Ceramic-Metal Systems
All-ceramic anterior restorations can appear very natural. Unfortunately, the ceramics used in these restorations are brittle and subject to fracture from high tensile stresses. Conversely, all-metal restorations are strong and tough but, from an esthetic viewpoint, acceptable only for posterior restorations. Fortunately, the esthetic qualities of ceramic materials can be combined with the strength and toughness of metals to produce restorations that have both a natural toothlike appearance and very good mechanical properties. As a result they are more successful as posterior restorations than all-ceramic crowns. A cross-section of a ceramic-metal anterior crown is shown in Fig. 19-1. The cast metal coping provides a substrate on which a ceramic coating is fused. The ceramics used for these restorations are porcelains, hence the common name porcelain-fused-to-metal restorations. These ceramic-metal restorations are highly popular and are used for most of the crown and bridge restorations made today.
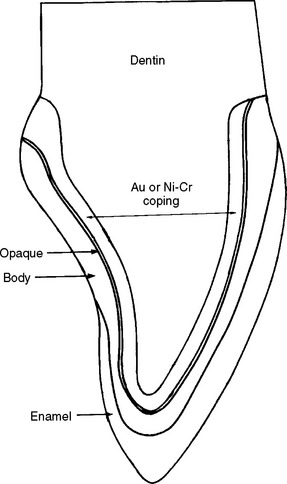
FIGURE 19-1 Cross-section of a ceramic-metal crown showing a gold alloy or base metal coping, the opaque body (dentin), and enamel ceramic layers.
REQUIREMENTS FOR A CERAMIC-METAL SYSTEM
1. The alloy must have a high melting temperature. The melting range must be substantially higher (>100° C) than the firing temperature of the ceramic and solders used to join segments of a bridge.
2. The ceramic must have a low fusing temperature. The fusing temperature must be lower than those of ceramics used for all-ceramic restorations so that no distortion of the coping takes place during fabrication.
3. The ceramic must wet the alloy readily when applied as a slurry in order to prevent voids forming at the interface. In general, the contact angle should be 60 degrees or less.
4. A good bond between the ceramic and metal is essential and is achieved by the interactions of the ceramic with metal oxides on the surface of metal (Fig. 19-2) and by the roughness of the metal coping.
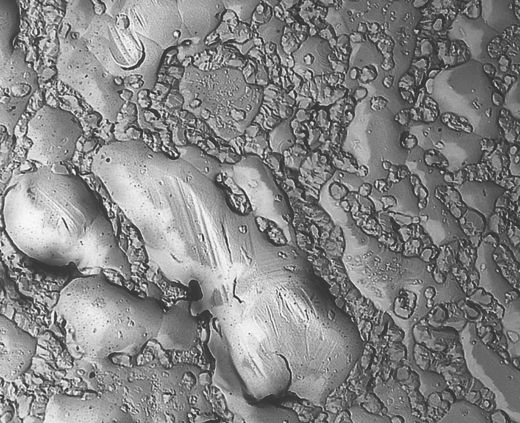
FIGURE 19-2 Electron micrograph of replicated oxidized surface of a Au-Pt-Pd alloy (×8000). (From Kelly M, Asgar K, O’Brien WJ: J Biomed Mater Res 3:403, 1969.)
5. Coefficients of thermal expansion of the ceramic and metal must be compatible so that the ceramic does not crack during fabrication. The system is designed so the value for the metal is slightly higher than for the ceramic, thus putting the ceramic in compression (where it is stronger) following cooling (Fig. 19-3).
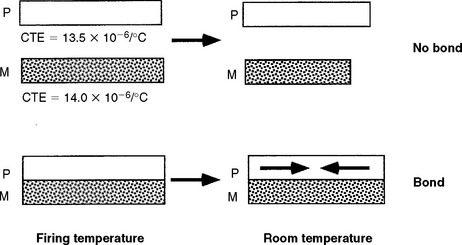
FIGURE 19-3 Diagram of the ceramic-metal bond at the firing temperature and at room temperature when the thermal coefficient of expansion of the metal is 0.5 × 106/° C greater than the ceramic, thus placing the ceramic in compression at room temperature. (From Craig RG, Powers JM, Wataha JC: Dental materials: properties and manipulation, ed 8, St Louis, 2004, Mosby.)
6. Adequate stiffness and strength of the alloy core are especially important for fixed bridges and posterior crowns. High stiffness in the alloy reduces stresses in the ceramic by reducing deflection and strain. High strength is essential in the interproximal regions in fixed bridges.
7. High sag resistance is essential. The alloy copings are relatively thin; no distortion should occur during firing of the ceramic, or the fit of the restoration will be compromised.
8. An accurate casting of the metal coping is required even with the higher melting range of the alloy.
9. Adequate design of the restoration is critical. The preparation should provide for adequate thickness of alloy (see item 5) as well as enough space for an adequate thickness of ceramic to yield an esthetic restoration. In some instances, a ceramic-metal restoration has an advantage over an all-ceramic restoration because less tooth structure needs to be removed to provide adequate bulk for the all-ceramic restoration. However, in cases of small, lower, anterior teeth, an all-ceramic restoration has an advantage with respect to esthetics, because with a ceramic-metal restoration it is difficult to remove enough tooth structure to provide space for the coping and the esthetic ceramic layer. The geometry of the shoulder should be flat with a rounded angle to allow enough bulk of ceramic and to avoid fracture in this area. If full ceramic coverage is not used (e.g., a metal occlusal surface), the position of the ceramic-metal joint should be located as far as possible from areas of contact with opposing teeth.
CERAMIC-METAL BONDING
The bond strength between the ceramic and metal is perhaps the most important requirement. In general, the bond is a result of chemisorption by diffusion between the surface oxides on the alloy and in the ceramic. These oxides are formed during wetting of the alloy by the ceramic and firing of the ceramic. The most common mechanical failure of these restorations is ceramic debonding from the metal. Many factors control ceramic-metal adhesion: the formation of strong chemical bonding, mechanical interlocking between the two materials, and residual stresses. In addition, as noted earlier, the ceramic must wet and fuse to the surface to form a uniform interface with no voids. These factors are also important for ceramic coatings on metallic implants.
An interface between a metal and a ceramic with many strong chemical bonds between them, with the bonds acting as tags that hold the two materials together, would obviously lead to strong bonding. However, methods producing a ceramic-metal interface with strong chemical bonding have not been developed. The formation of oxides on the surface of the metal has been proved to contribute to the formation of strong bonding. Noble metals, which are resistant to oxidizing, must have other, more easily oxidized elements added, such as indium (In) and tin (Sn), to form surface oxides. When these more easily oxidized elements are added, bonding is improved. The common practice of “degassing” or preoxidizing the metal coping before ceramic application creates surface oxides that improve bonding.
Base-metal alloys contain elements, such as nickel (Ni), chromium (Cr), and beryllium (Be), that form oxides easily during degassing, and care must be taken to avoid too thick an oxide layer. Manufacturers specify conditions to form the optimal oxide and often indicate the color of the oxide. Oxides rich in NiO tend to be dark gray, whereas those rich in Cr2O3 are greenish. These oxides dissolve in the ceramic during fixing and may discolor it or be visible through thin layers of ceramic near the gingival edge of the restoration.
The oxides are not completely dissolved during the fusion of the ceramic, and thus, the oxide-alloy interface can be the site of mechanical failure. This situation is especially true with some alloys that form layers rich in Cr2O3, which does not adhere well to the alloy. These alloys typically require the application of a bonding agent to the alloy surface to modify the type of oxide formed.
Alloys containing Be normally form welladhering oxides. BeO is a slow-growing oxide that does not delaminate from the surface of the alloy. Rare-earth elements such as yttrium can be added to the alloy to improve adherence, tying the alloy to the oxide layer.
From both theoretical and practical standpoints, the roughness of a ceramic-metal interface has a large effect on adhesion. The ceramic penetrating into a rough metal surface can mechanically interlock with the metal, like Velcro, improving adhesion. The increased area associated with a rougher interface also provides more room for chemical bonds to form. However, rough surfaces can reduce adhesion if the ceramic does not penetrate into the surface and voids are present at the interface; this may happen with improperly fired porcelain or metals that are poorly wetted by the porcelain. Airborne particle abrasion is often used to remove excess oxide and to roughen the surface of the metal coping to improve the bonding of the ceramic.
High residual stresses between the metal and ceramic can lead to failure. If the metal and ceramic have different thermal expansion coefficients, the two materials will contract at different rates during cooling and strong residual stresses will form across the interface. If these stresses are strong enough, the ceramic on the restoration will crack or separate from the metal. Even if the stresses are less strong and do not cause immediate failure, they can still weaken the bond. To avoid these problems the ceramics and alloys are formulated to have closely matched thermal expansion coefficients. Most porcelains have coefficients of thermal expansion between 13.0 and 14.0 × 10−6/° C, and metals between 13.5 and 14.5 × 10−6/° C. The difference of 0.5 × 10−6/° C in thermal expansion between the metal and ceramic causes the metal to contract slightly more than does the ceramic during cooling after firing. This condition puts the ceramic under slight residual compression, which makes it less sensitive to tensile stress induced by mechanical loading.
Wetting is important to the formation of good ceramic-metal bonding. During firing, the ceramic must wet and flow over the metal surface. The contact angle between the ceramic and metal is a measure of the wetting and, to some extent, the quality of the bond that forms. The wetting of the alloy surface by the fused ceramic indicates an interaction between surface atoms in the metal with the ceramic. Low contact angles indicate good wetting. The contact angle of ceramic on a gold (Au) alloy is about 60 degrees. The surface of the noble alloys containing Sn and In after heating have these oxides present, and they diffuse into and interact with the ceramic, forming an adhesive bond. The oxide surface of a Au-platinum (Pt)-palladium (Pd) 98% noble alloy is shown at high magnification in Fig. 19-2.
EVALUATION OF CERAMIC-METAL BONDING
Many tests have been used to determine the bond strength between ceramics and metals; however, the ideal test currently does not exist. In addition, data obtained from different tests are often not comparable. One of the established bond strength tests is the planar shear test. Other commonly used tests are the flexural tests. The flexural tests require layers of ceramic to be bonded to a strip or plate of metal. The coated metal plate is flexed in a controlled manner until the ceramic fractures. In the 3-point flexure test, ceramic is fired to one side of a rectangular strip of metal. The ceramic-metal strip is supported by two knife edges, and the specimen is loaded in the center with the ceramic surface down until failure occurs.
An adequate bond occurs when the fracture stress is above 25 MPa; however, with many ceramic-metal systems values of 40 to 60 MPa are common. In a variant of this test, opaque and body ceramics are applied and fired to a thickness of approximately 1 mm on a 20-mm × 5-mm × 0.5-mm alloy sheets. The specimen is then bent over a 1-cm-diameter rod (with the ceramic on the outside) and then straightened. The surface is viewed under low magnification and the percentage of the surface with retained ceramic is reported. Tests based on tensile and torsional loading schemes have also been used.
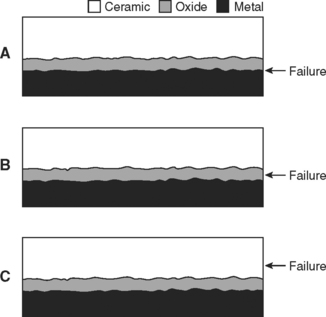
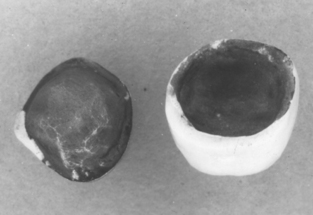
A ceramic-metal bond may fail in any of three possible locations (Fig. 19-4). Knowing the location of the fracture provides considerable information. The highest strength ceramic-metal specimens will fracture in the ceramic when tested (see Fig. 19-4, C); this is observed with some alloys that were properly prepared and had ceramic applied and fused. Testing these high-strength specimens using the push-through shear test shows the maximum strengths are approximately the same as the shear strength of the ceramic. Fractures through the oxide (see Fig. 19-4, B) and metal-metal oxide fracture (see Fig. 19-4, A) are commonly observed with poor bonding. Base-metal alloys commonly fracture through the oxide (Fig. 19-5) if an excessively thick oxide layer is present. Interfacial fracture is observed with metals that are resistant to forming surface oxides, such as pure gold or platinum.
CERAMICS FOR CERAMIC-METAL RESTORATIONS
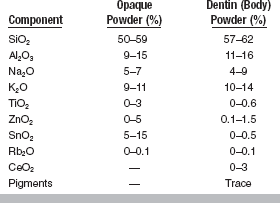
The ceramics used for ceramic-metal restorations must fulfill five requirements: (1) they must simulate the appearance of natural teeth, (2) they must fuse at relatively low temperatures, (3) they must have thermal expansion coefficients compatible with the metals used for ceramic-metal bonding, (4) they must withstand the oral environment, and (5) they must not unduly abrade opposing teeth. The ceramic is carefully formulated to achieve these requirements. These ceramics are composed of crystalline phases in an amorphous and glassy (vitreous) matrix. They comprise primarily SiO2, Al2O3, Na2O, and K2O (Table 19-1). Opacifiers (TiO2, ZrO2, SnO2) and various heat-stable pigments are also added to the ceramic. Because of their composition, they can be considered a type of glass. To match the appearance of tooth structures, small amounts of fluorescing pigments such as rare earth oxides (CeO2) are added. The nature of ceramics, with their glassy matrix and crystalline phases, produces a translucency much like that of teeth, whereas pigments and opacifiers control the color and translucency of the restoration. The ceramic is supplied as a fine powder.
In developing ceramics for ceramic-metal bonding, a major breakthrough was formulating products that had sufficiently high thermal expansion coefficients to match those of dental alloys. This higher expansion was made possible by the addition of potassium oxide and the formation of a high-expansion phase called leucite (KAlSi2O6). This phase increased the thermal expansion of the porcelain so it could match that of dental alloys.
These materials have other qualities that make them well suited for ceramic-metal restorations. They fuse at lower temperatures than do many other ceramic materials, lessening the potential of distorting the metal coping. Sodium and potassium oxides in the glassy matrix are responsible for lowering the fusing temperatures to the range 930° to 980° C; low-fusing ceramics have hydroxyl groups and more Na2O to lower fusing temperatures to as low as 660° C. Recently, ceramics having a low fusing temperature (760° to 780° C) and high coefficient of thermal expansion (15.8 × 10−6/° C) have become available. This material is designed to be compatible for bonding to yellow high-Au alloys, which have coefficients of thermal expansion between 16.1 and 16.8 × 10−6/° C). These ceramics can, however, be abrasive to opposing teeth because of their hardness; this becomes a significant problem if the ceramic surface is rough due to improper processing or becomes rough in the oral environment. Newer products have been shown to be less abrasive to natural teeth. These new ceramics are also strong in compression, which permits their use on the occlusal surfaces of the restorations. The ceramics used to bond to metals have tensile strengths of 35 MPa, compressive strengths of 860 MPa, shear strengths of 120 MPa, and flexural strengths of 60 MPa.
ALLOYS FOR CERAMIC-METAL RESTORATIONS
Chronologically the alloys developed for ceramic-metal restorations were Au-Pt-Pd, Ni-Cr, Co-Cr, Au-Pd-Ag, Pd-Ag, Au-Pd, Pd-Cu, and Ti.
COMPOSITION AND PROPERTIES OF NOBLE-METAL ALLOYS
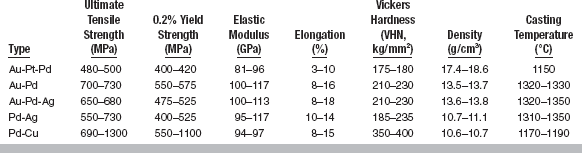
The composition ranges and colors of five types of noble alloys for ceramic-metal restorations are listed in Table 19-2. The properties of these alloys are given in Table 19-3.
Au-Pt-Pd Alloys
These alloys contain very high noble-metal content, mainly gold with platinum and palladium to increase the melting range. The high-noble content provides good corrosion resistance. Indium, tin, and iron (Fe) are present and form oxides to produce a ceramic-metal bond. Rhenium (Re) is added as a grain refiner. Hardening of Au-Pt-Pd alloys results from solid solution hardening and the formation of a FePt3 precipitate. Optimum heat treatment for hardening is 30 minutes at 550° C, but practically the hardening occurs during firing of the ceramic. During the casting of these alloys some of these base elements are lost; it is therefore recommended that 50% new alloy be used with a sprue button if it is used to make a second casting. The new alloy will provide enough of the base elements so adequate oxides and hardening result.
From Table 19-3 it is seen that these alloys have high stiffness (elastic modulus), strength, and hardness and reasonable elongation; however, they have somewhat low sag resistance. The alloys are very costly because of their high noble-metal content and high density; they are sold on a weight basis but used on a volume basis. The casting temperature is reasonably high, and although reasonably easy to solder, care must be taken because the soldering temperature is only about 50° C below the melting range of the alloys. Finally, although considerable platinum and palladium are present, these alloys are still yellow, which makes producing pleasing esthetics with the ceramic easier than with white alloys.
Au-Pd Alloys
These high-noble alloys with good corrosion resistance have decreased gold but increased palladium content. These alloys contain no platinum or iron and thus are solution-rather than precipitation-hardened. They contain indium for bonding, gallium (Ga) to decrease the fusion temperature, rhenium for grain refining, and ruthenium (Ru) for castability. Be-cause of their high palladium content, the alloys are white (some call it gray) rather than yellow, even though they contain about 50% gold. This color causes increased difficulty in producing esthetic restorations.
These alloys are stronger, stiffer, and harder than the Au-Pt-Pd alloys and have higher elongation (more ductile) and casting temperatures (easier to solder). They have lower densities, and when introduced they were less costly than the Au-Pt-Pd alloys, because palladium was cheaper than gold. With palladium being more costly than gold, there is no longer a cost advantage. The decrease in density indicates more care should be taken during casting because of the decrease in the force with which the alloy enters the casting ring. However, these alloys are easy to cast, and soldering is easy because of the higher casting temperature.
Au-Pd-Ag Alloys
These alloys contain less palladium than the Au-Pd alloys; the decrease is made up by adding silver. However, they still have good corrosion resistance. Again, indium and tin are added for bonding with the ceramics, ruthenium for castability, and rhenium for grain refining. Hardening results from solution hardening. As seen in Table 19-3, the properties of the Au-Pd-Ag alloys are similar to those of the Au-Pd alloys.
Pd-Ag Alloys
These alloys, which contain no gold and have a moderately high silver content, have the lowest noble-metal content of the five noble alloys. They contain indium and tin for bonding and ruthenium for castability. Their properties are similar to those of the Au-Pd-Ag alloys, except they are less dense (∼11 g/cm3 versus 14 g/cm3). They were developed at a time when the cost of gold was about $800/oz and the cost of palladium was low; those conditions no longer exist. Some ceramics used with these high-gold alloys resulted in what was called “greening,” really a color shift toward yellow. Contamination and technique were blamed to some extent for this problem.
Pd-Cu Alloys
These alloys contain very high palladium content with 10% to 15% copper. They contain indium for bonding and gallium for controlling casting temperature. These alloys have high strength and hardness, moderate stiffness and elongation, and low density. However, they have low sag resistance and form dark oxides. They are white alloys, like all the other metals except the yellow Au-Pt-Pd alloys.
COMPOSITION AND PROPERTIES OF BASE-METAL ALLOYS
The range of compositions of base metal alloys for ceramic-metal restorations are given in Table 19-4, and typical properties of these alloys are listed in Table 19-5. Considerable variation in composition and properties are shown in these tables.
Ni-Cr Alloys
Chromium provides tarnish and corrosion resistance, whereas alloys containing aluminum (Al) are strengthened by the formation of coherent precipitates of Ni3Al. Molybdenum (Mo) is added to decrease the thermal coefficient of expansion, and beryllium to improve castability (by decreasing the melting range) and hardening. Note that because of the wide differences in atomic weight of beryllium, nickel, and chromium, 2 wt% is roughly equal to 6 at%. The use of beryllium may cause some toxicity problems and surface oxidation at high temperatures.
These alloys are harder than noble alloys but usually have lower yield strengths. They also have higher elastic moduli, and it was hoped thinner copings and frameworks could result. They have much lower densities (7 to 8 g/cm3) and generally higher casting temperatures. Adequate casting compensation is at times a problem, as is the fit of the coping.
Co-Cr Alloys
Again, chromium provides tarnish and corrosion resistance. Unlike Co-Cr partial-denture alloys, the alloys for ceramic-metal restorations are strengthened by solution hardening rather than carbide formation. Molybdenum helps lower the coefficient of expansion, and ruthenium improves castability. Co-Cr alloys are stronger and harder than noble and Ni-Cr alloys and have roughly the same densities and casting temperature as Ni-Cr alloys. Casting and soldering of these alloys is more difficult than for noble alloys, as is obtaining a high degree of accuracy in the castings.
Ti and Ti Alloys
Pure titanium (Ti) and titanium alloyed with aluminum and vanadium (Ti-6Al-4V) may become important for cast restorations in the future, but they are currently important only in implant and orthodontic wire applications. They have superior biocompatibility compared with the other base metal alloys, but Ti and Ti-6Al-4V present processing difficulties, as indicated by their casting temperatures of 1760° to 1860° C, low densities, and ease of oxidation. Newer techniques, such as machine duplication and spark erosion to fabricate copings, may increase the use of these metals.
In summary, the noble alloys have good corrosion resistance, but only the Au-Pt-Pd alloys have a desirable yellow color. The other metals are white (gray), which is more difficult to mask with the ceramic. The Au-Pd, Au-Pd-Ag, and Pd-Ag alloys have excellent mechanical properties coupled with high fusion temperatures and ease of casting and soldering. However, the Pd-Ag alloys have caused some problems with discoloration of the ceramic. The Pd-Cu alloys are characterized by the formation of dark oxides, which may cause problems with masking by the ceramic. The Ni-Cr and Co-Cr alloys are noted for high hardness and elastic modulus, although some Ni-Cr alloys have lower yield strengths. They are also noted for high casting temperatures. In general titanium and its alloys have superior biocompatibility compared with the other base metal alloys but are difficult to process. Good bonding of ceramic and alloy can be achieved with all alloys, but bonding with some base metal alloys is more technique sensitive.
PREPARATION OF CERAMIC-METAL RESTORATIONS
The processing of the metal coping for ceramic-metal restorations is much like that of all-metal crowns and bridges. One significant difference relates to the reuse of metal. As mentioned earlier, when the metal is melted and cast, certain alloying elements can be lost, especially the elements that readily form oxides. These elements are important for bonding of noble-metal alloys. To conserve metal, portions of the casting are commonly remelted. Each time the metal is remelted, some of these easily oxidized elements are lost. Therefore, a certain portion of new alloy (usually half) should be added each time the metal is reused to replenish the lost alloying elements.
Surface treatment of the metal coping before ceramic application is important for good bonding. These treatments are used to roughen the coping surface and form surface oxides. The surface may be roughened by abrading with airborne particles (25 to 50 μm alumina); in some cases this results in a large increase in bond strength. In most cases the metal coping is heat-treated either in air or under partial vacuum to produce a surface oxide to improve bonding. In some Pd alloys, the heat treatment forms not only surface oxides but also internal oxides that penetrate the metal from the surface and effectively roughen the surface, thereby improving bonding. Some base-metal alloys tend to form excessively thick interfacial oxides, which weaken the ceramic-metal bond (see Fig. 19-5). With these alloys, the coping is heat-treated and then air abraded to remove enough oxide to achieve higher bonding. If this process is not used, failure through the oxide may occur.
Ceramic application is similar to that described in Chapter 18. However, there are several important considerations. The first layer of ceramic is especially important with ceramic-metal restorations because it must hide the metal; special opaque ceramic must be used (see Table 19-1). After the opaque layer has been applied and fired the dentin (or body) ceramic, which contains less of the opaque oxides (such as SnO2 and ZnO2), pigments, and fluorescing oxides, is applied and fired. Finally, once the correct contour has been established, an essentially transparent glaze layer is applied and fired. The ceramic-alloy compatibility is another important consideration. As pointed out earlier, the thermal expansion must be matched and the ceramic firing temperature must be low enough that the alloy will not sag; the manufacturer usually supplies compatibility information. Ti alloys require special ceramics, otherwise processing of the ceramic is similar to that of the other alloys.
EFFECT OF DESIGN ON CERAMIC-METAL RESTORATIONS
Because ceramics are weak in tension and can withstand very little strain before fracturing, the alloy copings must be rigid to minimize deformation of the ceramic. However, the coping should be as thin as possible to allow space for the ceramic to hide the color of the alloy without overcontouring the ceramic. This consideration is especially true for alloys that are white (gray). This might lead to the conclusion that Ni-Cr or Co-Cr alloys would be superior to the noble alloys because their moduli (stiffness) are 1.5 to 2 times greater and the thickness of the coping could be halved. However, loading the restoration places it in bending, and the bending deflection is a function of only the first power of the modulus, whereas it is a function of the cube of the thickness. It can be shown that for a typical dental ceramic-metal restoration, the thickness of a base-metal coping can be reduced only about 7% because of the higher elastic modulus. Thus, the advantage of the higher modulus for the base-metal alloys is minimal.
The labial margin of ceramic-metal prostheses is a critical area regarding design because there is little thickness at the margin to mask the appearance of the metal coping or to resist fracture of the ceramic. When there is confidence in the impression accuracy, the shoulder of the crown should be flat with a rounded angle. This “butt-joint” design provides for bulk of ceramic and minimizes fracture compared with a shoulderless “knife-edge” geometry. However, a ceramic butt-joint design should not be used when the impression is incomplete or has defects. If the shoulder is built too low, a premature contact in the margin area can cause fracture formation under the induced flexural stress. A beveled margin provides tolerance for impression inaccuracy and variation in cement layer thickness. In any case, sharp angles in the ceramic are to be avoided.
When using partial coverage by ceramics, such as when a metal occlusal surface is desired, the position of the ceramic-metal joint is critical. Because of the large difference in modulus of the ceramic and metal, stresses occur at the interface when the restoration is loaded. These stresses can be minimized by placing the ceramic-metal joint as far away as possible from contact with opposing teeth.
In the design of a ceramic-metal bridge, the geometry of the interproximal area between the crown and pontic is critical. The occlusal-gingival thickness of the joint should be as long as clinically feasible; because deflection is decreased as the cube of the thickness, greater thickness will minimize deflection of the ceramic. It should be remembered that the bridge is not a uniform beam; maximum deflection on loading will occur at the thinnest cross-section, the interproximal area.
FAILURE AND REPAIR OF CERAMIC-METAL RESTORATIONS
Ceramic-metal restorations remain the most popular materials combination selected for crown and bridge applications. Despite the challenges inherent in achieving acceptable bonding between ceramics and metal alloys, ceramic-metal crowns have a 10-year success rate of over 80% for vital teeth and over 50% for nonvital teeth. The majority of re-treatments are due to biological failures, such as tooth fracture, periodontal disease, and secondary caries. Prosthesis fracture and esthetic failures account for only 20% of re-treatment cases for single-unit restorations. For metal-ceramic bridges, prosthesis fracture is the most common reason for re-treatment, with long bridges (five or more units) having approximately twice the incidence of failure as short bridges.
When metal-ceramic prosthesis fracture does occur it is usually a case of adhesive failure between the ceramic and coping or within the ceramic near the ceramic-metal interface. Ideally, debonded surfaces should be cleaned, and a new oxide layer should be formed on the exposed area of metal for fusing to the ceramic. However, this cannot be achieved intraorally, and removal of the prosthesis is both unpleasant for the patient and time consuming. Thus, a variety of techniques have been developed for re-attaching ceramic fragments using dental composite. Any of these techniques present the challenge of bonding chemically dissimilar materials. A silane bonding agent can be used to achieve good adhesion between the composite and porcelain; however, metal alloys have no such bonding agent. Acid etching and priming with organic sulfur compounds have proved to be ineffective. Therefore, systems are available for coating the metal surface with silica particles through airborne particle abrasion. The particles are embedded in the metal surface upon im-pact, then a silane bonding agent can be applied. Alternatively, base metal alloys can be coated with tin followed by the application of an acidic primer. Both methods achieve high bond strength and may delay the eventual need for remaking the prosthesis.
Anthony, DH, Burnett, AP, Smith, DL, et al. Shear test for measuring bonding in cast gold alloy-porcelain composites. J Dent Res. 1970;49:27.
Anusavice, KJ. Reducing the failure potential of ceramic-based restorations. Part 1: Metal-ceramic crowns and bridges. Gen Dent. 1996;44:492.
Anusavice, KJ, Dehoff, PH, Fairhurst, CW. Comparative evaluation of ceramic-metal bond tests using finite element stress analysis. J Dent Res. 1980;59:608.
Anusavice, KJ, Hojjatie, B. Stress distribution in metal-ceramic crowns with a facial porcelain margin. J Dent Res. 1987;66:1493.
Anusavice, KJ, Ringle, RD, Fairhurst, CW. Bonding mechanism evidence in a ceramic non-precious alloy system. J Biomed Mater Res. 1977;11:701.
Anusavice, KJ, Ringle, RD, Fairhurst, CW. Identification of fracture zones in porcelain-veneered-to-metal bond test specimens by ESCA analysis. J Prosthet Dent. 1979;42:417.
Anusavice, KJ, Ringle, RD, Morse, PK, et al. A thermal shock test for porcelain metal systems. J Dent Res. 1981;60:1686.
Baran, GR. Phase changes in base metal alloys along metal-porcelain interfaces. J Dent Res. 1979;58:2095.
Baran, GR. Oxide compounds on Ni-Cr alloys. J Dent Res. 1984;63:1332.
Baran, GR, Meraner, M, Farrell, P. Transient oxidation of multiphase Ni-Cr base alloys. Oxides of Metals. 1988;29:409.
Baran, GR, Woodland, EC. Forming of cast precious metal alloys. J Dent Res. 1981;60:1767.
Bartolotti, RL, Moffa, JP. Creep rate of porcelain-bonding alloys as a function of temperature. J Dent Res. 1980;59:2062.
Butel, EM, DiFiore, PM. Crown margin design: a dental school survey. J Prosthet Dent. 1991;65:303.
Council on Dental Materials and Devices. How to avoid problems with porcelain-fused-to-metal restorations. J Am Dent Assoc. 1977;95:818.
Dent, RJ, Preston, JD, Moffa, JP, et al. Effect of oxidation on ceramometal bond strength. J Prosthet Dent. 1982;47:59.
Donachie MJ, Jr., eds. Titanium, a technical guide. Metals Park, OH: ASM International, 1988.
Donovan, T, Prince, J. An analysis of margin configurations for metal-ceramic crowns. J Prosthet Dent. 1985;53:153.
Dorsch P, Ingersoll C: A review of proposed standards for metal-ceramic restorations, Ivoclar-Vivadent Report, Ivoclar AG, Schoen, Liechtenstein, Feb 4, 1988.
Duncan, JD. Casting accuracy of nickel-chromium alloys: marginal discrepancies. J Dent Res. 1980;59:1164.
Duncan, JD. The casting accuracy of nickel-chromium alloys for fixed prostheses. J Prosthet Dent. 1982;47:63.
Duncanson, MG, Jr. Nonprecious metal alloys for fixed restorative dentistry. Dent Clin North Am. 1976;20:422.
Eden, GT, Franklin, OM, Powell, JM, et al. Fit of porcelain fused-to-metal crown and bridge casting. J Dent Res. 1979;58:2360.
Fairhurst, CW, Anusavice, KJ, Hashinger, DT, et al. Thermal expansion of dental alloys and porcelains. J Biomed Mater Res. 1980;14:435.
Farah, JW, Craig, RG. Distribution of stresses in porcelain-fused-to-metal and porcelain jacket crowns. J Dent Res. 1975;54:255.
Faucher, RR, Nicholls, JI. Distortion related to margin design in porcelain-fused-to-metal restorations. J Prosthet Dent. 1980;43:149.
German, RM. Hardening reactions in a high-gold content ceramo-metal alloy. J Dent Res. 1980;59:1960.
Haselton, DR, Diaz-Arnold, AM, Dunne, JT. Shear bond strengths of 2 intraoral porcelain repair systems to porcelain or metal substrates. J Prosthet Dent. 2001;86:526.
Huget, EF, Dvivedi, N, Cosner, HE, Jr. Characterization of gold-palladium-silver and palladium-silver for ceramic-metal restoration. J Prosthet Dent. 1976;36:58.
Huget, EF, Dvivedi, N, Cosner, HE, Jr. Properties of two nickel-chromium crown and bridge alloys for porcelain veneering. J Am Dent Assoc. 1977;94:87.
Jones, DW, Coatings of ceramics on metals. Ducheyne, P, Lemons, JE, eds. Bioceramics: materials characteristics versus in vivo behavior, 523. New York: New York Academy of Science, 1988.
Könönen, M, Kivilahti, J. Bonding of low-fusing dental porcelain to commercially pure titanium. J Biomed Mater Res. 1994;28:1027.
Lautenschlager, EP, Greener, EH, Elkington, WE. Microprobe analysis of gold-porcelain bonding. J Dent Res. 1969;48:1206.
Leibrock, A, Degenhart, M, Behr, M, et al. In vitro study of the effect of thermo- and load-cycling on the bond strength or porcelain repair systems. J Oral Rehabil. 1999;26:130.
Lenz, J, Schwarz, S, Schwickerath, H, et al. Bond strength of metal-ceramics systems in three-point flexure bond test. J Appl Biomater. 1955;6:55.
Lubovich, RP, Goodkind, RJ. Bond strength studies of precious, semiprecious, and non-precious ceramic-metal alloys with two porcelains. J Prosthet Dent. 1977;37:288.
Mackert, JR, Jr., Twiggs, SW, Evans-Williams, AL. Isothermal anneal effect on leucite content in dental porcelains. J Dent Res. 1995;74:1259.
Malhotra, ML, Maickel, LB. Shear bond strength of porcelain-fused-to-alloys of varying noble metal contents. J Prosthet Dent. 1980;44:405.
Meyer, JM, Payan, J, Nally, JM. Evaluation of alternative alloys to precious ceramic alloys. J Oral Rehabil. 1979;6:291.
Näpänkangas, R, Salonen-Kemppi, MAM, Raustia, AM. Longevity of fixed metal ceramic bridge prostheses: a clinical follow-up study. J Oral Rehabil. 2002;29:140.
O’Brien, WJ. Ceramics. Dent Clin North Am. 1985;29:851.
Ohno, H, Kanzawa, I, Kawashima, I, et al. Structure of high-temperature oxidation zones of gold alloys for metal-porcelain bonding containing small amounts of In and Sn. J Dent Res. 1983;62:774.
Özcan, M, Niedermeier, W. Clinical study on the reasons for and location of failures of metal-ceramic restorations and survival of repairs. Int J Prosthodont. 2002;15:299.
Ringle, RD, Fairhurst, CW, Anusavice, KJ. Microstructures in non-precious alloys near the porcelain-metal interaction zone. J Dent Res. 1979;58:1987.
Robin, C, Scherrer, SS, Wiskott, HWA, et al. Weibull parameters of composite resin bond strengths to porcelain and noble alloy using the Rocatec system. Dent Mater. 2002;18:389.
Saxton, PL. Post soldering of non-precious alloys. J Prosthet Dent. 1980;43:592.
Shell, JS, Nielsen, JP. Study of the bond between gold alloys and porcelain. J Dent Res. 1962;41:1424.
Shimoe, S, Tanoue, N, Yanagida, H, et al. Comparative strength of metal-ceramic and metal-composite bonds after extended thermocycling. J Oral Rehabil. 2004;31:689.
Smith, DL, Burnett, AP, Brooks, MS, Anthony, DH. Iron-platinum hardening in casting golds for use with porcelain. J Dent Res. 1970;49:283.
Valega TM, ed.. Alternatives to gold alloys in dentistry proceedings of a conference held at NIH, January. DHEW Publication No (NIH): Bethesda, MD, 1977:77–1227.
Vermilyea, SG, Huget, EF, Vilca, JM. Observations on gold-palladium-silver and gold-palladium alloys. J Prosthet Dent. 1980;44:294.
Walton, TR. A 10-year longitudinal study of fixed prosthodontics: clinical characteristics and outcome of single-unit metal-ceramic crowns. Int J Prosthodont. 1999;12:519.