Resin Composite Restorative Materials
The concept of a composite biomaterial, introduced in Chapter 4, can be described as a solid that contains two or more distinct constituent materials or phases when considered at greater than an atomic scale. In these materials, mechanical properties such as elastic modulus are significantly altered in comparison with a homogenous material consisting of either of the phases alone. Enamel, dentin, bone, and reinforced polymers are considered composites, but alloys such as brass are not. The ability to change properties of the macro scale object based on control of the individual constituents is a large advantage for use of composite materials. In dentistry, the term “resin composite” generally refers to a reinforced polymer used for restoring enamel and dentin. The proper materials science term is “polymer matrix composite” or for those composites with filler particles often used as direct-placed restorative composites, “particulate-reinforced polymer matrix composite.” In this chapter, the term “resin composite” will refer to the reinforced polymer matrix materials used as restorative materials. It is important to remember, however, that most biological materials including enamel, dentin, bone, connective tissue, muscle, and even cells are classified as composites within the broad range of biological engineering materials.
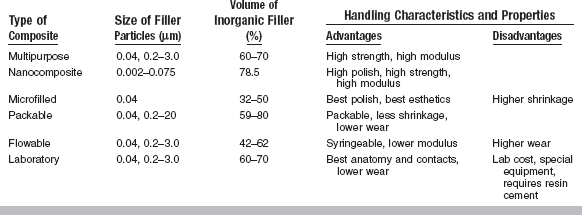
Resin composites are used to replace missing tooth structure and modify tooth color and contour, thus enhancing facial esthetics. A number of resin composites are commercially available for various applications. Some are optimized for esthetics and others are designed for higher stress bearing areas. Dental composites are generally classified by the particle size and percentage distribution of the inorganic filler, and may contain fine irregularly shaped particles from 0.4 to 3 μm in diameter, microfine particles having a diameter of 0.04 to 0.2 μm, nanometer-scale particles and clusters, or blends (microhybrids) of these particles sizes. Various types of composites are discussed in this chapter including those designed to be directly placed and indirectly or laboratory fabricated, core composites, provisional composites, and compomers. Direct filling materials such as glass and hybrid ionomers, used primarily because of their fluoride release, are discussed in Chapter 8. Light-activation methods are also discussed in this chapter, because most composites and compomers contain light-sensitive components that are used to initiate polymerization.
Silicates, introduced in 1878, were the first polymeric direct restorative materials followed by acrylic resins, then resin composites. Silicates were based on a cement-forming reaction between alumina-silica glass powder and phosphoric acid–based liquid. Although silicates provided an anticariogenic feature, their use subsided in the 1960s because of early clinical failure, which was most frequently related to dissolution in oral fluids, loss of translucency, surface crazing, and a lack of adequate mechanical properties. Acrylic restorative resins were unfilled, low-molecular-weight polymers and lacked the reinforcement provided by the ceramic filler particles used in composites. Early clinical failure of acrylics was related directly to dimensional instability, resulting in unsightly stains and recurrent caries.
The development of composites about 1960 resulted in higher mechanical properties, lower thermal coefficient of expansion, lower dimensional change on setting, and higher resistance to wear, thereby improving clinical performance. Early composites were chemically activated followed by photo-activated composites initiated with ultraviolet (UV) wavelengths. These were later replaced by composites activated in the visible wavelengths. Continued development of bonding agents for bonding composites to tooth structure (see Chapter 10) has also improved the longevity and performance of composite restorations.
A classification of preparation type and recommended composite category is listed in Table 9-1. Characteristics of these composite categories are summarized in Table 9-2.
TABLE 9-1
Types of Restorations and Recommended Resin Composites
| Type of Restoration | Recommended Resin Composite |
| Class 1 | Multipurpose, nanocomposite, packable microfilled (posterior),* compomer (posterior)* |
| Class 2 | Multipurpose, nanocomposite, packable, laboratory, microfilled (posterior),* compomer (posterior)* |
| Class 3 | Multipurpose, nanocomposite, microfilled, compomer |
| Class 4 | Multipurpose, nanocomposite |
| Class 5 | Multipurpose, nanocomposite, microfilled, compomer |
| Class 6 (MOD) | Packable, nanocomposite |
| Cervical lesions | Flowable, compomer |
| Pediatric restorations | Flowable, compomer |
| 3-unit bridge or crown | Laboratory (with fiber reinforcement) |
| Alloy substructure | Laboratory (bonded) |
| Core build-up | Core |
| Temporary restoration | Provisional |
| High caries-risk patients | Glass ionomers, hybrid ionomers (see Chapter 8) |
*Special microfilled composites and compomers are available for posterior use.
MULTIPURPOSE COMPOSITES
Overview
A resin composite is composed of four major components: organic polymer matrix, inorganic filler particles, coupling agent, and the initiator-accelerator system. The organic polymer matrix in most commercial composites today is either an aromatic or urethane diacrylate oligomer. Oligomers are viscous liquids, the viscosity of which is reduced to a useful clinical level by the addition of a diluent monomer.
The dispersed inorganic particles may consist of several inorganic materials such as glass or quartz (fine particles), colloidal silica (microfine particles), or zirconia-silica nanoclusters and silica nanoparticles. Two-dimensional diagrams of fine and microfine particles surrounded by polymer matrix are shown in Fig. 9-1.
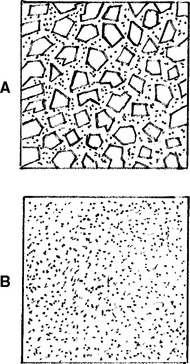
FIGURE 9-1 Two-dimensional diagrams of composites with, A, fine and, B, microfine particles. (From Craig RG, Powers JM, Wataha JC: Dental materials: properties and manipulation, ed 8, St. Louis, 2004, Mosby.)
The coupling agent, an organosilane (silane), is applied to the inorganic particles by the manufacturer before being mixed with the unreacted oligomer. The silane contains functional groups (such as methoxy), which hydrolyze and react with the inorganic filler, as well as unsaturated organic groups that react with the oligomer during polymerization. Silanes are called coupling agents, because they form a bond between the inorganic and organic phases of the composite.
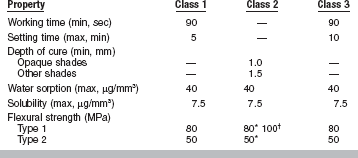
Composites are formulated to contain accelerators and initiators that allow self-curing (chemically activated), light curing (light activated), and dual curing (chemically and light activated). ISO 4049 for polymer-based filling, restorative, and luting materials (ANSI/ADA No. 27) describes two types and three classes of composites, as shown by the following:
Oligomers
The two most common oligomers that have been used in dental composites are dimethacrylates (Bis-GMA) 2,2-bis [4(2-Hydroxy-3-methacryloxy-propyloxy)-phenyl] propane and urethane dimethacrylate (UDMA). These oligomers are described in Chapter 7. Both contain reactive carbon double bonds at each end that can undergo addition polymerization. A few products use both Bis-GMA and UDMA oligomers.
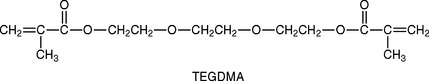
The viscosity of the oligomers, especially Bis-GMA, is so high that diluents must be added, so a clinical consistency can be reached when they are compounded with the filler. Low-molecular-weight compounds with difunctional carbon double bonds, usually triethylene glycol dimethacrylate (TEGDMA), shown below, are added by the manufacturer to reduce and control the viscosity of the compounded composite.
New monomers such as siloranes are being developed to reduce shrinkage and internal stress build-up resulting from polymerization in an effort to improve clinical durability.
Fillers
A helpful method of classifying dental composites is by the particle size, shape, and distribution of filler. Early composites contained large (20 to 30 μm) spherical particles, followed by products containing large irregularly shaped particles, microfine particles (0.04 to 0.2 μm), fine particles (0.4 to 3 μm), and finally blends (microhybrids) containing mostly fine particles with some microfine particles. Based on the type of filler particles, composites are currently classified as microhybrid and microfilled products. Recently, nanofabrication technologies have been used to produce nanosized fillers based on molecular scale building blocks. This process has produced a commercial product with 2- to 20-nm silica nanoparticles and 0.6-μm zirconia-silica nanoclusters.
Microhybrid composites contain irregularly shaped glass (borosilicate glass; lithium or barium aluminum silicate; strontium or zinc glass), quartz, or zirconia particles of fairly uniform diameter. Typically, composites have a distribution of two or more sizes of fine particles plus microfine filler (5% to 15%). This distribution permits more efficient packing, whereby the smaller particles fill the spaces between the larger particles. Microhybrid composites may contain 60% to 70% filler by volume, which, depending on the density of the filler, translates into 77% to 84% by weight in the composite. Most manufacturers report filler concentration in weight percent (wt %). A micrograph of a typical, fine glass filler is shown in Fig. 9-2, A.
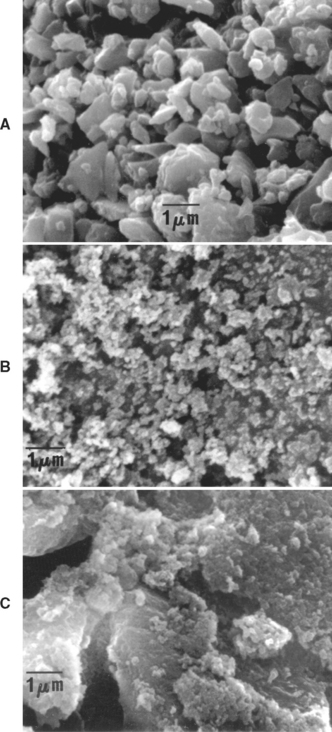
FIGURE 9-2 Scanning electron micrographs of types of filler. A, Fine inorganic filler; B, microfine silica filler; C, microfine silica in organic polymer filler.
Microfilled composites contain silica with a very high surface area (100 to 300 m2/g) having particle diameters of 0.04 to 0.2 μm. Because of the high surface area, only 25% by volume or 38% by weight can be added to the oligomer to keep the consistency of the paste sufficiently low for clinical applications. Fillers consisting of microfine silica in polymerized oligomers are prepared and ground into particles 10 to 20 μm in diameter. These reinforced fillers may be added to the oligomer in concentrations, so the inorganic content can be increased to 32% to 50% by volume or about 50% to 60% by weight. A variation of this modification is used in which most of the filler is reinforced filler, with smaller amounts of silica added to the oligomer. Another modification (homogeneous microfill) has no reinforced filler, but rather microfine silica dispersed in the oligomer. A typical microfine silica filler is shown in Fig. 9-2, B, and a reinforced filler containing microfine silica is shown in Fig. 9-2, C.
Coupling Agents
For a composite to have successful properties, a good bond must form between the inorganic filler and the organic oligomer during setting. Bonding is achieved during processing by the manufacturer, who treats the surface of the filler with a coupling agent before mixing it with the oligomer. The most common coupling agents are organic silicon compounds called silanes. A typical silane is shown in the following equation:
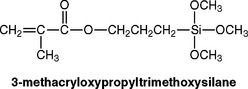
During the deposition of the silane on the filler, the methoxy groups hydrolyze to hydroxy groups that react with adsorbed moisture or —OH groups on the filler. They can also condense with —OH groups on an adjacent hydrolyzed silane to form a homopolymer film on the surface of the filler. During the setting reaction of the oligomer, the carbon double bonds of the silane react with the oligomer, thus forming a bond from the filler through the coupling agent to the polymer matrix (see the following schematic sketch).
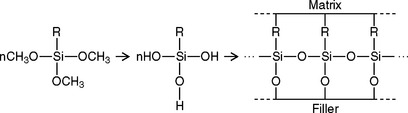
This coupling reaction binds the filler and the oligomer to enable stresses to be transferred from particle to adjacent particle through the lower stiffness polymer matrix. As a result, the strength of the composite is intermediate to that of the filler and the polymer separately. This bond degrades when water is absorbed by the composite intraorally.
Initiators and Accelerators
Composites are light activated or chemically activated, with the former being more common. Light activation is accomplished with blue light at a peak wavelength of about 470 nm, which is absorbed usually by a photo-activator, such as camphorquinone, added to the monomer by the manufacturer in amounts varying from 0.2% to 1.0%. The reaction is accelerated by the presence of an organic amine containing a carbon double bond (see Chapter 7). The amine and the camphorquinone are stable in the presence of the oligomer at room temperature, as long as the composite is not exposed to light. Although camphorquinone is the most common photo-activator, others are sometimes used to accommodate special esthetic considerations. Camphorquinone adds a slight yellow tint to the uncured composite paste. Although the color neutralizes during cure, some clinicians find shade matching difficult with the color shift.
Chemical activation is accomplished at room temperature by an organic amine (catalyst paste) reacting with an organic peroxide (universal paste) to produce free radicals, which in turn attack the carbon double bonds, causing polymerization. Once the two pastes are mixed, the polymerization reaction proceeds rapidly.
Some composites, such as core and provisional products, are dual cured. These formulations contain initiators and accelerators that allow light activation followed by self-curing or self-curing alone.
POLYMERIZATION REACTION
The basic chemistry and general curing reactions for free-radical addition polymerization are presented in Chapter 7. The polymerization reaction of self-cured composites is chemically initiated with a peroxide initiator and an amine accelerator. Polymerization of light-cured composites is initiated by visible blue light. Dual-cured products use a combination of chemical and light activation to carry out the polymerization reaction.
The polymerized resin is highly cross-linked because of the presence of the difunctional carbon double bonds. The degree of polymerization varies, depending on whether it is in the bulk or in the air-inhibited layer of the restoration. The polymerization of light-cured composites varies according to the distance from the composite to the light and the duration of light exposure. The percentage of the double bonds that react may vary from 35% to 80%. The degree of polymerization is higher for laboratory composites that are post-cured at elevated temperatures and light intensities.
PACKAGING OF COMPOSITES
These composites are supplied in various shades in spills, syringes, and compules, examples of which are shown in Fig. 9-3. The syringes are made of opaque plastic to protect the material from exposure to light and thus provide adequate shelf life. If packaged as a compule, the compule is placed on the end of a syringe, and the paste is extruded after removal of the protective tip. The advantages of compules are ease of placement of the composite paste, decrease in cross-infection, and protection of the paste from exposure to ambient light.
Self-Cured and Dual-Cured Composites
Self- and dual-cured composites are typically packaged in syringes or tubs of paste and catalyst and require mixing. An example of a two-paste core composite is shown in Fig. 9-4.
PROPERTIES OF COMPOSITES
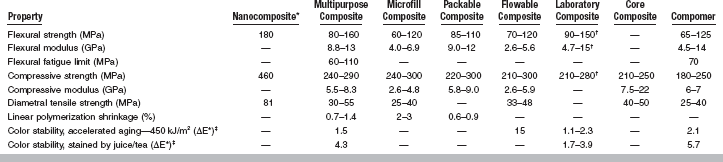
Important properties of composites include working and setting times, polymerization shrinkage, thermal properties, water sorption and solubility, mechan-ical properties, color stability, and radiopacity. Selected properties of multipurpose, microfilled, packable, flowable, laboratory, and core composites plus compomers are listed in Table 9-3. Values of properties for polymer-based filling and restorative materials based on ISO 4049 (ANSI/ADA No. 27) are summarized in Table 9-4.
PHYSICAL PROPERTIES
For light-cured composites, curing is considered to be “on demand.” Polymerization is initiated when the composite is first exposed to light. Stiffening takes place within seconds after light exposure by a high-intensity curing light source. Although the composite restoration appears hard and fully cured after exposure to the curing light source, the curing reaction continues for a period of 24 hours. Not all of the available unsaturated carbon double bonds react; studies report that about 25% remain unreacted in the bulk of the restoration. If the surface of the restoration is not protected from air by a transparent matrix, polymerization is inhibited; the number of unreacted carbon double bonds may be as high as 75% in the tacky surface layer. Although the restoration can be finished with abrasives and is functional after 10 minutes, optimum physical properties are not reached until about 24 hours after the reaction is initiated.
For most composites that are initiated by visible light, bright operatory lights can initiate cure if the composite is left unprotected on a mixing pad. Within 60 to 90 seconds after exposure to ambient light in an operatory, the surface of the composite may lose its capability to flow readily against tooth structure, and further work with the material becomes difficult. The dispensed paste can be covered with an opaque or orange cover to present premature exposure to light.
The setting times for chemically activated composites range from 3 to 5 minutes. These short setting times have been accomplished by controlling the concentration of initiator and accelerator.
Polymerization Shrinkage
The linear polymerization shrinkage of typical composites is listed in Table 9-3. Free volumetric polymerization shrinkage is a direct function of the amount of oligomer and diluent, and thus microhybrid composites shrink only 0.6% to 1.4%, compared with shrinkage of microfilled composites of 2% to 3%. This shrinkage creates polymerization stresses as high as 13 MPa between the composite and tooth structure. These stresses severely strain the interfacial bond between the composite and the tooth, leading to a very small gap that can allow marginal leakage of saliva. This stress can exceed the tensile strength of enamel and result in stress cracking and enamel fractures along the interfaces. The potential for this type of failure is even greater with microfilled composites, in which there is a much higher volume percent of polymer present, and polymerization shrinkage is greater. Because the development of shrinkage stress depends on the volumetric shrinkage strain and the stiffness of the composite at the time of shrinkage, low-shrinkage composites might exhibit high stress if the composite has a high filler fraction and correspondingly high elastic modulus. Adding the composite in 2-mm increments and polymerizing each increment independently can reduce the net effect of polymerization shrinkage. Net shrinkage is less because a smaller volume of composite is allowed to shrink before successive additions.
Thermal Properties
The linear coefficient of thermal expansion (α) of composites ranges from 25 to 38 × 10−6/° C for composites with fine particles to 55 to 68 × 10−6/° C for composites with microfine particles. The α values for composites are less than the mean of its constituents added together; however, the values are higher than those for dentin (8.3 × 10−6/° C) and enamel (11.4 × 10−6/° C). The higher values for the microfilled composites are related mostly to the greater amount of polymer present. Certain glasses may be more effective in reducing the effect of thermal change than are others, and some resins have more than one type of filler to compensate for differential rates.
Thermal stresses place an additional strain on the bond to tooth structure, which adds to the detrimental effect of the polymerization shrinkage. Thermal changes are also cyclic in nature, and although the entire restoration may never reach thermal equilibrium during the application of either hot or cold stimuli, the cyclic effect can lead to material fatigue and early bond failure. If a gap forms, the difference between the thermal coefficient of expansion of composites and teeth could permit percolation of oral fluids.
The thermal conductivity of composites with fine particles (25 to 30 × 10−4 cal/sec/cm2 [° C/cm]) is greater than that of composites with microfine particles (12 to 15 × 10−4 cal/sec/cm2 [° C/cm]) because of the higher conductivity of the inorganic fillers compared with the polymer matrix. However, for highly transient temperatures the composites do not change temperature as fast as tooth structure and this difference does not present a clinical problem.
Water Sorption
The water sorption of composites with hybrid particles (5 to 17 μg/mm3) is lower than that of composites with microfine particles (26 to 30 μg/mm3) because of the lower volume fraction of polymer in the composites with fine particles. The quality and stability of the silane coupling agent are important in minimizing the deterioration of the bond between the filler and polymer and the amount of water sorption. Expansion associated with the uptake of water from oral fluids could relieve some polymerization stress, but water sorption is a slow process when compared to polymerization shrinkage and stress development. In the measurement of hygroscopic expansion starting 15 minutes after the initial polymerization, most resins required 7 days to reach equilibrium and about 4 days to show the majority of expansion. Because composites with fine particles have lower values of water sorption than composites with microfine particles, they exhibit less expansion when exposed to water.
Solubility
The water solubility of composites varies from 0.25 to 2.5 mg/mm3. Inadequate light intensity and duration can result in insufficient polymerization, particularly at greater depths from the surface. Inadequately polymerized composites have greater water sorption and solubility, possibly manifested clinically with early color instability.
During the storage of microhybrid composites in water, the leaching of inorganic ions can be detected; such ions are associated with a breakdown in interfacial bonding. Silicon leaches in the greatest quantity (15 to 17 μg/mL) during the first 30 days of storage in water and decreases with time of exposure. Microfilled composites leach silicon more slowly and show a 100% increase in amount during the second 30-day period (14.2 μg/mL). Boron, barium, and strontium, which are present in glass fillers, are leached to various degrees (6 to 19 μg/mL) from the various resin-filler systems. Breakdown and leakage can be a contributing factor to the reduced resistance to wear and abrasion of composites.
Color and Color Stability
The color and blending of shades for the clinical match of esthetic restorations are important. The characteristics of color are discussed in Chapter 3, and these principles can be applied specifically to composites for determining appropriate shades for clinical use. Universal shades vary in color among currently marketed products.
Change of color and loss of shade match with surrounding tooth structure are reasons for replacing restorations. Stress cracks within the polymer matrix and partial debonding of the filler to the resin as a result of hydrolysis tend to increase opacity and alter appearance. Discoloration can also occur by oxidation and result from water exchange within the polymer matrix and its interaction with unreacted polymer sites and unused initiator or accelerator.
Color stability of current composites has been studied by artificial aging in a weathering chamber (exposure to UV light and elevated temperatures of 70° C) and by immersion in various stains (coffee/tea, cranberry/grape juice, red wine, sesame oil). As shown in Table 9-3, composites are resistant to color changes caused by oxidation but are susceptible to staining.
MECHANICAL PROPERTIES
Values of compressive and flexural strength and modulus for composites are listed in Table 9-3. The flexural and compressive strengths of the various composites are similar. The flexural and compressive moduli of microfilled and flowable composites are about 50% lower than values for multipurpose hybrids and packable composites, which reflects the lower volume percent filler present in the microfilled and flowable composites (see Table 9-2). For comparison, the modulus of elasticity in compression is about 62 GPa for amalgam, 19 GPa for dentin, and 83 GPa for enamel.
Knoop Hardness
Knoop hardness for composites (22 to 80 kg/mm2) is lower than enamel (343 kg/mm2) or dental amalgam (110 kg/mm2). The Knoop hardness of composites with fine particles is somewhat greater than values for composites with microfine particles because of the hardness and volume fraction of the filler particles. These values indicate a moderate resistance to indentation under functional stresses for more highly filled composites, but this difference does not appear to be a major factor in resisting functional wear.
A microhardness measurement such as Knoop can be misleading on composites with large filler particles (>10 μm in diameter), in which the small indentation could be made solely on the organic or the inorganic phase. However, with most current products, filler particle sizes are much smaller (<1 μm), and the microhardness values appear more reliable.
Bond Strength to Dental Substrates
Bonding of composites to tooth structure and other dental substrates is discussed in detail in Chapter 10.
Enamel and Dentin
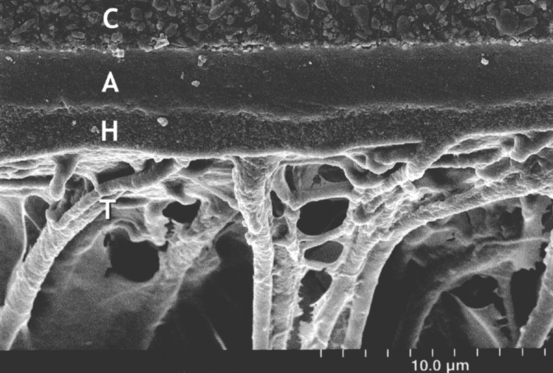
The bond strength of composites to etched enamel and primed dentin is typically between 20 and 30 MPa. Bonding is principally a result of micromechanical retention of the bonding agent into the etched surfaces of enamel and primed dentin. In dentin, a hybrid layer of bonding resin and collagen is often formed, and the bonding adhesive penetrates the dentinal tubules (Fig. 9-5).
Other Substrates
Composite can be bonded to existing composite restorations, ceramics, and alloys when the substrate is roughened and appropriately primed (see Chapter 10). In general, the surface to be bonded is sandblasted (microetched) with 50-μm alumina and then treated with a resin-silane primer for composite, a silane primer for ceramic, or a special alloy primer. Bond strengths to treated surfaces are typically greater than 20 MPa.
CLINICAL PROPERTIES
Clinical requirements for composites accepted for unrestricted use, including cuspal replacement in posterior teeth, as defined by American Dental Association (Proposed) Guidelines for Resin-based Composites for Posterior Restorations, are listed in Table 9-5.
TABLE 9-5
Clinical Requirements for Resin Composites Accepted for Unrestricted Use, Including Cuspal Replacement, in Posterior Teeth
| Property | Criteria |
| Maintenance of color (18 mo) | No more than 10% charlie |
| Marginal discoloration (18 mo) | No more than 10% charlie |
| Marginal integrity (18 mo) | No more than 5% charlie |
| Caries—recurrent or marginal (18 mo) | No more than 5% charlie |
| Maintenance of interproximal contact (18 mo) | 95% showing no observable broadening of contacts |
| Postoperative sensitivity | Thorough history of adverse sensitivity to hot, cold, and biting stimuli |
| Failure (18 mo) | No more than 5% |
| Wear between 6 and 18 mo | No more than 50 μm |
*Proposed American Dental Association guidelines for resin-based composites for posterior restorations.
Depth of Cure (Light-Cured Composites)
Light intensity decreases as the light source is moved away from the surface of an object and as the light travels through a scattering medium. The depth of light penetration into a composite restoration depends on the wavelength of light, its irradiance, and the scattering that takes place within the restoration. A number of factors influence the degree of polymerization at given depths from the surface after light curing. The concentration of photo-initiator or light absorber in the composite must be such that it will react at the proper wavelength and be present in sufficient concentration. Both filler content and particle size are critical to dispersion of the light beam. For this reason, microfilled composites with smaller and more numerous particles scatter more light than microhybrid composites with larger and fewer glass particles. Longer exposure times are needed to obtain adequate polymerization of microfilled composites.
Light intensity at the surface is a critical factor in completeness of cure at the surface and within the material. The tip of the light source should be held within 1 mm of the surface to provide optimum exposure. More opaque shades reduce light transmission and cure only to minimal depths (1 mm). A standard exposure time using most visible lights is 20 seconds. In general, this is sufficient to cure a light shade of resin to a depth of 2 or 2.5 mm, assuming that the light guide is immediately adjacent to the restoration surface. The anatomy of the tooth often precludes the positioning of the light guide close to the restoration surface. A 40-second exposure improves the degree of cure at all depths, and is required to obtain sufficient cure with the darker shades. Because the light beam is partially collimated and does not spread sufficiently beyond the diameter of the tip at the emitting surface, it is necessary to “step” the light across the surface of large restorations so the entire surface receives a complete exposure. Larger tips have been manufactured for placement on most light-curing units. However, as the light beam is distributed over a larger surface area, the intensity at a given point is reduced. A longer exposure time of up to 60 seconds should be used when larger emitting tips are used.
To evaluate the effective depth of cure of a specific light-curing unit, a small 5 to 10 mm section is cut from a clear straw and placed on a glass slide. The section is loaded with composite and exposed to light from top surface for 20 seconds. The straw is removed and the uncured composite at the based of the cylinder is scraped with a sharp knife. The length of the remaining specimen is measured. Effective depth of cure is estimated by dividing the length by two (ISO 4049).
Radiopacity
It is very difficult to locate enamel-composite margins radiographically because of the relatively low radiopacity of composites. Modern composites include glasses having atoms with high atomic numbers, such as barium, strontium, and zirconium. Some fillers, such as quartz, lithium-aluminum glasses, and silica, are not radiopaque and must be blended with other fillers to produce a radiopaque composite. Even at their highest volume fraction of filler, the amount of radiopacity seen in composites is noticeably less than that exhibited by a metallic restorative like amalgam. The microhybrid composites achieve some radiopacity by incorporating very finely divided heavy-metal glass particles.
Aluminum is used as a standard reference for radiopacity. A 2-mm thickness of dentin is equivalent in radiopacity to 2.5 mm of aluminum, and enamel is equivalent to 4 mm of aluminum. To be effective, a composite should exceed the radiopacity of enamel, but international standards accept radiopacity equivalent to 2 mm of aluminum. Amalgam has a radiopacity greater than 10 mm of aluminum, which exceeds all the composite materials available.
Wear Rates
Clinical studies have shown that composites are ideal for anterior restorations in which esthetics is essential and occlusal forces are low. Wear rates are a larger concern in the posterior segments where occlusal forces and lateral excursive contacts are higher than in the anterior segment. Although earlier generations of composites exhibited attrition and abrasion wear, newer formulations minimize the problem. Marginal degradation is still evident and is attributed to improper preparation design, inadequate adhesion, polymerization contraction of the composite, and marginal microcracks. Marginal degradation and stain are sometimes interpreted as recurrent caries, although this is not always the case. Currently accepted composites for posterior applications require clinical studies that demonstrate, over an 18-month period, a loss of surface contour less than 50 μm.
Biocompatibility
Details about the biocompatibility of composites are discussed in Chapter 5, but some of the central issues are discussed here. Nearly all of the major components of composites (Bis-GMA, TEGDMA, and UDMA, among others) are cytotoxic in vitro if used in pure form, but the biological liability of a cured composite depends on the release of these components from the composite. Although composites release some levels of components for weeks after curing, there is considerable controversy about the biological effects of these components. The amount of release depends on the type of composite and the method and efficiency of the cure of the composite. A dentin barrier markedly reduces the ability of components to reach pulpal tissues, but these components can traverse dentin barriers, albeit at reduced concentrations. The effects of low-dose, long-term exposures of cells to resin components are not generally known. On the other hand, the use of composite materials as direct pulp-capping agents poses a higher risk for adverse biological responses, because no dentin barrier exists to limit exposure of the pulp to the released components.
The effects of released components from composites on oral or other tissues are not known with certainty, although no studies have documented any adverse biological effects. The tissue at highest risk from this type of release would appear to be the mucosa in close, long-term contact with composites. Components of composites are known allergens, and there has been some documentation of contact allergy to composites. Most of these reactions occur with dentists or dental personnel who regularly handle uncured composite and, therefore, have the greatest exposure. There are no good studies documenting the frequency of allergy to composites in the general population.
Finally, there has been some controversy about the ability of components of composites to act as xenoestrogens. Studies have proved that bisphenol A and its dimethacrylate are estrogenic in in vitro tests that measure this effect using breast cancer cell growth. Trace levels of these components have been identified in some commercial composites; however, estrogencity from cured commercial composites has not been demonstrated. Furthermore, there is considerable controversy about the accuracy and utility of in vitro tests using breast cancer cells to measure a true estrogenic effect. An early study in this area, which claimed that dental sealants and composites were estrogenic in children, has since been discredited.
MANIPULATION OF COMPOSITES
Because composite pastes are sticky, they are easily contaminated with debris and moisture. For best results, a rubber dam should be used when placing composite restorations to isolate the tooth and simplify the control of moisture and debris contamination.
PULPAL PROTECTION
If a deep cavity exists after preparation, the pulp should be protected with a glass ionomer or hybrid ionomer. Liners and bases are described in Chapter 20.
ETCHING AND BONDING
To provide a bond between composite and tooth structure, the enamel and dentin surfaces are etched with phosphoric acid or an acidic primer. Application of the bonding agent will penetrate the prepared enamel and dentin surfaces and provide micromechanical retention of the restoration. Bonding agents and their interactions with tooth structure are discussed more fully in Chapter 10.
DISPENSING
Only the mass of composite needed to fill the cavity preparation should be dispensed onto a paper pad. Most composite syringes use a screw-driven plunger. Because composites are viscoelastic the composite will continue to flow out of the syringe after the plunger is twisted. The plunger should be reversed when sufficient composite has been extruded, to prevent further flow. The cap should be cleaned of excess composite so the syringe is ready for the next use.
Self- and Dual-Cured Composites
An example of a self-cured composite for core fabrication supplied as two pastes is shown in Fig. 9-4. One syringe contains the peroxide initiator or catalyst and the other syringe includes the amine accelerator. Mix equal amounts of universal and catalyst pastes thoroughly for 20 to 30 seconds. Plastic or coated metal spatulas should be used because the inorganic filler particles in the composite are abrasive. Uncoated metal spatulas can be abraded by the filler particles and result in discoloration of the composite.
PLACEMENT
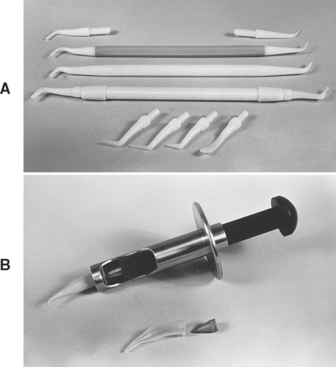
The composite can be placed into the cavity preparation by several methods. A plastic or coated metal instrument, such as one of those shown in Fig. 9-6, A, will make placement easier because the composite will not stick to the instrument as readily. It is not recommended to dip or otherwise coat the instrument with alcohol or unfilled resin. The solvent might mix with the composite and cause deterioration of the mechanical properties. The composite may also be placed in the plastic tip of a syringe, such as shown in Fig. 9-6, B, and then injected into the cavity preparation. Some composites are available prepackaged in syringe tips known as compules. The syringe or compule reduces the problem of incorporating voids in the composite during insertion and facilitates precise placement of the material. Heating units are available that raise the temperature of the composite slightly above room temperature to improve flow and adaptation.
POLYMERIZATION
Exposure times for polymerization vary depending on the type of light-curing unit and the type, depth, and shade of the composite. Times may vary from 20 to 60 seconds for a 2-mm-thick restoration. Microfilled composites require longer exposure than microhybrid composites because the small filler particles scatter more light. Darker shades or more opaque composites require longer exposure times (up to 60 seconds longer) than lighter shades or more translucent composites. In deep restorations, the composite should be placed and cured in 2-mm-thick layers. One layer bonds to another without any loss of strength as long as the previous layer has not been contaminated. The surface of the composite will appear glossy immediately after cure because of air inhibition of the surface.
The curing time of light-activated composite and the depth of cure within a given mass depend on the intensity, wavelength, and penetration of the light. A material with a low absorption coefficient cures to the greatest depth. The presence of UV absorbers for color stabilization, fluorescent dyes for esthetics, or excessive initiator concentration has a detrimental effect on extent of cure.
Self- and Dual-Cured Composites
After mixing, the self-cured composite has a working (or insertion) time of 1 to 1½ minutes. The mix will begin to harden, and the material should not be disturbed until the setting time of about 4 to 5 minutes from the start of the mix. Chemically activated composites cure at a much slower rate than their light-activated, cure-on-demand counterparts. It is thought that this slow curing process allows for shrinkage stresses to relax and be compensated by flow. Chemically activated composites can be placed in one increment. Some clinicians choose to place chemically activated composites at the gingival margin of the proximal areas of the restoration to minimize the effect of polymerization shrinkage at the critical, gingival regions. This is followed by placement of a light-activated composite in the remaining regions of the restoration.
Dual-cured composites contain chemical accelerators and light activators, so polymerization can be initiated by light and then continued by the self-cured mechanism. The dual-activation mechanisms are necessary when parts of the composite are not accessible to light such as under opaque restorations.
FINISHING AND POLISHING
Finishing should be done carefully in a wet field with a water-soluble lubricant to prevent damage to the newly formed enamel/dentin/composite bond and composite restoration. For gross reduction, diamonds, carbide finishing burs, finishing disks, or strips of alumina can be used. For final finishing of either microhybrid or microfilled composites, abrasive-impregnated rubber rotary instruments or a rubber cup with various polishing pastes are helpful.
Polishing is the final step of finishing and is usually performed with aluminum oxide or micro-diamond abrasives with progressively finer grit sizes. Polishing of composites is important, because a smooth surface is desired to prevent retention of plaque and is needed to maintain good oral hygiene.
A measure of the quality of polishing is surface roughness. A comparison of surface roughness of various composites is listed in Table 9-6. The smoothest surface is achieved by use of a Mylar matrix. Carbide burs produce smoother surfaces than diamond burs, but after polishing the surface roughness is similar.
TABLE 9-6
Surface Roughness (μm) of Various Types of Resin Composites
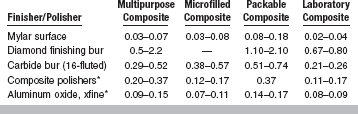
*After finishing with 16-fluted carbide bur.
Glazes and coatings are available for composite restorations. These are resins with small amounts of filler particles. They are designed to fill small voids and defects on the surface of the restoration and at the enamel margins.
COMPOSITES FOR SPECIAL APPLICATIONS
These composites are recommended for use in Class 3 and Class 5 restorations, in which a high polish and esthetics are most important. One product has been used successfully in posterior restorations. They are composed of light-activated, dimethacrylate resins with 0.04-μm colloidal silica fillers with filler loading of 32% to 50% by volume (see Table 9-2).
Typical properties of microfilled composites are listed in Table 9-3. Because they are less highly filled, microfilled composites exhibit more polymerization shrinkage, water sorption, and thermal expansion than microhybrid composites.
PACKABLE COMPOSITES
“Packable” is a term used for composite pastes that have very high viscosity and low surface tackiness. These materials are not condensable like amalgams, but can be compressed and forced to flow using flat-faced instruments. These composites (see Table 9-1) are recommended for use in Classes 1 and 2 cavity preparations. They are composed of light-activated, dimethacrylate resins with fillers (porous or irregular particles) that have filler loading of 66% to 70% by volume (see Table 9-2). The interaction of the filler particles and modifications of the resin cause these composites to be packable.
Typical properties of packable composites are listed in Table 9-3. Important properties include greater depth of cure, lower polymerization shrinkage, radiopacity, and lower wear rate (3.5 μm/year). Several packable composites are packaged in unit-dose compules. A bulk-fill technique is recommended by manufacturers but has not yet been demonstrated effective in clinical studies.
FLOWABLE COMPOSITES
These light-activated, low-viscosity composites are recommended for cervical lesions, pediatric restorations, and other small, low- or non-stress-bearing restorations (see Table 9-1). They contain dimethacrylate resin and inorganic fillers with a particle size of 0.4 to 3.0 μm and filler loading of 42% to 53% by volume (see Table 9-2).
Typical properties of flowable composites are listed in Table 9-3. Flowable composites have a low modulus of elasticity, which may make them useful in cervical abfraction areas. Because of their lower filler content, they exhibit higher polymerization shrinkage and lower wear resistance than microhybrid composites. The viscosity of these composites allows them to be dispensed by a syringe for easy handling.
LABORATORY COMPOSITES
Crowns, inlays, and veneers bonded to metal copings can be prepared with composites processed in the laboratory (see Table 9-1), using various combinations of light, heat, pressure, and vacuum to increase the degree of polymerization, density, mechanical properties, and wear resistance.
Typical properties of laboratory composites are listed in Table 9-3. For increased strength and rigidity, laboratory composites can be combined with fiber reinforcement. Restorations are usually bonded with resin cements.
CORE COMPOSITES
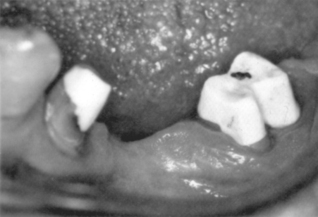
If sufficient tooth structure remains to retain and support a full coverage restoration, but extensive regions of dentin have been lost to disease, the core of the tooth can be restored before final preparation and impression. Amalgam is the most common core material, but composite is becoming popular. Composite core materials are typically two-paste, self-cured composites (see Fig. 9-4), although light-cured and dual-cured products are available. Core composites are usually tinted (blue, white, or opaque) to provide a contrasting color with the tooth structure. Some products release fluoride. An example of a composite core build-up is shown in Fig. 9-7. Typical properties of core composites are listed in Table 9-3.
Composite cores have the following advantages as compared with amalgam: they can be bonded to dentin, can be finished immediately, are easy to contour, and can have a more natural color under ceramic restorations. Composite cores are bonded to remaining enamel and dentin using bonding agents. A bonding agent recommended by the manufacturer of the core material should be used because some self-cured composite core materials are incompatible with some light-cured bonding agents. Retention of the final restoration should not rely on the composite structure alone because adhesion of the composite core to remaining dentin alone is insufficient to resist rotation and dislodgement of the crown.
PROVISIONAL COMPOSITES
Provisional restorations maintain the position of the prepared tooth, seal and insulate the preparation and protect the margins, establish proper vertical dimension, aid in diagnosis and treatment planning, and help to evaluate candidates for esthetic replacements. Provisional inlays, crowns, and fixed partial dentures are usually fabricated from acrylic resins or composites. Provisional restorations fabricated from composites are generally harder, stiffer, and more color stable than those made from acrylics. The properties of acrylic and composite provisional materials are compared in Table 9-7.
TABLE 9-7
Properties of Provisional Composites and Acrylics
| Property | Provisional Composite | Provisional Acrylic |
| Flexural strength (MPa) | 35–70 | 45–80 |
| Flexural modulus (GPa) | 0.8–2.5 | 0.8–2.6 |
| Compressive strength (MPa) | 130–260 | — |
| Linear polymerization shrinkage (%) | 2.5–3.3 | 2.7–7.0 |
| Color stability, accelerated aging—60 kJ/m2 (ΔE)*† | 0.5–9.5 | 2.0–8.0 |
| Color stability, staining (ΔE)*† | 4.9–11 | 1.2–3.6 |
COMPOMERS
Compomers or poly acid–modified composites are used for restorations in low stress–bearing areas, although a recent product is recommended by the manufacturer for Class 1 and Class 2 restorations in adults (see Table 9-1). Compomers are recommended for patients at medium risk of developing caries.
COMPOSITION AND SETTING REACTION
Compomers contain poly acid–modified monomers with fluoride-releasing silicate glasses and are formulated without water. Some compomers have modified monomers that provide additional fluoride release. The volume percent filler ranges from 42% to 67% and the average filler particle size ranges from 0.8 to 5.0 μm. Compomers are packaged as single-paste formulations in compules and syringes, as shown in Fig. 9-8.

FIGURE 9-8 Examples of compomers (packaged in compules and syringes) and a hybrid ionomer (packaged in capsules with applier) for restoring cervically eroded teeth. (From Craig RG, Powers JM, Wataha JC: Dental materials: properties and manipulation, ed 8, St. Louis, 2004, Mosby.)
Setting occurs primarily by light-cured polymerization, but an acid-base reaction also occurs as the compomer absorbs water after placement and upon contact with saliva. Water uptake is also important for fluoride transfer.
PROPERTIES
Typical properties of compomers are listed in Table 9-3. Compomers release fluoride by a mechanism similar to that of glass and hybrid ionomers. Because of the lower amount of glass ionomer present in compomers, the amount of fluoride release and its duration are lower than those of glass and hybrid ionomers. Also, compomers do not recharge from fluoride treatment or brushing with fluoride dentifrices as much as glass and hybrid ionomers.
LIGHT-CURING UNITS
The most common light sources used in dentistry to photoactivate composites is quartz-tungsten-halogen and blue LED (light-emitting diode). Definitions of terms used to describe light sources used to activate dental resins are listed in Table 9-8.
TABLE 9-8
Definitions of Terms Used to Describe Light Sources for Polymerization of Dental Resins
| Term | Unit | Definition |
| Spectral emission | nm | Effective bandwidth of wavelengths emitted by light source |
| Spectral requirement | nm | Bandwidth of wavelengths required to activate photo-initiator(s) of dental resin |
| Flux | mW | Number of photons per second emitted by light source |
| Irradiance or radiant exitance | mW/cm2 | Number of photons per second emitted by light source per unit area of curing tip |
| Energy | J* | Flux × time |
| Energy density | J/cm2 | Radiant exitance × time |
QUARTZ-TUNGSTEN-HALOGEN LIGHT-CURING UNITS
An example of a quartz-tungsten-halogen (QTH) light-curing unit used to activate polymerization of composites is shown in Fig. 9-9. The peak wavelength varies among units from about 450 to 490 nm. Typically, the irradiance ranges from 400 to 800 mW/cm2, but higher-intensity QTH units are available. Some units can be controlled to provide two or three different intensities (step cure) or at a continuously increasing (ramp cure) intensity. A typical 2-mm thick resin composite restoration requires a radiant exposure of 8 J/cm2 (400 mW/cm2 × 20 s = 8000 mW s/cm2) for proper polymerization.
FIGURE 9-9 Visible-light sources for photo-initiation of composition. (From Craig RG, Powers JM, Wataha JC: Dental materials: properties and manipulation, ed 8, St. Louis, 2004, Mosby.)
A QTH light source consists of a broad-spectrum light bulb (typically 75 W), several filters, a reflector, a fan, a power supply, and a light guide. The broad-spectrum output of the QTH bulb is clipped by a blue bandpass filter that only allows a narrow band of wavelengths centered around 470 to 480 nm (blue wavelengths) to be transferred to the light guide. A UV filter blocks passage of UV wavelengths. A dichroic reflector focuses the light on the end of the light guide. The reflector also enables infrared wavelengths to dissipate as heat through the back of the housing. Because substantial heat is generated by the 75 W bulb, a fan is necessary to cool the bulb and assembly.
A decrease in line voltage of 6% shows a corresponding reduction in output of about 25% in intensity in some lamps, but only 10% in lamps with voltage regulators in their circuitry. In general, the output from the various lamps decreases with continuous use and the intensity is not uniform for all areas of the light tip, being greatest at the center. Also, the intensity of the light decreases with distance from the source. Although the intensity is important with respect to the depth of cure, it has been shown for some products that a threefold difference in intensity had only a 15% difference in the depth of cure. Bulb life ranges from 50 to 75 hours.
Although there is minimal potential for radiation damage to surrounding soft tissue inadvertently exposed to visible light, caution should be used to prevent retinal damage to the eyes. Because of the high intensity of the light, the operator should not look directly at the tip or the reflected light from the teeth. A number of devices are marketed to filter the visible-light beam so the operator can directly observe the curing procedure and to protect the patient and staff. These orange-tinted devices are available as eyeglasses, flat shields that can be held over the field of vision, and curved shields that attach directly to the handpiece delivering the light beam (Fig. 9-10).
FIGURE 9-10 Eye protection devices that can be used with a polymerizing visible-light device. Top to bottom: glasses, an instrument shield, and a flat plate.
Some lamps produce considerable heat at the curing tip, which can produce pulpal irritation. Too much heat is being generated if one cannot hold a finger 2 to 3 mm from the tip for 20 seconds.
Maintenance of QTH lights must be provided on a regular basis, as summarized in Table 9-9.
TABLE 9-9
Factors Causing Decrease in Intensity of Light from Quartz-Tungsten-Halogen (QTH) Light-Curing Units and Maintenance Hints
| Factors | Maintenance Hints |
| Dust or deterioration of reflector | Clean or replace reflector |
| Burn-out of bulb filament | Replace bulb |
| Darkening/frosting of bulb | Replace bulb |
| Age of components | Monitor intensity, replace unit |
| Chipping of light tip | Replace light tip |
| Resin deposit on light tip | Clean or replace light tip |
| Change in line voltage | Get built-in voltage regulator |
| Lack of uniformity across light tip | Overlap curing on larger surface |
| Increased distance of tip from material to be cured | Keep light tip close to material |
PLASMA-ARC LIGHT-CURING UNITS
Plasma-arc (PAC) lights are high-intensity light-curing units. Light is obtained from an electrically conductive gas (plasma) that forms between two tungsten electrodes under pressure. The output is filtered to minimize transmission of infrared and UV energy. The energy transmitted is in the visible range between 380 and 500 nm, with an output peak near 480 nm. Because of the high intensity of light available at lower wavelengths, PAC lights are able to photoactivate composites with photo-initiators other than camphorquinone.
Properties of composites cured with PAC lights depend on the composite and light-curing unit. In general, PAC lights produce equal or lower degrees of conversion, depths of cure, and flexural moduli, but flexural strengths equal to QTH light-curing units.
BLUE LIGHT-EMITTING DIODES
Solid-state light-emitting diodes (LEDs) use junctions of doped semiconductors (p-n junctions) based on gallium nitride to emit blue light. The spectral output of blue LEDs falls between 450 and 490 nm, so these units are effective for curing materials with camphorquinone photo-initiators. Early models were not effective in activating photo-initiators with peak absorption below 450 nm. LED units do not require a filter, have a long life span, and do not produce as much heat as QTH devices. Early LED sources exhibited lower intensities than their QTH counterparts, but higher intensities are now seen in devices with larger arrays of LEDs. Heat becomes a concern even with LED sources, when large arrays are used. Because the output spectrum of blue LEDs matches the absorption spectrum of camphorquinone more closely than QTH sources, it is thought that blue LED sources are more efficient. For QTH sources, most of the light energy is discarded because QTH is a broad-spectrum source and only the wavelengths absorbed by camphorquinone are desired. For LED sources, the emission is not filtered. This, however, does not make LED sources more efficient in activating the camphorquinone photo-initiator than QTH. Creation of photons is dependent only on the energy applied in the absorbable wavelengths.
Composites cured with LED units have flexural properties similar to those cured with QTH units. Depth of cure with LED units appears to be higher.
British Dental Association Museum, http://www.bda-dentistry.org.uk/museum/story.cfm?
Wilson, AD, Batchelor, RF. Dental silicate cements. I. The chemistry of erosion. J Dent Res. 1967;46:1075.
Fletcher T: British Patent 3028, 1878, Dental silicate cement.
Fletcher T: German Patent 8202, 1879, Dental silicate cement.
Asmussen, E. Clinical relevance of physical, chemical, and bonding properties of composite resins. Oper Dent. 1985;10:61.
Bayne, SC, Thompson, JY, Swift, EJ, Jr., et al. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567.
Braem, M, Davidson, CL, Lambrechts, P, et al. In vitro flexural fatigue limits of dental composites. J Biomed Mater Res. 1994;28:1397.
Braem, M, Finger, W, Van Doren, VE, et al. Mechanical properties and filler fraction of dental composites. Dent Mater. 1989;5:346.
Braga, RR, Ferracane, JL. Alternatives in polymerization contraction stress management. Crit Rev Oral Biol Med. 2004;15:176.
Chantler, PM, Hu, X, Boyd, NM. An extension of a phenomenological model for dental composites. Dent Mater. 1999;15:144.
Choi, KK, Condon, JR, Ferracane, JL. The effects of adhesive thickness on polymerization contraction stress of composite. J Dent Res. 2000;79:812.
Condon, JR, Ferracane, JL. Factors affecting dental composite wear in vitro. J Biomed Mater Res. 1997;38:303.
Condon, JR, Ferracane, JL. In vitro wear of composite with varied cure, filler level, and filler treatment. J Dent Res. 1997;76:1405.
Condon, JR, Ferracane, JL. Reduction of composite contraction stress through non-bonded microfiller particles. Dent Mater. 1998;14:256.
Council on Scientific Affairs. Posterior resin-based composites. J Am Dent Assoc. 1998;129:1627.
Cross, M, Douglas, WH, Fields, RP. The relationship between filler loading and particle-size distribution in composite resin technology. J Dent Res. 1983;62:850.
Dauvillier, BS, Feilzer, AJ, de Gee, AJ, et al. Visco-elastic parameters of dental restorative materials during setting. J Dent Res. 2000;79:818.
DeWald, J, Ferracane, JL. A comparison of four modes of evaluating depth of cure of light-activated composites. J Dent Res. 1987;66:727.
Dietschi, D, Holy, J. A clinical trial of four light-curing posterior composite resins: two-year report. Quint Int. 1990;21:965.
Doray, PG, Wang, X, Powers, JM, et al. Accelerated aging affects color stability of provisional restorative materials. J Prosthodont. 1997;6:183.
El Hejazi, AA, Watts, DC. Creep and visco-elastic recovery of cured and secondary-cured composites and resin-modified glass-ionomers. Dent Mater. 1999;15:138.
Eldiwany, M, Friedl, K-H, Powers, JM. Color stability of light-cured and post-cured composites. Am J Dent. 1995;8:179.
Eldiwany, M, Powers, JM, George, LA. Mechanical properties of direct and post-cured composites. Am J Dent. 1993;6:222.
Farah, JW, Powers, JM. Core materials. Dent Advis. 2004;21(2):1.
Farah, JW, Powers, JM. Layered resin composites. Dent Advis. 2003;20(7):1.
Farah, JW, Powers, JM. Composite finishing and polishing. Dent Advis. 2003;20(6):1.
Farah, JW, Powers, JM. Laboratory composites. Dent Advis. 2005;22(3):1.
Farah, JW, Powers, JM. Microhybrid composites. Dent Advis. 2000;17(10):1.
Farah, JW, Powers, JM. Update: flowable composites. Dent Advis. 2005;22(4):1.
Fay, R-M, Servos, T, Powers, JM. Color of restorative materials after staining and bleaching. Oper Dent. 1999;24:292.
Feilzer, AJ, de Gee, AJ, Davidson, CL. Setting stress in composite resin in relation to configuration of the restoratives. J Dent Res. 1987;66:1636.
Feilzer, AJ, de Gee, AJ, Davidson, CL. Quantitative determination of stress reduction by flow in composite restorations. Dent Mater. 1990;6:167.
Ferracane, JL. Elution of leachable components from composites. J Oral Rehabil. 1994;21:441.
Ferracane, JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302.
Ferracane, JL, Mitchem, JC, Condon, JR, et al. Wear and marginal breakdown of composites with various degrees of cure. J Dent Res. 1997;76:1508.
Ferracane, JL, Moser, JB, Greener, EH. Rheology of composite restoratives. J Dent Res. 1981;60:1678.
Ferracane, JL. Developing a more complete understanding of stresses produced in dental composites during polymerization. Dent Mater. 2005;21:36.
Filho, HN, D’Azevedo, MTFS, Nagem, HD, Marsola, FP. Surface roughness of composite resins after finishing and polishing. Braz Dent J. 2003;14:37.
Gerzina, TM, Hume, WR. Effect of dentine on release of TEGDMA from resin composite in vitro. J Oral Rehabil. 1994;21:463.
Geurtsen, W. Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med. 2000;11:333.
Hanks, CT, Strawn, SE, Wataha, JC, et al. Cytotoxic effects of composite resin components on cultured mammalian fibroblasts. J Dent Res. 1991;70:1450.
Hanks, CT, Wataha, JC, Parsell, RR, et al. Permeability of biological and synthetic molecules through dentine. J Oral Rehabil. 1994;21:475.
Hu, X, Harrington, E, Marquis, PM, et al. The influence of cyclic loading on the wear of a dental composite. Biomaterials. 1999;20:907.
Hu, X, Marquis, PM, Shortall, AC. Two-body in vitro wear study of some current dental composites and amalgams. J Prosthet Dent. 1999;82:214.
Kalachandra, S. Influence of fillers on the water sorption of composites. Dent Mater. 1989;5:283.
Kim, K-H, Park, J-H, Imai, Y, et al. Fracture toughness and acoustic emission behavior of dental composite resins. Engin Fract Mech. 1991;40:811.
Labella, R, Lambrechts, P, Van Meerbeek, B, et al. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dent Mater. 1999;15:128.
Lee, Y-K, Powers, JM. Calculation of colour resulting from composite/compomer layering techniques. J Oral Rehabil. 2004;31:1102.
Lee, Y-K, El Zawahry, M, Noaman, KM, et al. Effect of mouthwash and accelerated aging on the color stability of esthetic restorative materials. Am J Dent. 2000;13:159.
Leinfelder, KF. Posterior composite resins: the materials and their clinical performance. J Am Dent Assoc. 1995;126:663.
Letzel, H. Survival rates and reasons for failure of posterior composite restorations in multicentre clinical trial. J Dent. 1989;17:S10.
Lu, H, Roeder, LB, Powers, JM. Effect of polishing systems on the surface roughness of microhybrid composites. J Esthet Restor Dent. 2003;15:297.
Manhart, J, Kunzelmann, K-H, Chen, HY, et al. Mechanical properties and wear behavior of light-cured packable composite resins. Dent Mater. 2000;16:33.
Mitchem, JC, Gronas, DG. The continued in vivo evaluation of the wear of restorative resins. J Am Dent Assoc. 1985;111:961.
Mitra, SB, Wu, D, Holmes, BN. An application of nanotechnology in advanced dental materials. J Am Dent Assoc. 2003;134:1382.
Ortengren, U, Wellendorf, H, Karlsson, S, et al. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J Oral Rehabil. 2001;28:1106.
Oysaed, H, Ruyter, IE. Water sorption and filler characteristics of composites for use in posterior teeth. J Dent Res. 1986;65:1315.
Paravina, RD, Ontiveros, JC, Powers, JM. Accelerated aging effects on color and translucency of bleaching-shade composites. J Esthet Restor Dent. 2004;16:117.
Paravina, RD, Ontiveros, JC, Powers, JM. Curing-dependent changes in color and translucency parameter of composite bleach shades. J Esthet Restor Dent. 2002;14:158.
Paravina, RD, Roeder, L, Lu, H, et al. Effect of finishing and polishing procedures on surface roughness, gloss and color of resin-based composites. Am J Dent. 2004;17:262.
Park, Y-J, Chae, K-H, Rawls, HR. Development of a new photoinitiation system for dental light-cure composite resins. Dent Mater. 1999;15:120.
Perry, R, Kugel, G, Kunzelmann, K-H, et al. Composite restoration wear analysis: conventional methods vs. three-dimensional laser digitizer. J Am Dent Assoc. 2000;131:1472.
Powers, JM. Lifetime prediction of dental materials: an engineering approach. J Oral Rehabil. 1995;22:491.
Powers, JM, Burgess, JO. Performance standards for competitive dental restorative materials. Trans Acad Dent Mater. 1996;9:68.
Powers, JM, Dennison, JB, Lepeak, PJ. Parameters that affect the color of direct restorative resins. J Dent Res. 1978;57:876.
Powers, JM, Hostetler, RW, Dennison, JB. Thermal expansion of composite resins and sealants. J Dent Res. 1979;58:584.
Powers, JM, Smith, LT, Eldiwany, M, et al. Effects of post-curing on mechanical properties of a composite. Am J Dent. 1993;6:232.
Price, RB, Felix, CA, Andreou, P. Knoop hardness of ten resin composites irradiated with high-power LED and quartz-tungsten-halogen lights. Biomaterials. 2005;26:2631.
Price, RB, Derand, T, Loney, RW, et al. Effect of light source and specimen thickness on the surface hardness of resin composite. Am J Dent. 2002;15:47.
Pratten, DH, Johnson, GH. An evaluation of finishing instruments for an anterior and a posterior composite. J Prosthet Dent. 1988;60:154.
Rathbun, MA, Craig, RG, Hanks, CT, et al. Cytotoxicity of a BIS-GMA dental composite before and after leaching in organic solvents. J Biomed Mater Res. 1991;25:443.
Roeder, LB, Powers, JM. Surface roughness of resin composite prepared by single-use and multi-use diamonds. Am J Dent. 2004;17:109.
Roeder, LB, Tate, WH, Powers, JM. Effect of finishing and polishing procedures on the surface roughness of packable composites. Oper Dent. 2000;25:534.
Sakaguchi, RL, Berge, HX. Reduced light energy density decreases post-gel contraction while maintaining degree of conversion in composites. J Dent. 1998;26:695.
Sakaguchi, RL, Peters, MCRB, Nelson, SR, et al. Effects of polymerization contraction in composite restorations. J Dent. 1992;20:178.
Sakaguchi, RL, Wiltbank, BD, Murchison, CF. Prediction of composite elastic modulus and polymerization shrinkage by computational micromechanics. Dent Mater. 2004;20:397.
Sakaguchi, RL, Wiltbank, BD, Shah, NC. Critical configuration analysis of four methods for measuring polymerization shrinkage strain of composites. Dent Mater. 2004;20:388.
Sakaguchi, RL, Wiltbank, BD, Murchison, CF. Cure induced stresses and damage in particulate reinforced polymer matrix composites: a review of the scientific literature. Dent Mater. 2005;21:43.
Sarrett, DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9.
Sideridou, I, Tserki, V, Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials. 2003;24:655.
Soderholm, K-JM. Leaking of fillers in dental composites. J Dent Res. 1983;62:126.
Soderholm, K-J, Zigan, M, Ragan, M, et al. Hydrolytic degradation of dental composites. J Dent Res. 1984;63:1248.
Stanford, CM, Fan, PL, Schoenfeld, CM, et al. Radiopacity of light-cured posterior composite resins. J Am Dent Assoc. 1987;115:722.
Stansbury, JW, Trujillo-Lemon, M, Lu, H, et al. Conversion-dependent shrinkage stress and strain in dental resins and composites. Dent Mater. 2005;21:56.
Suh, BI, Ferber, C, Baez, R. Optimization of hybrid composite properties. J Esthetic Dent. 1990;2:44.
Tate, WH, Friedl, K-H, Powers, JM. Bond strength of composites to hybrid ionomers. Oper Dent. 1996;21:147.
Tate, WH, Powers, JM. Surface roughness of composites and hybrid ionomers. Oper Dent. 1996;21:53.
Tirtha, R, Fan, PL, Dennison, JB, et al. In vitro depth of cure of photo-activated composites. J Dent Res. 1982;61:1184.
Trajtenberg, CP, Powers, JM. Bond strengths of repaired laboratory composites using three surface treatments and three primers. Am J Dent. 2004;17:123.
Trajtenberg, CP, Powers, JM. Effect of hydrofluoric acid on repair bond strength of a laboratory composite. Am J Dent. 2004;17:173.
Van Dijken, JWV. A clinical evaluation of anterior conventional, microfiller, and hybrid composite resin fillings: a 6-year follow-up study. Acta Odontol Scand. 1986;44:357.
Vandewalle, KS, Ferracane, JL, Hilton, TJ, et al. Effect of energy density on properties and marginal integrity of posterior resin composite restorations. Dent Mater. 2004;20:96.
Wataha, JC, Hanks, CT, Strawn, SE, et al. Cytotoxicity of components of resin and other dental restorative materials. J Oral Rehabil. 1994;21:453.
Weinmann, W, Thalacker, C, Guggenberger, R. Siloranes in dental composites. Dent Mater. 2005;21:68.
Wendt, SL, Jr. The effect of heat used as a secondary cure upon the physical properties of three composite resins. I. Diametral tensile strength, compressive strength, and a marginal dimensional stability. Quint Int. 1987;18:265.
Wendt, SL, Jr., Leinfilder, KF. The clinical evaluation of heat-treated composite resin inlays. J Am Dent Assoc. 1990;120:177.
Xu, HHK. Whisker-reinforced heat-cured dental resin composites: effects of filler level and heat-cure temperature and time. J Dent Res. 2000;79:1392.
Cattani-Lorente, MA, Dupuis, V, Moya, F, et al. Comparative study of the physical properties of a polyacid-modified composite resin and a resin-modified glass ionomer cement. Dent Mater. 1999;15:21.
Albers, HF. Resin polymerization. Adept Report. 2000;6:1.
Farah, JW, Powers, JM. LED light-curing units. Dent Advis. 2004;21(6):1.
Farah, JW, Powers, JM. Light-curing units. Dent Advis. 2002;19(4):1.
Ferracane, JL, Ferracane, LL, Musanje, L. Effect of light activation method on flexural properties of dental composites. Am J Dent. 2003;16:318.
Harrington, E, Wilson, HJ. Determination of radiation energy emitted by light activation. J Oral Rehabil. 1995;22:377.
Jandt, KD, Mills, RW, Blackwell, GB, et al. Depth of cure and compressive strength of dental composites cured with blue light emitting diodes (LEDs). Dent Mater. 2000;16:41.
Kirkpatrick, SJ. A primer on radiometry. Dent Mater. 2005;21:21.
Mills, RW, Jandt, KD, Ashworth, SH. Dental composite depth of cure with halogen and blue light emitting diode technology. Br Dent J. 1999;186:388.
Peutzfeldt, A, Sahafi, A, Asmussen, E. Characterization of resin composites polymerized with plasma arc curing units. Dent Mater. 2000;16:330.
Price, RB, Felix, CA, Andreou, P. Effects of resin composite composition and irradiation distance on the performance of curing lights. Biomaterials. 2004;25:4465.
Price, RB, Ehrnford, L, Andreou, P, et al. Comparison of quartz-tungsten-halogen, light-emitting diode, and plasma arc curing lights. J Adhes Dent. 2003;5:193.
Sakaguchi, RL, Berge, HX. Reduced light energy density decreases postgel contraction while maintaining degree of conversion in composites. J Dent. 1998;26:695.
Sakaguchi, RL, Wiltbank, BD, Murchison, CF. Contraction force rate of polymer composites is linearly correlated with irradiance. Dent Mater. 2004;20:402.
Satrom, KD, Morris, MA, Crigger, LP. Potential retinal hazards of visible light photopolymerization units. J Dent Res. 1987;66:731.
Stahl, F, Ashworth, SH, Jandt, KD, et al. Light emitting diodes (LED) polymerisation of dental composites: flexural properties and polymerisation potential. Biomaterials. 2000;21:1379.
Watts, DC, Al Hindi, A. Intrinsic “soft-start” polymerisation shrinkage-kinetics in an acrylic-based resin-composite. Dent Mater. 1999;15:39.
Wataha, JC, Lockwood, PE, Lewis, JB, et al. Biological effects of blue light from dental curing units. Dent Mater. 2004;20:150.
Chung, K, Lin, T, Wang, F. Flexural strength of a provisional resin material with fibre addition. J Oral Rehabil. 1998;25:214.
Doray, PG, Eldiwany, MS, Powers, JM. Effect of resin surface sealers on improvement of stain resistance for a composite provisional material. J Esthet Restor Dent. 2003;15:244.
Doray, PG, Li, D, Powers, JM. Color stability of provisional restorative materials after accelerated aging. J Prosthodont. 2001;10:212.
Farah, JW, Powers, JM. Provisional materials. Dent Advis. 2002;19(9):1.
Grajower, R, Shaharbani, S, Kaufman, E. Temperature rise in pulp chamber during fabrication of temporary self-curing resin crowns. J Prosthet Dent. 1979;41:535.
Ireland, MF, Dixon, DL, Breeding, LC, et al. In vitro mechanical property comparison of four resins used for fabrication of provisional fixed restorations. J Prosthet Dent. 1998;80:158.
Lepe, X, Bales, DJ, Johnson, GH. Retention of provisional crowns fabricated from two materials with the use of four temporary cements. J Prosthet Dent. 1999;81:469.
Lui, JL. Hypersensitivity to a temporary crown and bridge material. J Dent. 1979;7:22.
Robinson, FB, Hovijitra, S. Marginal fit of direct temporary crowns. J Prosthet Dent. 1982;47:390.