers of extracted teeth. The micro-tensile bond strength test (Fig. 10-6) was devised to be a more clinically relevant test. It is claimed that the test reduces the probability of crack initiation and propagation within individual specimens because of the small bonded area (1 mm2). A disadvantage of the test is that using multiple specimens from the same tooth leads to difficulties in determining the statistical meaning of the results.
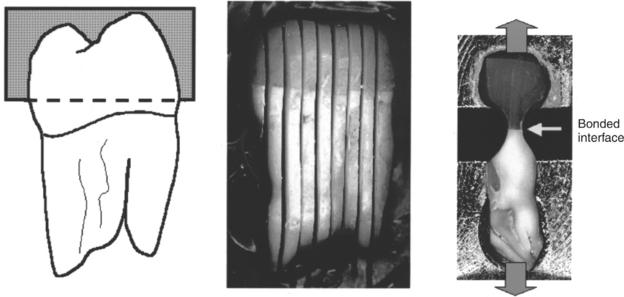
FIGURE 10-6 Production and testing of microtensile bond strength specimens. An extracted tooth with the roots embedded in resin is flattened across the occlusal surface and attached to composite using a bonding agent. The tooth is sectioned parallel to the long axis. A longitudinal section in the region near the bonded interface is reduced in size with a high-speed bur to generate a small dumbbell-shaped section for tensile testing. The ends of the section are cemented to the test equipment and then pulled in tension. (Courtesy B Rosa, São Paulo, Brazil.)
For all bond strength tests, faster loading rates tend to increase the observed bond strengths. Higher test temperatures (including thermocycling) may contribute to post-curing and strengthen the adhesive. Wet specimens are generally weaker than dry ones, as a result of the plasticizing effect of absorbed water. Fig. 10-7 demonstrates the variability reported by different laboratories attempting to test the same bonding agent.
CHARACTERIZATION OF HUMAN ENAMEL AND DENTIN
Adhesion in dentistry involves a wide range of substrates, but most applications involve adhesion to enamel and dentin. Challenges for adhesive procedures are related directly to the structure of these tissues. The following information is therefore provided as background for bonding to enamel and dentin.
STRUCTURE AND MORPHOLOGY OF ENAMEL
Enamel prisms are filled with millions of hydroxyapatite crystals, which occupy about 89% by volume of the entire enamel structure. Between the crystals are small amounts or remnant protein structure and water. The general composition of enamel and dentin by weight and volume are reported in Table 10-2. Enamel crystals are packed with their long axes roughly aligned with the long axis of the enamel prism. Crystals are hexagonal in cross-section and elongated. Looking down along the long axis of the enamel prism, the crystals are perpendicular to the view in the center of the prism but are more tipped toward the periphery. The crystals in the tail are the least densely packed and tipped at perhaps as much as 30 degrees away from perpendicular. Chemical properties of the prism vary between the center and perimeter.
TABLE 10-2
Composition of Human Enamel and Dentin in Weight and Volume Percent

Adapted from LeGeros RZ: Calcium phosphates in oral biology and medicine, Monogr Oral Sci 15:108–113, 1991.
Enamel prisms are essentially parallel to each other and run from the dentoenamel junction (DEJ) outward in a radial pattern. In the neighborhood of enamel cusps, the prisms are perpendicular to the DEJ. Near the cementoenamel junction, the prisms are highly tipped. It is crucial to avoid undermining enamel rods during cavity preparation, or bonding will tend to dislodge the enamel prisms in those regions during mechanical loading.
Etching of enamel with phosphoric acid eliminates smear layers associated with cavity preparation, dissolves persisting layers of prismless enamel in deciduous teeth, and differentially dissolves enamel crystals in each prism. The pattern of etched enamel is categorized as Type 1 (preferential prism core etching), Type 2 (preferential prism periphery etching, Fig. 10-8, C), and Type 3 (mixed). There appears to be no difference in micro-mechanical bonding of the different etching patterns. In a standard cavity preparation for a composite, the orientation of the enamel surfaces being etched could be perpendicular to enamel prisms (perimeter of the cavity outline), oblique cross-section of the prisms (beveled occlusal or proximal margins), and axial walls of the prisms (cavity preparation walls).
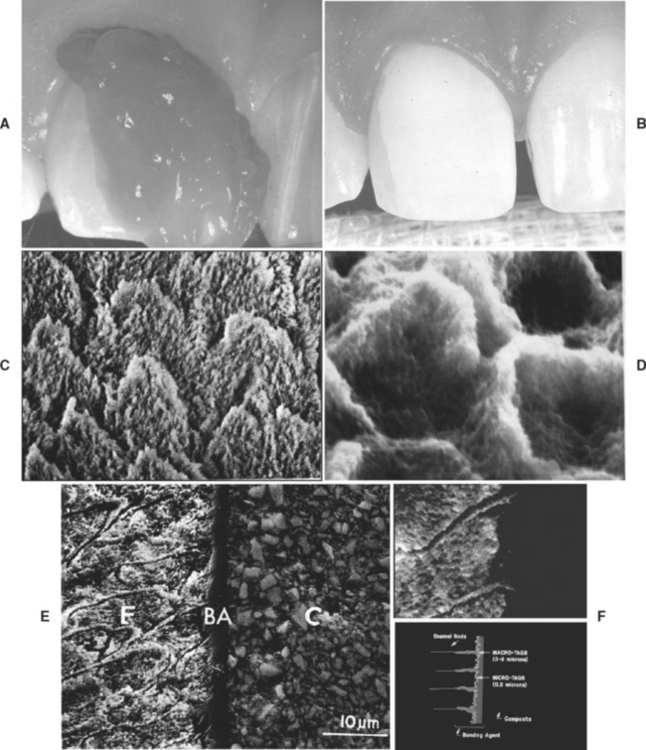
FIGURE 10-8 Micromechanical retention to human enamel. A, Gel etchant dispensed from a syringe onto the enamel portion of the cavity preparation. B, Frosty or dull appearance of enamel after etching, rinsing, and drying. C, Magnified view of etched enamel with Type 2 etching. D, Magnified view of bonding agent revealed by dissolving enamel. E, Scanning electron photomicrograph of macrotags and microtags in enamel formed by bonding system. F, Schematic representation of macrotags and microtags. (Courtesy SC Bayne, University of North Carolina School of Dentistry, Chapel Hill, NC.)
During the early stages of etching, when only a small amount of enamel crystal dissolution occurs, it may be difficult or impossible to detect the extent of the process. However, as the etching pattern begins to develop, the surface etched with phosphoric acid develops a frosty appearance (Fig. 10-8, B), which has been used as the traditional clinical landmark for sufficient etching. Unfortunately, acidic primers found in self-etching bonding agents (described later in this chapter) do not produce this frosty appearance.
STRUCTURE AND MORPHOLOGY OF DENTIN
Dentin’s composition is much more rich in organic material than enamel. Only about 50% by volume of dentin is mineralized with hydroxyapatite crystals (see Table 10-2). A large proportion of the crystals is interspersed among collagen fibers. Within collagen fibers, individual fibrils contain crystals at their ends as well. The lower mineral content of dentin permits more elastic deformation during loading.
Dentin tubules are 0.5 to 1.5 μm in diameter and are formed at a slight angle to the DEJ and pulp chamber. There is a higher density of tubules along the inner or deep dentin (43,000 tubules/mm2) than at the middle dentin (35,000 tubules/mm2) or the outer, superficial dentin (15,000 tubules/mm2). A labyrinth of secondary tubules among neighboring tubules often interconnects primary tubules. Fig. 10-9 is a scanning electron micrograph of dentin prepared for bonding. It reveals the primary tubule in the center with secondary (lateral) tubules branching off from the tubule. Intertubular dentin comprises collagen, hydroxyapatite, and water. Under normal circumstances, the tubule is filled with the odontoblastic process.
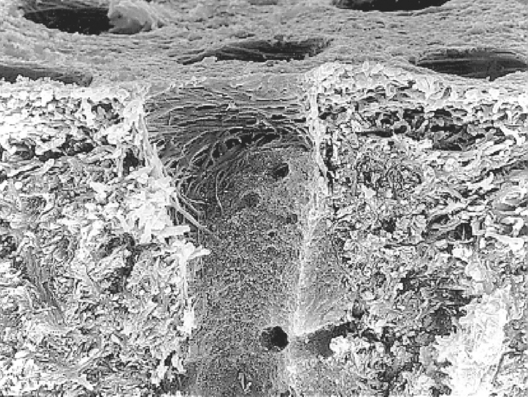
FIGURE 10-9 Scanning electron photomicrograph of fractured human dentin revealing intertubular (collagen, hydroxyapatite, water), peritubular (collagen, hydroxyapatite), and tubular dentin. The surface has been etched with phosphoric acid to remove the smear layer and dissolve hydroxyapatite crystals in a superficial zone of about 2 μm. The lateral orientation of most collagen fibers is revealed along the top edge of the dentinal tubule. Smaller orifices of lateral tubules are seen easily within the primary dentinal tubule. (Courtesy J Perdigão, University of Minnesota, Minneapolis, MN.)
Etching of dentin surfaces primarily dissolves hydroxyapatite crystals within the surface of the intertubular dentin and along the surface of the outermost peritubular dentin. A smear layer exists from cavity preparation (Fig. 10-10, A and B) that is typically 1 to 2 μm thick with smear plugs within the ends of the tubules (Fig. 10-10, C). The smear layer is almost entirely hydroxyapatite debris from high-speed cutting during cavity preparation. High surface temperatures along the cutting interface pyrolize most of the organic material.
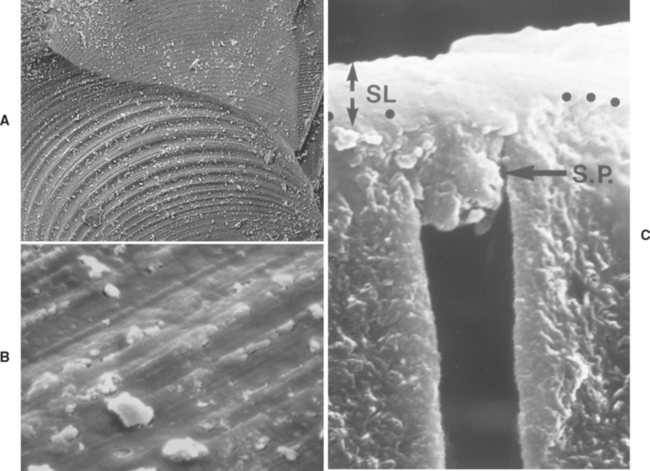
FIGURE 10-10 Smear layer on dentin. A, Scanning electron photomicrograph of cavity preparation showing dentin smear layer with some loose debris on surface. B, High-magnification scanning electron microscopic (SEM) view of smear layer on same surface as A showing some cracks within the compacted debris layer. C, Cross-sectional view of smear layer (1 to 2 μm in thickness) with smear plugs compacted into dentinal tubule openings. (A and B courtesy SC Bayne, University of North Carolina School of Dentistry, Chapel Hill, NC; C courtesy D Pashley, Medical College of Georgia, Augusta, GA.)
When the etchant first contacts the smear layer, it begins to dissolve and penetrate it. Immediately the underlying intertubular dentin, peritubular dentin, and tubules come into contact with acid. Intertubular dentin contains about 50% hydroxyapatite crystals; these crystals are the same size as in enamel and are embedded within the spaces between collagen fibers. This material is relatively quickly dissolved. Peritubular dentin is about 80% to 90% hydroxyapatite crystals by volume and is readily dissolved in acid. The dentinal fluid is highly buffered. Therefore acid that mixes with dentinal fluid is quickly neutralized. Effects of acid etching are typically limited to 0.1 to 5 μm of the superficial region of intertubular dentin and about 2 to 10 μm along the walls of peritubular dentin (Fig. 10-11, A). The result of the etching process is the creation of a demineralized zone of dentin between the tubules and along the outer mouth of individual dentinal tubules. This surface is porous and allows primer penetration for tag formation. Fig. 10-11, B, is an atomic force microscope image of the etching sequence.
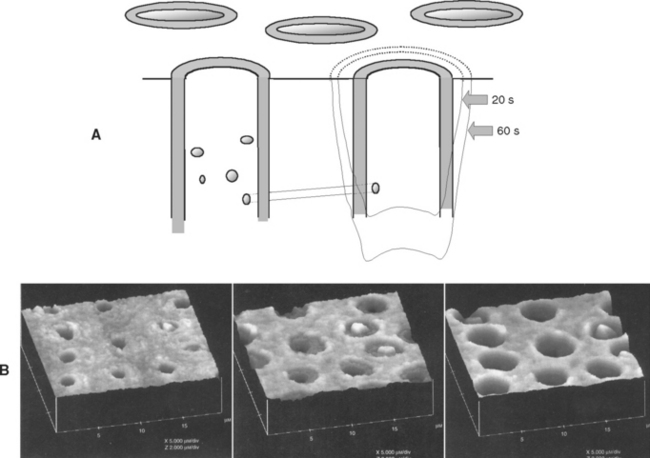
FIGURE 10-11 Acid-etching effects on dentin. A, Schematic view of the progression of acid etching on dentin. B, Atomic force microscope image of the acid-etching effects of dentin, showing progress after 20s, 60s, and 100s, respectively, with citric acid etching. (Courtesy GW Marshall, UCSF School of Dentistry, San Francisco, CA.)
BONDING AGENTS FOR DIRECT RESIN COMPOSITES
A chronology of the development of bonding agents used to place direct composites is shown in Table 10-3. Modern bonding agents contain three major ingredients (etchant, primer, adhesive) that may be packaged separately or combined. Box 10-1 presents a summary of the components of currently available fourth-, fifth-, sixth-, and seventh-generation bonding agents. Typical compositions of these components are summarized in Table 10-4.
TABLE 10-4
Representative Compositions of Major Components of Bonding Agents
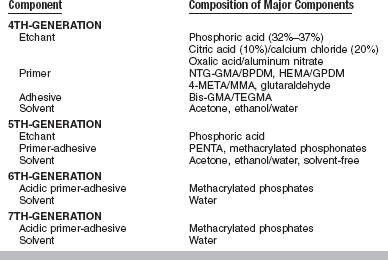
4-META, 4-methacryloxyethyl trimellitic anhydride; Bis-GMA, 2,2-bis[4(2-hydroxy-3-methacry-loxy-propyloxy)-phenyl]propane; BPDM, biphenyl dimethacrylate; GPDM, glycerophosphoric acid dimethacrylate; HEMA, 2-hydroxyethylmethacrylate; MMA, methyl methacrylate; NTG-GMA, N-tolylglycine-glycidyl methacrylate; PENTA, dipentaerythritol pentacrylate phosphoric acid ester; TEGDMA, triethyleneglycoldimethacrylate.
Etchants are relatively strong acid solutions that are mainly based on phosphoric acid. Primers contain hydrophilic monomers to produce good wetting. Adhesives include typical dimethacrylate oligomers that are found in composites. Most fourth- and fifth-generation bonding agents are remarkably similar.
COMPOSITION
A wide range of organic (maleic, tartaric, citric, EDTA, acidic monomers), polymeric (polyacrylic acid), and mineral (hydrochloric, nitric, hydrofluoric) acids have been investigated as etchants, but phosphoric acid solutions and gels (37%, 35%, 10%) have been shown to produce the most reliable etching patterns. Acid etchants are also called conditioners to disguise the fact that most are relatively strong acids (pH ≅ 1.0).
Originally, etching solutions were free-flowing liquids and were difficult to control during placement. Gel etchants were developed by the addition of small amounts of microfiller or cellulose thickening agents. These gels flow under slight pressure but do not flow under their own weight.
Primers
Primers are hydrophilic monomers (see Table 10-4) usually carried in a solvent. Acidic primers containing carboxylic acid groups are used in self-etching bonding agents. The solvents used in primers are acetone, ethanol-water, or primarily water. In some primers, the solvent levels can be as high as 90%. A few fourth- and fifth-generation bonding agents are solvent-free. Therefore, primers have different evaporation rates, drying patterns, and penetration characteristics, all of which can influence the resulting bond strength. Advantages and disadvantages of primers with various solvents are listed in Table 10-5.
TABLE 10-5
Advantages and Disadvantages of Primers with Various Solvents
| Solvent | Advantages | Disadvantages |
| Acetone | Dries quickly | Evaporates quickly after being dispensed; can evaporate from container; sensitive to wetness of dentin; multiple coats may be required; offensive odor |
| Ethanol/water | Evaporates less quickly, less sensitive to wetness of dentin | Extra drying time |
| Water | Slow evaporation, not sensitive to wetness of dentin | Long drying time; water can interfere with adhesive if not removed |
| Solvent-free | No drying, single coat | Higher film thickness |
Adhesives
Adhesives are generally hydrophobic, dimethacrylate oligomers (see Table 10-4) that are compatible with monomers used in the primer and composite. These oligomers are usually diluted with lower-molecular weight monomers.
Initiators and Accelerators
Most bonding agents are light-cured and contain an activator such as camphorquinone and an organic amine. Dual-cured bonding agents include a catalyst to promote self-curing.
Fillers
Although most bonding agents are unfilled, some products contain inorganic fillers ranging from 0.5% to 40% by weight. Filler particles include micro-fillers, also called nanofillers, and sub-micron glass. Filled bonding agents may be easier to place on the tooth and may produce higher in vitro bond strengths.
PROPERTIES
Bond Strength: Most bonding agents produce bond strengths to human enamel and superficial dentin of 15 to 35 MPa. Bond strengths determined for deep dentin tend to be lower than those for superficial dentin. A variety of clinical problems can reduce bond strength. Some clinical problems and suggested solutions are listed in Table 10-6.
TABLE 10-6
Clinical Problems That Can Decrease Bond Strength of Bonding Agents and Suggested Solutions
| Problem | Solution |
| Dentin surface too dry | Use moist cotton pellet to rehydrate surface |
| Dentin surface too wet | Gently air dry to achieve glistening surface |
| Contamination with saliva or blood | Rinse, re-etch if contamination is moderate or greater |
| Contamination with caries detector, handpiece lubricant or hemostatic agent | Rinse and re-etch |
| Contamination by eugenol | Avoid eugenol-containing provisional materials and temporary cements |
| Surface does not glisten after application of primer | Apply additional coasts of primer |
| Self-cured composite or resin cement debonds from adhesive | Use dual-cured bonding agent with self-cured composite or resin cement |
| Bonding agent undercured | Cure recommended time with properly maintained light-curing unit, be sure bonding agent is compatible with light-curing unit |
| Recent bleaching procedure | Wait 1 week after bleaching |
Fatigue Strength: Over long periods (>10 years), the bonded interface will undergo extensive fatigue cycling. Combinations of mechanical and thermal cycling stresses may produce as many as 1 million cycles of loading on the interfaces per year. Weak or compromised interfaces could debond and allow microleakage and fluid flow. The latter is detectable as pain. Only a limited number of low-cycle fatigue tests have been performed on dentin bonding systems in the laboratory. Fatigued systems failed at the bonded interface and displayed 50% reduction in bond strength even after only 1000 cycles of loading. To date there are no laboratory data that would strongly support long-term success of bonding agents.
Biological Properties
Solvents and monomers in bonding agents are typically skin irritants. Certain material, such as 2-hydroxyethylmethacrylate (HEMA), is not considered biocompatible as a monomer. Bonding agents may produce local and systemic reactions in dentists and dental assistants sufficient to preclude their further use in the dental office. It is critical that dental personnel protect themselves from recurring exposure. Protective techniques include wearing gloves, immediately replacing contaminated gloves, using high-volume evacuation where the materials are being used, keeping all bottles tightly closed or using unit-dose systems, and disposing of materials in such a way that the monomers cannot evaporate into the office air. Even with double gloves, contact with these aggressive solvents and monomers will produce actual skin contact in a few minutes. Follow all reasonable precautions, and if unwanted contact occurs, immediately flush affected areas with copious amounts of water and soap. Once the materials are polymerized, there is very little risk of side effects. Although patients should be protected during bonding operations, properly polymerized materials have not been shown to create any subsequent patient hazard.
Clinical Properties
Enamel bonding has a long history of success when enamel is etched appropriately, microtags are formed, and components are cured. Clinical evaluations of the performance of bonding agents were initiated in the late 1970s but were primarily focused on the ability of enamel bonding agents to suppress microleakage. There were no standardized clinical procedures for evaluating bonding systems until the ADA developed “Guidelines for Dental Adhesion” in 1994. These guidelines require the testing of adhesives for bonding restorations to nonretentive Class 5 lesions. The lesions, which may be saucer-shaped or notch-shaped, are found on enamel margins along the coronal margin and dentin along the apical margin (Fig. 10-12, A). The success of a bonding agent is evaluated indirectly by examining the performance of the restorations for (1) postoperative sensitivity, (2) interfacial staining, (3) secondary caries, and (4) retention or fracture from insertion to 18 months. These clinical trials test short-term retention and initial sealing.
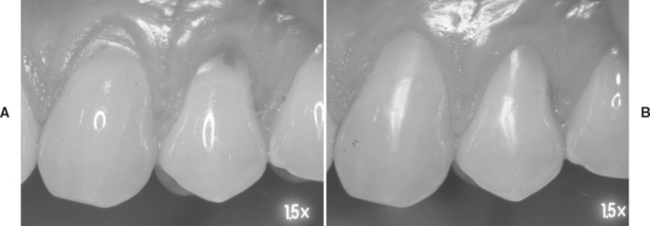
FIGURE 10-12 Clinical testing of bonding systems by restoring nonretentive Class 5 lesions. A, Clinical examples of Class 5 lesions. B, Clinical examples of the same lesions restored with a bonding agent and composite restorations.
Most commercial products for enamel and dentin bonding are successful in clinical trials. However, these clinical trials generally combine enamel and dentin bonding. There is no acceptable clinical regimen for critically testing dentin bonding only in nonretentive preparations. Because clinical trials are usually highly controlled, they are often not predictive of average clinical usage in general practice. Longevity of bonding in general practice may be only 40% of that achieved in clinical trials because of technique or other failures. Long-term clinical performance of bonding systems (>10 years) for a wide range of materials has not yet been reported.
Sites of failure for most bonded restorations occur along cervical margins where the bonding is primarily to dentin. Examination of bonded composites in Class 2 restorations demonstrates that 95% of all secondary caries associated with composite occurs interproximally. These margins are the most difficult to fabricate during placement of the restoration, are typically bonded to dentin and cementum rather than enamel, and are hard to access with visible light for adequate polymerization.
MANIPULATION
Bonding to enamel occurs primarily by micro-mechanical retention after acid etching is used to remove smear layers and preferentially dissolve hydroxyapatite crystals in the outer surface of the interface. Fluid adhesive constituents penetrate into the newly produced surface irregularities and become locked into place after polymerization of the adhesive.
Gel etchants (typically phosphoric acid) are dispensed from a syringe onto tooth surfaces to be etched (see Fig. 10-8, A). Etching times for enamel vary depending on the type and quality of enamel. Generally, a 15-second etch with 37% phosphoric acid is sufficient to produce microtags. However, until macro-spaces are evident, the characteristic clinical endpoint of a frosty enamel appearance will not develop. Deciduous enamel generally contains some prismless enamel that has not yet worn away. It takes longer to etch through this outer layer and to reveal characteristic etch patterns on the underlying prism enamel. It is therefore commonplace to recommend 120 seconds of acid etching for primary enamel.
Some enamel may have been rendered more insoluble as a result of fluorosis. In those cases, extended etching times are required to ensure that sufficient micro-mechanical bonding can occur. It is not uncommon to extend etching times for several minutes to accomplish an adequate etch. The only caution is that dentin should be protected from exposure to acid for this long a time.
After the intended etching time with fourth- and fifth-generation bonding systems, the materials are rinsed away and the tooth structure is maintained in a moist surface condition for the next stage of bonding. Then, primer can be flowed onto the surface to penetrate into the available surface irregularities. Primer and adhesive that flow into larger irregularities, such as the prism peripheries shown in Fig. 10-8, D, produce resin tags once the adhesive is cured. These tags are actually “macrotags.” Inspection of the details of the surfaces for a single prism reveals that smaller tags, “microtags,” form where adhesive flows into the spaces between partially dissolved individual hydroxyapatite crystals (see Fig. 10-8, E and F). Microtags are much more numerous and contribute to most of the micro-mechanical retention.
Dentin Bonding
Dentin bonding involves three distinct processes—etching (conditioning), priming, and bonding. In contrast to enamel, dentin contains more water (see Table 10-2) and is strongly hydrophilic. To manage this problem, primers have hydrophilic components that wet dentin and penetrate the surface. The goal is to produce microtags for micro-mechanical adhesion.
Modern bonding agents are applied to moist dentin surfaces. Any drying must be done cautiously. Once the hydroxyapatite component of the outer layer of dentin is removed, dentin contains about 50% unfilled space and about 20% remaining water. Even a short air blast from an air-water spray can inadvertently dehydrate the outer surface and cause the remaining collagen sponge to collapse onto itself. Once this happens, the collagen mesh readily excludes the penetration of primer and bonding will fail. It is then necessary to rehydrate the dentin surface before priming. Hold a cotton pellet moistened with water against the surface for about 10 to 15 seconds or apply a rewetting agent (surfactant solutions in water). Do not bond to dentin unless the surface looks glistening. This is the critical clinical clue that the surface is sufficiently hydrated. An overly moist condition is equally bad. Excess moisture tends to dilute the primer and interfere with resin interpenetration. In the total-etch technique, etch enamel and dentin simultaneously with phosphoric acid. Apply etchant to enamel first and then to dentin. Rinse excess material from the surfaces. Use gentle air-drying to check the enamel for a frosty appearance, but guard against overdrying etched dentin while air-drying. If necessary, rehydrate the dentin to a glistening appearance before applying primer. With self-etching primers or self-etching adhesives, phosphoric acid is not used (except for etching of unground enamel). Apply the self-etching system directly to the enamel and dentin with no rinsing.
The process of resin impregnation of etched dentin by the primer is called hybrid layer formation, as described in Fig. 10-13. The result of this process has been called the resin-interpenetration zone or resin-interdiffusion zone. This layer is critical to bonding and creates the necessary microtags. The hybrid layer is revealed to be quite variable in thickness (see Fig. 10-13, D). Concurrent with hybrid layer formation is the penetration of primer into the fluid-filled or open dentinal tubules. This generates quite large macrotags. However, these appear to be of little value to overall bonding at the present time. This material is generally undercured and behaves as soft flexible tags. If dentin is dehydrated before priming and bonding, these macrotags are more likely to be quite extensive.
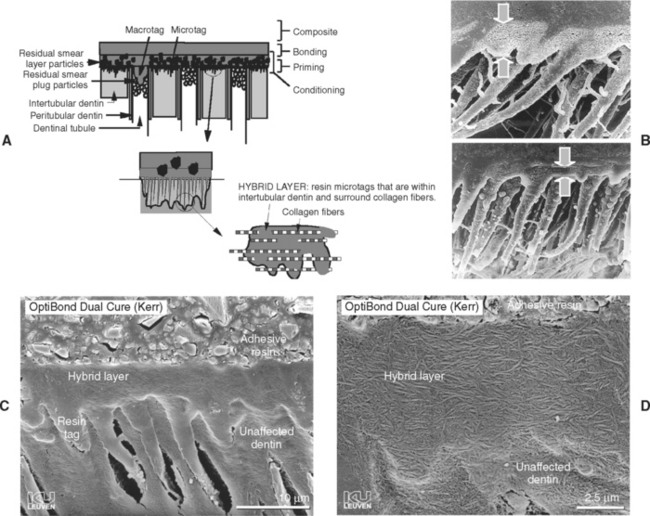
FIGURE 10-13 Hybrid layer formation. A, Etching removes hydroxyapatite crystals within intertubular dentin and along peritubular dentin. Primer penetrates intertubular spaces and fluid-filled tubular spaces. Cured primer forms microtags within intertubular dentin and macrotags within tubules. B, Scanning electron photomicrographs indicating the varying thickness of the hybrid layer. C, SEM of restoration and hybrid layer after removal of dentin structure. D, Higher magnification of region of hybrid layer showing interpenetration of polymerized primer and collagen. (B Courtesy J Perdigão, University of Minnesota, Minneapolis, MN; C and D, courtesy B van Meerbeek, Catholic University of Leuven, Belgium.)
The thickness of a hybrid layer is not a critical requirement for success. Dentin bond strength is probably proportional only to the number of microtags, not the length of the microtags. Effective etching and priming of dentin does not require long times to produce acceptable dentin bond strengths. Inadequate etching or priming, however, is a concern; therefore the tendency is to err routinely on the side of longer-than-needed etching times. If the etched zone is too deep, it is more challenging to fill the zone with primer. A potential problem presents if decalcified dentin is not fully impregnated. The etched but not impregnated space may reside as a mechanically weak zone and promote nanoleakage. While this zone has been detected in laboratory experiments, the clinical results of this process have never been demonstrated to be a problem.
Primers contain solvents to enhance their wetting and to co-solubilize the range of monomers involved. During application of the primer, most of the solvent evaporates quickly. Very little of the original primer that is applied actually remains as polymerizable material to produce dentin impregnation. Thus, several layers usually must be applied. The rule of thumb is that one should apply as many layers as are necessary to produce a persisting glistening appearance on dentin.
Once the primer is applied adequately, an adhesive is applied and light cured. Surfaces of the cured bonding agents are initially air inhibited and do not immediately react. However, as composite is placed against the surface, the air is displaced and copolymerization occurs.
BONDING SYSTEMS FOR OTHER SUBSTRATES
For bonding to dental amalgam, the objective is to cause the unset bonding agent and unset amalgam to intermingle before they set. Because dental amalgam is opaque, the bonding system must be chemically cured.
A bonding agent is applied to the cavity preparation. Dental amalgam is condensed against the unset bonding system. The thickness of the bonding layer must be increased by using multiple layers of bonding agent or by adding thickening agents to the bonding agent. One bonding agent uses an admixture of small, poly(methyl methacrylate) powder particles in the bonding agent to thicken it. Generally, the thickness of the bonding layer is increased to 20 to 50 μm. Fig. 10-14, A, portrays this process schematically. Fig. 10-14, B, provides a scanning electron photomicrograph of the resulting interface. In the absence of suitable commingling, the bond strength might be only 4 to 8 MPa. However, with micro-mechanical interlocking at the interface, the bond strength may be 20 MPa. In most amalgam restorations, bonding agents simply seal the cavity against fluid flow and microleakage. Bonding agents provide little or no retention for set amalgams, even if they are roughened, because the bonding systems do not wet or adapt to the set amalgam surfaces very well.
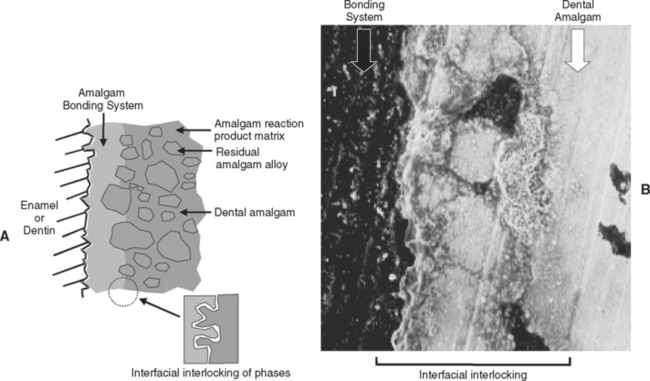
FIGURE 10-14 Mechanism of bonding amalgam to tooth structure. A, Schematic of thickened layer of bonding agent to permit intermingling with fluid dental amalgam along the interface during amalgam placement and before setting. B, Scanning electron photomicrograph of interface between bonding agent and dental amalgam. (B Adapted from Ramos JC, Perdigão J: Am J Dent 10:152, 1997.)
LABORATORY COMPOSITES
Restorations made with laboratory composites (see Chapter 9) include inlays, onlays, veneers, and milled restorations, and are fabricated indirectly in a dental laboratory and then bonded into existing cavity preparations. Bonding requires agents for both the tooth structure and the undersurfaces of the indirect restoration. Resin composite cements are usually used to fill the space between the two surfaces. Bonding to the tooth structure is straightforward and reliable.
Bonding to the indirect composite surfaces is difficult. The goal is to swell the outer surfaces of the resin matrix and allow new monomers from the bonding agent to penetrate spaces among existing polymer chains. At the time of curing, the new polymer chains become micro-mechanically intertwined with the existing polymer chains, producing relatively strong bonding. Bonding can be enhanced by blasting (micro-etching) with particles of aluminum oxide, etching with hydrofluoric acid gel, or treating with primers. Sandblasting roughens the surface. Etching removes smear layers and partially dissolves glass filler particles. Primers provide good wetting and potential chemical bonding to exposed glass filler particle surfaces. Commercial primers for laboratory composites contain silane, unfilled resin monomers, or silane-monomer combinations.
Bonding composite cement to laboratory composites generally produces bond strengths in the range of 20 to 35 MPa.
CERAMIC
Ceramic restorations bonded to enamel and dentin include a number of bonded interfaces. Enamel and dentin are treated with whatever bonding agent is desirable. The undersurface of the all-ceramic inlay, onlay, crown, bridge, or veneer is treated with 5% to 9% hydrofluoric acid gel. The pH of this gel is low and capable of removing any smear layers while etching the phase boundaries and the more soluble silicate glass phases. Although this process does not work on alumina- or zirconia-based ceramics, it is effective for other ceramics. Another popular option is to blast (micro-etch) the surface with 50-μm aluminum oxide particles.
Once the ceramic surface is prepared, an aqueous and acidified solution of silane is applied to enhance wetting and potentially function as a chemical coupling agent. Silane is a difunctional molecule capable of reacting to the hydroxyls on the silicate phases along the treated surface’s restoration. The other end of the silane is capable of copolymerizing with resin cement, which is used to attach the two treated surfaces and fill in the typically 100 μm of intervening space after seating (Fig. 10-15).
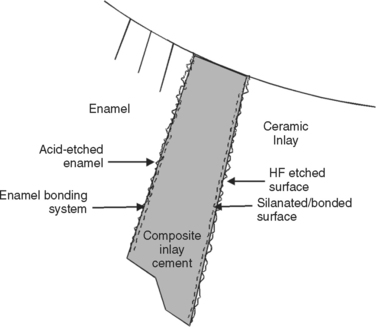
FIGURE 10-15 Schematic of materials and interfaces involved in bonding all-ceramic restorations to tooth structure.
Bonding systems used with resin cements for all-ceramic systems can be light-cured but are often dual-cured or self-cured. Bond strengths for a bonded ceramic restoration to tooth structure are generally in the range of 20 to 40 MPa.
COMPOSITE BONDED TO CAST ALLOYS
It is sometimes desirable to bond a resin composite to a cast alloy substrate rather than ceramic. Traditionally, resin bonding to metal substrates has relied on macro-mechanical retention (latticework, mesh, beads, or posts along the metal surface). Another approach to resin-metal adhesion was introduced in 1984 and trademarked “Silicoating.” A silicon oxide coating is generated to create a surface that is capable of chemical bonding. This technique has been applied to gold, cobalt-chromium, silver-palladium, and titanium alloys. For Silicoating, a metal surface is roughened by sandblasting with 250-μm Al2O3, cleaned, silica-coated, chemically silanated, primed, and the composite is bonded.
Other techniques for treating alloy surfaces to be bonded with composite include thermally applied silica and ceramic blasting (Box 10-2). Recently, liquid primers composed of thiophosphate monomers have become available. These techniques are equally effective in providing a relatively strong bond (18 to 30 MPa) of composites to alloy substrates.
REPAIR OF COMPOSITE, CERAMIC, AND CERAMIC-METAL RESTORATIONS
It is quite common to encounter restorations that require repair because of long-term wear, unwanted color change, the appearance of small surface defects, or chipping. These situations can be easily managed by veneering the surface with fresh composite. The damaged surface is removed to a depth of 1 to 1.5 mm below the final intended surface employing small burs, and the excavation is extended to neighboring enamel margins. The margins are roughened and etched to remove any smear layer and provide micro-mechanical retention. Priming produces tags into the enamel and diffusion into the surface of the old restoration. It is critical that the old composite is not overdried because water in the old restoration tends to hold open the old polymer network and allow some hybrid layer formation. The bonding agent is cured. Restorative material is added, cured, and polished. Excellent repair bond strengths (20 to 35 MPa) are possible.
Abdalla, AI, Davidson, CL. Bonding efficiency and interfacial morphology of one-bottle adhesives to contaminated dentin surfaces. Am J Dent. 1998;11:281.
Agostini, FG, Kaaden, C, Powers, JM. Bond strength of self-etching primers to enamel and dentin of primary teeth. Pediatr Dent. 2001;23:481.
Barakat, MM, Powers, JM. In vitro bond strength of cements to treated teeth. Aust Dent J. 1986;31:415.
Bayne, SC, Fleming, JE, Faison, S. SEM-EDS analysis of macro and micro resin tags of laminates. J Dent Res. 1982;6lA:304.
Bayne, SC, Swift, EJ, Jr. Solvent analysis of three reduced-component dentin bonding systems. Trans Acad Dent Mater. 1997;1:156.
Boghosian, A. Clinical evaluation of a filled adhesive system in Class 5 restorations. Compend Contin Educ Dent. 1996;17:750–752. 754
Bouillaguet, S, Wataha, JC, Hanks, CT, et al. In vitro cytotoxicity and dentin permeability of HEMA. J Endod. 1996;22:244.
Bouillaguet, S, Wataha, JC, Virgillito, M, et al. Effect of sub-lethal concentrations of HEMA (2-hydroxyethyl methacrylate) on THP-1 human monocyte-macrophages, in vitro. Dent Mater. 2000;16:213.
Bowen, RL, Eick, JD, Henderson, DA, et al. Smear layer: removal and bonding considerations. Oper Dent Suppl. 1984;3:30.
Bozalis, WG, Marshall, GW, Jr., Cooley, RO. Mechanical pretreatments and etching of primary-tooth enamel. ASDC J Dent Child. 1979;46:43.
Buonocore, MG. Simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34:849.
Choi, KK, Condon, JR, Ferracane, JL. The effects of adhesive thickness on polymerization contraction stress of composite. J Dent Res. 2000;79:812.
Clemmensen, S. Sensitizing potential of 2-hydroxyethylmethacrylate. Contact Dermatitis. 1985;12:203.
Costa, CA, Teixeira, HM, do Nascimento, AB, et al. Biocompatibility of an adhesive system and 2-hydroxyethylmethacrylate. ASDC J Dent Child. 1999;66:337.
Davidson, CL, Feilzer, AJ. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J Dent. 1997;25:435.
Drummond, JL, Sakaguchi, RL, Racean, DC, et al. Testing mode and surface treatment effects on dentin bonding. J Biomed Mater Res. 1996;32:533.
Eick, JD, Wilko, RA, Anderson, CH, et al. Scanning electron microscopy of cut tooth surfaces and identification of debris by use of the electron microprobe. J Dent Res. 1970;49(Suppl):1359.
Eick, JD. Smear layer—materials surface. Proc Finn Dent Soc. 1992;88(Suppl 1):225.
el-Kalla, IH, Garcia-Godoy, F. Saliva contamination and bond strength of single-bottle adhesives to enamel and dentin. Am J Dent. 1997;10:83.
Eiriksson, SO, Pereira, PN, Swift, EJ, et al. Effects of blood contamination on resin-resin bond strength. Dent Mater. 2004;20:184.
Eiriksson, SO, Pereira, PN, Swift, EJ, Jr., et al. Effects of saliva contamination on resin-resin bond strength. Dent Mater. 2004;20:37.
Farah, JW, Powers, JM. Bonding agents. Dent Advis. 2003;20(8):1.
Farah, JW, Powers, JM. Update on bonding agents. Dent Advis. 2004;21(8):1.
Farah, JW, Powers, JM. Update: 6th- and 7thgeneration bonding agents. Dent Advis. 2005;22(9):1.
Fissore, B, Nicholls, JI, Yuodelis, RA. Load fatigue of teeth restored by a dentin bonding agent and a posterior composite resin. J Prosthet Dent. 1991;65:80.
Frankenberger, R, Kramer, N, Petschelt, A. Fatigue behaviour of different dentin adhesives. Clin Oral Investig. 1999;3:11.
Fritz, UB, Finger, WJ, Stean, H. Salivary contamination during bonding procedures with a one-bottle adhesive system. Quint Int. 1998;29:567.
Fusayama, T. [Cavity preparation for a new adhesive restorative resin]. Shikai Tenbo. 1981;57:1223.
Fusayama, T. A simple pain-free adhesive restorative system by minimal reduction and total etching. Tokyo: Ishiyaku EuroAmerica, 1993.
Garberoglio, R, Brannstrom, M. Scanning electron microscopic investigation of human dentinal tubules. Arch Oral Biol. 1976;21:355.
Kaaden, C, Powers, JM, Friedl, K-H, et al. Bond strength of self-etching adhesives to dental hard tissue. Clin Oral Investig. 2002;6:155.
Kaaden, C, Schmalz, G, Powers, JM. Morphological characterization of the resin-dentin interface in primary teeth. Clin Oral Investig. 2003;7:235.
Katsuno, K, Manabe, A, Hasegawa, T, et al. Possibility of allergic reaction to dentin primer—application on the skin of guinea pigs. Dent Mater J. 1992;11:77.
LeGeros, RZ. Calcium phosphates in oral biology and medicine Monographs in Oral Science, 15 . Karger: Basel, 1992.
Marshall, GW, Jr., Wu-Magidi, IC, Watanabe, LG, et al. Effect of citric acid concentration on dentin demineralization, dehydration, and rehydration: atomic force microscopy study. J Biomed Mater Res. 1998;42:500.
May, KN, Jr., Swift, EJ, Jr., Bayne, SC. Bond strengths of a new dentin adhesive system. Am J Dent. 1997;10:195.
Mjor, IA. Frequency of secondary caries at various anatomical locations. Oper Dent. 1985;10:88.
Munksgaard, EC. Permeability of protective gloves to (di)methacrylates in resinous dental materials. Scand J Dent Res. 1992;100:189.
Nakabayashi, N, Ashizawa, M, Nakamura, M. Identification of a resin-dentin hybrid layer in vital human dentin created in vivo: durable bonding to vital dentin. Quint Int. 1992;23:135.
Nakabayashi, N, Nakamura, M, Yasuda, N. Hybrid layer as a dentin-bonding mechanism. J Esthet Dent. 1991;3:133.
Nakabayashi, N. Dentinal bonding mechanisms. Quint Int. 1991;22:73.
Nakabayashi, N. The hybrid layer: a resin-dentin composite. Proc Finn Dent Soc. 1992;88(Suppl 1):321.
O’Keefe, KL, Powers, JM. Adhesion of resin composite core materials to dentin. Int J Prosthodont. 2001;14:451.
Pashley, DH, Carvalho, RM, Sano, H, et al. The microtensile bond test: a review. J Adhes Dent. 1999;1:299.
Pashley, DH. Smear layer: overview of structure and function. Proc Finn Dent Soc. 1992;88(Suppl 1):215.
Pashley, DH. Smear layer: physiological considerations. Oper Dent Suppl. 1984;3:13.
Pashley, DH. Dentin: a dynamic substrate—a review. Scan Microsc. 1989;3:161.
Perdigão, J, Lambrechts, P, van Meerbeek, B, et al. Morphological field emission-SEM study of the effect of six phosphoric acid etching agents on human dentin. Dent Mater. 1996;12:262.
Powers, JM, Finger, WJ, Xie, J. Bonding of composite resin to contaminated human enamel and dentin. J Prosthodont. 1995;4:28.
Powers, JM, O’Keefe, KL, Pinzon, LM. Factors affecting in vitro bond strength of bonding agents to human dentin. Odontology. 2003;91:1.
Rose, EE, Joginder, L, Williams, NB, et al. The screening of materials for adhesion to human tooth structure. J Dent Res. 1955;34:577.
Sano, H, Ciucchi, B, Matthews, WG, et al. Tensile properties of mineralized and demineralized human and bovine dentin. J Dent Res. 1994;73:1205.
Sano, H, Takatsu, T, Ciucchi, B, et al. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995;20:18.
Sano, H, Yoshiyama, M, Ebisu, S, et al. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Oper Dent. 1995;20:160.
Silverstone, LM, Saxton, CA, Dogon, IL, et al. Variation in the pattern of acid etching of human dental enamel examined by scanning electron microscopy. Caries Res. 1975;9:373.
Tate, WH, You, C, Powers, JM. Bond strength of compomers to dentin using acidic primers. Am J Dent. 1999;12:235.
Tate, WH, You, C, Powers, JM. Bond strength of compomers to human enamel. Oper Dent. 2000;25:283.
Trajtenberg, CP, Pereira, PNR, Powers, JM. Resin bond strength and micromorphology of human teeth prepared with an Erbium:YAG laser. Trans Acad Dent Mater. 2001;15:171.
van Meerbeek, B, Dhem, A, Goret-Nicaise, M, et al. Comparative SEM and TEM examination of the ultrastructure of the resin-dentin interdiffusion zone. J Dent Res. 1993;72:495.
Xie, J, Flaitz, CM, Hicks, MJ, et al. In-vitro bond strength of composite to sound dentin and artificial carious lesions in dentin. Am J Dent. 1996;9:31.
Xie, J, Powers, JM, McGuckin, RS. In vitro bond strength of two adhesives to enamel and dentin under normal and contaminated conditions. Dent Mater. 1993;9:295.
Yoshii, E. Cytotoxic effects of acrylates and methacrylates: relationships of monomer structures and cytotoxicity. J Biomed Mater Res. 1997;37:517.
Crumpler, DC, Bayne, SC, Sockwell, S, et al. Bonding to re-surfaced posterior composites. Dent Mater. 1989;5:417.
DeSchepper, EJ, Cailletea, JG, Roeder, L, et al. In vitro tensile bond strengths of amalgam to treated dentin. J Esthet Dent. 1991;3:117.
Hansson, O, Moberg, LE. Evaluation of three silicoating methods for resin-bonded prostheses. Scand J Dent Res. 1993;101:243.
Hero, H, Ruyter, IE, Waarli, ML, et al. Adhesion of resins to Ag-Pd alloys by means of the silicoating technique. J Dent Res. 1987;66:1380.
Hummel, SK, Pace, LL, Marker, VA. A comparison of two silicoating techniques. J Prosthodont. 1994;3:108.
Masil, R, Tiller, HJ. The adhesion of dental resin to metal surfaces: the Kulzer Silicoater technique. Wehrbeim: Kulzer and Co, 1984;9.
Mazurat, RD, Pesun, S. Resin-metal bonding systems: a review of the Silicoating and Kevloc systems. J Can Dent Assoc. 1998;64:503.
Miller, BH, Nakajima, H, Powers, JM, et al. Bond strength between cements and metals used for endodontic posts. Dent Mater. 1998;14:312.
Mukai, M, Fukui, H, Hasegawa, J. Relationship between sandblasting and composite resin-alloy bond strength by a silica coating. J Prosthet Dent. 1995;74:151.
NaBadalung, DP, Powers, JM, Connelly, ME. Comparison of bond strengths of three denture base resins to treated nickel-chromium-beryllium alloy. J Prosthet Dent. 1998;80:354.
O’Keefe, KL, Miller, BH, Powers, JM. In vitro tensile bond strength of adhesive cements to new post materials. Int J Prosthodont. 2000;13:47.
Pesun, S, Mazurat, RD. Bond strength of acrylic resin to cobalt-chromium alloy treated with the Silicoater MD and Kevloc systems. J Can Dent Assoc. 1998;64:798.
Ramos, JC, Perdigão, J. Shear bond strengths and SEM morphology of dentin-amalgam adhesives. Am J Dent. 1997;10:152.
Roulet, JF, Soderholm, KJ, Longmate, J. Effects of treatment and storage conditions on ceramic/composite bond strength. J Dent Res. 1995;74:381.
Schneider, W, Powers, JM, Pierpont, HP. Bond strength of composites to etched and silica-coated porcelain fusing alloys. Dent Mater. 1992;8:211.
Shahverdi, S, Canay, S, Sahin, E, et al. Effects of different surface treatment methods on the bond strength of composite resin to porcelain. J Oral Rehabil. 1998;25:699.
Stokes, AN, Tay, WM, Pereira, BP. Shear bond of resin cement to post-cured hybrid composites. Dent Mater. 1993;9:370.
Sturdevant, JR, Swift, EJ, Jr., Bayne, SC. Cement bond strength to millable composite for CAD/CAM restorations. J Dent Res. 2000;79:453.
Suliman, AH, Swift, EJ, Jr., Perdigão, J. Effects of surface treatment and bonding agents on bond strength of composite resin to porcelain. J Prosthet Dent. 1993;70:118.
Tate, WH, Friedl, K-H, Powers, JM. Bond strength of composites to hybrid ionomers. Oper Dent. 1996;21:147.
Thompson, VP, Del Castillo, E, Livaditis, GJ. Resin-bonded retainers. Part I: resin bond to electrolytically etched nonprecious alloys. J Prosthet Dent. 1983;50:771.
Trajtenberg, CP, Powers, JM. Bond strengths of repaired laboratory composites using three surface treatments and three primers. Am J Dent. 2004;17:123.
Trajtenberg, CP, Powers, JM. Effect of hydrofluoric acid on repair bond strength of a laboratory composite. Am J Dent. 2004;17:173.
Watanabe, I, Kurtz, KS, Kabcenell, JL, et al. Effect of sandblasting and silicoating on bond strength of polymer-glass composite to cast titanium. J Prosthet Dent. 1999;82:462.
Wolf, DM, Powers, JM, O’Keefe, KL. Bond strength of composite to etched and sandblasted porcelain. Am J Dent. 1993;6:155.